Abstract
A series of sixteen β-carbolines, bearing chalcone moiety at C-1 position, were prepared from easily accessible 1-acetyl-β-carboline and various aldehydes under basic conditions followed by N2-alkylation using different alkyl bromides. The prepared compounds were evaluated for in vitro cytotoxicity against a panel of human tumor cell lines. N2-Alkylated-β-carboline chalcones 13a–i represented the interesting anticancer activities compared to N2-unsubstituted β-carboline chalcones 12a–g. Off the prepared β-carbolines, 13g exhibited broad spectrum of activity with IC50 values lower than 22.5 μM against all the tested cancer cell lines. Further, the N2-alkylated-β-carboline chalcone 13g markedly induced cell death in MDA-MB-231 cells by AO/EB staining assay. The most cytotoxic compound 13g possessed a relatively high drug score of 0.48. Additionally, the prepared β-carboline chalcones displayed moderate antibacterial activities against tested bacterial strains.
Keywords: β-Carboline chalcones, β-Carbolinium chalcone bromides, Anticancer activity, Apoptosis, Antibacterial activity
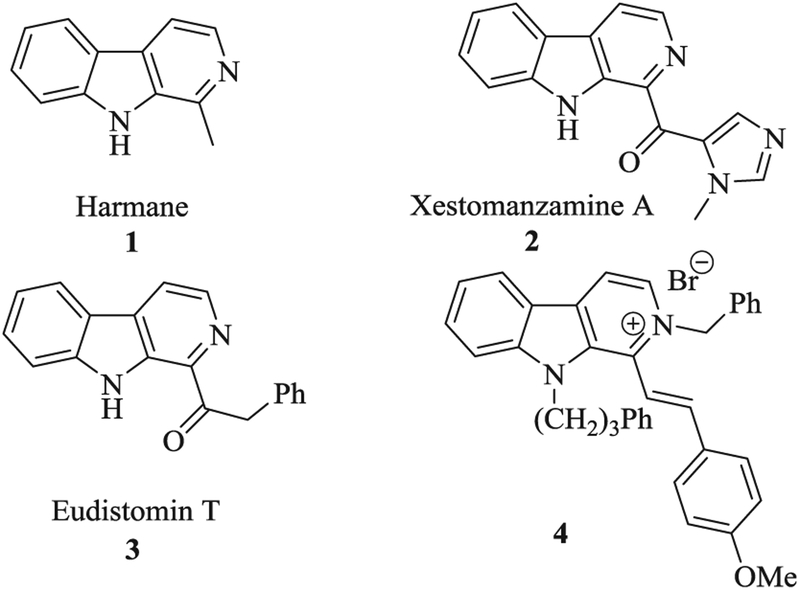
β-Carbolines are an important class of nitrogen containing heterocycles due to their widespread biological and pharmacological applications.1 Particularly, C-1 substituted β-carbolines possess remarkable antimalarial, anti-HIV, antimicrobial, antileishmania, and antitumoral properties.2–6 Recently, interest in β-carboline alkaloids was stimulated by their potential antitumor and antibacterial activities.7–9 β-carbolines exhibit anticancer properties through DNA intercalation, tubulin polymerization, topoisomerase and kinase inhibition.10,11 For instance, natural β-carboline derivative Harmane 1, isolated from cured tobacco and its smoke,12 exhibited moderate anticancer activity through DNA intercalation in addition to anti-bacterial activities.13,14 Kobayashi et al. reported isolation and cytotoxicity of novel manzamine alkaloid Xestomanzamine A (2) from Okinawan marine sponges of Xestospongia sp. which exhibited weak cytotoxicity against KB cell line.15 Cardellina group isolated Eudistomin T (3) from Eudistoma olivaceum, which was endowed with antimicrobial and weak phototoxicity.16–18 In 2008, Cao research group prepared 1-benzylidine-N2-benzylated-β-carbolinium bromides 4 and 5 with interesting cytotoxic activities (Figs. 1 and 2).19–21 From a series of novel β-carboline-based chalcones, Chauhan et al. identified compound 6 with high cytotoxicity (IC50 = 2.25 μM; MCF-7) against breast cancer cell lines.22
Fig. 1.
