Abstract
Bladder dysfunction is associated with fibrosis-mediated aging, but the corresponding mechanism remains to be elucidated. Activation of the NACHT, LRR and PYD domains-containing protein 3 (NLRP3) inflammasome is related to chronic diseases associated with aging, including organ fibrosis. The present study aimed to explore the role of NLRP3/interleukin 1β in aging-associated bladder dysfunction. Female Sprague-Dawley rats were divided into the following two groups (n=10 rats/group): 2-month-old group (young group) and 24-month-old group (old group). Urodynamics were performed to assess the bladder function of the rats. The histological alterations were identified using Masson's trichrome staining. The protein expression of the NLRP3 inflammasome and NAD-dependent protein deacetylase sirtuin-3, mitochondrial (SIRT3) were detected by western blot analysis, and immunohistochemistry was used to examine a senescence marker (p21) and the NLRP3 inflammasome in the bladder. The localization of the key molecule Caspase1 was determined using immunofluorescence. The voiding time was longer in the old group compared with the young group. The expression levels of SIRT3 were reduced in the bladders of the old group, while those of the NLRP3 inflammasome and the senescence marker were significantly higher in the bladders of the old group compared with the young group. Increased collagen deposition leads to chronic bladder fibrosis with increased NLRP3. In the histological examination, the bladders of the old group displayed increased collagen deposition, urothelial thinning and detrusor shrinkage compared with the young group. Tissue fibrosis and urothelial alterations are the principal causes of bladder dysfunction during aging. Downregulated SIRT3 and upregulated expression of the NLRP3 inflammasome are involved in the degradation of aging bladders. Inflamm-aging is a novel mechanism underlying bladder dysfunction.
Keywords: aging, urinary bladder, inflammasomes, urothelium, lower urinary tract symptoms
Introduction
The prevalence of bladder dysfunction increases with age and it strongly influences the quality of life of the elderly population. Aging male patients with prostate hyperplasia are prone to suffer from lower urinary tract symptoms (LUTS), and elderly female patients are susceptible to overactive bladder with absence of bladder outlet obstruction (BOO) (1,2). Therefore, BOO is not the only factor causing bladder dysfunction. Transurethral resection of the prostate is an effective approach to relieve obstruction in males, but complete bladder function is not recovered following prostate resection (3), which may be due to the irreversible bladder damage mediated by aging. Therefore, female rats without BOO were used in the present study. The urodynamics and bladder morphology of aging rats requires investigation. It was speculated that a molecular mechanism associated with the aging process may explain these alterations (4,5).
Age-associated alterations have been investigated more extensively in previous studies, but these have focused primarily on bladder contraction in vivo and in vitro (5) The exact pathophysiological mechanisms of bladder aging remain to be elucidated (6). Inflamm-aging, which offers new insights into the aging process, involves chronic inflammatory cytokine production and functional decline (7). Accumulating evidence demonstrates that aging is closely associated with chronic low-level inflammation. Meanwhile, a recent study confirmed that activation of the NACHT, LRR and PYD domains-containing protein 3 (NLRP3) inflammasome, which includes cleaved Caspase1, is regulated through the NAD-dependent protein deacetylase sirtuin-3, mitochondrial (SIRT3)-superoxide dismutase 2, mitochondrial (SOD2) signaling pathway (8). Our previous study demonstrated that the NLRP3 inflammasome is involved in endothelial cellular senescence (9). Compared with the majority of other types of epithelial cells, the urothelium may underlie this mechanism in the bladder. However, the involvement of NLRP3 in urothelial alterations and bladder dysfunction with advancing age remains poorly understood.
The proposed etiologies of LUTS involve a number of factors, including myogenic and neurogenic factors, but remodeling of the urothelium serves an equal role in the progression of LUTS (10). A previous study demonstrated that the urotheliogenic factor and its interactions with the detrusor muscle and neurons may explain the mechanism underlying bladder dysfunction with advancing age (11). The urothelium, which is characterized by sensory innervation, serves a critical role in regulating micturition (12). The expression level of NLRP3, which is principally located in the urothelium, is induced by bladder injury from noxious stimuli in the urine and may trigger the urothelial inflammatory response (13). Aging process was demonstrated to be accelerated by senescent cells (14) and urothelial senescence may be responsible for bladder degradation. However, there is no evidence to confirm that alterations in the urothelium are associated with a decline in bladder function with age. It was hypothesized that inflamm-aging may serve an important role in the bladder, particularly in the urothelium, with advancing age. Therefore, in order to validate this hypothesis in the present study, the senescence marker p21 (15) was identified by immunohistochemical staining, and differences in inflammasome expression were determined by immunofluorescence and western blot analysis between young and old rats. Cystometry was used to assess detrusor activity.
Materials and methods
Animals and sample preparation
The animal experiments were approved by the Ethics Committee of Chengdu University (Chengdu, China). A total of 20 female Sprague-Dawley (SD) rats were obtained from The Dashuo Laboratory Animal Co., Ltd. (Chengdu, China) and divided into the following two groups (n=10 rats/group): 2-month-old group (young group, 271±28 g), and 24-month-old group (old group, 412±47 g). A total of two rats were housed in each cage at room temperature (20±2°C) and saturated humidity (50±2%), with access to food and water ad libitum. The room had a constant 12-h light/dark cycle. The rats were anesthetized with 3% isoflurane inhalant, and the peritoneal cavity was opened. The bladder was quickly excised and the rat was subsequently sacrificed. The tissue from the upper part of the bladder were placed in 10% formalin for fixation at room temperature for 2 days, and used for histological analysis. The remaining bladder tissues were cut into small pieces of ~5 mm and placed in liquid nitrogen for western blot analysis.
