Abstract
Background:
Lactate clearance has been developed into a marker of resuscitation in trauma, but no study has compared the predictive power of the various clearance calculations. Our objective was to determine which method of calculating lactate clearance best predicted 24-hour and in-hospital mortality after injury.
Study design:
Retrospective chart review of patients admitted to a Level-1 trauma center directly from the scene of injury from 2010 to 2013 who survived >E15 min, had an elevated lactate at admission (≥3 mmol/L), followed by another measurement within 24 h of admission. Lactate clearance was calculated using five models: actual value of the repeat level, absolute clearance, relative clearance, absolute rate, and relative rate. Models were compared using the areas under the respective receiver operating curves (AUCs), with an endpoint of death at 24 h and in-hospital mortality.
Results:
3910 patients had an elevated admission lactate concentration on admission (mean = 5.6 ± 3.0 mmol/L) followed by a second measurement (2.7 ± 1.8 mmol/L). Repeat absolute measurement best predicted 24-hour (AUC = 0.85, 95% CI: 0.84–0.86) and in-hospital death (AUC = 0.77; 95% CI, 0.76–0.78). Relative clearance was the best model of lactate clearance (AUC = 0.77, 95% CI: 0.75–0.78 and AUC = 0.705, 95% CI: 0.69–72, respectively) (p < 0.0001 for each). A sensitivity analysis using a range of initial lactate measures yielded similar results.
Conclusions:
The absolute value of the repeat lactate measurement had the greatest ability to predict mortality in injured patients undergoing resuscitation.
Keywords: Lactate, Clearance, Calculation, Trauma, Resuscitation, Survival
1. Introduction
Injury is one of the leading causes of loss of life across the world [1]. Hemorrhage and shock continue to be the most common causes of death in injured patients [2]. Unfortunately, traditional heart rate and blood pressure vital signs are relatively insensitivemethods of detecting shock [3]. This is especially true in the elderly, in whom a diminished cardiovascular response to hypoperfusion may be blunted by medications and comorbidities [4,5]. The peripheral venous lactate concentration has been shown to be elevated in these patients, revealing a highmortality cohortwith normal vital signs [6–8]. Abramson and colleagues reported that critically injured patients who were able to reduce or “clear” an initially elevated lactate concentration in response to resuscitation all survived to discharge [9].
In more recent work, we demonstrated that poor relative lactate clearance is a better predictor of early death than severe brain injury or abnormal vital signs [10]. Over the past two decades, lactate has been developed as a method of detecting at-risk patients [11,12], and lactate clearance has been used as a marker of resuscitation in both traumatic and septic shock [13,14]. Measuring lactates is inexpensive [15], fast [16], portable [17], and there aremultipleways to obtain equivalent samples [18–21], making lactate an ideal biomarker. Furthermore, lactate predicts mortality as well, or better than, base deficit [22–24], including patients with normal base deficits [25].
However, only recently has anyone studied alternate ways lactate clearance can be calculated. Puskarich and colleagues [26] sought to determine which method of calculating lactate clearance best predicted survival in a cohort of patients with sepsis undergoing resuscitation. This is an important question because clinicians are often faced with having to interpret small changes in a patient’s lactate concentration, where the absolute or relative lactate clearance may be discordant. A hierarchy of how to interpret changes in lactate could help clinicians determine when the patient is improving, or when they need further interventions. The purpose of this investigation is to determine which method of calculating lactate clearance provides the best predictions of 24-hour and in-hospital mortality after traumatic injury.