Pharmacologically interesting C-1 substituted β-carbolines.
Fig. 2.
Rational design of β-carboline chalcones and their bromide salts.
On the other hand, compounds with enone system continue to be of great interest because of their simple chemistry, easy synthesis and biological importance and usefulness as building blocks in synthetic chemistry.23 Particularly, wide variety of biological activities associated with chalcones have encouraged organic and medicinal chemists to undertake their structural modifications and chemistry.24 Chalcones exhibit anti-cancer activities through various mechanisms such as inhibition of multi-drug resistance channels such as ABCG2, BCRP, p-glycoprotein and inhibition of protein deacetylation.25 Also, many research groups either isolated or synthesized natural chalcones with antibacterial as well as anticancer activities. For example, Licochalcone A (7) isolated from Glycyrrhiza inflate is known to exhibit potent antibacterial activity especially towards Bacillus subtilis, Staphylococcus aureus and Micrococcus luteus.26 In recent past, Kumar et al. reported synthesis and antitumor activity of indole-based chalcones 8 with IC50 values ranging 0.03–0.09 μM against human pancreatic (PaCa-2) carcinoma cells.27
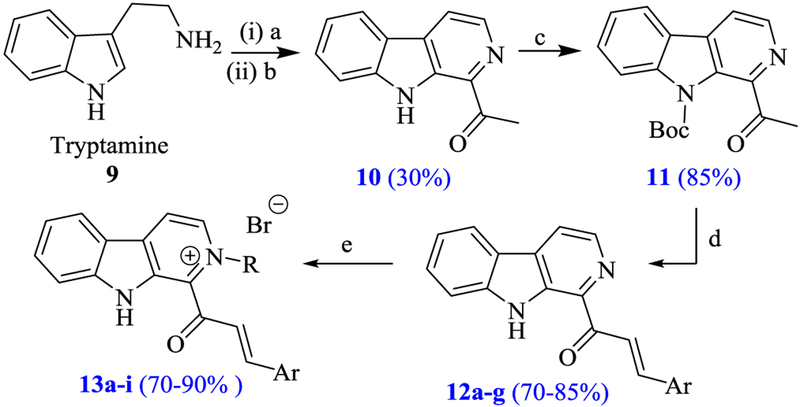
In continuation of our efforts to develop potent anticancer agents, recently we have identified indole analogues possessing significant cytotoxicity.28–33 Inspired by the attractive anticancer properties of β-carboline and chalcone units, herein, we have designed a diverse series of β-carboline chalcones 12a–g and their bromide salts 13a–i by incorporating amazing features of β-carboline and chalcone in a single molecule (Fig. 2).
Synthesis of novel β-carboline chalcone analogues 12a–g and 13a–i is depicted in Scheme 1.22,34–36 Initilly 1-acetyl-β-carboline 10 was prepared by the Pictet–Spengler reaction of tryptamine with pyruvaldehyde under acidic conditions followed by in situ aromatization with 10% palladium on carbon. Later the N—H protection of 1-acetyl-β-carboline with di-tert-butyl dicarbonate in the presence of DMAP produced N-boc-1-acetyl-β-carboline 11. Further, reaction of 11 with respective aldehydes in the presence of NaOH solution (20%) in aqueous ethanol for 16 h afforded β-carboline chalcone analogues 12a–g in good to excellent yields. Finally, the N2-alkylation of three β-carboline chalcones 12c–e with various alkylbromides led to the corresponding bromide salts 13a–i with >80% yields.
Scheme 1.
Synthesis of β-carboline chalcones 12a–g and their bromide salts 13a–i. Reagents and conditions: (a) Pyruvaldehyde, TFA, DCM, 30 °C, 48 h, N2; (b) 10% Pd/C, Xylene, reflux, 24 h; (c) Di-tert-butyldicarbonate, DMAP, THF, 24 h; (d) ArCHO, 20% NaOH, ethanol:water (1:1), 0 °C − 25 °C, 16 h; (e) RBr, DMF, 50 °C, 12 h.
