Abstract
Our previous studies suggested that paeonol, the active constituent of the traditional Chinese medicine Cortex Moutan, may be an effective treatment for inflammatory disorders. In the present study, the therapeutic potential of paeonol on atopic dermatitis (AD) was investigated using animal and cell experiments. AD-like lesions were induced by repeated application of 1-chloro-2,4-dinitrobenzene (DNCB) to the shaved dorsal skin of BALB/c mice, and P815 cells were used for in vitro assays. The skin lesions, serum and spleens of the mice were analyzed using lesion severity scoring, histological analysis, flow cytometry, reverse transcription-quantitative polymerase chain reaction, western blotting and ELISA, in order to investigate the anti-AD effects of paeonol. In addition, western blotting and ELISA were conducted for in vitro analysis of P815 cells. The results demonstrated that oral administration of paeonol inhibited the development of DNCB-induced AD-like lesions in the BALB/c mice by reducing severity of the lesions, epidermal thickness and mast cell infiltration; this was accompanied by reduced levels of immunoglobulin E and inflammatory cytokines [interleukin (IL)-4, histamine, IL-13, IL-31 and thymic stromal lymphopoietin], along with regulation of the T helper (Th) cell subset (Th1/Th2) ratio. Application of paeonol also reduced the protein expression levels of phosphorylated (p)-p38 and p-extracellular signal-regulated kinase (ERK) in skin lesions. In vitro, paeonol reduced the expression levels of tumor necrosis factor-α and histamine in P815 cells, and inhibited p38/ERK/mitogen-activated protein kinase signaling. The present findings indicated that paeonol may relieve dermatitis by acting on cluster of differentiation 4+ T and mast cells; therefore, paeonol may represent a potential therapeutic strategy for the treatment of allergic inflammatory conditions via immunoregulation.
Keywords: paeonol, traditional Chinese medicine, atopic dermatitis, cluster of differentiation 4+ T cells, Th1/Th2, mast cells
Introduction
Atopic dermatitis (AD) is a common type of chronic inflammatory disease of the skin, characterized by erythema, dryness and recurrent pruritus, which seriously impairs the quality of daily life (1). The prevalence of AD has been steadily increasing year-on-year in developed and developing countries, and it affects up to 15–20% of children and 1–3% of adults (2,3). To the best of our knowledge, the mechanism underlying AD remains unknown; however, an overactive immune system is strongly correlated with the natural course of the disease. In particular, the abnormal activation of T helper (Th)2 cells is associated with progression of the disease, which contributes to a predominant Th2 response, and an imbalance between Th1 and Th2 responses (4,5). Increasing infiltration of Th2 cells and related cytokines, including interleukin (IL)-4, IL-13 and IL-31, mediates the secretion of immunoglobulin E (IgE). High serum IgE levels are an important characteristic of AD and are associated with the severity of allergic diseases (6,7). Previous studies have demonstrated that regulating the balance between Th1 and Th2 by shifting it to Th1 dominance can markedly inhibit the immune reaction, which indicates an effective strategy for the treatment of AD (8,9).
Mast cells are widely recognized as another crucial factor responsible for allergic inflammatory reactions (10). A previous study demonstrated that high IgE levels activate mast cells. The IgE receptor FcεRI sits on the cell surface and mast cells, as effector cells, release histamines and other biologically active products that trigger allergic inflammatory symptoms (11). Therefore, methods that reduce the number of mast cells and inhibit their activation may ameliorate the symptoms of AD.