2. Materials and methods
2.1. Patients and parameters
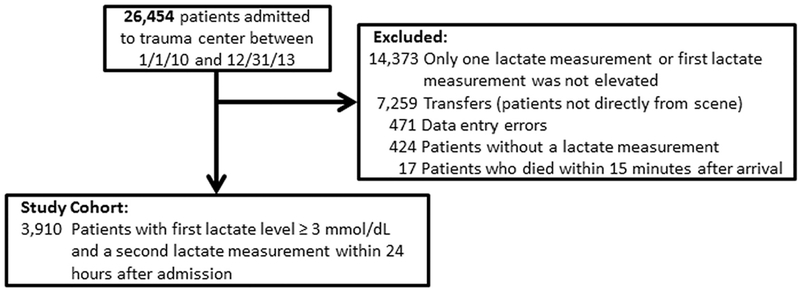
The subjects for this single-center retrospective chart review were patients admitted to a Level I trauma center between January 1, 2010, and December 31, 2013. All of the data in this study was abstracted electronically from our trauma registry. All patients underwent resuscitation according to our institutional guidelines, which includes crystal-loid and blood transfusions at the direction of the attending physician, a venous lactate concentration measured on arrival. To be included in the main analysis, subjects had to 1) be transported directly from the scene of injury, 2) have two lactate measurements within the first 24 h of admission, and 3) their initial lactate level was critically elevated (≥3.0 mmol/L). Lactates were measured using a gas chromatograph in our hospital laboratory, where lactates ≤2.0 mmol/dL are considered normal. For this analysis, the second lactate result received was considered the second measurement of lactate. Death at 24 h and in-hospital mortality were used as endpoints for all calculations [27]. Patients were excluded if they had been transferred from another facility, died within 15 min after arrival, had incomplete records, did not have serial lactate measurements, or did not have an initial elevated lactate concentration (see consort diagram, Fig. 1). This study protocol was approved by the Institutional Review Board at the medical facility with which the authors are affiliated.
Fig. 1.
CONSORT Diagram for the population studied.
2.2. Statistical methods
Demographics and baseline characteristics of the study population was presented. Normally distributed values were summarized using mean and standard deviation; all other values were reported as median and interquartile range (IRQ). All tests used a significance level of 0.05 and analyses were completed using SAS 9.3 and MedCalc (v12.7.7.0 for Windows 8, copyright ©1993–2013). Demographic information, documentation of death at 24 h, death in-hospital, lactate levels, and timing of test results were extracted from our patient registry.
2.3. Definition of lactate clearance models
Serial lactate measurements were used to calculate five models of clearance for each subject: actual value of the repeat level (Lactate2, mmol/L), absolute clearance (Lactate1-Lactate2, mmol), relative clearance ([Lactate1-Lactate2]/Lactate1, %), absolute rate ([Lactate1- Lactate2]/[timeLactate1-lactate2], mmol/h), and relative rate ([Lactate1-Lactate2]/[Lactate1 × timeLactate1-Lactate2], %/h). The test characteristics of the first lactate taken on admission was included for context.
2.4. Comparison of lactate clearance models
We compared the different models using the area under the receiver operating curves (AUCs) [28]. These dependent AUCs were compared using Delong’s method [29] and then Bonferronni-corrected for multiple comparisons. Cutoff values that optimized the sensitivity, specificity, and positive and negative likelihood ratios for each model were calculated. ORs for the cutoff values for the best performing measures of clearance were also calculated.
2.5. Sensitivity analysis
The definition of elevated initial lactate measurement may differ between institutions. We sought to demonstrate the robustness of the highest-performing models by recalculating the AUCs and optimal cutoffs for the highest-performing lactate clearance models using the following patient populations: 1) any patient with two lactate measurements (regardless of level), 2) any patient with two lactates, where the first was ≥2 mmol/dL, and 3) any patient with two lactates, with the first ≥4 mmol/dL.
3. Results
3.1. Characteristics of the study cohort
Of the 26,454 patients seen during the study period, 3910 patients met the study criteria (Fig. 1). The majority of excluded subjects had only one lactate measurement (7732 [29.1%]) or were transfers who were resuscitated prior to arrival at our center (7259 [27.4]). Those patients who were excluded for having only a single lactate measurement had an in-hospital mortality of 1.6%. The subjects included in the study had an in-hospital mortality of 7.3%. The demographic, clinical, and laboratory data at the time of admission of the study subjects are shown in Table 1. Within the study population, the mean lactate level on admission for survivors was significantly lower than for nonsurvivors (5.5 ± 3.0 vs 8.5 ± 4.3 mmol/L, respectively [p b 0.0001]). Repeat lactate measurements also differed between survivors and nonsurvivors (2.7 ± 1.8 vs 7.8 ± 4.8 [p < 0.0001]). Survival was high (99.4%) among the minority of patients (1504 [38.5%]) whose second lactate measurement was normal (≤2.0 mmol/dL). The initial lactate measurements were resulted and available to clinicians a median 13.0 min after patients’ arrival (IRQ, 9–19 min). Repeat measurements were reported a median of 4.2 h later (IRQ, 2.6–6.7 h).
