Abstract
Polyunsaturated fatty acids are oxidized by non-enzymatic or enzymatic reactions. The oxidized products are multifunctional. In this study, we investigated how oxidized fatty acids inhibit cell proliferation in cultured cells. We used polyunsaturated and saturated fatty acids, docosahexaenoic acid (DHA; 22:6), eicosapentaenoic acid (EPA; 20:5), linoleic acid (LA; 18:2), and palmitic acid (16:0). Oxidized fatty acids were produced by autoxidation of fatty acids for 2 days in the presence of a gas mixture (20% O2 and 80% N2). We found that oxidized polyunsaturated fatty acids (OxDHA, OxEPA and OxLA) inhibited cell proliferation much more effectively compared with un-oxidized fatty acids (DHA, EPA and LA, respectively) in THP-1 (a human monocytic leukemia cell line) and DLD-1 (a human colorectal cancer cell line) cells. In particular, OxDHA markedly inhibited cell proliferation. DHA has the largest number of double bonds and is most susceptible to oxidation among the fatty acids. OxDHA has the largest number of highly active oxidized products. Therefore, the oxidative levels of fatty acids are associated with the anti-proliferative activity. Moreover, caspase-3/7 was activated in the cells treated with OxDHA, but not in those treated with DHA. A pan-caspase inhibitor (zVAD-fmk) reduced the cell death induced by OxDHA. These results indicated that oxidized products from polyunsaturated fatty acids induced apoptosis in cultured cells. Collectively, the switch between cell survival and cell death may be regulated by the activity and/or number of oxidized products from polyunsaturated fatty acids.
Keywords: polyunsaturated fatty acids, non-enzymatic oxidation, cell death, apoptosis, lipotoxicity
Introduction
Fatty acids are structural components of the cellular membrane, and a source of energy. Numerous studies have been conducted on fatty acids to analyze their effect on diseases and health (1,2). Consumption of a high amount of unsaturated fatty acids is thought to be one of the main risk factors for certain cancers. Fatty acids, such as docosahexaenoic acid (DHA), eicosapentaenoic acid (EPA), linoleic acid (LA), and palmitic acid (PA), have various cellular roles. Especially, polyunsaturated fatty acids play a key role in inflammatory processes (3). In addition, some fatty acids suppress cancer growth in vitro and in vivo (4–10). A mixture of fatty acids (EPA+arachidonic acid (AA) or DHA+AA) decreases the viability and proliferation of breast cancer cell lines (MDA-MB-231 and MCF7) (11). Saturated fatty acids (PA and stearic acid) also induce death in human cancer cells (12,13). Not only fatty acids, but also fatty acid-analogues have been shown to be potent in anti-cancer therapies (14). However, the mechanism of the multifunctional effects of fatty acids is not clear.
Polyunsaturated fatty acids are oxidized by non-enzymatic or enzymatic reactions. In non-enzymatic reaction, lipid peroxidation is an autoxidation process initiated by the attack of free radicals, such as reactive oxygen and nitrogen species (·OH and ONOO−). After a radical chain reaction, various bioactive oxidized products are produced from fatty acids (15). Paradoxically, these products exhibit both pro- and anti-inflammatory effects. The oxidized 1-palmitoyl-2-arachidonoyl-sn-glycero-3-phosphocholine (OxPAPC) physiologically exerts the multiple functions (15,16).
15-Deoxy-Δ12,14-prostaglandin J2, an oxidized fatty acid, induces death in breast cancer cells (17). Synthetic ether phospholipid hexadecyl azelaoyl phosphatidylcholine causes mitochondrial dysfunction and apoptosis in HL-60 cells (18). 13-Hydroxy ocatadecadienoic acid is a highly potent inducer of reactive oxygen species (ROS) production in human aortic endothelial cells (19). DHA hydroperoxide is a potential inducer of apoptosis via mitochondrial dysfunction in human neuroblastoma SH-SY5Y cells (20). In contrast, 15-deoxy-Δ12,14-prostaglandin J2 protects SIN-1-induced death in PC12 cells (21). EPA, but not AA, attenuates dexamethasone-induced cell apoptosis in murine bone marrow-derived mesenchymal stem cells (22). The mixture of OxEPA and OxDHA ameliorates the loss of viability induced by 2,2′, 4,4′-tetrabromodiphenyl ether in HepG2 cells (23). Oleic acid (0.1–1.5 mM) has a protective effect against the cytotoxicity induced by tert-butyl hydroperoxide, a oxidative stress inducer, in mouse 3T3-L1 fibroblasts, while DHA (>0.1 mM) enhanced this cytotoxicity (24). However, the relationship between oxidized fatty acids and cell death is controversial.
In this study, we aimed to investigate how oxidized fatty acids affect the viability and death of cultured cells. We showed that of all the fatty acids used in this study, OxDHA had the most anti-proliferative effect in cultured cells. Moreover, OxDHA, but not DHA induced apoptosis through caspase activation in THP-1 cells (human monocytic leukemia cell line). Among fatty acids, DHA has the most number of double bonds, and is most susceptible to oxidation. These results suggested that oxidized products from highly unsaturated fatty acids have a potent anti-proliferative and pro-apoptotic activity. The switch between cell survival and cell death may be regulated by the activity and/or number of oxidized products from polyunsaturated fatty acids.
Materials and methods
Cell culture
THP-1 (ATCC) and DLD-1 (Cell Resource Center) cells were cultured in RPMI medium (Invitrogen/Life technologies, Tokyo, Japan) supplemented with 10% fetal bovine serum (GE Healthcare Life Sciences, Tokyo, Japan), penicillin (Nacalai Tesque, Kyoto, Japan), streptomycin (Nacalai Tesque, Kyoto, Japan), and amphotericin B (GE Healthcare Life Sciences, Tokyo, Japan) at 37°C with 5% CO2.
Preparation of human lymphocytes
Cell culture analysis, using peripheral blood mononuclear cells (PBMC) from healthy volunteers, was approved by the Institutional Review of Committee of Seikei University. Informed consent was obtained from volunteers in accordance with the Declaration of Helsinki. Peripheral blood samples from four healthy volunteers were transferred into polystyrene centrifuge tubes containing EDTA and then gently mixed. The same volume of PBS was added into the tubes. The diluted blood samples (3 ml each) were layered on 3 ml of Lymphosepar I (Immuno-Biological Laboratories, Gunma, Japan). Lymphocytes were isolated by centrifugation at room temperature for 30 min at 1,800 rpm (400 × g). The lymphocyte layer was collected and washed with PBS, and then resuspended in RPMI-1640 medium supplemented with 10% fetal bovine serum, penicillin, streptomycin, and amphotericin B. Lymphocytic blastogenesis was induced by treatment with 20 µg/ml phytohemagglutinin-P (PHA) (Sigma-Aldrich, Tokyo, Japan). PHA is able to stimulate lymphocyte proliferation by binding on the cell surface and activating RNA and protein synthesis.