Blood chemical measurement
In total, 1 ml blood was collected from the abdominal aorta of 20 rats following bladder extraction and the serum was extracted by centrifugation at 13,000 × g for 10 min at 4°C. The serum was stored at 4°C and the oxidative stress markers including malondialdehyde and superoxide dismutase, and the level of blood lipids were detected within 12 h. The biochemical data were detected with a full-automatic biochemical analyzer (Chemray 240; Rayto Life and Analytical Sciences Co., Ltd., Shenzhen, China) using routine enzymatic assays.
Urodynamic analysis
Rats from the young group and the old group (n=4 rats/group) were anesthetized via inhalation of 3% isoflurane. A PE50 polyethylene catheter (internal diameter, 0.58 mm; outer diameter, 0.96 mm) was implanted into the bladder dome and buried subcutaneously at the back of the neck as described by Zhao et al (4). A total of 3 days after the surgery, cystometry was performed through the polyethylene catheter that was connected to a pressure transducer (Bonito XL; Laborie Medical Technologies Inc., Mississauga, ON, Canada) and a syringe pump (Jian Yuan Medical Technology Co., Ltd., Changsha, China), which administered a warm saline infusion at a rate of 10 ml/h. The rats remained awake without anesthetization and were restricted to a small cage. Urodynamic parameters, including the maximum bladder capacity (MBC), maximum voiding pressure (MVP), bladder leak point pressure (BLPP), voiding volume (VV), voiding time (VT) and residual volume (RV), were analyzed. The BLPP was recorded when micturition occurred in a phase of high-frequency oscillations.
Histomorphology and immunohistochemical staining
The paraffin-embedded bladders were cut into sections (5 µm) perpendicular to the longitudinal axis of the bladder. Masson's trichrome staining was performed on a number of the sections, according to the manufacturer's instructions (Sigma-Aldrich; Merck KGaA, Darmstadt, Germany). The remaining sections were deparaffinized and rehydrated in xylene and a gradient alcohol series, and antigen retrieval was performed by heating the sections at ~95°C for 8 min with sodium citrate antigen retrieval solution (pH 6.0). Subsequently, endogenous peroxidase was blocked with 3% hydrogen peroxide. PBS containing 3% bovine serum albumin (BSA; cat. no. G5001; Wuhan Servicebio Technology Co., Ltd., Wuhan, China) was used for blocking of non-specific binding at room temperature for 30 min. The sections were incubated with primary antibodies, including those for rabbit p21 (1:100; cat. no. 10355-1-1AP; Wuhan Sanying Biotechnology, Wuhan, China) and NLRP3 (1:100; cat. no. 19771-1-AP; Wuhan Sanying Biotechnology), at 4°C overnight, and subsequently with secondary antibodies (1:1,000; cat. no. GB23302; Wuhan Servicebio Technology Co., Ltd.) incubated at room temperature for 50 min and labelled with horseradish peroxidase. 3,3′-Diaminobenzidine chromogenic reagent was used to stain the nucleus at room temperature until the nuclei exhibited positive staining. Subsequently, the samples were counterstained with hematoxylin at room temperature for 3 min. Following dehydration, the sections were mounted with resin medium. Samples were observed and imaged using an ortho-fluorescence microscope (Nikon Corporation, Tokyo, Japan; magnification, 100× or 200×). The detrusor muscle and collagen content were quantified using ImagePro Plus 6.0 software (Media Cybernetics, Inc., Rockville, MD, USA).
Western blot analysis
The bladder tissues were lysed with radioimmunoprecipiation assay-150 lysis buffer and complete protease inhibitor (Roche Applied Science, Penzberg, Germany). The protein concentration was detected by bicinchoninic acid protein assay kit; cat. no. CW0014; CoWin Biosciences Co., Ltd., Beijing, China) and 50 µg total protein was loaded in each lane. Proteins were separated on a 10% SDS-PAGE gel and transferred to a PVDF membrane (EMD Millipore, Billerica, MA, USA). Subsequently, the membrane was blocked with 5% BSA in TBS with Tween-20 for 1 h at room temperature and incubated with the primary antibodies, including interleukin (IL)1β (1:100; cat. no. 12242S; Cell Signaling Technology, Inc., Danvers, MA, USA), Caspase1 (1:100; cat. no. 3866S; Cell Signaling Technology, Inc.), SIRT3 (1:200; cat. no. 1099-1-AP; Wuhan Sanying Biotechnology) and GAPDH (1:1,000; cat. no. 10494-1-AP; Wuhan Sanying Biotechnology, Wuhan, China) overnight at 4°C. The membranes were incubated with the horseradish peroxidase-conjugated secondary antibodies (1:1,000; cat. nos. 7074P2 and 7076P2; Cell Signaling Technology, Inc.) for 1 h at room temperature. The images were detected by a ChemiDoc XRS+ system (Bio-Rad Laboratories, Inc., Hercules, CA, USA) using an enhanced chemiluminescence kit (Thermo Fisher Scientific, Inc.). The densities of the western blot bands were analyzed using Image Lab Software 5.2.1 (Bio-Rad Laboratories, Inc.).