Table 1.
Demographics, injury severity, injury mechanism, and admission laboratory results and vital signs of cohort of patients with serial lactates (Overall Cohort, N = 3910).
| Age, mean (SD) | 39.1 (17.9) |
| Male sex, N (%) | 3094 (79.1) |
| Race, N, (%) | |
| White | 2044 (52.3) |
| Black | 1601 (40.9) |
| Hispanic | 93 (2.4) |
| Other | 160 (4.1) |
| ISS, N (%) | |
| <9 | 1173 (30.0) |
| 9–15 | 924 (23.6) |
| 16–25 | 694 (17.7) |
| >25 | 1073 (27.4) |
| Mechanism, N (%) | |
| MVC | 1659 (42.4) |
| Falls | 726 (18.6) |
| Stabbing | 416 (10.6) |
| Gunshot | 472 (12.1) |
| Admission Laboratory Values | |
| Hemoglobin, mg/L | 13.5 (2.0) |
| Creatinine, mg/L | 1.1 (0.5) |
| Total bilirubin, mg/L | 1.1 (0.9) |
| First lactate (mmol/L), mean (SD) | 5.6 (3.0) |
| Second lactate (mmol/L), mean (SD) | 2.7 (1.8) |
| Time to second lactate measurement (hours), median (IRQ) | 4.16 (2.6–6.7) |
| Admission GCS score, N (%) | |
| 3–8 | 618 (15.8) |
| 9–13 | 431 (11.0) |
| 14–15 | 2861 (73.2) |
| Admission Shock Index, mean (SD) | 0.7 (0.2) |
3.2. Assessment of approaches of calculating lactate clearance
The average absolute rate of lactate clearance was 0.70 mmol/dL/h (25th–75th percentile: 0.07–0.72 mmol/dL/h). Absolute rate was no better than chance at predicting 24-hour or in-hospital mortality (AUC = 0.57, 95% CI: 0.55–0.58, AUC = 0.54, 95% CI: 0.52–0.55, respectively). The average absolute difference was 2.08 mmol/dL (25th–75th percentile: 0.60–2.80 mmol/dL) and the relative rate of clearance was 10.40%/h (25th–75th percentile: 2.47–15.01%/h). Absolute difference (AUC = 0.68, 95% CI: 0.66–0.69, AUC = 0.63, 95% CI: 0.62–0.65, respectively) and relative rate (AUC = 0.59, 95% CI:0.58–0.61, AUC = 0.55, 95% CI:0.53–0.57) were also poor predictors of 24-h and in-hospital mortality. The value of the repeat lactate measurement had the highest AUC for both 24-h and in-hospital mortality (Table 2), which was significantly higher than all other models of calculating clearance (p < 0.0001 for all comparisons). Relative lactate clearance had the next highest AUC for early- and overall hospital mortality (AUC = 0.77 and AUC = 0.71 respectively), which was significantly higher than the AUC calculated using absolute clearance, relative clearance rate, and absolute clearance rate (p b 0.0001 for all comparisons). Table 2 also demonstrates the diagnostic value added by repeated lactates, as both the relative lactate clearance and the second lactate value are stronger of predictors of 24-h and in-hospital survival than the value of the first lactate.
Table 2.