All the prepared chalcones 12a–g and their bromide salts 13a–i were fully characterized using infra-red (IR), NMR (1H and 13C) and mass spectral data. In IR spectra, two characteristic bands at ~3370 cm−1 (N—H) and ~1650 cm−1 (C=O) were observed for all compounds. The 1H NMR spectra for chalcones 12a–g showed broad singlet at δ ~10.5–12.0 ppm (β-carboline N—H proton) and two doublets at δ ~8.4 ppm and ~7.9 ppm (enone HC=CH protons). 13C NMR spectra of 12a–g and 13a–i exhibited signals for the carbonyl carbon at δ ~190 ppm. HPLC analysis (Column: Waters-C18; 250 × 4.6 mm; condition: 0.01% TFA in acetonitrile; flow rate = 1 mL/min; and UV detector) showed the purity of the β-carbolines 12a–g and 13a–i >97%.
After synthesis and characterization, we studied the in vitro anticancer activity of β-carboline chalcones 12a–g and their bromide salts 13a–i against six different cancer cell lines using MTT assay. The tumor cell line panel consisted of pancreatic cancer (BxPC-3), cervical cancer (HeLa), castration-resistant prostate cancer (C4–2), human prostate cancer (PC-3), human embryonic kidney 293 (HEK293T) and breast carcinoma (MDA-MB-231) cells. Doxorubicin was used as the reference drug. Table 1 summaries the cytotoxicity results in terms of IC50 values. It is evident that most of the derivatives exhibited moderate to good cytotoxicity against the tested cancer cell lines. The structural changes by varying substituents on β-carboline (R) and aryl (Ar) moieties on enone part produced the sixteen compounds with a wide ranging anticancer activity with IC50 values ranging from 15.9 μM to >100 μM. Compound 12a without any substituents on β-carboline and enone moieties displayed moderate anticancer activity against a panel of cancer cell lines (IC50 = 65.5–93.0 μM). Replacement of phenyl group of enone moiety with tolyl, p-methoxyphenyl and 3,4-dimethoxyphenyl led to inactive compounds 12b–d (IC50 = >100 μM). Introduction of 3,4,5-trimethoxyphenyl moiety resulted in compound 12e with improved activity. Cytotoxicity of compounds 12f–g could not be tested due to solubility problem.
Table 1.
In vitro cytotoxicity of β-carboline chalcones 12a–g and their bromides 13a–i against a panel of cancer cells and a non-cancerous cell line (IC50 in μM).
 | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Compd | Ar | R | BxPC-3 | HeLa | C4–2 | PC-3 | HEK293T | MDA-MB-231 | NIH3T3 |
| 12a | C6H5 | 72.02 ± 2.75 | 82.36 ± 3.43 | 80.25 ± 5.46 | 93.02 ± 3.54 | 65.58 ± 2.84 | 71.11 ± 4.12 | >100 | |
| 12b | 4-CH3C6H4 | >100 | >100 | >100 | >100 | >100 | >100 | >100 | |
| 12c | 4-CH3OC6H4 | >100 | >100 | >100 | >100 | >100 | >100 | >100 | |
| 12d | 3,4-(CH3O)2C6H3 | >100 | >100 | >100 | >100 | >100 | >100 | >100 | |
| 12e | 3,4,5-(CH3O)3C6H2 | 70.78 ± 4.98 | 75.2 ± 3.6 | 71.6 ± 4.87 | >100 | 82.1 ± 5.09 | 71.54 ± 4.3 | >100 | |
| 12f | 4-CF3C6H4 | ND | ND | ND | ND | ND | ND | ND | |
| 12 g | 4-(CH3)2NC6H4 | ND | ND | ND | ND | ND | ND | ND | |