Immunofluorescent staining
Sections from the paraffin-embedded bladders were deparaffinized and rehydrated, and antigen retrieval was performed. The slides were incubated with spontaneous fluorescence quenching reagent for 5 min and subsequently with 5% BSA for 30 min. The excess BSA was washed off and the sections were incubated with the primary antibody anti-Caspase1 (1:200; cat. no. 1872; Abcam, Cambridge, UK) overnight at 4°C. The sections were rinsed with PBS (pH 7.4) and then covered with the secondary antibody for 50 min in the dark. When the nuclei had been stained with a DAPI counterstain for 10 min at room temperature, antifade mounting medium and a coverslip were added to the slide. Samples were observed and imaged using an ortho-fluorescent microscopy (Nikon Corporation, Tokyo, Japan; magnification, 100× or 400×).
Statistical analysis
The data are presented as the mean ± standard deviation. Each experiment was repeated at least three times. Statistical analysis was performed using GraphPad Prism software 5.01 (GraphPad Software, Inc., La Jolla, CA, USA). Comparisons between two groups were performed using Student's t-tests and P<0.05 was considered to indicate a statistically significant difference.
Results
Changes in bladder weight and morphology
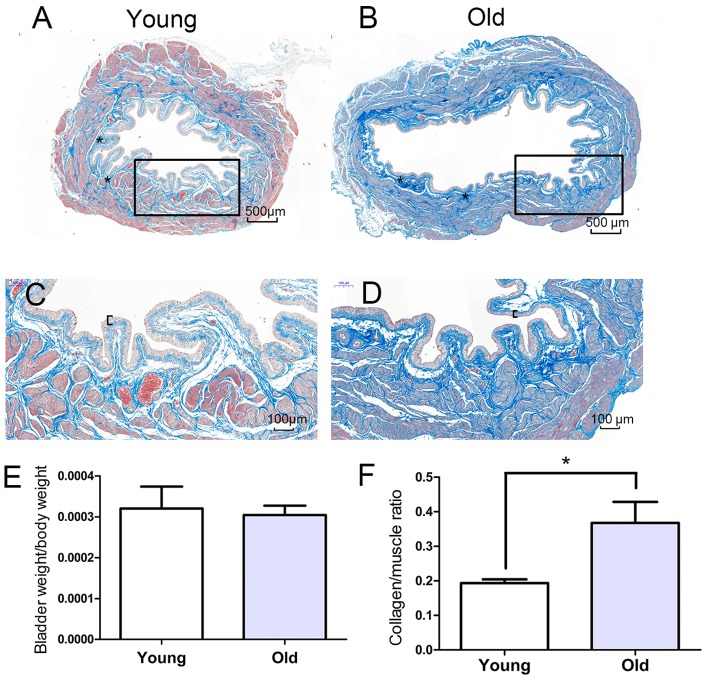
The morphological alterations in the bladders were evaluated using Masson's trichrome staining, and representative images are presented in Fig. 1A-D. Compared with the young bladders, the old bladders displayed pathological characteristics of aging, including increased collagen deposition, urothelial thinning and muscle shrinkage, which contribute to weakening of detrusor contraction. The weights of the bladders in the old group (0.126±0.021 g) were significantly higher compared with those of the young group (0.086±0.010 g). Furthermore, the body weight was increased in the old group compared with the young group and the ratio of bladder weight to body weight was not significantly different (P>0.05) between the two groups (Fig. 1E). The ratio of collagen content to muscle was higher in the old group compared with the young group (Fig. 1F).
Figure 1.
Masson's trichrome staining of representative bladder sections. Sections from (A) young and (B) old rats (magnification, 100×). Higher magnification of the outlined area from sections of (C) young and (D) old rats (magnification, 200×). The black brackets indicate the urothelium thickness and the asterisks indicate vessels in the submucosa. There was collagen deposition (blue) in the lamina propria and muscle layer. (E) The bladder to body weight ratio in each group. (F) The collagen to muscle ratio in each group. *P<0.05.
Urodynamic parameters
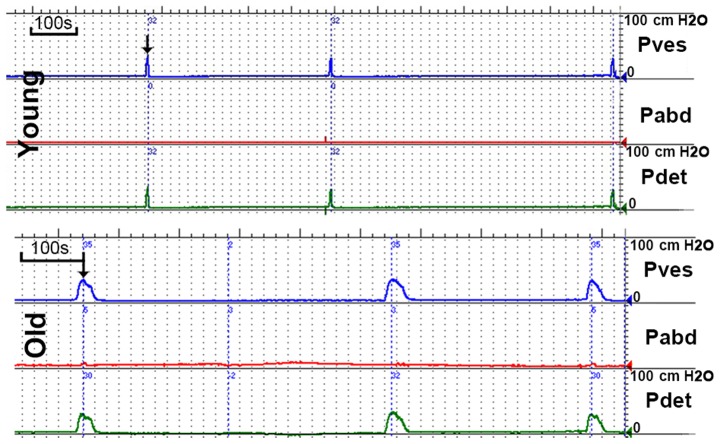
Representative cystometrogram recordings and urodynamic parameters are presented in Fig. 2 and Table I, respectively. The results of the cystometry indicated that the MVP and BLPP were similar in the two groups. However, the MBC and RV were significantly increased in the old group and the VT was prolonged in the old group compared to the young group. Marked unstable contraction was not observed prior to the voiding peak pressure.
Figure 2.
Representative cystometrogram recordings. The voiding time was prolonged in the old group compared with the young group. The bladder capacity and residual urine were increased in the old group compared with the young group. The arrows indicate the maximum vesical pressure. Pdet, detrusor pressure; Pabd, abdominal pressure; Pves, vesical pressure.
Table I.