Highest-performing models of lactate clearance and optimal cut-offs for predicting mortality at 24 h and in-hospital mortality after injury.
| Model | Mean, 25th–75th percentile | Endpoint | AUC (95% CI) | Optimal cutoff | Sensitivity (95% CI) | Specificity (95% CI) | +LR | −LR |
|---|---|---|---|---|---|---|---|---|
| First lactatea | 4.6, 2.8–5.3 mmol/dL | 24-hour | 0.73 (0.72–0.75) | >5.4 mmol/dL | 71.6 (62.1–79.8) | 66.3 (64.8–67.8) | 2.1 | 0.4 |
| In-hospital | 0.63 (0.61–0.64) | >5.1 mmol/dL | 58.3 (52.3–64.0) | 62.8 (61.2–64.4) | 1.6 | 0.7 | ||
| Second value | 2.5, 1.5–3.0 mmol/dL | 24-hour | 0.85 (0.84–0.86) | >3.7 mmol/dL | 76.2 (67.0–83.8) | 82.8 (81.6–84.0) | 4.2 | 0.3 |
| In-hospital | 0.77 (0.76–0.78) | >3.6 mmol/dL | 61.1 (55.1–66.7) | 83.3 (82.1–84.5) | 3.7 | 0.5 | ||
| Relative difference | 37%, 18%–63% | 24-hour | 0.77 (0.75–0.78) | <32% | 76.1 (67.0–83.8) | 69.6 (68.1–71.1) | 2.5 | 0.3 |
| In-hospital | 0.71 (0.66–0.69) | <32% | 62.1 (55.1–66.7) | 70.1 (68.6–71.6) | 2.1 | 0.5 |
Carried forward to show the diagnostic value of the various models of lactate clearance.
3.3. Resuscitation benchmarks and sensitivity analysis
The cut-offs that optimize sensitivity and specificity for each model and mortality endpoint, as well as their associated positive and negative likelihood ratios, are shown in Table 2. Table 3 shows the results of the sensitivity analysis. Over a range of selection criteria, we found that patients had increased chances for survival if their second lactate <3.6 mmol/dL or if their lactate level had decreased by about a third.
Table 3.
Sensitivity analysis of lactate clearance using various selection criteria.
| Model | Endpoint | Selection criteria | N | Mortality (N, %) | Threshold | AUC (95% CI) |
|---|---|---|---|---|---|---|
| Second value | 24-hour mortality | Any two lactates | 6641 | 127 (1.9) | >3.7 | 0.84 (0.83 to 0.85) |
| First lactate ≥2 | 5653 | 121 (2.1) | >3.7 | 0.85 (0.84 to 0.86) | ||
| First lactate ≥3 | 3910 | 109 (2.8) | >3.7 | 0.85 (0.84 to 0.86) | ||
| First lactate ≥4 | 2488 | 94 (3.8) | >5.1 | 0.88 (0.86 to 0.89) | ||
| In-hospital | Any two lactates | 6641 | 391 (5.9) | >3.6 | 0.72 (0.71 to 0.74) | |
| First lactate ≥2 | 5653 | 355 (6.3) | >3.6 | 0.74 (0.73 to 0.76) | ||
| First lactate ≥3 | 3910 | 285 (7.3) | >3.6 | 0.77 (0.76 to 0.78) | ||
| First lactate ≥4 | 2488 | 221 (8.9) | >3.7 | 0.79 (0.77 to 0.81) | ||
| Relative difference | 24-hour mortality | Any two lactates | 6641 | 127 (1.9) | <19% | 0.69 (0.68 to 0.70) |
| First lactate ≥2 | 5653 | 121 (2.1) | <32% | 0.72 (0.71 to 0.74) | ||
| First lactate ≥3 | 3910 | 109 (2.8) | <32% | 0.77 (0.75 to 0.78) | ||
| First lactate ≥4 | 2488 | 94 (3.8) | <32% | 0.83 (0.81 to 0.84) | ||
| In-hospital | Any two lactates | 6641 | 391 (5.9) | <29% | 0.64 (0.63 to 0.65) | |
| First lactate ≥2 | 5653 | 355 (6.3) | <29% | 0.67 (0.65 to 0.68) | ||
| First lactate ≥3 | 3910 | 285 (7.3) | <32% | 0.71 (0.69 to 0.72) | ||
| First lactate ≥4 | 2488 | 221 (8.9) | <32% | 0.75 (0.74 to 0.77) |
4. Discussion
In this large, single-center study, we compared the various methods of calculating lactate clearance in a large retrospective chart review of injured patients. The current study complements work by Puskarich and colleagues, who found that relative lactate clearance and lactate normalization were strong predictors of survival in septic patients undergoing protocolized resuscitation [31]. In our study of trauma patients, all of the models of clearance were significantly better than chance, but relative clearance and simply taking the second value were the best predictors of 24 h and in-hospital mortality. Similar to other studies, patients with decreasing lactate values during resuscitation were more likely to survive [9,10,30]. In this study population, the AUC of both the second lactate value and relative lactate clearance were greater than that of the AUC of the initial lactate. This implies that there was a group of patients that was at risk of dying that was not identified by a single lactate on admission, and there is diagnostic value to repeating lactates through a patient’s resuscitation. The sensitivity analysis shows that the optimal thresholds of the second value of lactate and relative lactate clearance are generally constant in this study population across multiple categories of initial lactates. This suggests that these results may be generalizable to other trauma centers with different definitions of abnormal lactate concentration.
