SUMMARY
Background:
CD200 and its receptor CD200R are both type I membrane glycoproteins that modulate the activity of myeloid and lymphoid cells, and their interaction is functionally important in the suppression of effector T-cell responses by regulatory T-cells. We aimed to investigate the extent of expression of CD200 and CD200R1 on CD4+ T-cells in blood of children with ulcerative colitis (UC) and Crohn’s disease (CD) and to explore their correlations with effector T cell subsets, regulatory T cells (Treg), and routine clinical and serological markers.
Methods:
The frequencies of blood CD4+ expressing CD200 and CD200R1 as well as T-helper CD4+CD25+Foxp3+ Treg, CD4 + IL- 17+ (Th17), CD4+ IFN-γ + (Th1), and CD4+IL-4+ (Th2) were estimated by flow cytometry in 23 pa-tients with CD, 14 with UC, and 14 healthy volunteers (HCs). The clinical and inflammatory markers were also in-vestigated.
Results:
IBD patients showed decreased CD4+CD200R1+ T-cells, whereas, CD4+CD200+ T-cells were significantly higher in patient groups compared with healthy controls. Treg cells were found significantly decreased in the pa-tients with UC and CD compared with healthy controls (both at p < 0.01). The percentage of Th17 was found sig-nificantly increased in CD (p < 0.05) compared with UC patients and healthy subjects (p = 0.014). CD200+CD4+ T-cells showed significant positive correlations with ESR, Th1, and Th17 (r = 0.438, p < 0.05; r = 0.411, p < 0.05; r = 0.492, p < 0.01, respectively). CD200R1+CD4+ T-cells correlated positively with Th2 and Treg (r = 0.482, p < 0.01, and r = 0.457, p < 0.01, respectively) and negatively with ESR (r = −0.387, p < 0.01).
Conclusions:
Our study demonstrates an aberrant expression of CD200/CD200R1 on CD4+ T-cells in IBD patients and these data may have potent pathological significance in IBD pathophysiology.
Keywords: inflammatory bowel disease, ulcerative colitis, Crohn’s disease, T-helper lymphocytes, effector T-cells, regulatory T-cells
INTRODUCTION
Inflammatory bowel diseases (IBD) is a group of chronic autoimmune disorders affecting the large intestine with repeated episodes of inflammation and ulceration of the bowel that requires proactive medical management and accurate diagnosis. IBD comprises two major disorders, ulcerative colitis (UC) and Crohn’s disease (CD). Both conditions are chronic and relapsing and can result in significant long-term morbidity, increased cancer risk, and mortality especially at pediatric IBD [1,2]. Recent epidemiological studies suggest an increase in the incidence of pediatric IBD worldwide, with about 10% of cases with IBD occurring in children less than 10 years old [3,4]. The incidence of pediatric IBD in Saudi Arabian children is lower than suggested in the Western literature; however, there is a significantly increasing trend over time [5].
The specific etiology of IBD is unknown; however, increasing evidence suggests that the loss of tolerance to enteric and food antigen or breakdown in intestinal barrier functions allows the access of inflammatory bacterial products to the immune system and potentially triggers an inappropriate immune responses to gut microflora and eventually developing IBD [6,7]. In addition, it has been demonstrated that lymphoid effector cells of the gut mucosa and their products including cytokines and other mediators have an inherent capacity to migrate to other organs that may contribute to their inflammation [8,9]. Moreover, cell transfer studies have indicated that certain subsets of T-helper lymphocytes (CD4+ T-cells) induce colitis in SCID, suggesting that CD4+ T-cells play a prominent role in the pathogenesis of IBD [10,11]. These studies provided a direct demonstration that CD4+ T-cells reactive with enteric bacterial flora can mediate chronic inflammatory bowel disease. However, normal hosts are genetically programmed to resist the effects of these cells through induction of peripheral tolerance mediated by regulatory T-cells (Treg) and its cytokines to prevent inflammatory immune responses [12]. In addition, T-cells are covered with various inhibitory receptors that regulate the initiation and termination of effective immune responses to infections while limiting exaggerated inflammatory responses [13].
CD200 and CD200R, type I transmembran-glycoproteins are inhibitory molecules that are involved in various cellular activities including down-regulation of myeloid and lymphoid cell activity, regulation of differentiation, adhesion, and chemotaxis of various lymphoid cell subsets, cytokines production, and maintenance of immune homeostasis [14]. Knock-out mice lacking CD200 and normal mice in which CD200R was being blocked by its antagonists showed an increased production of Th1/Th17 specific cytokines, such as IL-6, IL-10, and IL-17 and precipitated susceptibility to external stimuli that contribute to development of autoimmune disease [15]. On the other hand, the interaction between CD200 and CD200R was implicated in the induction of Treg [16] and attenuates the production of proinflammatory cytokines IL-6, tumor necrosis factor-α, and nitric oxide [17].
The present study was designed to investigate the expression of CD200 and CD200R1 on human CD4+ T-cells and to characterize their correlation with other effector T-cell subsets and regulatory T-cells in pediatric patients with IBD and healthy volunteers.