Oxidized fatty acid preparation in an in vitro cell free system
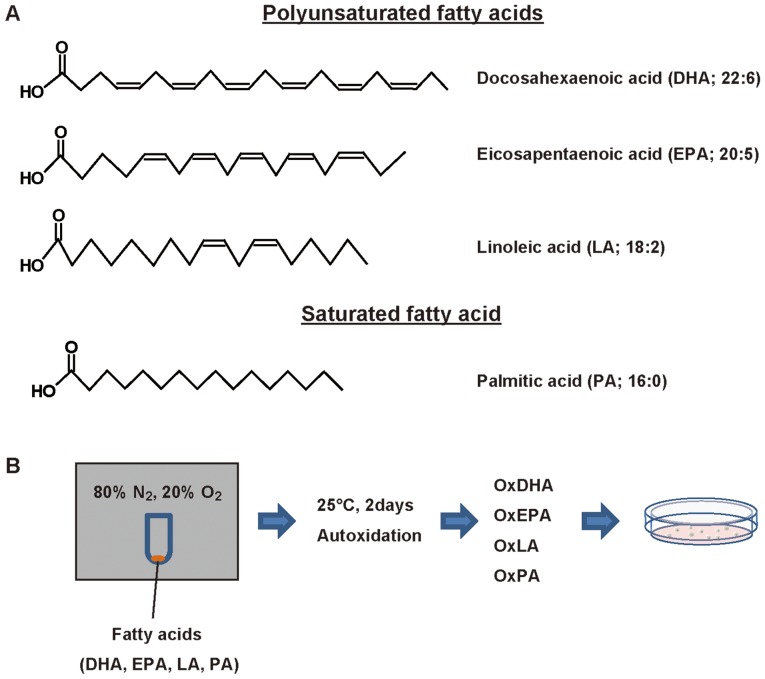
Oxidized fatty acids were produced by autoxidation as described previously (25). DHA, EPA, LA, and PA were purchased from Funakoshi (Tokyo, Japan). 2.5 mg of DHA, EPA, LA, or PA in 500 µl of ethanol was transferred to a glass tube, and dried under a gentle stream of nitrogen. The fatty acid residue was allowed to autoxidize for 2 days in the presence of the gaseous mixture of 80% N2 and 20% O2 at 25°C in a closed aluminum bag (Fig. 1). Then, the oxidized fatty acids were suspended in PBS containing 10% ethanol.
Figure 1.
Structure of fatty acids and scheme for fatty acid oxidation. (A) Structure of polyunsaturated and saturated fatty acids. Scheme for air oxidation of fatty acid. (B) Fatty acids were autoxidized for 2 days in the presence of 80% N2 and 20% O2. Ox, oxidized.
Treatment of cells with fatty acids or oxidized fatty acids
Cells were seeded at a concentration of 0.5-1×105 cells/ml, incubated for the indicated times, and then treated with the fatty acids or oxidized fatty acids at the indicated concentrations. We used the concentrations of fatty acids used in our experiments (0.6 to 5 µg/ml), based on the previous reference (26) described the human plasma concentrations of DHA.
Cellular viability of cells treated with oxidized fatty acids
Cell viability was measured using a Cell Counting Kit-8 (CCK-8, Dojindo Laboratories, Kumamoto, Japan), according to the manufacture's protocol. The cells were treated with oxidized fatty acids. Then, the CCK-8 reagent was added and the cells were incubated for 4 h at 37°C. Absorbance was measured at 450 nm using a microplate reader (Food Mark, Bio-Lad, CA, USA).
Caspase-3/7 activation assay
To determine the activity of caspase-3/7, CellEvent™ Caspase-3/7 Green ReadyProbes reagent (Invitrogen/Life Technologies, Tokyo, Japan) was used. This reagent consists of a four-amino acid peptide (DEVD) conjugated to a nucleic acid-binding dye. After 1 h of incubation, the fluorescence of the nuclei was observed using a fluorescence microscope (Leica DM IL LED; Leica Microsystems, Wetzlar, Germany).
Propidium iodide exclusion assay
Cell death was determined by the ability of the cells to exclude propidium iodide (PI) (Sigma-Aldrich). THP-1 cells were treated with vehicle, DHA, or OxDHA in the absence or presence of 20 µM zVAD-fmk (American Peptide Company, CA, USA), and stained with 50 µg/ml PI. The cells were then analyzed using the Cell Lab Quanta™ SC flow cytometer (Beckman Coulter, Brea, CA, USA).
Determination of apoptotic cells treated with OxDHA
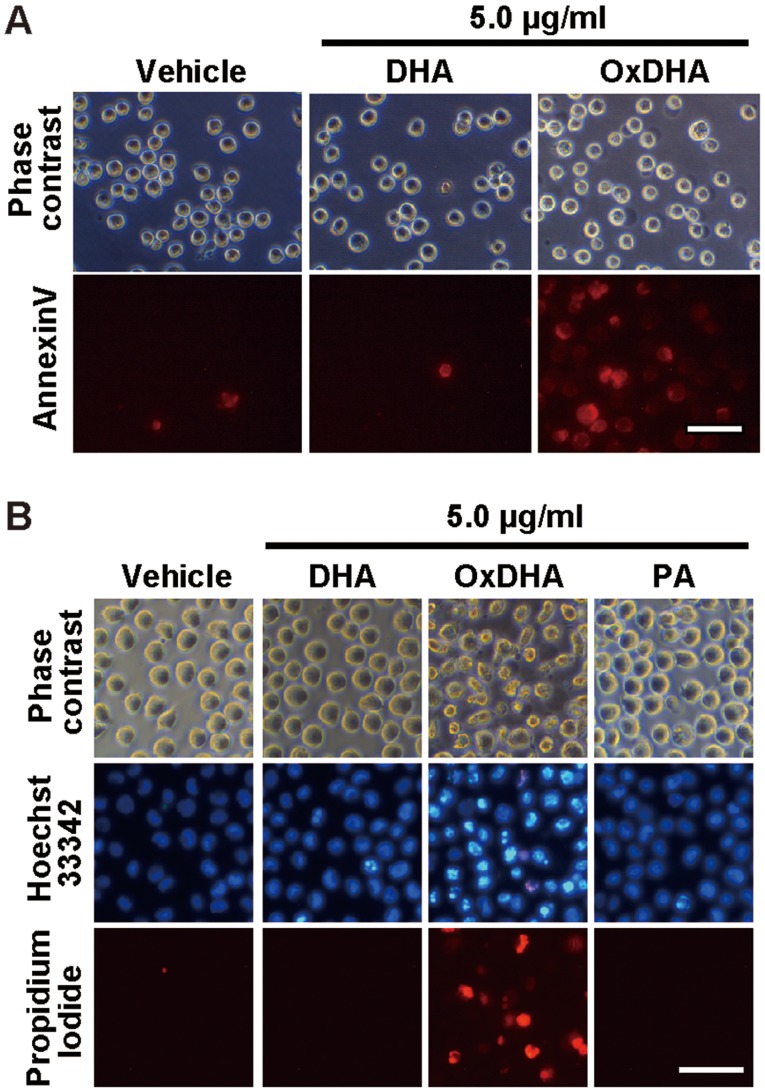
Apoptotic cell death assay was performed by Hoechst 33342 and propidium iodide staining, as described previously (27). The cells were treated with oxidized fatty acids for the indicated times. The cells were stained with Hoechst 33342 (1 µg/ml) (Dojindo Laboratories, Kumamoto, Japan) and propidium iodide (1 µg/ml), and observed under a fluorescence microscope (Leica DM IL LED; Leica Microsystems). The apoptotic cell death was determined by morphological changes of chromatin (condensed and/or fragmented chromatin).