Absolute lactate clearance was the least predictive of the models studied, perhaps because multiple interpretations can be drawn from a single absolute difference. For example, a drop of 4 mmol/L in the lac-tate level would be considered an improvement in most patients, but if a patient started at a lactate of 8 mmol/L, he or she is still at high risk of dying if the second lactate value is 4 mmol/L. Similarly, a drop of 2 mmol/L might be considered a poor response in a patient with an initial lactate of 8 mmol/L but would be considered to indicate normalization if the initial concentration was 4 mmol/L. Large absolute decreases in lactate (e.g., >10 mmol/L) are almost certainly an unambiguous improvement, but these are infrequent cases. Small drops of 2 to 4 are much more common. The multiple different interpretations of these small drops in lactate concentration likely diminishes the predictive performance of absolute lactate clearance as a marker of resuscitation.
The second lactate measurement, taken as a single value without context, had the best predictive value of the models studied, including the first lactate taken on admission. Similar to patients with septic shock [14], trauma patients with a lactate concentration close to 4.0 mmol/L are at increased risk of death. As shown in a separate analysis, the second lactate measurement performed as well as admission lactate (AUC = 0.85) [10]. Both of the time-dependent models had no predictive value, similar to the work by Puskarich [26]. One possible explanation is that oxygen delivery to the tissues might fluctuate rapidly as patients decompensate or improve in response to multiple simultaneous resuscitative interventions. Given that the half-life of lactate is 15 to 30 min in healthy subjects [32] and that the first and second lac-tate measurements were separated by >4 h, there could have been large variations in lactate levels that were not captured in our testing interval. This implies that each lactate measurement is simply a “snapshot in time” and indicative of the patient’s current oxygen delivery status, independent of prior performance.
We excluded those patients who did not survive N15 min because these patients are clinically obvious and lactate would have been of little additional diagnostic value. In addition, patients who survived N15 min and only one lactate measurement had excellent survival (99.1%). This suggests our study exclusion criteria was appropriate and the majority of the critically-ill patients were properly funneled into the final study group.
5. Limitations
This study selected a series severely injured patients with high lac-tate concentrations out of the general population of patients seen at our trauma center. This approach allowed us to create a natural experiment, but it generated biases. These results should be replicated, ideally by a prospective trial of injured patients undergoing resuscitation with lactate measurements taken at regular timed intervals.
We could not control for the timing of the lactate measurements. The first measure was uniformly reported shortly after admission. The majority of the second lactate measurements were completed within 6 h of admission, similar to other studies of lactate clearance [9,13,26,30,33]. We eliminated a large proportion of the patients seen over the study period to reduce the heterogeneity of the data set. This approach may have inadvertently excluded a group of high-risk patients. However, the in-hospital mortality of this excluded group was low (1.6%).