MATERIALS AND METHODS
Patients
Thirty-seven IBD patients presented to the pediatric clinics of King Abdulaziz University Hospital (KAUH). IBD patients, 14 UC and 23 CD, were included in the study after written consent from one of the parents. Patients were excluded if they were referred with cancer. Serum and peripheral blood mononuclear cells (PBMCs) were collected from the same patients. Only patients with a confirmed diagnosis of UC or CD according to established criteria [23] were included into the study. Fourteen age-matched pediatric volunteers (5 males and 9 females), who were clinically and laboratory free of any autoimmune diseases, served as a healthy control group. The study protocol was approved by the Ethics Committee of the KAUH, and the study was conducted according to the principles expressed in the Declaration of Helsinki. Patients or one of their parents were informed regarding the nature of the investigation and were instructed to complete the questionnaire. The clinical characteristics of the patients are shown in Table 1.
Table 1.
Characteristics of IBD patients and healthy controls.
| Ulcerative Colitis (n = 14) |
Crohn’s Disease (n = 23) |
Healthy Control (n = 14) |
|
|---|---|---|---|
| Age (years) | 13.25 ± 2.94 | 13.73 ± 3.10 | 15.21 ± 2.41 |
| Gender (M/F) | 4/10 | 9/14 | 5/9 |
| Weight (Kg) | 21.43 ± 1.51 * | 33.79 ± 3.876 * | 47.9 ± 4.73 |
| Height (cm) | 114 ± 3.088 * | 136.95 ± 5.071 * | 152.8 ± 7.67 |
| Rectal Bleeding | 14 (100%) | 13 (56.5%) | - |
| Diarrhea | 14 (100%) | 19 (82.6%) | |
| Disease Duration (months) | |||
| < 6 months | 50% | 39% | - |
| Paris classification | |||
| Location | left-sided 4 (28.6%) | terminal ileum 8 (34.8%) | - |
| extensive 2 (14,3%) | colon 4 (17.4%) | ||
| pancolitis 8 (57.1%) | ileocolon in 11 (47.8%) | ||
| Behavior | N/A | non-stricturing, non-penetrating 16 (69.6%) | - |
| stricturing 4 (17.4%) | |||
| Penetrating 3 (13.04%) | |||
| Mayo Score | Mild 0 | N/A | - |
| Moderate 5 (35.7%) | |||
| Sever 9 (64.3%) | |||
| Therapy | |||
| Mesalamine | 14 (100%) | 18 (78.3%) | |
| Prednisone | 12 (85.7%) | 23 (100%) | |
| Azathioprine | 12 (85.7%) | 23 (100%) | |
| Sulphasalazine | 0 | 10 (43.5%) | |
N/A - not applicable.
Sample preparations
Ten milliliters of blood were drawn from each participant. Each blood sample was divided into two equal parts, 5 mL of blood were collected in serum separator tubes, centrifuged, and assayed immediately for routine biochemistry tests. The other part of the blood sample was collected into tubes with the anticoagulant ethylenediaminetetraacetic acid (EDTA) for isolation of PBMCs from whole blood using Ficoll-Paque (GE Health Care) density gradient centrifugation. Cells were washed once with RPMI-1640 (BioWhittaker) and prepared for flow cytometry assays.
Laboratory assessments
Serum concentrations of C-reactive protein (CRP), erythrocyte sedimentation rate (ESR), ALB (albumin), ALT (alanine aminotransferase), AST (aspartate amino-transferase), ALP (alkaline phosphatase), and GGT (glutamyl transferase) were assayed using commercially available kits according to the manufacturer’s instructtion. A standard ELISA technique was employed to detect the IgG and IgA antibodies to ASCA in accordance with the manufacturer’s instructions (Inova Diagnostics Inc., San Diego, CA, USA). pANCA was analyzed by ELISA and indirect immunofluorescence, as previously described [18].
Isolation of peripheral blood mononuclear cells (PBMCs)
Peripheral venous blood samples were collected from all study participants after overnight fast. Peripheral blood mononuclear cells (PBMCs) were isolated by density-gradient centrifugation using Ficoll-Paque Plus (Amersham Biosciences, Little Chalfont, UK). Cells were washed once with RPMI-1640 (BioWhittaker) and prepared for flow cytometry assays.