Paeonol (2′-hydroxy-4′-methoxyacetophenone) is a constituent of the traditional Chinese medicine (TCM) Cortex Moutan, which has been used to treat inflammatory conditions and immune disorders due to its numerous pharmacological activities, including anti-oxidant, anti-inflammatory, anticancer, apoptosis-inducing and anti-diabetic effects (12,13). Several animal models and cell experiments have reported that paeonol may suppress inflammatory responses (14,15). The results of our previous study demonstrated that paeonol alleviates psoriasis-like lesions in a mouse model and relieves inflammatory reactions (16). It was therefore hypothesized that paeonol may ameliorate allergic diseases induced by activated Th2 and mast cells. Therefore, the present study focused on the effects of paeonol on 1-chloro-2,4-dinitrobenzene (DNCB)-induced AD-like lesions and mast cells (P815 cells), in order to determine the potential efficiency and underlying mechanism of paeonol for the treatment of AD.
Materials and methods
Experimental animals
A total of 48 female BALB/c mice (age, 6–8 weeks; weight, 18–22 g) were obtained from Beijing HFK Bioscience Co., Ltd. (Beijing, China; certification no. SCXK Jing 2017-0001). The mice were housed in individual ventilated cages under a 12-h light/dark cycle at a temperature of 23–25°C and relative humidity of 55–65%. The mice were fed a standard diet and had free access to purified water. All experimental procedures were performed in accordance with the Research and Ethical Guidelines of the Committee of the International Association for the Study of Pain (17) and were approved by the Animal Ethics Committee of Capital Medical University (Beijing, China).
Materials, reagents and instruments
Paeonol was provided by the National Institutes for Food and Drug Control (Beijing, China). DNCB (cat. no. 237329; Sigma-Aldrich; Merck KGaA, Darmstadt, Germany) was dissolved in acetone and olive oil (3:1 v/v). Paeonol was dissolved in normal saline to achieve various concentrations for oral administration. Prednisolone (Pred; cat. no. BP464; Sigma-Aldrich; Merck KGaA) was dissolved in normal saline to a concentration of 10 mg. TRIzol® was purchased from Invitrogen (Thermo Fisher Scientific, Inc., Waltham, MA, USA). The NucleoSpin® RNA Clean-up kit was obtained from Macherey-Nagel GmbH & Co. KG (Düren, Germany). The real-time polymerase chain reaction (RT-PCR) FastStart Universal Master mix was purchased from Roche Diagnostics (Indianapolis, IN, USA) and the AffinityScript Multiple Temperature cDNA Synthesis kit was purchased from Agilent Technologies, Inc. (Santa Clara, CA, USA).
Induction of DNCB-induced AD-like skin lesions
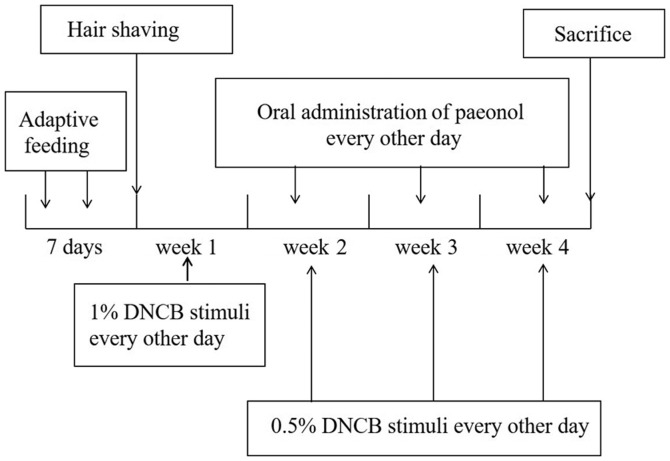
AD-like skin lesions were induced by repeated application of DNCB onto the hairless dorsal skin of the mice, which was based on a previous study (18); the experimental schedule is shown in Fig. 1. The mice were randomly divided into six groups (n=8) after being allowed to acclimate for 1 week. The groups were as follows: Control, model, Pred (Pred at 10 mg/kg), paeonol-high (PH; 200 mg/kg), paeonol-medium (PM; 100 mg/kg) and paeonol-low (PL; 50 mg/kg). Preoperative dorsal hair removal was conducted under isoflurane anesthesia, and pectin feed containing the analgesic carprofen was available for 3 days following shaving in individually ventilated cage. The shaved dorsal skin of the mice was sensitized with 200 µl 1% DNCB every other day for the first week, excluding the control group in which acetone and olive oil (3:1 v/v) were topically applied. Subsequently, DNCB (0.5%) was applied twice a week for a further 3 weeks to stimulate and maintain inflammation. Olive oil was used to moisturize the skin lesions. Mice in the drug therapy groups were intragastrically administered the corresponding treatments every other day alongside DNCB for the last 3 weeks. Meanwhile, the model group was exposed to identical stimuli alongside saline treatment. Following 4 weeks, the mice were weighed and anesthetized via intraperitoneal injection of 70 mg/kg pentobarbital sodium (cat. no. P3761; Sigma-Aldrich; Merck KGaA), blood samples were collected from the eye socket, and the mice were sacrificed by cervical dislocation after 24 h. Samples of blood, ear skin and spleens were obtained for pathological analysis.