| 13a | 4-CH3OC6H4 | Benzyl | 50.25 ± 4.91 | 60.14 ± 4.65 | 65.5 ± 5.58 | 88.3 ± 7.1 | 41.4 ± 3.6 | 52.2 ± 4.95 | 85.3 ± 5.1 |
| 13b | 4-CH3OC6H4 | n-Butyl | 50.79 ± 4.91 | 44.99 ± 4.32 | 38.79 ± 4.91 | 64.99 ± 5.5 | 35.18 ± 4.1 | 40.9 ± 3.96 | 74.6 ± 2.50 |
| 13c | 4-CH3OC6H4 | Propargyl | >100 | >100 | >100 | >100 | >100 | >100 | >100 |
| 13d | 3,4-(CH3O)2C6H3 | Benzyl | 55.3 ± 3.48 | 45.25 ± 4.41 | 42.05 ± 4.35 | 78.95 ± 6.4 | 55.14 ± 5.8 | 48.23 ± 4.69 | 82.25 ± 3.33 |
| 13e | 3,4-(CH3O)2C6H3 | n-Butyl | >100 | >100 | >100 | >100 | >100 | >100 | >100 |
| 13f | 3,4-(CH3O}2C6H3 | Propargyl | 30.14 ± 3.41 | 25.32 ± 2.79 | 24.4 ± 2.64 | 29.6 ± 3.1 | 19.14 ± 2.78 | 21.2 ± 3.1 | 72.1 ± 4.12 |
| 13 g | 3,4,5-(CH3O)3C6H2 | Benzyl | 20 ± 2.1 | 22.1 ± 3.23 | 16.13 ± 4.2 | 22.02 ± 3.25 | 17.18 ± 2.98 | 15.95 ± 3.41 | 55.23 ± 5.8 |
| 13 h | 3,4,5-(CH3O)3C6H2 | n-Butyl | 25.1 ± 3.65 | 35.56 ± 2.2 | 39.65 ± 2.98 | 40.13 ± 3.5 | 34.4 ± 3.89 | 32.05 ± 4.0 | 68.6 ± 6.54 |
| 13i | 3,4,5-(CH3O)3C6H2 | Propargyl | 72.02 ± 5.3 | 70.1 ± 4.9 | 65.5 ± 5.74 | 60.98 ± 6.1 | 55.07 ± 4.4 | 59.61 ± 4.81 | 88 ± 3.85 |
| Doxorubicin | 13.56 ± 2.21 | 5.23 ± 1.92 | 3.2 ± 1.1 | 10.2 ± 0.98 | 3.59 ± 1.1 | 7.85 ± 1.59 | 21.32 ± 2.56 | ||
The activity data represent mean values ± SD of experiments conducted in triplicates at three independent times
To improve the cytotoxic activity, N2-alkylated derivatives of β-carbolines 13a–i were prepared. Interestingly, as shown in Table 1, compared with parent β-carboline chalcones 12c–e (12c: IC50 > 100 μM, 12d: IC50 > 100 μM, 12e: IC50 = 70–100 μM), the N2-alkylated-β-carbolines 13a–i showed enhanced cytotoxic activity (IC50 = 15–100 μM). Incorporation of benzyl at N2 of β-carbolines 12c–e yielded compounds 13a, 13d and 13g shown good to moderate activity (IC50 = 15–88 μM). Notably, compound 13g found to be the most potent analogue of the series with broad cytotoxicity against all the tested cell lines (IC50 = 15.9–22.1 μM). Introduction of propargyl moiety in β-carbolines 12c–e also resulted with similar activity (13c, 13f and 13i, IC50 = 19.1->100 μM). However, butylated derivatives 13b, 13e and 13h were inferior in the activity (IC50 25.1->100 μM) when compared to N2-benzylated analogues 13g. The activity results suggested that N2-alkylation of β-carboline moiety is beneficial for the anticancer activity.
We also investigated potential toxicity of compounds 12a–g and 13a–i against murine fibroblast NIH3T3 cell line. The results indicate that all the tested compounds 12a–g and 13a–i exhibit lower toxicity than the standard drug, Doxorubicin.
Next to determine the preliminary mechanism of cell death, we performed acridine orange/ethidium bromide assay.37–39 Acridine orange is a vital dye and stains both live and dead cells. Ethidium bromide stains cells that have lost membrane integrity and tinge the nucleus red. Thus, live cells appear uniformly green in acridine orange/ethidium bromide assay. Fig. 3 shows that control cells possess normal healthy morphology with intact nuclear architecture and are green in colour.