Flow cytometry Assays
Expression of CD200 and CD200R1
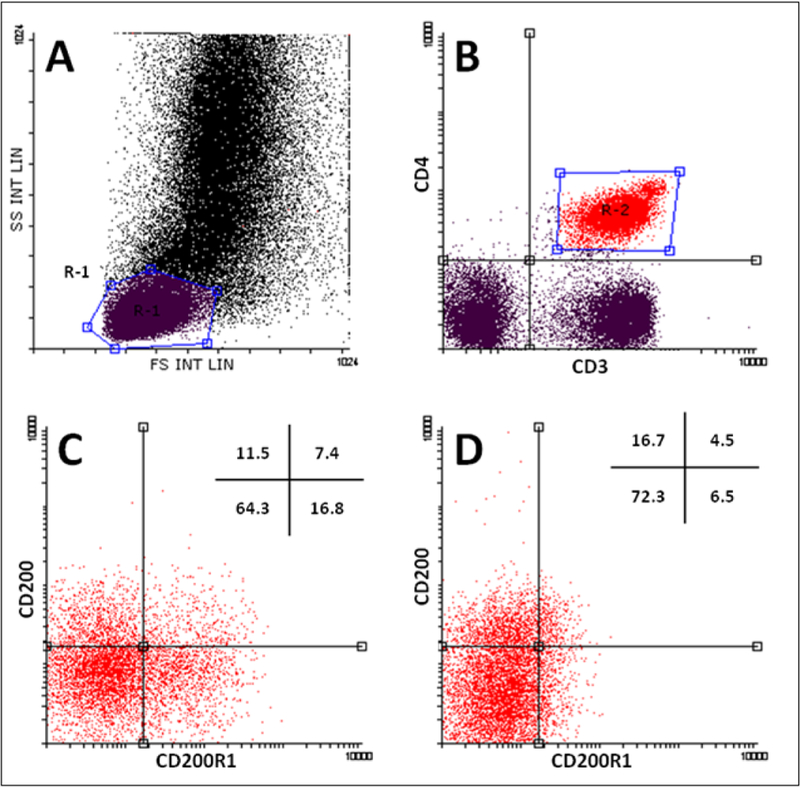
The expression of CD200 and CD200R1 was identified on T-cell subsets using multiparametric flow cytometry as described previously with slight modifications [19]. Briefly, 100 µL of PBMCs were stained with FITC-CD3 Clone UCHT1, R&D systems), PE-CD200R1 (Clone 380525, R&D systems), and APC-CD200 (Clone 325516, R&D systems), and AF-700 CD4 (Clone 11830, R&D systems). Isotype-matched control mAbs were used to determine the nonspecific binding. Cells were gated by their forward- and side-scatter properties and identified further by specific surface markers (Figure 1A). Percentages of CD200 and CD200R1 were determined after gating on CD3+CD4+ and CD3+CD4- cells (Figure 1).
Figure 1.
Representative flow cytometry data to illustrate the gating strategy for FACS analysis of T-cell subsets.
Dot plot with a gate (R1) encompassing the lymphocyte population (A), CD4+ lymphocytes was further gated (R2) (B), dot plots represent the expression of CD200/CD200R1 on CD3 +CD4+ cells of healthy controls (C) and of IBD patients (D), respectively. HC - healthy controls, UC - ulcerative colitis patients, CD - Crohn’s Disease patients, FS - forward scatter, SS - side scatter.
Regulatory T-cells analysis
For regulatory T-cell analysis, 1 × 106 PBMCs were resuspended in 100 μL flow cytometry staining buffer (R&D Systems, Minneapolis, MN, USA). Cells were incubated with FITC-labelled anti-CD4 (clone FAB3791F, R&D Systems) and APC-labelled anti-CD25 (clone BC96, R&D Systems) antibodies for 30 minutes at 4°C in the dark. For intracellular staining, after permeabilization with fixation/permeabilization buffer (R&D Systems), PE-labelled anti-Foxp3 antibody (clone IC8214P, R&D Systems) was added and incubated for 30 minutes at 4°C in the dark. FITC- and APC-conjugated mouse IgG2a and PE-conjugated rabbit IgG antibodies were used as the isotype control antibodies.
Effector T cell subsets
The frequency of effector T cell subsets Th1 (CD4 +IFN-γ+), Th2 (CD4+IL-4+), and Th17 (CD4+IL17A+) were determined after brief stimulation by flow cytometry following intracellular staining with anti-cytokine antibodies. Briefly, PBMCs (106/mL) were stimulated in duplicate with 50 ng/mL of phorbol myristate acetate (PMA) and 1.0 µg/mL of ionomycin (Sigma, St. Louis, MO, USA) in 10% fetal bovine serum in RPMI 1640 medium at 37°C in a humidified incubator with 95% air and 5% CO2 for 2 hours and then cultured for another 4 hours in the presence of 0.5 µg/mL of brefeldin A (BFA, Sigma). The control PBMCs were cultured in medium alone. The stimulated PBMCs were harvested and stained with APC-labeled anti-CD4. After 30 minutes incubation, cells were fixed with the Perm/Fix solution, and permeabilized, followed by staining with PerCP-labeled anti-IL-17 (Clone 41802, R&D Systems), PE IFN-γ (Clone 25723, R&D Systems), and Fluorescein IL-4 (Clone 3007, R&D Systems, Minneapolis, MN, USA). Appropriate conjugated IgG antibodies were used as isotype controls. All flow cytometry data were acquired on a Beckman-Coulter Navios cytometer and analyzed with Summit software (Beckman Coulter Inc., USA).
Statistical analysis
All data were statistically analyzed using Statistical Package for Social Sciences (SPSS) V20.0. The data were expressed as means ± standard deviation (SD). Correlations were done using Pearson’s tests, and analyses of variance were done using an ANOVA test followed by Bonferroni correction. P -value less than or equal to 0.05 is considered as being statistically significant.