Figure 1.
Experimental scheme for the DNCB-induced atopic dermatitis-like mouse model. DNCB, 1-chloro-2,4-dinitrobenzene.
Cell culture
The mouse mastocytoma cell line P815 (China Infrastructure of Cell Line Resources, Beijing) was cultured in Dulbecco's modified Eagle's medium (HyClone; GE Healthcare Life Sciences, Logan, UT, USA) supplemented with 10% fetal bovine serum and 1% penicillin-streptomycin (both from Gibco; Thermo Fisher Scientific, Inc.) in a 5% CO2 atmosphere at 37°C. The mast cells were seeded in 6-well plates and maintained overnight. Compound 48/80 (10 µg/ml; C48/80; cat. no. C2313; Sigma-Aldrich; Merck KGaA) was used to activate the cells and various concentrations of paeonol (375, 750 and 1,500 µg/ml) were additionally added to the medium. The cells were co-treated in a 5% CO2 atmosphere at 37°C. Cells and supernatants were harvested after 12 h.
Dynamic evaluation of dermatitis
Lesion severity scoring was used to estimate the severity of the dermatitis on the back regions of the mice each week; this included scoring of the erythema/hemorrhage, edema, excoriation/erosion and scaling/dryness (18). The score for each was determined as follows: No symptoms, 0; mild symptoms, 1; moderate symptoms, 2; and severe symptoms, 3. The dermatitis score was calculated as the sum of the individual scores. Scratching behavior was recorded at the same time.
Histopathological alterations
Dorsal skin and ear samples were fixed in 10% buffered formalin for 24 h at room temperature, embedded in paraffin and sectioned into 5-µm slices. The skin sections were stained with hematoxylin and eosin (H&E) according to the manufacturer's protocol (cat. no. G1120; Beijing Solarbio Science & Technology Co., Ltd., Beijing, China), and the general tissue features were observed under a light microscope (Zeiss GmbH, Jena, Germany; magnification, ×200). Toluidine blue staining (cat. no. G1436; Beijing Solarbio Science & Technology Co., Ltd.) was applied for measurement of mast cell infiltration, and the number of mast cells was counted in five randomly selected fields of view under a light microscope (magnification, ×200). All procedures were performed according to the manufacturer's protocol at room temperature.