Fig. 3.
Morphological assessment of 13g-treated MDA-MB-231 cells.
Fluorescence microscopic image of MDA-MB-231 cells treated with 13g and reference drug-Doxorubicin clearly demonstrate morphological changes characteristic of apoptotic cells formation. This suggest that 13g induced apoptosis in MDA-MB-231 cancer cell line.
In the view of interesting antibacterial activities of chalcones, the newly prepared β-carboline derivatives 12a–g and 13a–i were screened for their antibacterial activity.23,24,40 In vitro antibacterial activity of sixteen β-carboline chalcones 12a–g and their bromide salts 13a–i was evaluated against two Gram-positive bacterial strains including Staphylococcus aureus (MTCC 96), Bacillus subtilis (MTCC 121) and three Gram-negative bacterial strains including Escherichia coli (MTCC 1652), Pseudomonas aeruginosa (MTCC 424) and Enterobacter cloacae (NAIMCC-B-02025) with respect to Chloramphenicol, a standard drug. The Zone of Inhibition (ZOI) and Minimum Inhibitory Concentrations (MICs) for compounds 12a–g and 13a–i were determined by the modified broth micro-dilution values method as given in Table 2. The activity results described that β-carboline chalcones exerted moderate antibacterial activity against tested bacterial strains. Among all the compounds, carboline derivative 13a was found to be most potent analogue against Gram-positive bacterial stain (S. aureus) with 15 mm of ZOI and MIC value of 440 μg/mL
Table 2.
In vitro antibacterial activity of β-carboline chalcones 12a–g and their bromides 13a–i against a panel of bacterial strains.
 | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Compd | Ar | R | Gram positive | Gram negative | ||||||
| B. subtilis | S. aureus | E. cloacae | E. coli | P. aeruginosa | ||||||
| ZOI (mm) | MIC (μM) | ZOI (mm) | MIC (μM) | ZOI (mm) | MIC (μM) | ZOI (mm) | ZOI (mm) | |||
| 12a | C6H5 | 9 | 838 | - | - | - | - | - | - | |
| 12b | 4-CH3C6H4 | 12 | 720 | 10 | 820 | 10 | 828 | - | - | |
| 12c | 4-CH3OC6H4 | 8 | 885 | 12 | 733 | 9 | 846 | - | 8 | |
| 12d | 3,4-(CH3O)2C6H3 | ND | ND | ND | ND | ND | ND | ND | ND | |
| 12e | 3,4,5-(CH3O)3C6H2 | - | - | 7 | >900 | 10 | 834 | - | - | |
| 12f | 4-CF3C6H4 | - | - | 11 | 710 | 11 | 726 | 10 | - | |
| 12 g | 4-(CH3)2NC6H4 | 8 | 870 | 11 | 715 | 8 | 880 | 8 | - | |
| 13a | 4-CH3OC6H4 | Benzyl | 8 | 875 | 15 | 440 | 8 | 874 | 8 | - |
| 13b | 4-CH3OC6H4 | n-Butyl | - | - | 9 | - | - | - | - | - |
| 13c | 4-CH3OC6H4 | Propargyl | - | - | 9 | - | 6 | - | - | 7 |
| 13d | 3,4-(CH3O)2C6H3 | Benzyl | 6 | - | 7 | - | 8 | - | - | - |
| 13e | 3,4-(CH3O)2C6H3 | n-Butyl | 7 | - | 9 | - | - | - | - | |
| 13f | 3,4-(CH3O)2C6H3 | Propargyl | 7 | - | 9 | - | 6 | - | - | 7 |
| 13 g | 3,4,5-(CH3O)3C6H2 | Benzyl | 7 | - | 9 | - | 12 | 726 | - | - |
| 13 h | 3,4,5-(CH3O)3C6H2 | n-Butyl | - | - | 10 | 812 | 10 | 806 | 8 | 12 |
| 13i | 3,4,5-(CH3O)3C6H2 | Propargyl | - | - | 9 | - | 13 | 590 | - | 9 |
| Chloramphenicol | 22 | 32 | 22 | 17 | 20 | 24 | 22 | 21 | ||
ZOI (in mm) and MIC (in μg/mL) values. Assay experiments were performed in duplicates at two independent times