RESULTS
Clinical and laboratory characteristics
Patient and control demographics and clinical characteristics are shown in Table 1. Thirty-seven consecutive unselected pediatric patients with IBD were included, 14 (4 female/10 male) had UC and 23 (9 female/14 male) had CD, with an age range of 9 – 15 years (mean age 13.25 ± 2.9). Fourteen healthy children with an age range of 13 – 16.6 years (mean age 15.2 ± 2.4) served as controls. IBD patients showed a significant weight reduction (p < 0·001) compared to control subjects. The duration of the disease was more than 6 months in 50% of UC children and in 61% of children with CD. In the UC group, the inflammation was left-sided in 4 (28.6%), extensive in 2 (14.3%), and it was spread throughout the large intestine (pancolitis) in 8 (57.1%) of the UC children. According to Mayo score classification of UC, 5 (35.7%) children showed moderate and 9 (64.3%) severe inflammation. In the CD group, inflammation was detected in the terminal ileum in 8 (34.8%), colon in 4 (17.4%), and ileocolon in 11 (47.8%) of the patients. According to the Montreal classification, the majority of CD patients (16; 69.6%) had non-stricturing non-penetrating inflammation, whereas it was stricturing in 4 (17.4%), and perforating in 3 (13.04%) patients. The majority of CD and UC patients had been treated with the combination of aminosalicylate (5-ASA) mesalamine with immunosuppressive drugs such as prednisolone, azathioprine, or infliximab.
Biochemical and Serological markers
The levels of erythrocyte sedimentation rate (ESR), Creactive protein (CRP), and alkaline phosphatase (ALP) are known to be good predictors of disease activity in IBD. CD patients showed significantly higher CRP than UC and control subjects (p < 0.05, 0.01, respectively). Patients with CD and UC had significantly higher levels of ESR and ALP compared with healthy controls, while no significant differences were detected between the two patient groups. Sera from patients and healthy controls were also tested for anti-Saccharomyces cerevisiae antibodies (ASCA) and perinuclear antineutrophilic cytoplasmic antibody (pANCA) and found that none of the control group had these autoantibodies. UC patients had significantly higher pANCA than CD patients (35.7 vs. 17.4%; p < 0.01). Both ASCA -IgG and ASCA-IgA were significantly higher in CD than UC patients (Table 2).
Table 2.
Biochemical and serological parameters.
| Ulcerative Colitis (n = 14) |
Crohn’s Disease (n = 23) |
Healthy Control (n = 14) |
|
|---|---|---|---|
| ALP (IU/L) | 175.29 ± 32.24 **, ∆ | 195.15 ± 27.05 ** | 67.2 ± 12.74 |
| C-RP (mg/dL) | 13.57 ± 4.46 **, ∆ | 34.05 ± 8.56 ** | 3.42 ± 1.07 |
| ESR (mm/H) | 30 ± 4.08 * | 35.5 ± 6.23 * | 11.2 ± 2.1 |
| pANCA | 5 (35.7)% ∆∆ | 4 (17.4%) | 0 |
| ASCA-IgA | 2 (14.2%) ∆∆ | 6 (26.1)% | 0 |
| ASCA-IgG | 3 (21.4%) ∆ | 7 (30.4%) | 0 |
- denotes statistically significant difference compared with control, * p < 0.05
p < 0.01
- denotes statistically significant compared with CD: ∆ p < 0.05
p < 0.01.
Flow cytometric detection of T-cell subsets
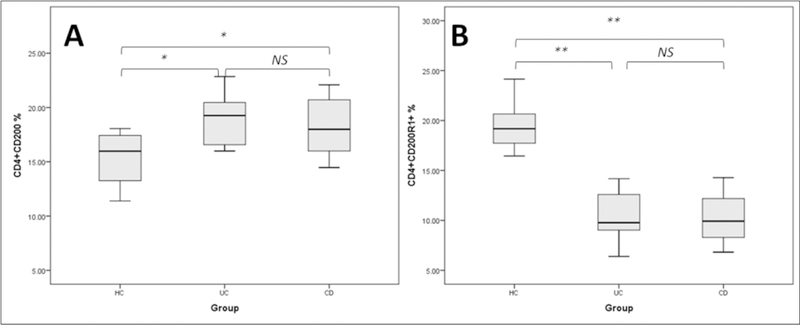
Representative dot-plots of flow cytometry analysis of CD4+ T-lymphocytes and their expression of CD200 and CD200R1 are shown in Figure 1. Significant differences in the percentage of circulating CD4+ T-cells were observed between studied groups (p < 0.02). The percentages of CD200R1+CD4+ T- cells were significantly lower in UC and CD patients (p < 0.05, one-way ANOVA), whereas CD4 + T-lymphocytes expressing CD200 were significantly higher in the two groups of patients than healthy controls (p < 0.01, one-way ANOVA) (Figure 2).
Figure 2.
Percentage of CD4 T-helper lymphocytes expressing CD200 and CD200R1.
HC - healthy controls, UC - ulcerative colitis patients, CD - Crohn’s Disease patients, NS - not significant, asterisks denote statistical significance, * p < 0.05, ** p < 0.01.