Characteristics of oxidative stress marker and urodynamic parameters in rats.
| Parameter | Young | Old |
|---|---|---|
| MDA, nmol/ml | 4.12±0.93 | 8.24±2.31a |
| SOD, U/ml, | 360.44±32.63 | 314.98±13.94a |
| MBC, ml | 1.08±0.21 | 1.55±0.17a |
| MBC/BW | 11.72±1.23 | 12.04±0.97 |
| BLPP, cmH2O | 23.62±3.85 | 24.94±5.32 |
| MVP, cmH2O | 36.37±2.56 | 34.94±3.54 |
| VV, ml | 0.97±0.19 | 1.28±0.22 |
| RV, ml | 0.09±0.04 | 0.26±0.11a |
| VT, sec | 9.75±2.21 | 16.50±4.65a |
P<0.05 vs. young. Values are presented as the mean ± standard deviation. MDA, malondialdehyde; SOD, superoxide dismutase; MBC, maximum bladder capacity; BLPP, bladder leak point pressure; MVP, maximum vesical pressure; BW, bladder weight; RV, residual urine volume; VV, voiding volume; VT, voiding time.
Identification of senescence markers in the bladder tissues and oxidative stress markers in the serum
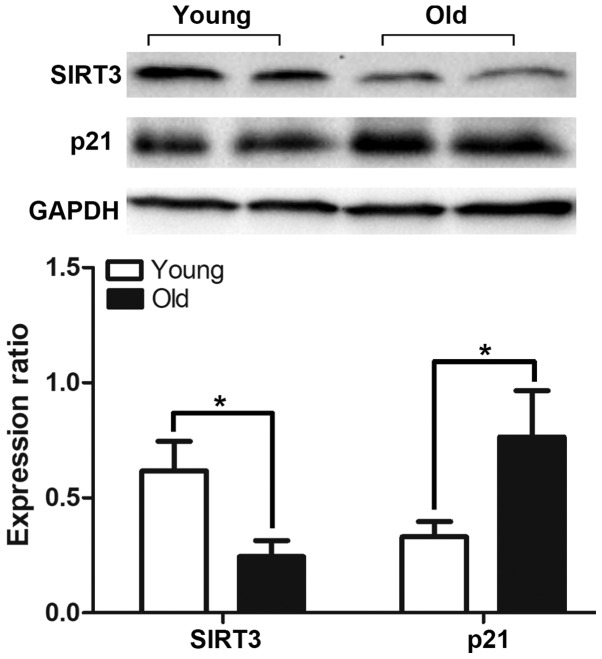
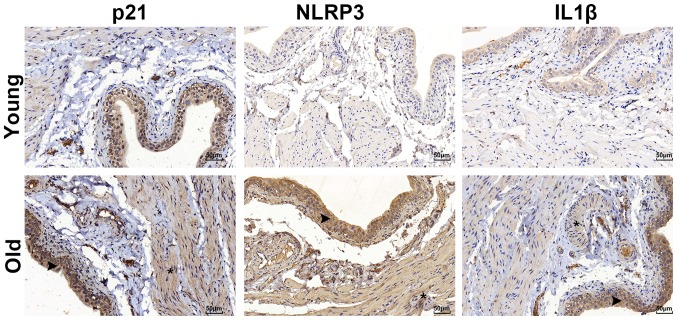
In order to determine the aging status of the bladders, the expression of the senescence marker p21 was detected by western blotting and immunohistochemical staining. The results demonstrated that the expression of p21 was significantly increased in the old group (Fig. 3), and there was positive expression of p21 in the bladders of the old group that was primarily located in the urothelium rather than the muscle layer (Fig. 4). Aging-associated degeneration of the mitochondrial protein SIRT3 was analyzed by western blotting, and the data indicated that the expression was significantly decreased in the bladders of the old group (Fig. 3). The serum biomarkers of oxidative stress were analyzed and the results demonstrated that the levels of malondialdehyde (MDA) were increased, while those of the antioxidant marker SOD were decreased in the old group compared with the young group (Table I). The level of blood lipids was not altered among groups.
Figure 3.
Western blot analysis for the detection of the expression levels of SIRT3 and p21 in the bladders of the old group compared with those of the young group. The band intensities were quantified relative to GAPDH in each group. *P<0.05. SIRT3, NAD-dependent protein deacetylase sirtuin-3, mitochondrial.
Figure 4.
Immunostaining for NLRP3, IL1β and p21. The positive staining (brown) was primarily located in the urothelia of the bladders. The arrows indicate the urothelium and the asterisks indicate the detrusor. NLRP3, NACHT, LRR and PYD domains-containing protein 3; IL1β, interleukin 1β.