Assessment of apoptotic cell death by Annexin V staining
After treatment with OxDHA, THP-1 cells were washed with PBS and resuspended in a staining solution containing the Annexin V-Cy3 reagent (BioVision, CA, USA) in 1×binding buffer. After a 15-min incubation in the dark at room temperature, the cells were washed with HEPES buffer, and observed using a fluorescence microscope (Leica DM IL LED; Leica Microsystems).
Statistical analysis
The data shown in the figures represent the mean values ± standard deviation of three or four independent determinations. Data were evaluated statistically by one-way analysis of variance followed by the Tukey's honest significant difference test. The statistical significance at different P-values is indicated in each figure.
Results
Oxidized polyunsaturated fatty acids inhibit proliferation of cultured cells
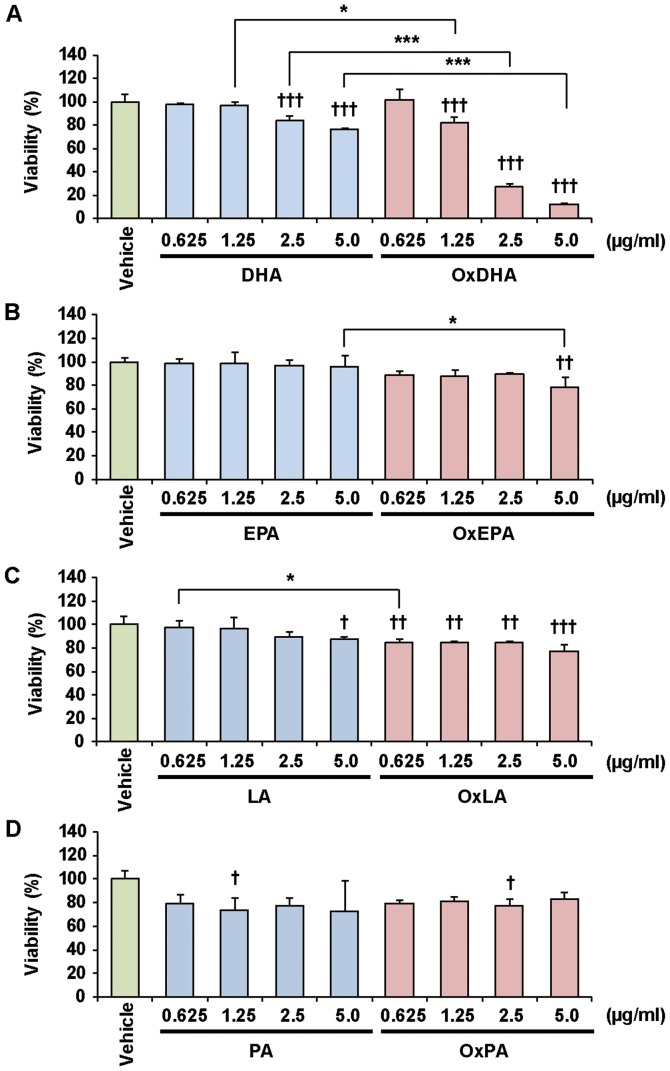
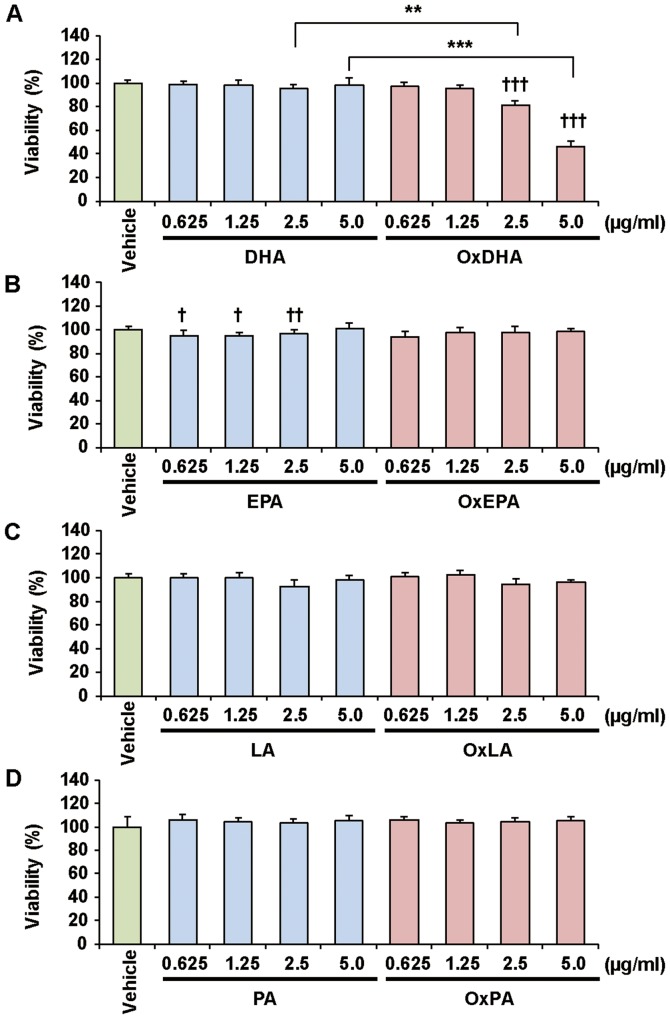
Previous studies showed that polyunsaturated fatty acids inhibit cancer cell growth in vitro (28). We first investigated the effect of fatty acids and oxidized fatty acids on the proliferation of various cultured cells, as determined by the CCK-8 assay (Figs. 2 and 3). Treatment with OxDHA significantly decreased the proliferation of THP-1 cells in a dose-dependent manner (Fig. 2A). Native DHA slightly decreased cell proliferation at high concentrations (>2.5 µg/ml DHA). OxEPA also decreased the proliferation of THP-1 cells dose-dependently, but EPA (except for 5.0 µg/ml EPA) did not (Fig. 2B). OxLA, as well as OxEPA, slightly decreased the proliferation of THP-1 cells dose-dependently, but LA (except for 5.0 µg/ml LA) did not (Fig. 2C). Neither PA nor OxPA inhibited the proliferation of THP-1 cells (Fig. 2D). As shown in Fig. 3, OxDHA but not DHA inhibited the proliferation of the DLD-1 cells. Proliferation in DLD-1 cells was hardly inhibited by EPA, LA, OxEPA, and OxLA, even at high concentrations (5.0 µg/ml) (Figs. 3B and 3C). PA and OxPA hardly decreased the proliferation of DLD-1 cells at all concentrations (Fig. 3C). As shown in Figs. 2 and 3, OxDHA had the most anti-proliferative effect among these fatty acids. These results indicated that the anti-proliferative effect of oxidized fatty acids is responsible for the activity and/or number of oxidized products.