Analyses of Treg cell and effector T-helper subsets
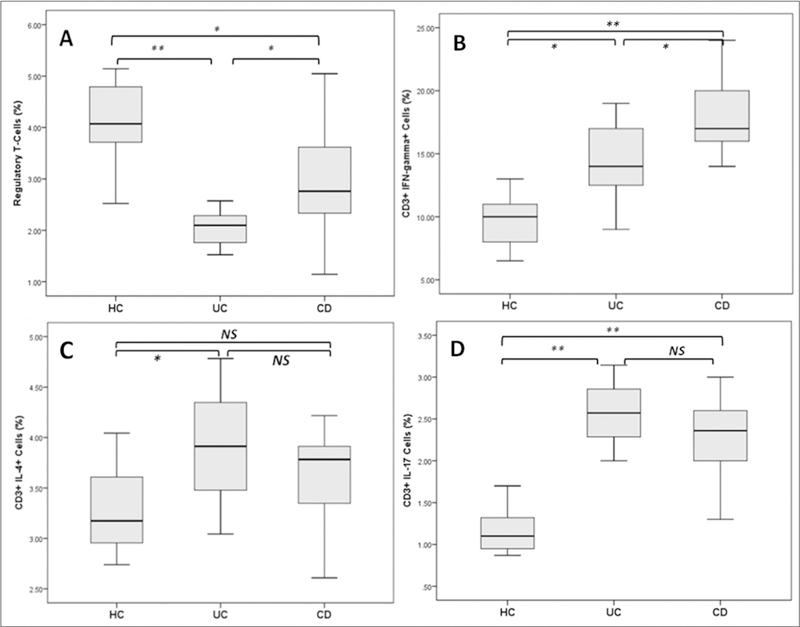
Regulatory T-cells as identified by flow cytometry based on the expression of CD4, CD25, and Foxp3 were found significantly decreased in the patients with UC and CD compared with healthy controls (both at p < 0.01) (Figure 3A). The Th1 cell subset as identified by flow cytometry based on the expression of intracellular cytokine IFN-γ was found increased significantly in CD compared with UC and controls. Levels of Th1 were also significantly higher in UC patients than controls (Figure 3B). The Th2 cell population was found increased significantly (p < 0.05) in the UC patient group compared with healthy controls. The CD patients group also showed higher Th2 cells than that of controls, although the difference did not reach statistical significance (Figure 3C) . Th17 cells were found significantly increased in CD (p < 0.05) compared with UC patients and healthy subjects (p < 0.05). Patients with UC displayed an increased percentage of Th17 cells when compared with that of healthy controls (p < 0.01) (Figure 3D).
Figure 3.
Quantification of Treg, TH1, TH2, and Th17 cells in the peripheral blood from patients with IBD. Th17 and lymphocytes analyzed by flow cytometry.
Boxplots show the percentage of (A) Treg (CD4+CD25+Foxp3+), (B) Th17 (CD4+ IL-17A+), (C) Th17/Treg ratio, (D) Th1 (CD4+ IFN-γ+), and (E) Th2 (CD4+ IL-4+) cells in the studied groups. HC - healthy controls, UC - ulcerative colitis patients, CD - Crohn’s Disease patients, NS - not significant, asterisks denote statistical significance, * p < 0.05, ** p < 0.01.
Correlations of T-helper cells with clinical and laboratory parameters
The relationships between the percentages of circulating CD4 expressing CD200 and CD200R1 cells and clinical parameters were studied and found not significant. Regarding laboratory parameters, CD200+CD4+ T-cells showed significant positive correlations with ESR, Th1, and Th17 (r = 0.438, p < 0.05; r = 0.411, p < 0.05; r = 0.492, p < 0.01, respectively). CD200R1+CD4+ T-cells correlated positively with Th2 and Treg (r = 0.482, p < 0.01 and r = 0.457, p < 0.01, respectively) and negatively with ESR (r = −0.387, p < 0.01).
DISCUSSION
The CD4 T-helper lymphocyte population is a group of heterogeneous cell subsets that play critical roles in mediating adaptive immunity to a variety of pathogens. They are also involved in autoimmunity, asthma, and allergic responses [20]. In animal studies, adoptive transfer of CD45RBhiCD4+ cells induces colitis in SCID mice, although the cotransfer of CD45RBhiCD4+ cells and CD45RBloCD4+ cells protects these mice from developing colitis [21]. Later cell transfer research has demonstrated that CD4 + expressing CD25+ but not CD45RB T cells are able to cure intestinal inflammation induced by coliotogenic CD4+ T cells [22]. Transfer of CD4+CD25+ T cells into mice with colitis led to resolution of the lamina propria infiltrate in the intestine and reappearance of normal intestinal architecture. These opposing effects of CD4 T-cell subsets suggests that a fine balance between cytopathic and regulatory CD4 subsets is required to maintain immune homeostasis in healthy individuals [23]. In addition, it has been suggested that a small proportion of these coliotogenic intramucosal lymphocytes may return to the blood flow and contribute to persistence of intestinal inflammation and several extra-intestinal manifestations (EIM) [24]. In this report, we have focused on a certain subset(s) of CD4+ T cells that express the cell surface glycoprotein CD200 and its receptor CD200R1 of IBD patients. The CD200/CD200R axis has been reported to be important in immunoregulation of lymphoid cells. Several studies demonstrate that interaction of CD200R by CD200 initiates an inhibitory pathway that displays immunosuppressive effects [25,26]. Dysregulation of this process has been reported to induce inflammation and autoimmunity [14,27]. Our phenotypic data demonstrated a significant increase in the percentage of CD4 expressing CD200 and a significant decrease of CD200R1 of IBD patients compared with controls. These findings suggest that the CD200/CD200R1 axis is disturbed in IBD. This is in agreement with the findings of Li and colleagues in patients with systemic lupus erythematosus (SLE), where a subset of patients was identified as having increased CD4 + T-cells expressing CD200, which appeared unrelated to disease activity [28] and Gao et al. who found increased expression of CD200 on follicular helper T cells that was also found to be independent of disease activity index (DAS28 score) [29]. Although SLE and RA are both systemic autoimmune diseases that preferentially affect different organ systems, they are considered common EIM in IBD, affecting approximately 10 and 30% of IBD patients, respectively [30, 31]. Moreover, our findings confirm and extend previous reports demonstrating that disrupted CD200 - CD200R interaction is implicated in the exaggerated immune response in several inflammatory and autoimmune disorders [19,32].