Differences in the expression of NLRP3 inflammasome components
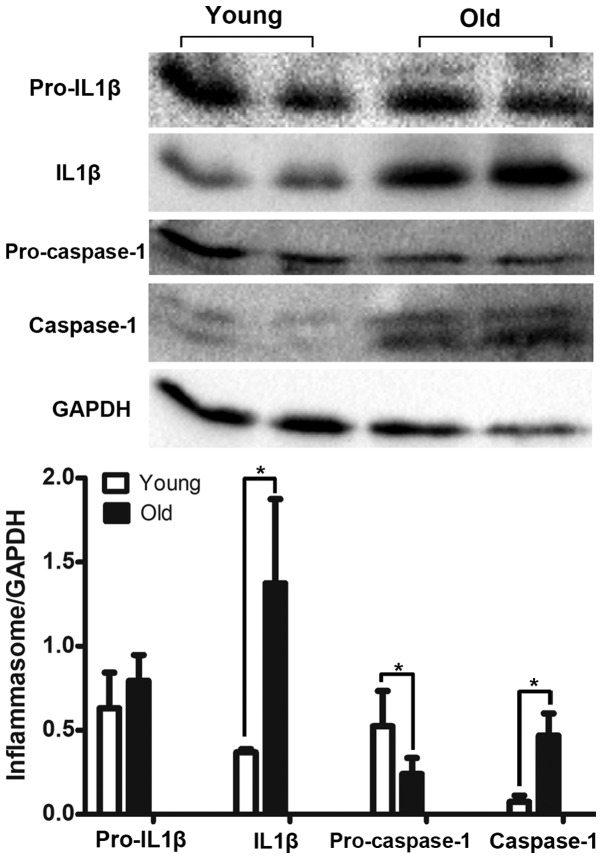
The expression of NLRP3 inflammasome components (NLRP3 and IL1β) was detected by immunohistochemical staining. The results revealed that NLRP3 and IL1β were primarily located in the urothelium and were markedly upregulated in the bladder tissues of the old group (Fig. 4). However, the inflammasome sensor NLRP3 was scarcely observed in the muscle layer, as illustrated in Fig. 4. Furthermore, the increased expression profile of the NLRP3 inflammasome components was examined using a western blot assay. The bladders in the old group had significantly elevated levels of Caspase1 and IL1β expression, while the expression levels of pro-Caspase1 were decreased in the old group (Fig. 5). In addition, the expression of pro-IL1β was not different between the two groups (Fig. 5).
Figure 5.
Western blot analysis for the detection of the expression levels of IL1β, Caspase1, pro-IL1β and pro-Caspase1 in the bladders of the old and young groups. The band intensities were quantified relative to GAPDH in each group. *P<0.05. IL1β, interleukin 1β.
Expression and distribution of Caspase1
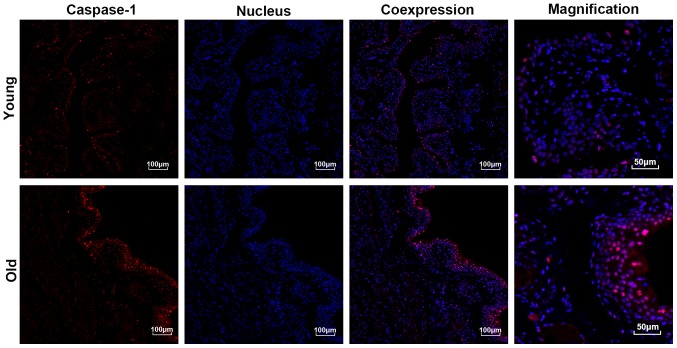
As presented in Fig. 6, the results of the immunofluorescent staining demonstrated that the expression levels of Caspase1, particularly in the urothelium, were significantly higher in the bladders of the old group compared with those in the young group. Moreover, the expression of active Caspase1 was increased and primarily localized to the nucleus. In accordance with the results of the immunofluorescent staining, the western blotting also indicated that the expression levels of Caspase1 were significantly increased in the bladders of the old group compared with the young group (Fig. 5).
Figure 6.
Immunofluorescent staining of the expression and location of Caspase1 in the bladders. Caspase1 (red) was primarily located in the urothelium. The nucleus (blue) was stained with DAPI.
Discussion
Aging mediates the progressive degenerative process closely associated with low-level inflammation and exacerbates the degenerative alterations that naturally occur. A previous study documented that chronic inflammation serves a crucial role in degenerative disorders in elderly patients (16). However, the exact mechanism behind the accelerated aging process of the bladder has rarely been studied. The aim of the present study was to examine the hypothesis that inflamm-aging contributes to bladder dysfunction. In young bodies, the immune response is rapid and transient, but aging bodies fail to respond in time, leading to chronic low-grade inflammation. NLRP3 is associated with chronic low-grade inflammation leading to functional decline in the process of aging (17). In addition, the bladder is a metabolically active organ and is chronically exposed to the toxic environment of urine, which readily induces bladder inflamm-aging. The present findings indicated that there is an association between the physiology of aging bladders and functional alterations in the NLRP3 inflammasome. Therefore, the present study focused on the molecular and morphological alterations associated with bladder dysfunction, in order to elucidate the effect of inflammation on aging of the bladder. The present study is the first, to the best of our knowledge, to demonstrate a crucial association between inflamm-aging and the aging process of bladder.
The present study demonstrated that the levels of serum MDA increased and those of antioxidative SOD decreased with advancing age. These results are consistent with those of a previous study, which reported that peroxidative injury is associated with the aging process (18). A previous report indicated that the generation of reactive oxygen species (ROS) is a crucial element in the activation of the NLRP3 inflammasome (19). A previous study suggested that atherosclerosis induces injury to larger vessels, which leads to chronic bladder ischemia that produces ROS (20). However, in the present study, it was observed that the rats without raised blood lipids (data not shown) exhibited morphological alterations in the small vessels of the bladder submucosa, which may also cause bladder ischemia and hypoxia. This may induce oxidative injury that results in aging bladder dysfunction, from hyperactivity to underactivity (21,22).
Subsequently, the expression of IL1β was detected in the bladder tissues. The results indicated that the levels of IL1β were distinctly increased in aged bladders compared with young ones. Furthermore, Masson's trichrome staining demonstrated that there was a mass of collagen fibers in the submucosa and detrusor layer in the bladders of the old group, which is a typical feature of age-induced bladder degeneration.