Flow cytometry
Spleens were harvested and weighed under sterile conditions. Single-cell suspensions were obtained after the isolated spleens were minced through a 70-µm mesh, in order to determine proportional changes in the Th1/Th2 balance. A total of 1×106 cells were suspended in fixation/permeabilization solution for 20 min at 4°C (Cytofix/Cytoperm Solution kit; BD Biosciences, Franklin Lakes, NJ, USA) and were then stained with fluorescein isothiocyanate (FITC)-conjugated mouse anti-cluster of differentiation (CD)4 (1:200; cat. no. 35-0041; Tonbo Biosciences, San Diego, CA, USA). Phorbol myristate acetate (10 ng/ml) in PBS and ionomycin (1 µg/ml) in PBS were used to stimulate the cells for intracellular cytokine detection at 37°C and 5% CO2 for 6 h. Subsequently, allophycocyanin (APC) anti-interferon (IFN)-γ (1:1,000; cat. no. 20-7311) and phycoerythrin anti-IL-4 (1:400; cat. no. 50-7041; both from Tonbo Biosciences) antibodies were applied for 20 min at room temperature. Samples were analyzed using a flow cytometer (FACSCalibur) and CellQuest Pro 5.1 software (both from BD Biosciences). In order to assess the effects of paeonol on immune function, the spleen index was calculated as: Spleen index = (spleen weight/body weight) ×100.
Reverse transcription-quantitative PCR (RT-qPCR)
RT-qPCR was conducted to detect the mRNA expression levels of various cytokines, according to the manufacturers' protocols, and the conditions were similar to those described in a previous study (16). Total RNA was isolated from the excised skin using TRIzol® and purified using the NucleoSpin® RNA Clean-up kit. Following generation of cDNA using the AffinityScript Multiple Temperature cDNA Synthesis kit according to the manufacturer's protocol. The real-time PCR FastStart Universal Master Mix (Roche Diagnostics) was used to determine the relative expression levels of genes with an ABI 7500 Fast Real-Time PCR system (Applied Biosystems; Thermo Fisher Scientific, Inc.). The gene-specific primers are listed in Table I. The cycle parameters were as follows: 95°C for 10 min, followed by 50 cycles at 95°C for 15 sec and 60°C for 60 sec. β-actin was used as a reference gene to normalize the data, which were quantitatively analyzed using the 2−ΔΔCq method (19).
Table I.
Primer sequences used for reverse transcription-quantitative polymerase chain reaction.
| Target gene | Direction | Sequence (5′-3′) | Amplicon size (bp) |
|---|---|---|---|
| IL-4 | Forward | TCGTCTGTAGGGCTTCCAAGGTGCT | 166 |
| Reverse | GTGGACTTGGACTCATTCATGGTGC | ||
| IL-13 | Forward | GTCAACAACCCACAGGTCCAG | 108 |
| Reverse | TCAGCAGCGACTCCTTTTCC | ||
| IL-31 | Forward | CCTCAGACTACCTCAACCGTTCC | 191 |
| Reverse | AGGCTCCCTCTTCAGGACCAG | ||
| TSLP | Forward | CTCAATCCTATCCCTGGCTG | 129 |
| Reverse | TGCCATTTCCTGAGTACCGT | ||
| β-actin | Forward | GCCTTCCTTCTTGGGTAT | 97 |
| Reverse | GGCATAGAGGTCTTTACGG |
IL, interleukin; TSLP, thymic stromal lymphopoietin.