For predicting the adsorption, distribution, metabolism and excretion (ADME) properties, computational studies for the compounds 12a–g, and 13a–i were performed. Lipinski’s rule of five and drug likeness score were used for predicting the physiochemical properties of the molecules.41–43 The newly prepared β-carboline derivatives 12a–g and 13a–i were evaluated for percentage absorption (% ABS) and drug-likeness score (Table 3). β-carboline chalcones 12a–g and their bromide salts 13a–i possess lower logP values suggesting them to be better candidates for bioavailability. This was in accordance with the better cytotoxicity of β-carboline chalcones 12a–g and 13a–i. All the β-carbolines, 12a–g and 13a–i showed topological polar surface area (TPSA) less than 160 Å2 but >40 Å2, which indicates good intestinal absorption property of these molecules than their Blood-Brain Barrier (BBB) penetration ability. Drug-likeness model score (a combined outcome of physicochemical properties, pharmacokinetics and pharmacodynamics of a compound is represented by a numerical value) was computed by MolSoft software for the synthesized compounds. Computed drug-likeness scores are presented in Table 3. β-carboline chalcones, 12c–e, 13a and 13d–i possessed a positive score in the range of 0.06–0.48, which implies them to be good drug candidates. The most cytotoxic compound 13g [IC50 = 15.9 μM against MDA-MB-231 cell line] possessed a relatively high drug score of 0.48.
Table 3.
Calculated drug-like properties of β-carboline chalcones 12a–g and β-carbolinium chalcone bromides 13a–i.
| Compd | Lipinski’s parameters | nRB | TPSAb | % ABSc | No. of violations | Drug-likeness Scored | |||
|---|---|---|---|---|---|---|---|---|---|
| nHBA (N&O) | nHBD (NH&OH) | LogPa | Molecular weight | ||||||
| 12a | 3 | 1 | 4.49 | 298.35 | 3 | 45.75 | 93.22 | 0 | −0.28 |
| 12b | 3 | 1 | 4.93 | 312.37 | 3 | 45.75 | 93.22 | 0 | −0.15 |
| 12c | 4 | 1 | 4.54 | 328.37 | 4 | 54.99 | 90.03 | 0 | 0.06 |
| 12d | 5 | 1 | 4.13 | 358.40 | 5 | 64.22 | 86.84 | 0 | 0.40 |
| 12e | 6 | 1 | 4.12 | 388.42 | 6 | 73.46 | 83.65 | 0 | 0.29 |
| 12f | 3 | 1 | 5.38 | 366.34 | 4 | 45.75 | 93.22 | 1 | −0.27 |
| 12g | 4 | 1 | 4.59 | 341.41 | 4 | 48.99 | 92.10 | 0 | −0.32 |
| 13a | 4 | 1 | 1.72 | 419.50 | 6 | 45.98 | 93.13 | 0 | 0.14 |
| 13b | 4 | 1 | 1.56 | 385.49 | 7 | 45.98 | 93.13 | 0 | −0.05 |
| 13c | 4 | 1 | 0.28 | 367.43 | 5 | 45.98 | 93.13 | 0 | 0.00 |
| 13d | 5 | 1 | 1.31 | 449.53 | 7 | 55.22 | 89.95 | 0 | 0.33 |
| 13e | 5 | 1 | 1.15 | 415.51 | 8 | 55.22 | 89.95 | 0 | 0.16 |
| 13f | 5 | 1 | −0.13 | 397.45 | 6 | 55.22 | 89.95 | 0 | 0.21 |
| 13g | 6 | 1 | 1.29 | 479.56 | 8 | 64.45 | 86.76 | 0 | 0.48 |
| 13h | 6 | 1 | 1.13 | 445.54 | 9 | 64.45 | 86.76 | 0 | 0.13 |
| 13i | 6 | 1 | −0.14 | 427.48 | 7 | 64.45 | 86.76 | 0 | 0.16 |
LogP = Lipophilicity calculated using molinspiration cheminformatics software
TPSA = Topological polar surface area calculated using molinspiration cheminformatics software
% ABS = Percentage absorption calculated using the formula %ABS = 109 − (0.345 × TPSA).