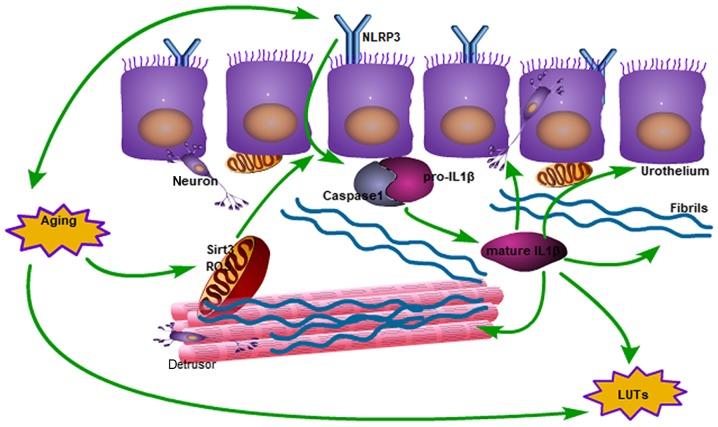
In a previous study, inflammasome activation was exclusive to immune cells such as macrophages, but recent research has confirmed that IL1β is secreted from the lung epithelium (23) and renal tubular epithelium (24). Compared with the majority of other types of epithelial cells, the urothelium may be a source of IL1β. In the present study, it was verified that IL1β comes from the urothelium via immunohistochemical staining. Notably, this is in accordance with the idea that IL1β infiltrates the laminar and detrusor layer, where it triggers collagen deposition in BOO (25). Thus, older bladders become more rigid. Another previous study demonstrated that IL1β causes denervation in the bladder to induce an overactive bladder and mediate the detrusor-impaired contractility responsible for LUTS (26). The NLRP3 inflammasome, which is upstream of IL1β, is located in the urothelium and is not expressed in the other layers of the bladder (27). Although this phenomenon has been identified in BOO, the results of the present study demonstrated that IL1β also has an impact on bladder dysfunction in the absence of BOO (Fig. 7).
Figure 7.
Schematic diagram of the impact of aging on bladder function degeneration, from mitochondrial damage to activated IL1β. In the mitochondria, the expression of SIRT3 decreases and ROS increases, which results in activation of the inflammasome. Subsequently, IL1β induces collagen deposition and neurological disorders, which interacts with the aging process of the bladder. ROS, reactive oxygen species; LUTs, lower urinary tract symptoms; IL1β, interleukin 1β; SIRT3, NAD-dependent protein deacetylase sirtuin-3, mitochondrial; NLRP3, NACHT, LRR and PYD domains-containing protein 3.
The present study evaluated the state of the older bladders using western blotting and immunohistochemical staining for p21, which was localized to the urothelium and muscle layer. The difference between the young and old groups principally existed in the urothelium rather than the detrusor, which may mean that the urothelium is more prone to aging compared with the detrusor. In other types of cells, p21 serves an important role in age-imposed tissue degeneration (28). It is thought that p21 may be used as a predictor of a senescent bladder.
Activated NLRP3, as a pathogen sensor, is able to cleave and promote Caspase1, which facilitates the processing of pro-IL1β to mature IL1β (17). The results of the present study demonstrated that Caspase1 serves a crucial role in the urothelium. This is consistent with previous research, which reported that NLRP3 is located in the urothelium and is not expressed in other layers of the bladder (27). Furthermore, the urothelium maintains a strong defensive barrier and serves a crucial role in sensory nerve function, but this defense system is susceptible to weakening with advancing age (29). The urothelium is exposed to the toxic environment of urine, and it is vulnerable to aberrant urothelial differentiation and a decline in urothelial function. Daly et al (30) demonstrated that degeneration of the bladder with age is due to altered purinergic signaling in the urothelium, and afferent neurons are affected. However, another study reported that IL1β damages the afferent and efferent neurons that mediate detrusor contraction due to urothelial damage and has a direct apoptotic effect on neurons (31). The present study demonstrated that IL1β expression is upregulated in older bladders and is primarily located in the urothelium, which demonstrates the crucial role of IL1β in the senescence process of the bladder.
In the present study, it was observed that the MBC and RV were increased in the old group compared with the young group, suggesting that the bladder function had degenerated, which was similar to the results of a previous study (4). However, catheter implantation may destroy the integrity of the bladder, which decreases bladder capacity and weakens contractility. This may explain the differences in the maximum capacity and voiding pressure. In order to make adjustments for a number of the urodynamic parameters, bladder weight was taken into account when significantly different changes in the MBC were evaluated. However, the results indicated that there was no significant difference in the MBC to bladder weight ratio between the young group and old group. Bladder capacity may increase with bladder weight and collagen deposition, which does not rapidly occur in SD female rats before 24 months of age (4). The present findings demonstrated that the VT was prolonged in old female rats without BOO, which indicated that the bladder contractility is weakened in old rats compared with young rats. In addition, unstable contraction was not observed, although this has been reported in male rats (32). This difference may be explained by the fact that male rats have greater urethral resistance compared with females.
SIRT3, which acts upstream of NLRP3, is located in the mitochondria and regulates ROS generation, which serves an important role in weakening the NLRP3 inflammasome (8). Notably, a study demonstrated that SIRT3 is involved in longevity and neurodegeneration (33). The present study is the first, to the best of our knowledge, to confirm that SIRT3 expression is reduced in older bladders compared with young bladders. Brown et al (34) reported that aging-associated degeneration may be reversed via the SIRT3 pathway. Therefore, the association of SIRT3 with aging of the bladder requires further investigation.