Figure 2.
Effect of FA and OxFA on THP-1 cell proliferation. (A) Effect of DHA or OxDHA on cell proliferation. THP-1 cells were treated with DHA or OxDHA at the indicated concentrations for 24 h. Cell growth was determined by a Cell Counting Kit-8 assay, according to the manufacturer's protocol. (B) Effect of EPA or OxEPA on cell proliferation. (C) Effect of LA or OxLA on cell proliferation. (D) Effect of PA or OxPA on cell proliferation. n=3-4. †P<0.05, ††P<0.01, †††P<0.001 vs. vehicle; *P<0.05, ***P<0.001. FA, fatty acid; Ox, oxidized; DHA, docosahexaenoic acid; EPA, eicosapentaenoic; LA, linoleic acid; PA, palmitic acid.
Figure 3.
Effect of FA and OxFA on DLD-1 cell proliferation. (A) Effect of DHA or OxDHA on cell proliferation. DLD-1 cells were treated with DHA or OxDHA at the indicated concentrations for 24 h. Cell growth was determined by a Cell Counting Kit-8 assay. (B) Effect of EPA or OxEPA on cell proliferation. (C) Effect of LA or OxLA on cell proliferation. (D) Effect of PA or OxPA on cell proliferation. n=4. †P<0.05, ††P<0.01, †††P<0.001 vs. vehicle; **P<0.01, ***P<0.001. FA, fatty acid; Ox, oxidized; DHA, docosahexaenoic acid; EPA, eicosapentaenoic; LA, linoleic acid; PA, palmitic acid.
Oxidized DHA, but not DHA induces death of THP-1 cells
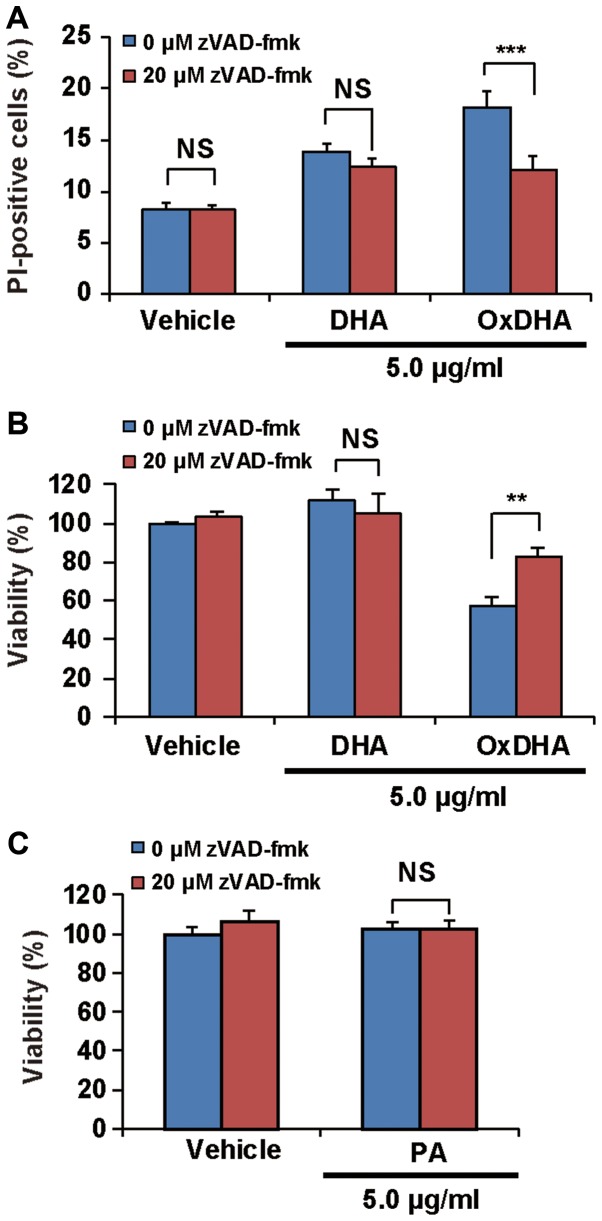
As shown above, treatment of cells with oxidized unsaturated fatty acids resulted in a decrease in their proliferation. To investigate whether the oxidized fatty acids induced death in the cultured cells, the THP-1 cells were analyzed using the propidium iodide (PI) exclusion assay. PI can only enter the dead cells, as their membranes are disrupted. As described in Fig. 4A, samples treated with OxDHA, but not with DHA contained PI-positive cells (dead cells). OxDHA-induced cell death was significantly suppressed by a pan-caspase inhibitor (zVAD-fmk) (Fig. 4A). As presented in Fig. 4B, the anti-proliferative effect of OxDHA was partially suppressed by zVAD-fmk (Fig. 4B). Palmitic acid did not inhibit cell proliferation in the presence of zVAD-fmk (Fig. 4C). In both the cell proliferation and the PI exclusion assay, the OxDHA-induced decrease of viability was suppressed by pre-treatment with zVAD-fmk. These results suggest that OxDHA decreased cell proliferation through the activation of caspases, followed by cell death.