Drug-likeness Score = calculated using Molsoft.
In conclusion, a library of sixteen simple β-carboline chalcones 12a–g and bromide salts 13a–i was prepared in good yields. In vitro antitumor activity of newly synthesized β-carbolines 12a–g and 13a–i was performed against a panel of cancer cell lines and compound 13g displayed fairly good anticancer activity against all the tested cancer cells with IC50 value ranges 15.9 to 22.1 μM. Activity of N2-benzylated-β-carboline chalcones were found to be better than that of N2-unsubstituted-β-carboline chalcones Preliminary mechanism of action studies suggests that 13g induces apoptosis in breast cancer cells and possess a relatively good drug score of 0.48. Additionally, β-carboline chalcones 12a–g and 13a–i demonstrate moderate antibacterial activity against the tested bacterial strains.
Supplementary Material
Acknowledgements
Authors are thankful to Council of Scientific & Industrial Research (CSIR, No. 02(0239)/17/EMR-II), New Delhi, India and National Institute of Health (R03 CA 166912 to KS) for providing financial support to carry out this work. Also, we would like to acknowledge BITS Pilani, Rajasthan and DST-FIST, New Delhi for NMR and HRMS facility.
Footnotes
A. Supplementary data
Supplementary data associated with this article can be found, in the online version, at https://doi.org/10.1016/j.bmcl.2018.03.033.
References
- 1.Cao R, Peng W, Wang Z, et al. Curr Med Chem. 2007;14:479–500. [DOI] [PubMed] [Google Scholar]
- 2.Laine AE, Lood C, Koskinen AM, et al. Molecules. 2014;19:1544–1567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lunagariya N, Gohil V, Kushwah V, Neelagiri S, Jain S, Singh S, Bhutani KK, et al. Planta Med. 2015;81:PM_84. [Google Scholar]
- 4.Olmedo GM, Cerioni L, González MM, et al. Food Microbiol. 2017;62:9–14. [DOI] [PubMed] [Google Scholar]
- 5.Quintana VM, Piccini LE, Zénere JDP, et al. Antivir Res. 2016;134:26–33. [DOI] [PubMed] [Google Scholar]
- 6.Lunagariya NA, Gohil VM, Kushwah V, et al. Bioorganic Med Chem Lett. 2016;26:789–794. [DOI] [PubMed] [Google Scholar]
- 7.Zhang J, Li L, Dan W, et al. Nat Prod Commun. 2015;10:899–902. [PubMed] [Google Scholar]
- 8.Samundeeswari S, Chougala B, Holiyachi M, et al. Eur J Med Chem. 2017;128:123–139. [DOI] [PubMed] [Google Scholar]
- 9.Zhang M, Sun D, et al. Anticancer Agents Med Chem. 2015;15:537–547. [DOI] [PubMed] [Google Scholar]
- 10.Da Silva CM, Silva MM, Reis FS, et al. J Photochem Photobiol B. 2017;172:129–138. [DOI] [PubMed] [Google Scholar]
- 11.Kumar S, Singh A, Kumar K, et al. Eur J Med Chem. 2017;142:48–73. [DOI] [PubMed] [Google Scholar]
- 12.Poindexter EH, Carpenter RD, et al. Phytochemistry. 1962;1:215–221. [Google Scholar]
- 13.Cao R, Chen H, Peng W, et al. Eur J Med Chem. 2005;40:991–1001. [DOI] [PubMed] [Google Scholar]
- 14.Kodani S, Imoto A, Mitsutani A, et al. J Appl Phycol. 2002;14:109–114. [Google Scholar]
- 15.Kobayashi M, Chen Y-J, Aoki S, et al. Tetrahedron. 1995;51:3727–3736. [Google Scholar]
- 16.VanWagenen BC, Cardellina JH, et al. Tetrahedron Lett. 1989;30:3605–3608. [Google Scholar]