Western blot analysis
In order to investigate the possible mechanism underlying the effects of paeonol on AD, the expression levels of proteins associated with the mast cell signaling pathway were analyzed. The skin samples and P815 cells were lysed in radioimmunoprecipitation assay lysis buffer (Cell Signaling Technology, Inc., Danvers, MA, USA) supplemented with phenylmethane sulfonyl fluoride (Thermo Fisher Scientific, Inc.) and protein concentrations were estimated using the bicinchoninic acid protein assay kit (Thermo Fisher Scientific, Inc.). Subsequently, 30 µg total protein was loaded in each lane. Proteins were separated using 10% SDS-PAGE and were transferred onto polyvinylidene fluoride membranes by electroblotting at 4°C. Following blocking using 5% bovine serum albumin (Amresco, LLC, Solon, OH, USA) for 30 min at room temperature, the membrane fractions were incubated with mouse anti-p-p38 mitogen-activated protein kinase (MAPK; 1:1,000; cat. no. 9216), rabbit anti-p-ERK1/2 (1:2,000; cat. no. 4370), rabbit anti-p38 MAPK (1:1,000; cat. no. 8690), mouse anti-ERK1/2 (1:2,000; cat. no. 4696) (all from Cell Signaling Technology, Inc.), mouse anti-GAPDH (1:20,000; cat. no. YM3029; ImmunoWay Biotechnology Company, Plano, TX, USA), rat anti-IL-4 (1:1,000; cat. no. sc-32242), mouse anti-IL-13 (1:1,000; cat. no. sc-393365) (both from Santa Cruz Biotechnology, Inc., Dallas, TX, USA), rabbit anti-IL-31 (1:1,000; cat. no. ab102750; Abcam, Cambridge, MA, USA) and rabbit anti-TSLP (1:500; cat. no. ab188766; Abcam) primary antibodies at 4°C overnight. Subsequently, IRDye 700DX- or 800DX-conjugated secondary antibodies immunofluorescence was assessed using an Odyssey infrared imaging system (LI-COR Biosciences, Lincoln, NE, USA). The secondary antibodies used were the following: Goat anti-mouse immunoglobulin G (IgG; conjugated to Alexa Fluor Plus 800; 1:10,000; cat. no. A32730; Invitrogen; Thermo Fisher Scientific, Inc.), goat anti-rabbit IgG (conjugated to Alexa Fluor Plus 680; 1:10,000; cat. no. A32734; Invitrogen; Thermo Fisher Scientific, Inc.) and goat anti-rat IgG (conjugated to Alexa Fluor Plus 680; 1:10,000; cat. no. A21096; Invitrogen; Thermo Fisher Scientific, Inc.). Image Pro Plus (version 6.0; Media Cybernetics, Inc., Rockville, MD, USA) was used for densitometry.
Cell viability assay
Cell viability was measured using the Cell Counting kit-8 (Dojindo Molecular Technologies, Inc., Kumamoto, Japan), according to the manufacturer's protocol. Briefly, P815 cells were seeded in 96-well plates (1×104 cells/well) with various concentrations of paeonol for 12, 24 and 48 h, and were then maintained in growth media in an atmosphere containing 5% CO2 at 37°C for 2 h. The mean optical density of the cells in each group was used to identify the non-toxic concentration of paeonol.
ELISA
The concentrations of IgE and inflammatory cytokines in the serum and supernatants were determined. Whole blood was collected and allowed to clot for 30 min at room temperature. Whole blood was centrifuged at 1,200 × g for 15 min and the serum samples were then stored at −80°C until use. The serum levels of IgE and inflammatory cytokines were determined using corresponding ELISA kits (IgE, cat. no. CSB-E07983m; IL-4, cat. no. CSB-E04634m; Cusabio Technology LLC, Houston, TX, USA), according to the manufacturer's protocols.
P815 cells were seeded in 12-well culture plates at a density of 1×106 cells/ml and pretreated with various concentrations (375, 750 and 1,500 µg/ml) paeonol for 24 h, followed by treatment with C48/80 (1 µg/ml) for 2 h. The expression levels of TNF-α (cat. no. CSB-E04741m) and histamine (cat. no. CSB-E07043m) (both from Cusabio Technology LLC) in the supernatants were determined using mouse ELISA kits, according to the manufacturer's protocols. Standard curves were generated using purified recombinant TNF-α and histamine at various dilutions.
Statistical analysis
All statistical analyses were performed using SPSS version 17.0 (SPSS, Inc., Chicago, IL, USA). The results are presented as the mean ± standard deviation. The experiments were performed at least three independent times. Statistical analysis of the results was performed using an independent t-test; when three or more groups were compared, one-way analysis of variance followed by least-significant difference post hoc test was used. P-values are two-sided and P<0.05 was considered to indicate a statistically significant difference.