There are a number of factors that may alter a patient’s lactate metabolism, independent of acute injury. Biguanides [34] and nucleoside reverse transcriptase inhibitors (HIV) [35], are two classes of medications that are known to cause lactic acidosis. HIV itself, as well as sepsis, seizures, carbon monoxide poisoning, strenuous exercise, and respiratory failure may all raise baseline lactate [36,37]. Liver dysfunction as the result of viral hepatitis, alcoholic cirrhosis, acetaminophen toxicity, and hepatic steatosis can all increase resting lactate through impaired lactate clearance [38,39]. Trauma patients often have high blood alcohol levels on admission, which decreases lactate metabolism in a dose-dependent manner [40]. This study did not examine these factors independently. Physicians should be cautious when applying our results to injured patients with these conditions.
6. Conclusions
The raw value of the second lactate measurement had the greatest ability to predict short-term mortality in our population of severely injured patients, followed by relative lactate clearance. Patients who had a second lactate level concentration <3.7 mmol/L, or who had a relative lactate clearance ≥32%, had improved likelihood for survival. These values could potentially be used as guides for determining whether an injured patient is improving in response to resuscitative efforts.
Acknowledgments
The authors have no conflicts of interest to disclose. Dr. Smith was supported by a grant from the U.S. National Institute on Alcohol Abuse and Alcoholism (R01AA18707). Dr. Hirshon was supported by a grant from the U.S. National Institute of Health Fogarty International Center (5D43TW007296). Drs. Yang, Hu and Mackenzie were supported by grant FA8650–11-2-6D01, US Air Force Medical Support Agency/Medical Modernization Directorate (SG9). Some of the information contained in this manuscript was presented as an abstract at the American College of Emergency Medicine Scientific Assembly in Boston, MA, 2015 (Dezman ZDW, et al., “Repeat Lactate Value, Not Lactate Clearance, Best Predicts 24-Hour Mortality in Injured Patients. Annals of Emergency Medicine. 2015 Oct 1;66(4):S108”).
References
- [1].Centers for Disease Control and Prevention. Injury: The Leading Cause of Death Among Persons Ages 1–44. Available online at: www.cdc.gov/injury/index.html. (Accessed on September 11, 2015).
- [2].Shackelford SA, Colton K, Stansbury LG, et al. Early identification of uncontrolled hemorrhage after trauma: current status and future direction. J Trauma Acute Care Surg 2014;77(3 suppl 2):S222–7. [DOI] [PubMed] [Google Scholar]
- [3].Basel KJ, Guse C, Gentilello LM, Nirula R. Heart rate: is it truly a vital sign? J Trauma 2007;62:812–7. [DOI] [PubMed] [Google Scholar]
- [4].Heffernan DS, Thakkar RK, Monaghan SF, et al. Normal presenting vital signs are unreliable in geriatric blunt trauma victims. J Trauma 2010;69:813–20. [DOI] [PubMed] [Google Scholar]
- [5].Zarzaur BL, Croce MA, Magnotti LJ, et al. Identifying life-threatening shock in the older injured patient: an analysis of the National Trauma Data Bank. J Trauma 2010;68:1134–8. [DOI] [PubMed] [Google Scholar]
- [6].Scalea TM, Holman M, Fuortes M, et al. Central venous blood oxygen saturation: an early accurate measurement of volume status during hemorrhage. J Trauma 1998; 28:725–32. [PubMed] [Google Scholar]
- [7].Callaway DW, Shapiro NI, Donnino MW, et al. Serum lactate and base deficit as predictors of mortality in normotensive elderly blunt trauma patients. J Trauma 2009; 66:1040–4. [DOI] [PubMed] [Google Scholar]