- 17.Kinzer KF, Cardellina JH, et al. Tetrahedron Lett. 1987;28:925–926. [Google Scholar]
- 18.McNulty J, Still IWJ, et al. Curr Org Chem. 2000;4:121–138. [Google Scholar]
- 19.Cao R, Yi W, Wu Q, et al. Bioorganic Med Chem Lett. 2008;18:6558–6561. [DOI] [PubMed] [Google Scholar]
- 20.Cao R, Fan W, Guo L, et al. Eur J Med Chem. 2013;60:135–143. [DOI] [PubMed] [Google Scholar]
- 21.Zhang G, Cao R, Guo L, et al. Eur J Med Chem. 2013;65:21–31. [DOI] [PubMed] [Google Scholar]
- 22.Chauhan SS, Singh AK, Meena S, et al. Bioorganic Med Chem Lett. 2014;24:2820–2824. [DOI] [PubMed] [Google Scholar]
- 23.Zhuang C, Zhang W, Sheng C, et al. Chem Rev. 2017;117:7762–7810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Singh P, Anand A, Kumar V, et al. Eur J Med Chem. 2014;85:758–777. [DOI] [PubMed] [Google Scholar]
- 25.Ciupa A, De Bank PA, Mahon MF, et al. MedChemComm. 2013;4:956–961. [Google Scholar]
- 26.Tsukiyama R-I, Katsura H, Tokuriki N, et al. Antimicrob Agents Chemother. 2002;46:1226–1230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kumar D, Kumar NM, Akamatsu K, et al. Bioorganic Med Chem Lett. 2010;20:3916–3919. [DOI] [PubMed] [Google Scholar]
- 28.Venkataramana Reddy PO, Mishra S, Tantak MP, et al. Bioorganic Med Chem Lett. 2017;27:1379–1384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tantak MP, Das Mukherjee D, Kumar A, et al. Anticancer Agents Med Chem. 2017;17:442–455. [DOI] [PubMed] [Google Scholar]
- 30.Tantak MP, Klingler L, Arun V, et al. Eur J Med Chem. 2017;136:184–194. [DOI] [PubMed] [Google Scholar]
- 31.Venkataramana Reddy PO, Tantak MP, Valdez R, et al. RSC Adv. 2016;6:9379–9386. [Google Scholar]
- 32.Das Mukherjee D, Kumar NM, Tantak MP, et al. Biochem. 2016;55:3020–3035. [DOI] [PubMed] [Google Scholar]
- 33.Vaddula BR, Tantak MP, Sadana R, et al. Sci Rep. 2016;6:23401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nemet I, Varga-Defterdarović L, et al. Bioorganic Med Chem. 2008;16:4551–4562. [DOI] [PubMed] [Google Scholar]
- 35.Nemet I, Varga-Defterdarović L, et al. Amino Acids. 2007;32:291–293. [DOI] [PubMed] [Google Scholar]
- 36.Skouta R, Hayano M, Shimada K, et al. Bioorganic Med Chem Lett. 2012;22:5707–5713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kasibhatla S, Amarante-Mendes GP, Finucane D, Brunner T, Bossy-Wetzel E, Green DR, et al. Cold Spring Harb. Protoc 2006;2006:pdb.prot4493. [Google Scholar]
- 38.Ribble D, Goldstein NB, Norris DA, et al. BMC Biotechnol. 2005;5:12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cohen JJ et al. Immunol Today. 1993;14:126–130. [DOI] [PubMed] [Google Scholar]
- 40.Kumar A, Gupta V, Singh S, et al. Asian J Res Chem. 2017;10:225–239. [Google Scholar]
- 41.Tibbitts J, Canter D, Graff R, et al. MAbs. 2016;8:229–245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Roller SG, CHAPTER 8 ADME Properties of Peptide Therapeutics in Drug Discovery and Development, Peptide-based Drug Discovery: Challenges and New Therapeutics, The Royal Society of Chemistry 2017, pp. 223–251. [Google Scholar]
- 43.Zhao YH, Abraham MH, Le J, et al. Pharm Res. 2002;19:1446–1457. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.