Figure 4.
OxDHA induces cell death through caspase activation in THP-1 cells. (A) THP-1 cells were treated with vehicle, DHA, or OxDHA in the presence or absence of zVAD-fmk for 24 h. Subsequently, cell death was assessed by PI staining. The THP-1 cells treated with vehicle, DHA, or OxDHA were analyzed for cell death using flow cytometry. (B) THP-1 cells were treated with vehicle, DHA, or OxDHA in the presence or absence of zVAD-fmk for 24 h. Cell growth was determined by a CCK-8 assay. n=3-4. (C) THP-1 cells were treated with vehicle or PA in the presence or absence of zVAD-fmk for 24 h. Cell growth was determined by CCK-8 assay. n=4. **P<0.01, ***P<0.001. Ox, oxidized; DHA, docosahexaenoic acid; PI, propidium iodide; CCK-8, Cell Counting Kit-8; PA, palmitic acid.
Oxidized DHA, but not DHA induces apoptosis in THP-1 cells
During early apoptosis, phosphatidylserine on the outer membrane of cells was exposed. As shown in Fig. 5A, the analysis using the Annexin V-Cy3 reagent, which stains phosphatidylserine residues, showed that the number of Annexin V-positive cells was high in the OxDHA-treated samples (Fig. 5A). Apoptosis is generally accompanied by chromatin shrinkage, chromatin condensation, and nuclear fragmentation. We analyzed the changes in chromatin morphology in THP-1 cells treated with OxDHA. As shown in Fig. 5B, THP-1 cells treated with OxDHA but not DHA or PA exhibited typical apoptotic features, such as chromatin condensation and fragmentation (Fig. 5B). These results suggested that OxDHA induced apoptotic cell death.
Figure 5.
OxDHA induces apoptosis of THP-1 cells. (A) THP-1 cells were treated with vehicle, DHA, or OxDHA for 24 h at the indicated concentrations. Subsequently, cell death was assessed using Annexin V assay. (B) Apoptotic nuclear morphological changes of THP-1 cells treated with OxDHA. THP-1 cells were treated with vehicle, DHA, OxDHA, or PA for 48 h at the indicated concentrations. Then, the cells were stained with Hoechst 33342 Propidium Iodide and observed under a fluorescence microscope. Apoptotic cells had condensed or fragmented chromatin. Scale bar, 50 µm. Ox, oxidized; DHA, docosahexaenoic acid; PA, palmitic acid.
Oxidized DHA but not DHA induces caspase-3/7 activation in THP-1 cells
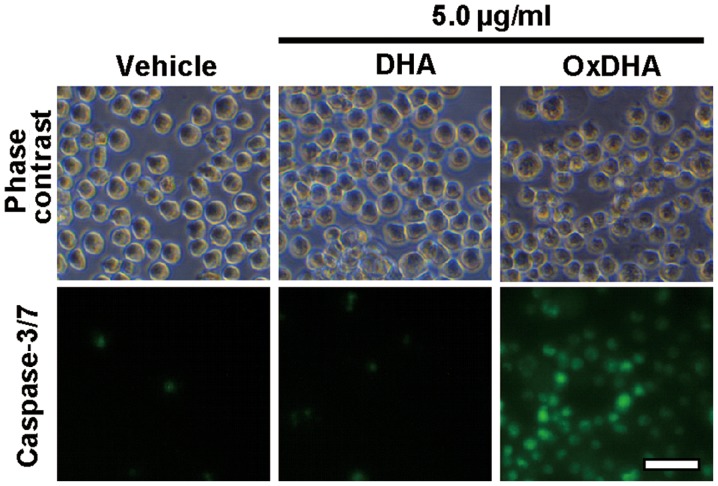
Fig. 4 showed that OxDHA-induced cell death was inhibited by a pan-caspase inhibitor. Next, we investigated whether the members of the caspase family are required for the induction of apoptosis by OxDHA. Caspase-3/7 were activated in THP-1 cells treated with OxDHA; this was not observed in THP-1 cells treated with DHA and vehicle (Fig. 6). These results indicate that caspase-3/7 activation is involved in apoptotic cell death induced by OxDHA.
Figure 6.
OxDHA induces caspase-3/7 activation in THP-1 cells. THP-1 cells were treated with vehicle, DHA, or OxDHA for 24 h at the indicated concentrations. Then, apoptotic cell death was detected by CellEvent caspase-3/7 Green Detection reagent, according to the manufacturer's protocol. Scale bar, 50 µm. Ox, oxidized; DHA, docosahexaenoic acid.
Discussion
In this study, the oxidized products from unsaturated fatty acids (DHA, EPA, and LA) exhibited an anti-proliferative activity in cultured human cells. Among the oxidized fatty acids, OxDHA had the most significant anti-proliferative activity. The numbers of double bonds of DHA, EPA, LA, or PA are 6, 5, 2, or 0, respectively (Fig. 1A). DHA has the most number of double bonds, and is most susceptible to oxidation. DHA has six double bonds and bis-allylic methylene groups, which have several possible positions for hydrogen abstraction. Therefore, DHA can be easily oxidized into highly active products in the air as well as under intracellular conditions. These chemical properties are the reason why OxDHA produces more toxic products (e.g., various hydroperoxides, aldehydes, and other products) than OxEPA and OxLA. Among these fatty acids, OxDHA has the most number of highly active oxidized products.
Suspension cells (THP-1 cells and lymphocytes) were sensitive to native and oxidized fatty acids (Figs. 2 and 7). However, only OxDHA induced a loss of viability of DLD-1 cells, which are adherent cells. Neither DHA nor OxDHA inhibited proliferation in Human Hepatoma HepG2 cells (data not shown). These results suggest that the sensitivity of blood cells to oxidized fatty acids is higher than those of solid cancer cells. In general, human leukemia cell lines are highly sensitive to ROS-generating agents.
Figure 7.