- [8].del Portal DA, Shofer F, Mikkelsen ME, et al. Emergency department lactate is associated with mortality in older adults admitted with and without infections. Acad Emerg Med 2010;17:260–8. [DOI] [PubMed] [Google Scholar]
- [9].Abramson D, Scalea TM, Hitchcock R, et al. Lactate clearance and survival following injury. J Trauma 1993;35:584–9. [DOI] [PubMed] [Google Scholar]
- [10].Dezman ZD, Comer AC, Smith GS, Narayan M, Scalea TM, Hirshon JM. Failure to clear elevated lactate predicts 24-hour mortality in trauma patients. J Trauma Acute Care Surg 2015;79(4):580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Lavery RF, Livingston DH, Tortella BJ, et al. The utility of venous lactate to triage injured patients in the trauma center. J Am Coll Surg 2000;190:656–64. [DOI] [PubMed] [Google Scholar]
- [12].Vandromme MJ, Griffin RL, Weinberg JA, et al. Lactate is a better predictor than systolic blood pressure for determining blood requirement and mortality: could prehospital measures improve trauma triage? J Am Coll Surg 2010;210:861–9. [DOI] [PubMed] [Google Scholar]
- [13].Jones AE, Shapiro NI, Trzeciak S, et al. , Emergency Medicine Shock Research Network (EMShockNet) Investigators. Lactate clearance vs central venous oxygen saturation as goals of early sepsis therapy: a randomized clinical trial. JAMA 2010;303:739–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Dellinger RP, Levy MM, Rhodes A, et al. , Surviving Sepsis Campaign Guidelines Committee including the Pediatric Subgroup. Surviving sepsis campaign: international guidelines for management of severe sepsis and septic shock: 2012. Crit Care Med 2013;41:580–637. [DOI] [PubMed] [Google Scholar]
- [15].Jansen TC, van Bommel J, Bakker J. Blood lactate monitoring in critically ill patients: a systematic health technology assessment. Crit Care Med 2009;37(10):2827–39. [DOI] [PubMed] [Google Scholar]
- [16].Aduen J, Bernstein WK, Khastgir T, Miller J, Kerzner R, Bhatiani A, et al. The use and clinical importance of a substrate-specific electrode for rapid determination of blood lactate concentrations. JAMA 1994;272(21):1678–85. [PubMed] [Google Scholar]
- [17].Saunders AC, Feldman HA, Correia CE, Weinstein DA. Clinical evaluation of a portable lactate meter in type I glycogen storage disease. J Inherit Metab Dis 2005;28(5): 695–701. [DOI] [PubMed] [Google Scholar]
- [18].Weil MH, Michaels S, Rackow EC. Comparison of blood lactate concentrations in central venous, pulmonary artery, and arterial blood. Crit Care Med 1987;15(5):489–90. [PubMed] [Google Scholar]
- [19].Younger JG, Falk JL, Rothrock SG. Relationship between arterial and peripheral venous lactate levels. Acad Emerg Med 1996;3(7):730–4. [DOI] [PubMed] [Google Scholar]
- [20].Gallagher EJ, Rodriguez K, Touger M. Agreement between peripheral venous and arterial lactate levels. Ann Emerg Med 1997;29(4):479–83. [PubMed] [Google Scholar]
- [21].Fauchère JC, Bauschatz AS, Arlettaz R, Zimmermann-Bär U, Bucher HU. Agreement between capillary and arterial lactate in the newborn. Acta Paediatr 2002;91(1): 78–81. [DOI] [PubMed] [Google Scholar]
- [22].Mikulaschek A, Henry SM, Donovan R, Scalea TM. Serum lactate is not predicted by anion gap or base excess after trauma resuscitation. Journal of Trauma-Injury Infection & Critical Care 1996;40(2):218–24. [DOI] [PubMed] [Google Scholar]
- [23].Husain FA, Martin MJ, Mullenix PS, Steele SR, Elliott DC. Serum lactate and base deficit as predictors of mortality and morbidity. Am J Surg 2003;185(5):485–91. [DOI] [PubMed] [Google Scholar]
- [24].Martin M, Murray J, Berne T, Demetriades D, Belzberg H. Diagnosis of acid-base derangements and mortality prediction in the trauma intensive care unit: the physio-chemical approach. J Trauma 2005;58(2):238–43. [DOI] [PubMed] [Google Scholar]