In our study, there was a significant decrease in percentage of CD4+CD25 +Foxp3+ Treg of IBD patients, with a slight reduction in UC compared to CD. This finding is in agreement with Maul, Loddenkemper [33] who reported that the frequency of Treg cells is decreased in peripheral blood of patients with IBD and that it varies with disease activity. Furthermore, the authors have demonstrated that IBD is not associated with a functional defect in Treg, but rather with a reduction of the peripheral blood Treg pool and only a moderate expansion in intestinal lesions. The positive association between frequency of CD200R+CD4+ T-cells and Treg support previous reports which demonstrated that the interaction between CD200 and CD200R is implicated in the induction of CD4+ CD25+ regulatory T cells [16,34]. Treg cells play an important role in preventing autoimmunity and controlling inflammation and immune response in the gut by inhibiting the proliferation and effector functions of other T cells. Maul, Loddenkemper [33] observed that IBD patients had increased Treg cells during remission but decreased during active disease. The reduction of Treg in IBD was found concomitant with increased levels of IL-1 β and IL-6 that mediate a range of inflammatory immune responses including the development of Th17 [35].
Th17 cells are a subset of IL-17-producing CD4+ T cells, which play a crucial role in clearing pathogens during host defense reactions and in inducing tissue inflammation in autoimmune diseases [36]. There is considerable evidence, both in humans and in mice, that skewing of responses towards Th17 or Th1 and away from Treg may be responsible for the development and/ or progression of inflammatory and autoimmune diseases [37,38]. In the present study, IBD patients showed increased numbers of Th17 cells compared with controls. These results are in agreement with findings of Annunziato, Cosmi [39] who also reported that CD4+ T cells from peripheral blood and tissues of Crohn’s disease patients express high levels of IL-17, with up to 40% co-expressing IFN-γ. In addition, our data support previous studies suggesting that Th17 cells are critical for the development of IBD and that their levels are associated with disease activity [40].
Moreover, our data demonstrate that CD patients have significantly higher Th1 percentage than UC and healthy controls. These results are in agreement with Sakuraba, Sato [41], who reported that Th1/Th17 type of immune response is most frequent in CD. Interestingly, we found that levels of circulating Th1 and Th17 correlate positively with CD200 expression on CD4+ T-cells. These data may provide further evidence for previous findings that expression of CD200 is enhanced in response to the immune system activation during the inflammatory response [42].
On the contrary, we found that IBD patients have a significant decrease in CD4+ expressing CD200R1 both in UC and CD patients. In addition, CD200R1+CD4+ T-cells showed significant positive correlation with Th2 and Treg. This is in accordance with previous studies on mice and humans which demonstrated increased expression of CD200R mRNA in T helper type 2-polarized T cells [43]. In addition, these results are supported by a previous in vitro study by Gorczynski, Khatri [16] which demonstrated the importance of CD200 and CD200R1 axis in the induction of regulatory t-cells.
CONCLUSION
Taken together, our results demonstrate that IBD patients have altered expressions of the immunomodulatory molecules CD200/CD200R1 on CD4 T-helper lymphocytes of IBD patients and that these alterations are associated with an imbalance between Treg and inflammatory effector cells Th1 and Th17. Finally, although limited by the small numbers of pediatric IBD patients, this is the first study to reveal an aberrant expression of CD200/CD200R1 on CD4+ T-cells in IBD patients and these data may have potent pathological significance in IBD pathophysiology.
Acknowledgement:
This project was funded by the Deanship of Scientific Research at King Abdulaziz University, Jeddah, under grant number RG/02/32. The authors, therefore, Acknowledge with thanks DSR for technical and financial support.
LIST OF ABBREVIATIONS
- IBD
inflammatory bowel diseases
- UC
ulcerative colitis
- CD
Crohn’s disease
- FACS
fluorescence activated cell sorter
Footnotes
Declaration of Interest:
None of the authors has any potential financial conflict of interest related to this manuscript.