Results
Paeonol ameliorates the morphological features of DNCB-induced AD-like lesions
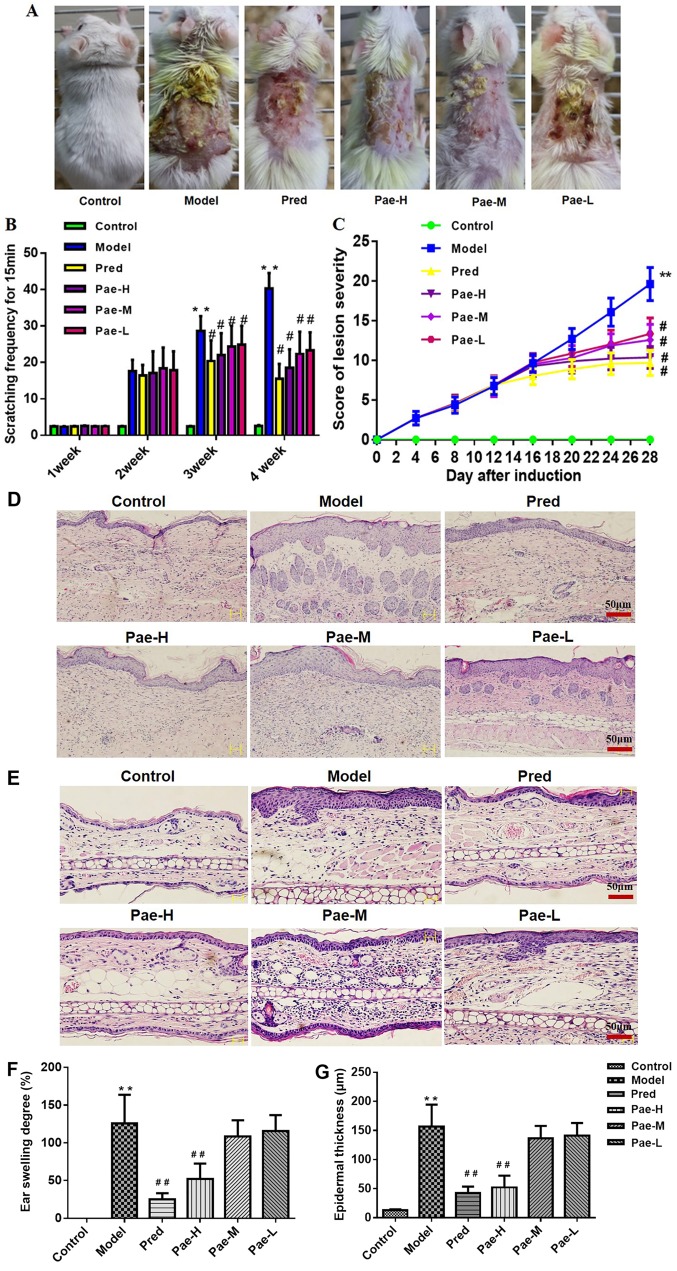
The skin of the model group exhibited AD-like signs and symptoms that began with infiltrative erythema, edema, pruritus and hemorrhage, and were followed by erosion, scratching, excoriation and dryness, which is similar to the clinical manifestation of AD. No alterations in the back skin of the control mice were identified. During the same period, the mice that were orally administered paeonol exhibited less severe pathological alterations in a dose-dependent manner (Fig. 2A-C).
Figure 2.
Oral administration of paeonol inhibits DNCB-induced AD-like skin lesions in BALB/c mice. (A) Typical pathological presentation of AD-like lesions induced by DNCB application. (B) Severity scoring of lesions was assessed daily using the Lesion severity scoring index. Five signs and symptoms (itching, erythema, edema, excoriation and dryness) were each scored on a scale between 0 and 3 (0, none; 1, mild; 2, moderate; and 3, severe). (C) Scratching behavior was observed daily for 15 min. Pathological observation of the (D) back skin and (E) ear tissues (hematoxylin and eosin staining; magnification, ×200; scale bar, 50 µm). (F) Ear swelling and (G) epidermal thickness in each group. Data are presented as the means ± standard deviation (n=8). **P<0.01 vs. the control group; #P<0.05, ##P<0.01 vs. the model group. AD, atopic dermatitis; DNCB, 1-chloro-2,4-dinitrobenzene; Pae-H, paeonol-high (200 mg/kg); Pae-L, paeonol-low (50 mg/kg); Pae-M, paeonol-medium (100 mg/kg); Pred, prednisolone.