- [25].Martin MJ, FitzSullivan E, Salim A, Brown CVR, Demetriades D, Long W. Discordance between lactate and base deficit in the surgical intensive care unit: which one do you trust? Am J Surg 2006;191(5):625–30. [DOI] [PubMed] [Google Scholar]
- [26].Puskarich MA, Trzeciak S, Shapiro NI, et al. Whole blood lactate kinetics in patients undergoing quantitative resuscitation for severe sepsis and septic shock. Chest 2013;143:1548–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Zink Karen A, et al. A high ratio of plasma and platelets to packed red blood cells in the first 6 hours of massive transfusion improves outcomes in a large multicenter study. Am J Surg 2009;197(5):565–70. [DOI] [PubMed] [Google Scholar]
- [28].Hanley JA, McNeil BJ. The meaning and use of the area under a receiver operating characteristic (ROC) curve. Radiology 1982;143:29–36. [DOI] [PubMed] [Google Scholar]
- [29].DeLong ER, DeLong DM, Clarke-Person DL. Comparing the areas under two or more correlated receiver operating characteristic curves: a nonparametric approach. Bio-metrics 1988;44:837–45. [PubMed] [Google Scholar]
- [30].Heinonen E, Hardcastle TC, Barle H, Muckart DJJ. Lactate clearance predicts outcome after major trauma. African J of Emerg Med 2014. Jun;4(2):61–5. [Google Scholar]
- [31].Nguyen HB, Rivers EP, Knoblich BP, et al. Early lactate clearance is associated with improved outcome in severe sepsis and septic shock. Crit Care Med 2004;32: 1637–42. [DOI] [PubMed] [Google Scholar]
- [32].Belcastro AN, Bonen A. Lactic acid removal rates during controlled and uncontrolled recovery exercise. J Appl Physiol 1975;39:932–6. [DOI] [PubMed] [Google Scholar]
- [33].Holcomb JB, del Junco DJ, Fox EE, Wade CE, Cohen MJ, Schreiber MA, et al. , PROMMTT Study Group. The prospective, observational, multicenter, major trauma transfusion (PROMMTT) study: comparative effectiveness of a time-varying treatment with competing risks. JAMA Surg 2013. February;148(2):127–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Stang M, Wysowski DK, Butler-Jones D. Incidence of lactic acidosis in metformin users. Diabetes Care 1999. June 1;22(6):925–7. [DOI] [PubMed] [Google Scholar]
- [35].Chariot P, Drogou I, de Lacroix-Szmania I, Eliezer-Vanerot MC, Chazaud B, Lombès A, et al. Zidovudine-induced mitochondrial disorder with massive liver steatosis, myopathy, lactic acidosis, and mitochondrial DNA depletion. J Hepatology 1999. January 31;30(1):156–60. [DOI] [PubMed] [Google Scholar]
- [36].Chattha G, Arieff AI, Cummings C, Tierney LM. Lactic acidosis complicating the acquired immunodeficiency syndrome. Annals of Int Med 1993. January 1;118(1):37–9. [DOI] [PubMed] [Google Scholar]
- [37].Mizock BA, Falk JL. Lactic acidosis in critical illness. Crit Care Med 1992. January 1;20(1): 80–93. [DOI] [PubMed] [Google Scholar]
- [38].Murphy ND, Kodakat SK, Wendon JA, Jooste CA, Muiesan P, Rela M, et al. Liver and intestinal lactate metabolism in patients with acute hepatic failure undergoing liver transplantation. Crit Care Med 2001. November 1;29(11):2111–8. [DOI] [PubMed] [Google Scholar]
- [39].Almenoff PL, Leavy J, Weil MH, Goldberg NB, Vega D, Rackow EC. Prolongation of the half-life of lactate after maximal exercise in patients with hepatic dysfunction. Crit Care Med 1989. September 1;17(9):870–3. [DOI] [PubMed] [Google Scholar]
- [40].Dezman ZD, Comer AC, Narayan M, Scalea TM, Hirshon JM, Smith GS. Alcohol consumption decreases lactate clearance in acutely injured patients. Injury 2016. September 30;47(9):1908–12. [DOI] [PMC free article] [PubMed] [Google Scholar]