In conclusion, IL1β is triggered by cleaved Caspase1 in the process of bladder aging and appears to serve a crucial role in the pathological alterations to the urothelium and detrusor, which may be an important risk factor for LUTS. Taken together, the present findings suggest a novel mechanism underlying the alterations in aging bladders via the NLRP3 inflammasome. NLRP3 inhibitors may become a novel therapy for bladder dysfunction triggered by inflamm-aging. However, a safe and effective NLRP3 inhibitor has not yet been produced to treat LUTS, and this may be a focus of future studies.
Acknowledgements
Not applicable.
Funding
The present study was supported by the National Natural Science Foundation of China (grant no. 81500577), the Research Projects of Chengdu Science and Technology (grant no. 2015HM0100580SF), the Research Projects of the Department of Science and Technology of Sichuan Province (grant no. 2017JY0097 and 19YYJC1427), the Research Projects of the Department of Education of Sichuan Province (grant nos. 16ZA0383 and 16ZA0388), the Research Projects of the Health and Family Planning Commission of Chengdu (grant no. 2015123), and the Research Projects of Sichuan Province Medical Association (grant no. S17002).
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Authors' contributions
JY and LC were responsible for protocol/project development. PH and LC performed the manuscript writing and data analysis. YY and KW performed data collection. BA, AS, YZ, ZW, SX and XL analyzed and interpreted the data, and edited the manuscript, revising it critically for important intellectual content.
Ethics approval and consent to participate
The animal experiments were approved by the Ethics Committee of Chengdu University (Chengdu, China).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Vahabi B, Wagg AS, Rosier PFWM, Rademakers KLJ, Denys MA, Pontari M, Lovick T, Valentini FA, Nelson PP, Andersson KE, Fry CH. Can we define and characterize the aging lower urinary tract?-ICI-RS 2015. Neurourol Urodyn. 2017;36:854–858. doi: 10.1002/nau.23035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Suskind AM. The aging overactive bladder: A review of aging-related changes from the brain to the bladder. Curr Bladder Dysfunct Rep. 2017;12:42–47. doi: 10.1007/s11884-017-0406-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Oelke M, Baard J, Wijkstra H, de la Rosette JJ, Jonas U, Hofner K. Age and bladder outlet obstruction are independently associated with detrusor overactivity in patients with benign prostatic hyperplasia. Eur Urol. 2008;54:419–426. doi: 10.1016/j.eururo.2008.02.017. [DOI] [PubMed] [Google Scholar]
- 4.Zhao W, Aboushwareb T, Turner C, Mathis C, Bennett C, Sonntag WE, Andersson KE, Christ G. Impaired bladder function in aging male rats. J Urol. 2010;184:378–385. doi: 10.1016/j.juro.2010.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lluel P, Palea S, Barras M, Grandadam F, Heudes D, Bruneval P, Corman B, Martin DJ. Functional and morphological modifications of the urinary bladder in aging female rats. Am J Physiol Regul Integr Comp Physiol. 2000;278:R964–R972. doi: 10.1152/ajpregu.2000.278.4.R964. [DOI] [PubMed] [Google Scholar]
- 6.Siroky MB. The aging bladder. Rev Urol. 2004;1:S3–S7. [PMC free article] [PubMed] [Google Scholar]
- 7.Baylis D, Bartlett DB, Patel HP, Roberts HC. Understanding how we age: Insights into inflammaging. Longev Healthspan. 2013;2:8. doi: 10.1186/2046-2395-2-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chen ML, Zhu XH, Ran L, Lang HD, Yi L, Mi MT. Trimethylamine-N-Oxide induces vascular inflammation by activating the NLRP3 inflammasome through the SIRT3-SOD2-mtROS signaling pathway. J Am Heart Assoc. 2017;6:e006347. doi: 10.1161/JAHA.117.006347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Xing SS, Li J, Chen L, Yang YF, He PL, Li J, Yang J. Salidroside attenuates endothelial cellular senescence via decreasing the expression of inflammatory cytokines and increasing the expression of SIRT3. Mech Ageing Dev. 2018;175:1–6. doi: 10.1016/j.mad.2017.12.005. [DOI] [PubMed] [Google Scholar]
- 10.Birder L, Andersson KE. Urothelial signaling. Physiol Rev. 2013;93:653–680. doi: 10.1152/physrev.00030.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Roosen A, Chapple CR, Dmochowski RR, Fowler CJ, Gratzke C, Roehrborn CG, Stief CG, Andersson KE. A refocus on the bladder as the originator of storage lower urinary tract symptoms: A systematic review of the latest literature. Eur Urol. 2009;56:810–819. doi: 10.1016/j.eururo.2009.07.044. [DOI] [PubMed] [Google Scholar]