References:
- 1.Persson P, Bernell O, Leijonmarck CE, Farahmand BY, Hellers G, Ahlbom A. Survival and cause-specific mortality in inflammatory bowel disease: a population-based cohort study. Gastroenterology 1996;110(5):1339–45. [DOI] [PubMed] [Google Scholar]
- 2.El Mouzan MI, Abdullah AM, Al Habbal MT. Epidemiology of juvenile-onset inflammatory bowel disease in central Saudi Arabia. J Trop Pediatr 2006;52(1):69–71. [DOI] [PubMed] [Google Scholar]
- 3.Benchimol EI, Manuel DG, Guttmann A, et al. Changing age demographics of inflammatory bowel disease in Ontario, Canada: a population-based cohort study of epidemiology trends. Inflamm Bowel Dis 2014;20(10):1761–9. [DOI] [PubMed] [Google Scholar]
- 4.Martin-de-Carpi J, Rodriguez A, Ramos E, et al. Increasing incidence of pediatric inflammatory bowel disease in Spain (1996–2009): the SPIRIT Registry. Inflamm Bowel Dis 2013;19(1):73–80. [DOI] [PubMed] [Google Scholar]
- 5.El Mouzan MI, Saadah O, Al-Saleem K, et al. Incidence of Pediatric Inflammatory Bowel Disease in Saudi Arabia: A Multicenter National Study. Inflamm Bowel Dis 2014;20(6):1085–90. [DOI] [PubMed] [Google Scholar]
- 6.Izcue A, Coombes JL, Powrie F. Regulatory T cells suppress systemic and mucosal immune activation to control intestinal inflammation. Immunol Rev 2006;212:256–71. [DOI] [PubMed] [Google Scholar]
- 7.Hermiston ML, Gordon JI. Inflammatory bowel disease and adenomas in mice expressing a dominant negative N-cadherin. Science 1995;270(5239):1203–7. [DOI] [PubMed] [Google Scholar]
- 8.Bliss SK, Bliss SP, Beiting DP, Alcaraz A, Appleton JA. IL-10 regulates movement of intestinally derived CD4+ T cells to the liver. J Immunol 2007;178(12):7974–83. [DOI] [PubMed] [Google Scholar]
- 9.Grant AJ, Lalor PF, Salmi M, Jalkanen S, Adams DH. Homing of mucosal lymphocytes to the liver in the pathogenesis of hepatic complications of inflammatory bowel disease. Lancet 2002;359 (9301):150–7. [DOI] [PubMed] [Google Scholar]
- 10.Do J-s, Visperas A, Dong C, Baldwin WM, Min B. Cutting edge: Generation of colitogenic Th17 CD4 T cells is enhanced by IL-17+ γδ T cells. J Immunol 2011;186(8):4546–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cong Y, Brandwein SL, McCabe RP, et al. CD4+ T cells reactive to enteric bacterial antigens in spontaneously colitic C3H/HeJBir mice: increased T helper cell type 1 response and ability to transfer disease. J Exp Med 1998;187(6):855–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dupaul-Chicoine J, Dagenais M, Saleh M. Crosstalk between the intestinal microbiota and the innate immune system in intestinal homeostasis and inflammatory bowel disease. Inflamm Bowel Dis 2013;19(10): 2227–37. [DOI] [PubMed] [Google Scholar]
- 13.Odorizzi PM, Wherry EJ. Inhibitory receptors on lymphocytes: insights from infections. J Immunol 2012;188(7):2957–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.1Holmannova D, Kolackova M, Kondelkova K, Kunes P, Krejsek J, Andrys C. CD200/CD200R paired potent inhibitory molecules regulating immune and inflammatory responses; Part I: CD200/ CD200R structure, activation, and function. Acta Medica (Hradec Kralove) 2012;55(1):12–7. [DOI] [PubMed] [Google Scholar]
- 15.Rygiel T, Karnam G, Goverse G, et al. CD200-CD200R signaling suppresses anti-tumor responses independently of CD200 expression on the tumor. Oncogene 2012; 31(24): 2979–88. [DOI] [PubMed] [Google Scholar]
- 16.Gorczynski R, Khatri I, Lee L, Boudakov I. An interaction between CD200 and monoclonal antibody agonists to CD200R2 in development of dendritic cells that preferentially induce populations of CD4+CD25+ T regulatory cells. J Immunol 2008;180(9):5946–55. [DOI] [PubMed] [Google Scholar]
- 17.Liu Y, Bando Y, Vargas-Lowy D, et al. CD200R1 agonist attenuates mechanisms of chronic disease in a murine model of multiple sclerosis. J Neurosci 2010;30(6):2025–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zholudev A, Zurakowski D, Young W, Leichtner A, Bousvaros A. Serologic testing with ANCA, ASCA, and anti-OmpC in children and young adults with Crohn’s disease and ulcerative colitis: diagnostic value and correlation with disease phenotype. Am J Gastroenterol 2004;99(11):2235–41. [DOI] [PubMed] [Google Scholar]
- 19.Ren Y, Yang B, Yin Y, et al. Aberrant CD200/CD200R1 expression and its potential role in Th17 cell differentiation, chemotaxis and osteoclastogenesis in rheumatoid arthritis. Rheumatology 2015;54(4):712–21. [DOI] [PubMed] [Google Scholar]
- 20.Zhu J, Yamane H, Paul WE. Differentiation of Effector CD4 T Cell Populations. Annu Rev Immunol 2010;28:445–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Powrie F, Leach MW, Mauze S, Caddle LB, Coffman RL. Phenotypically distinct subsets of CD4+ T cells induce or protect from chronic intestinal inflammation in C. B-17 scid mice. Int Immunol 1993;5(11):1461–71. [DOI] [PubMed] [Google Scholar]