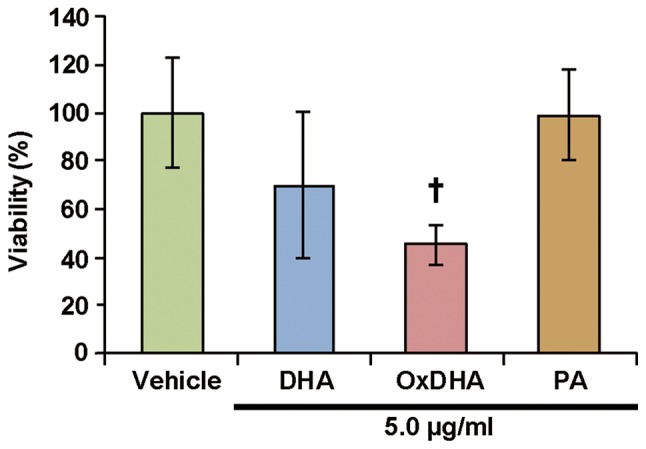
Effect of DHA and OxDHA on viabiltity of human lymphocytes. Human lymphocytes were treated with vehicle, DHA, OxDHA or PA for 24 h at the indicated concentrations. Cell growth was determined by Cell Counting Kit-8 assay, according to the manufacturer's protocol. n=3-4. †P<0.05 vs. vehicle. Ox, oxidized; DHA, docosahexaenoic acid; PA, palmitic acid.
As mentioned previously, OxDHA has the most potent anti-proliferative activity. DHA has the most number of double bonds; therefore, it has the most unsaturated fatty acids and sensitivity to ROS. In contrast, saturated fatty acids, such as PA, are resistant to oxidation when exposed to ROS. As shown in Figs. 2 and 3, neither PA nor OxPA had an anti-proliferative activity in this study. To date, the oxidation of unsaturated fatty acids generates many diverse oxidized products, such as 4-hydroxy-2-nonenal (HNE), malondialdehyde (MDA), acrolein, 13-hydroperoxylinoleic acid (13-HPODE), and F2-isoprostanes (29–33). In the present study, OxDHA and OxEPA had a light-yellow color, but native DHA and EPA did not (data not shown). It is reported that DHA is oxidized by oxidizing agents such as 2,2′-azobis- (2-amidinopropane) hydrochloride (AAPH) or copper ions. In the chemical reaction, a family of F4-isoprostanes were produced from DHA (34). A4/J4-neuroprostanes were identified by the mass spectrometry analysis of AAPH-treated DHA, which has a cytoprotective effect and an anti-inflammatory activity (35). Previously, using mass spectroscopy, we had described that a large number of bioactive oxidized lipids were produced by the autoxidation of PAPC in an in vitro cell-free system (25). Therefore, the OxDHA used in this study may also contain various bioactive oxidized fatty acids. The anti-proliferative activity of native polyunsaturated fatty acids may be dependent on their oxidized products in cells.
15d-PGJ2, a potent inhibitor of mitochondrial respiratory complex I, increases the rate of ROS production (36). Therefore, OxDHA may inhibit mitochondrial respiratory complexes. DHA induced apoptotic cell death through ROS production and caspase-8 activation (37). In our study, caspase-3/7 was activated in THP-1 cells treated with OxDHA. In general, the caspase-3/7 is activated by mitochondrial dysfunction followed by caspase-8 and/or caspase-9 activation. Collectively, these results and reports suggest that OxDHA may activate caspase-8 and/or caspase-9 via mitochondrial dysfunction.
DHA blocks the NF-κB pathway and significantly decreases cell proliferation (38). 13-HPODE upregulates the expression of the chemokine monocyte chemoattractant protein-1 via the activation of NF-κB (39). Lipid peroxidation products activate the NF-κB pathway and inactivate anti-apoptotic B-Cell Lymphoma-2 (Bcl-2) (40,41). The main cellular function of Bcl-2 is the regulation of cytochrome c release (42,43). Thus, the apoptotic cell death induced by OxDHA may be associated with NF-κB activation and the change in the level of Bcl-2.
In summary, we have demonstrated that oxidized fatty acids produced from unsaturated fatty acids play important roles in the apoptotic cell death pathway. The oxidized fatty acid products regulate not only apoptosis, but also non-apoptotic cell death induction (ferroptosis, autophagy, and regulated neutrophil cell death in immune response) (44–47). OxDHA may be useful for studying the mechanism of cell death induced by lipid peroxidation, which is involved in a wide variety of diseases.
Acknowledgements
Not applicable.
Funding
Financial support for this research was provided by Grants-in-Aid for Scientific Research of Seikei University (Tokyo, Japan; grant no. 2017) and Scientific Research from the Japan Society for the Promotion of Science (grant nos. 15K16524 and 18K11001 to KI).
Availability of data and materials
All data generated and/or analyzed during the current study are available from the corresponding author on reasonable request.
Authors' contributions
KI, ME, MS and HH conceived and designed the experiments. KI, ME, MS and CY performed the experiments. KI, ME, MS and HH contributed to data analysis. KI, ME, MS and HH contributed to manuscript preparation. All authors read and approved the final manuscript.