- 12.Birder LA, Ruggieri M, Takeda M, van Koeveringe G, Veltkamp S, Korstanje C, Parsons B, Fry CH. How does the urothelium affect bladder function in health and disease? ICI-RS 2011. Neurourol Urodyn. 2012;31:293–299. doi: 10.1002/nau.22195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Inouye BM, Hughes FM, Jr, Sexton SJ, Purves JT. The emerging role of inflammasomes as central mediators in inflammatory bladder pathology. Curr Urol. 2018;11:57–72. doi: 10.1159/000447196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.van Deursen JM. The role of senescent cells in ageing. Nature. 2014;509:439–446. doi: 10.1038/nature13193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hudgins AD, Tazearslan C, Tare A, Zhu Y, Huffman D, Suh Y. Age- and Tissue-Specific Expression of Senescence Biomarkers in Mice. Front Genet. 2018;9:59. doi: 10.3389/fgene.2018.00059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Howcroft TK, Campisi J, Louis GB, Smith MT, Wise B, Wyss-Coray T, Augustine AD, McElhaney JE, Kohanski R, Sierra F. The role of inflammation in age-related disease. Aging (Albany NY) 2013;5:84–93. doi: 10.18632/aging.100531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Youm YH, Grant RW, McCabe LR, Albarado DC, Nguyen KY, Ravussin A, Pistell P, Newman S Carter R, Laque A, et al. Canonical Nlrp3 inflammasome links systemic low-grade inflammation to functional decline in aging. Cell Metab. 2013;18:519–532. doi: 10.1016/j.cmet.2013.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Inal ME, Kanbak G, Sunal E. Antioxidant enzyme activities and malondialdehyde levels related to aging. Clin Chim Acta. 2001;305:75–80. doi: 10.1016/S0009-8981(00)00422-8. [DOI] [PubMed] [Google Scholar]
- 19.Tschopp J, Schroder K. NLRP3 inflammasome activation: The convergence of multiple signalling pathways on ROS production? Nat Rev Immunol. 2010;10:210–215. doi: 10.1038/nri2725. [DOI] [PubMed] [Google Scholar]
- 20.Andersson KE, Boedtkjer DB, Forman A. The link between vascular dysfunction, bladder ischemia, and aging bladder dysfunction. Ther Adv Urol. 2017;9:11–27. doi: 10.1177/1756287216675778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nomiya M, Yamaguchi O, Akaihata H, Hata J, Sawada N, Kojima Y, Andersson KE. Progressive vascular damage may lead to bladder underactivity in rats. J Urol. 2014;191:1462–1469. doi: 10.1016/j.juro.2013.10.097. [DOI] [PubMed] [Google Scholar]
- 22.Sunagawa M, Wolf-Johnston A, Nomiya M, Sawada N, Andersson KE, Hisamitsu T, Birder LA. Urinary bladder mucosal responses to ischemia. World J Urol. 2015;33:275–280. doi: 10.1007/s00345-014-1298-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Peeters PM, Perkins TN, Wouters EF, Mossman BT, Reynaert NL. Silica induces NLRP3 inflammasome activation in human lung epithelial cells. Part Fibre Toxicol. 2013;10:3. doi: 10.1186/1743-8977-10-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bakker PJ, Butter LM, Claessen N, Teske GJ, Sutterwala FS, Florquin S, Leemans JC. A tissue-specific role for Nlrp3 in tubular epithelial repair after renal ischemia/reperfusion. Am J Pathol. 2014;184:2013–2022. doi: 10.1016/j.ajpath.2014.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hughes FM, Jr, Sexton SJ, Jin H, Govada V, Purves JT. Bladder fibrosis during outlet obstruction is triggered through the NLRP3 inflammasome and the production of IL-1beta. Am J Physiol Renal Physiol. 2017;313:F603–F610. doi: 10.1152/ajprenal.00128.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lutolf R, Hughes FM, Jr, Inouye BM, Jin H, McMains JC, Pak ES, Hannan JL, Purves JT. NLRP3/IL-1beta mediates denervation during bladder outlet obstruction in rats. Neuroural Urodyn. 2018;37:952–959. doi: 10.1002/nau.23419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hughes FM, Jr, Hill HM, Wood CM, Edmondson AT, Dumas A, Foo WC, Oelsen JM, Rac G, Purves JT. The NLRP3 Inflammasome mediates inflammation produced by bladder outlet obstruction. J Urol. 2016;195:1598–1605. doi: 10.1016/j.juro.2015.12.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Li J, Han S, Cousin W, Conboy IM. Age-specific functional epigenetic changes in P21 and p16 in injury-activated satellite cells. Stem Cells. 2015;33:951–961. doi: 10.1002/stem.1908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Perse M, Injac R, Erman A. Oxidative status and lipofuscin accumulation in urothelial cells of bladder in aging mice. PloS One. 2013;8:e59638. doi: 10.1371/journal.pone.0059638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Daly DM, Nocchi L, Liaskos M, McKay NG, Chapple C, Grundy D. Age-related changes in afferent pathways and urothelial function in the male mouse bladder. J Physiol. 2014;592:537–549. doi: 10.1113/jphysiol.2013.262634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.He P. Lutolf R, Hughes FM, Jr., Inouye BM, Jin H, McMains JC, Pak ES, Hannan JL, Purves JT. NLRP3/IL-1beta mediates denervation during bladder outlet obstruction in rats. Neurourology and urodynamics 2017. Neurourol Urodyn. 2018;37:1506. doi: 10.1002/nau.23478. [DOI] [PubMed] [Google Scholar]
- 32.Kohan AD, Danziger M, Vaughan ED, Jr, Felsen D. Effect of aging on bladder function and the response to outlet obstruction in female rats. Urol Res. 2000;28:33–37. doi: 10.1007/s002400050007. [DOI] [PubMed] [Google Scholar]
- 33.Haigis MC, Sinclair DA. Mammalian sirtuins: Biological insights and disease relevance. Annu Rev Pathol. 2010;5:253–295. doi: 10.1146/annurev.pathol.4.110807.092250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Brown K, Xie S, Qiu X, Mohrin M, Shin J, Liu Y, Zhang D, Scadden DT, Chen D. SIRT3 reverses aging-associated degeneration. Cell Rep. 2013;3:319–327. doi: 10.1016/j.celrep.2013.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.