- 22.Mottet C, Uhlig HH, Powrie F. Cutting edge: cure of colitis by CD4+CD25+ regulatory T cells. J Immunol 2003;170(8):3939–43. [DOI] [PubMed] [Google Scholar]
- 23.Cools N, Ponsaerts P, Van Tendeloo VF, Berneman ZN. Balancing between immunity and tolerance: an interplay between dendritic cells, regulatory T cells, and effector T cells. J Leukoc Biol 2007;82(6):1365–74. [DOI] [PubMed] [Google Scholar]
- 24.Kanai T, Watanabe M, Hibi T. Systemically circulating colitogenic memory CD4+T cells may be an ideal target for the treatment of inflammatory bowel diseases. Keio J Med 2009;58(4): 203–9. [DOI] [PubMed] [Google Scholar]
- 25.Chen DX, He H, Gorczynski RM. Synthetic peptides from the N-terminal regions of CD200 and CD200R1 modulate immunosuppressive and anti-inflammatory effects of CD200-CD200R1 interaction. Int Immuno 2005;17(3):289–96. [DOI] [PubMed] [Google Scholar]
- 26.Fallarino F, Asselin-Paturel C, Vacca C, et al. Murine plasmacytoid dendritic cells initiate the immunosuppressive pathway of tryptophan catabolism in response to CD200 receptor engagement. J Immunol 2004;173(6):3748–54. [DOI] [PubMed] [Google Scholar]
- 27.Gorczynski RM. CD200 and its receptors as targets for immunoregulation. Curr Opin Investig Drugs 2005;6(5):483–8. [PubMed] [Google Scholar]
- 28.Li Y, Zhao LD, Tong LS, et al. Aberrant CD200/CD200R1 expression and function in systemic lupus erythematosus contributes to abnormal T-cell responsiveness and dendritic cell activity. Arthritis Res Ther 2012;14(3):R123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chakera A, Bennett SC, Morteau O, Bowness P, Luqmani RA, Cornall RJ. The phenotype of circulating follicular-helper T cells in patients with rheumatoid arthritis defines CD200 as a potential therapeutic target. Clin Dev Immunol 2012;2012:948218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lanna CCD, Ferrari MdLA, Rocha SL, Nascimento E, de Carvalho MAP, da Cunha AS. A cross-sectional study of 130 Brazilian patients with Crohn’s disease and ulcerative colitis: analysis of articular and ophthalmologic manifestations. Clin Rheumatol 2008;27(4):503–9. [DOI] [PubMed] [Google Scholar]
- 31.De Jager PL, Graham R, Farwell L, et al. The role of inflammatory bowel disease susceptibility loci in multiple sclerosis and systemic lupus erythematosus. Genes Immun 2006;7(4):327–34. [DOI] [PubMed] [Google Scholar]
- 32.Hernangomez M, Carrillo-Salinas FJ, Mecha M, et al. Brain innate immunity in the regulation of neuroinflammation: therapeutic strategies by modulating CD200-CD200R interaction involve the cannabinoid system. Curr Pharm Des 2014;20(29):4707–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Maul J, Loddenkemper C, Mundt P, et al. Peripheral and intestinal regulatory CD4+ CD25 (high) T cells in inflammatory bowel disease. Gastroenterology 2005;128(7):1868–78. [DOI] [PubMed] [Google Scholar]
- 34.Gorczynski RM, Lee L, Boudakov I. Augmented Induction of CD4+CD25+ Treg using monoclonal antibodies to CD200R. Transplantation 2005;79(9):1180–3. [PubMed] [Google Scholar]
- 35.Eastaff-Leung N, Mabarrack N, Barbour A, Cummins A, Barry S. Foxp3+ regulatory T cells, Th17 effector cells, and cytokine environment in inflammatory bowel disease. J Clin Immunol 2010;30(1):80–9. [DOI] [PubMed] [Google Scholar]
- 36.Korn T, Bettelli E, Oukka M, Kuchroo VK. IL-17 and Th17 Cells. Annu Rev Immunol 2009;27:485–517. [DOI] [PubMed] [Google Scholar]
- 37.Noack M, Miossec P. Th17 and regulatory T cell balance in autoimmune and inflammatory diseases. Autoimmun Rev 2014;13(6):668–77. [DOI] [PubMed] [Google Scholar]
- 38.Bettelli E, Carrier Y, Gao W, et al. Reciprocal developmental pathways for the generation of pathogenic effector TH17 and regulatory T cells. Nature 2006;441(7090):235–8. [DOI] [PubMed] [Google Scholar]
- 39.Annunziato F, Cosmi L, Santarlasci V, et al. Phenotypic and functional features of human Th17 cells. J Exp Med 2007;204(8):1849–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jiang W, Su J, Zhang X, et al. Elevated levels of Th17 cells and Th17-related cytokines are associated with disease activity in patients with inflammatory bowel disease. Inflamm Res 2014;63 (11):943–50. [DOI] [PubMed] [Google Scholar]
- 41.Sakuraba A, Sato T, Kamada N, Kitazume M, Sugita A, Hibi T. Th1/Th17 immune response is induced by mesenteric lymph node dendritic cells in Crohn’s disease. Gastroenterology 2009;137(5): 1736–45. [DOI] [PubMed] [Google Scholar]
- 42.Mukhopadhyay S, Plüddemann A, Hoe JC, et al. Immune inhibitory ligand CD200 induction by TLRs and NLRs limits macrophage activation to protect the host from meningococcal septicemia. Cell Host Microbe 2010;8(3):236–47. [DOI] [PubMed] [Google Scholar]
- 43.Wright GJ, Cherwinski H, Foster-Cuevas M, et al. Characterization of the CD200 receptor family in mice and humans and their interactions with CD200. J Immunol 2003;171(6):3034–46. [DOI] [PubMed] [Google Scholar]