Ethics approval and consent to participate
The present study was approved by the Institutional Review of Committee of Seikei University (Tokyo, Japan). Written informed consent was obtained from volunteers in accordance with the Declaration of Helsinki.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Wang W, Zhu J, Lyu F, Panigrahy D, Ferrara KW, Hammock B, Zhang G. ω-3 polyunsaturated fatty acids-derived lipid metabolites on angiogenesis, inflammation and cancer. Prostaglandins Other Lipid Mediat. 2014;113-115:13–20. doi: 10.1016/j.prostaglandins.2014.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Niki E. Biomarkers of lipid peroxidation in clinical material. Biochim Biophys Acta. 2014;1840:809–817. doi: 10.1016/j.bbagen.2013.03.020. [DOI] [PubMed] [Google Scholar]
- 3.Calder PC. Polyunsaturated fatty acids and inflammation. Biochem Soc Trans. 2005;33:423–427. doi: 10.1042/BST0330423. [DOI] [PubMed] [Google Scholar]
- 4.Zajdel A, Wilczok A, Chodurek E, Gruchlik A, Dzierzewicz Z. Polyunsaturated fatty acids inhibit melanoma cell growth in vitro. Acta Pol Pharm. 2013;70:365–369. [PubMed] [Google Scholar]
- 5.Yee LD, Young DC, Rosol TJ, Vanbuskirk AM, Clinton SK. Dietary (n-3) polyunsaturated fatty acids inhibit HER-2/neu-induced breast cancer in mice independently of the PPARgamma ligand rosiglitazone. J Nutr. 2005;135:983–988. doi: 10.1093/jn/135.5.983. [DOI] [PubMed] [Google Scholar]
- 6.Notarnicola M, Tutino V, De Nunzio V, Dituri F, Caruso MG, Giannelli G. Dietary ω-3 polyunsaturated fatty acids inhibit tumor growth in transgenic ApcMin/+ mice, correlating with CB1 receptor Up-Regul. Int J Mol Sci. 2017;18(pii):E485. doi: 10.3390/ijms18030485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Notarnicola M, Messa C, Refolo MG, Tutino V, Miccolis A, Caruso MG. Polyunsaturated fatty acids reduce fatty acid synthase and hydroxy-methyl-glutaryl CoA-reductase gene expression and promote apoptosis in HepG2 cell line. Lipids Health Dis. 2011;10:10. doi: 10.1186/1476-511X-10-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yin Y, Sui C, Meng F, Ma P, Jiang Y. The omega-3 polyunsaturated fatty acid docosahexaenoic acid inhibits proliferation and progression of non-small cell lung cancer cells through the reactive oxygen species-mediated inactivation of the PI3K/Akt pathway. Lipids Health Dis. 2017;16:87. doi: 10.1186/s12944-017-0474-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.D'Eliseo D, Velotti F. Omega-3 fatty acids and cancer cell cytotoxicity: Implications for multi-targeted cancer therapy. J Clin Med. 2016;5(pii):E15. doi: 10.3390/jcm5020015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pizato N, Luzete BC, Kiffer LFMV, Corrêa LH, de Oliveira Santos I, Assumpção JAF, Ito MK, Magalhães KG. Omega-3 docosahexaenoic acid induces pyroptosis cell death in triple-negative breast cancer cells. Sci Rep. 2018;8:1952. doi: 10.1038/s41598-018-20422-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mansara PP, Deshpande RA, Vaidya MM, Kaul-Ghanekar R. Differential ratios of omega fatty acids (AA/EPA+DHA) modulate growth, lipid peroxidation and expression of tumor regulatory MARBPs in breast cancer cell lines MCF7 and MDA-MB-231. PLoS One. 2015;10:e0136542. doi: 10.1371/journal.pone.0136542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mu YM, Yanase T, Nishi Y, Tanaka A, Saito M, Jin CH, Mukasa C, Okabe T, Nomura M, Goto K, Nawata H. Saturated FFAs, palmitic acid and stearic acid, induce apoptosis in human granulosa cells. Endocrinology. 2001;142:3590–3597. doi: 10.1210/endo.142.8.8293. [DOI] [PubMed] [Google Scholar]
- 13.Zhang Y, Xue R, Zhang Z, Yang X, Shi H. Palmitic and linoleic acids induce ER stress and apoptosis in hepatoma cells. Lipids Health Dis. 2012;11:1. doi: 10.1186/1476-511X-11-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Murray M, Dyari HR, Allison SE, Rawling T. Lipid analogues as potential drugs for the regulation of mitochondrial cell death. Br J Pharmacol. 2014;171:2051–2066. doi: 10.1111/bph.12417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bochkov VN, Oskolkova OV, Birukov KG, Levonen AL, Binder CJ, Stöckl J. Generation and biological activities of oxidized phospholipids. Antioxid Redox Signal. 2010;12:1009–1059. doi: 10.1089/ars.2009.2597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Miller YI, Shyy JY. Context-dependent role of oxidized lipids and lipoproteins in inflammation. Trends Endocrinol Metab. 2017;28:143–152. doi: 10.1016/j.tem.2016.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Clay CE, Monjazeb A, Thorburn J, Chilton FH, High KP. 15-Deoxy-delta12,14-prostaglandin J2-induced apoptosis does not require PPARgamma in breast cancer cells. J Lipid Res. 2002;43:1818–1828. doi: 10.1194/jlr.M200224-JLR200. [DOI] [PubMed] [Google Scholar]
- 18.Chen R, Yang L, McIntyre TM. Cytotoxic phospholipid oxidation products. Cell death from mitochondrial damage and the intrinsic caspase cascade. J Biol Chem. 2007;282:24842–24850. doi: 10.1074/jbc.M702865200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wang L, Gill R, Pedersen TL, Higgins LJ, Newman JW, Rutledge JC. Triglyceride-rich lipoprotein lipolysis releases neutral and oxidized FFAs that induce endothelial cell inflammation. J Lipid Res. 2009;50:204–213. doi: 10.1194/jlr.M700505-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Liu X, Shibata T, Hisaka S, Kawai Y, Osawa T. DHA hydroperoxides as a potential inducer of neuronal cell death: A mitochondrial dysfunction-mediated pathway. J Clin Biochem Nutr. 2008;43:26–33. doi: 10.3164/jcbn.2008040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lim SY, Jang JH, Na HK, Lu SC, Rahman I, Surh YJ. 15-Deoxy-Delta12,14-prostaglandin J(2) protects against nitrosative PC12 cell death through up-regulation of intracellular glutathione synthesis. J Biol Chem. 2004;279:46263–46270. doi: 10.1074/jbc.M406555200. [DOI] [PubMed] [Google Scholar]
- 22.Gao B, Han YH, Wang L, Lin YJ, Sun Z, Lu WG, Hu YQ, Li JQ, Lin XS, Liu BH, et al. Eicosapentaenoic acid attenuates dexamethasome-induced apoptosis by inducing adaptive autophagy via GPR120 in murine bone marrow-derived mesenchymal stem cells. Cell Death Dis. 2016;7:e2235. doi: 10.1038/cddis.2016.144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yeh A, Kruse SE, Marcinek DJ, Gallagher EP. Effect of omega-3 fatty acid oxidation products on the cellular and mitochondrial toxicity of BDE 47. Toxicol In Vitro. 2015;29:672–680. doi: 10.1016/j.tiv.2015.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Haeiwa H, Fujita T, Saitoh Y, Miwa N. Oleic acid promotes adaptability against oxidative stress in 3T3-L1 cells through lipohormesis. Mol Cell Biochem. 2014;386:73–83. doi: 10.1007/s11010-013-1846-9. [DOI] [PubMed] [Google Scholar]
- 25.Iuchi K, Imoto A, Kamimura N, Nishimaki K, Ichimiya H, Yokota T, Ohta S. Molecular hydrogen regulates gene expression by modifying the free radical chain reaction-dependent generation of oxidized phospholipid mediators. Sci Rep. 2016;6:18971. doi: 10.1038/srep18971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Abdelmagid SA, Clarke SE, Nielsen DE, Badawi A, El-Sohemy A, Mutch DM, Ma DW. Comprehensive profiling of plasma fatty acid concentrations in young healthy Canadian adults. PLoS One. 2015;10:e0116195. doi: 10.1371/journal.pone.0116195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Eguchi Y, Shimizu S, Tsujimoto Y. Intracellular ATP levels determine cell death fate by apoptosis or necrosis. Cancer Res. 1997;57:1835–1840. [PubMed] [Google Scholar]
- 28.Schley PD, Jijon HB, Robinson LE, Field CJ. Mechanisms of omega-3 fatty acid-induced growth inhibition in MDA-MB-231 human breast cancer cells. Breast Cancer Res Treat. 2005;92:187–195. doi: 10.1007/s10549-005-2415-z. [DOI] [PubMed] [Google Scholar]
- 29.Perluigi M, Coccia R, Butterfield DA. 4-Hydroxy-2-nonenal, a reactive product of lipid peroxidation, and neurodegenerative diseases: A toxic combination illuminated by redox proteomics studies. Antioxid Redox Signal. 2012;17:1590–1609. doi: 10.1089/ars.2011.4406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Milne GL, Yin H, Hardy KD, Davies SS, Roberts LJ., II Isoprostane generation and function. Chem Rev. 2011;111:5973–5996. doi: 10.1021/cr200160h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Usatyuk PV, Natarajan V. Hydroxyalkenals and oxidized phospholipids modulation of endothelial cytoskeleton, focal adhesion and adherens junction proteins in regulating endothelial barrier function. Microvasc Res. 2012;83:45–55. doi: 10.1016/j.mvr.2011.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Esterbauer H, Schaur RJ, Zollner H. Chemistry and biochemistry of 4-hydroxynonenal, malonaldehyde and related aldehydes. Free Radic Biol Med. 1991;11:81–128. doi: 10.1016/0891-5849(91)90192-6. [DOI] [PubMed] [Google Scholar]
- 33.Meilhac O, Zhou M, Santanam N, Parthasarathy S. Lipid peroxides induce expression of catalase in cultured vascular cells. J Lipid Res. 2000;41:1205–1213. [PubMed] [Google Scholar]
- 34.Nourooz-Zadeh J, Liu EH, Anggård E, Halliwell B. F4-isoprostanes: A novel class of prostanoids formed during peroxidation of docosahexaenoic acid (DHA) Biochem Biophys Res Commun. 1998;242:338–344. doi: 10.1006/bbrc.1997.7883. [DOI] [PubMed] [Google Scholar]
- 35.Majkova Z, Layne J, Sunkara M, Morris AJ, Toborek M, Hennig B. Omega-3 fatty acid oxidation products prevent vascular endothelial cell activation by coplanar polychlorinated biphenyls. Toxicol Appl Pharmacol. 2011;251:41–49. doi: 10.1016/j.taap.2010.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Martínez B, Pérez-Castillo A, Santos A. The mitochondrial respiratory complex I is a target for 15-deoxy-delta12,14-prostaglandin J2 action. J Lipid Res. 2005;46:736–743. doi: 10.1194/jlr.M400392-JLR200. [DOI] [PubMed] [Google Scholar]
- 37.Kang KS, Wang P, Yamabe N, Fukui M, Jay T, Zhu BT. Docosahexaenoic acid induces apoptosis in MCF-7 cells in vitro and in vivo via reactive oxygen species formation and caspase 8 activation. PLoS One. 2010;5:e10296. doi: 10.1371/journal.pone.0010296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yun EJ, Song KS, Shin S, Kim S, Heo JY, Kweon GR, Wu T, Park JI, Lim K. Docosahexaenoic acid suppresses breast cancer cell metastasis by targeting matrix-metalloproteinases. Oncotarget. 2016;7:49961–49971. doi: 10.18632/oncotarget.10266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Dwarakanath RS, Sahar S, Reddy MA, Castanotto D, Rossi JJ, Natarajan R. Regulation of monocyte chemoattractant protein-1 by the oxidized lipid, 13-hydroperoxyoctadecadienoic acid, in vascular smooth muscle cells via nuclear factor-kappa B (NF-kappa B) J Mol Cell Cardiol. 2004;36:585–595. doi: 10.1016/j.yjmcc.2004.02.007. [DOI] [PubMed] [Google Scholar]
- 40.Timucin AC, Basaga H. Pro-apoptotic effects of lipid oxidation products: HNE at the crossroads of NF-κB pathway and anti-apoptotic Bcl-2. Free Radic Biol Med. 2017;111:209–218. doi: 10.1016/j.freeradbiomed.2016.11.010. [DOI] [PubMed] [Google Scholar]
- 41.Yadav UC, Ramana KV. Regulation of NF-κB-induced inflammatory signaling by lipid peroxidation-derived aldehydes. Oxid Med Cell Longev. 2013;2013:690545. doi: 10.1155/2013/690545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tsujimoto Y. Cell death regulation by the Bcl-2 protein family in the mitochondria. J Cell Physiol. 2003;195:158–167. doi: 10.1002/jcp.10254. [DOI] [PubMed] [Google Scholar]
- 43.Shimizu S, Tsujimoto Y. Proapoptotic BH3-only Bcl-2 family members induce cytochrome c release, but not mitochondrial membrane potential loss, and do not directly modulate voltage-dependent anion channel activity. Proc Natl Acad Sci USA. 2000;97:577–582. doi: 10.1073/pnas.97.7.3100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hill BG, Haberzettl P, Ahmed Y, Srivastava S, Bhatnagar A. Unsaturated lipid peroxidation-derived aldehydes activate autophagy in vascular smooth-muscle cells. Biochem J. 2008;410:525–534. doi: 10.1042/BJ20071063. [DOI] [PubMed] [Google Scholar]
- 45.Xie Y, Hou W, Song X, Yu Y, Huang J, Sun X, Kang R, Tang D. Ferroptosis: Process and function. Cell Death Differ. 2016;23:369–379. doi: 10.1038/cdd.2015.158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yotsumoto S, Muroi Y, Chiba T, Ohmura R, Yoneyama M, Magarisawa M, Dodo K, Terayama N, Sodeoka M, Aoyagi R, et al. Hyperoxidation of ether-linked phospholipids accelerates neutrophil extracellular trap formation. Sci Rep. 2017;7:16026. doi: 10.1038/s41598-017-15668-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Parisi LR, Morrow LM, Visser MB, Atilla-Gokcumen GE. Turning the spotlight on lipids in non-apoptotic cell death. ACS Chem Biol. 2018;13:506–515. doi: 10.1021/acschembio.7b01082. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data generated and/or analyzed during the current study are available from the corresponding author on reasonable request.