H&E staining of the back skin and ear tissue of the mice revealed hyperkeratosis, acanthosis and increased perivascular infiltration of inflammatory cells in the model group compared with the normal skin structure of the control group. Paeonol treatment significantly reduced epidermal thickness and the degree of ear swelling (Fig. 2D-G).
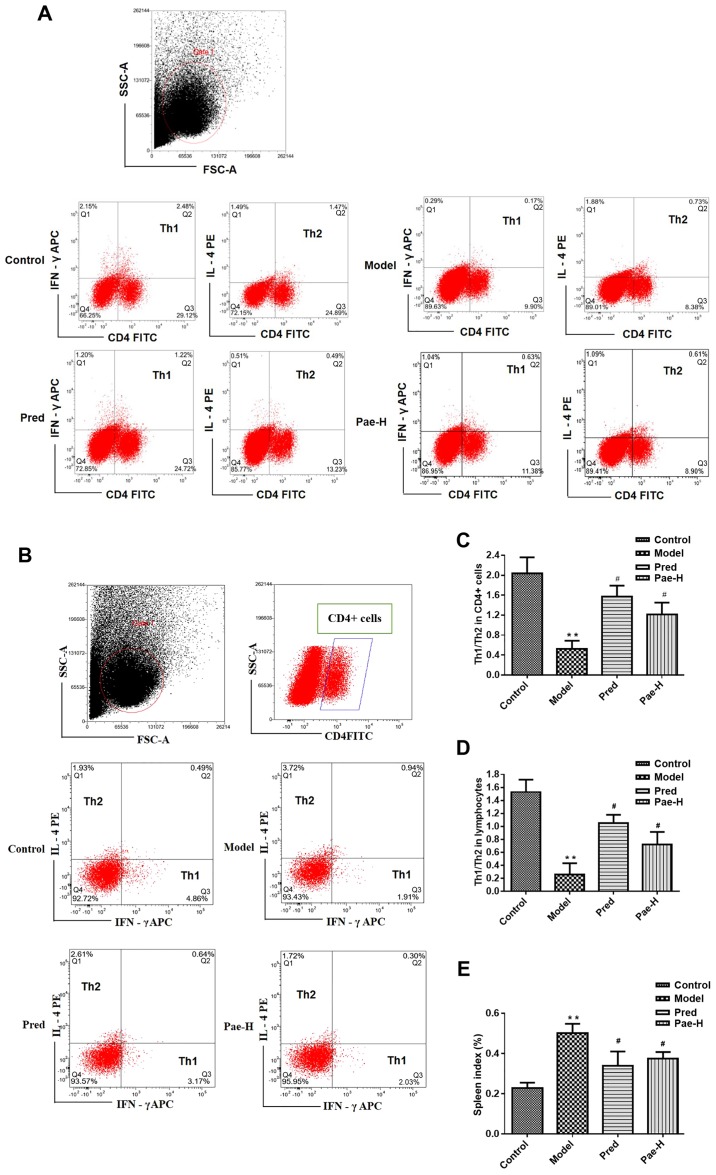
Paeonol redresses the Th1/Th2 cell balance
In order to investigate the ratio of Th1/Th2 cells in allergically inflamed skin, spleen cells were collected and the proportions of Th cell subsets were detected. There was an increased percentage of the Th2 subset in the model group, whereas treatment with the high dose of paeonol corrected the imbalance by inhibiting the Th2 immune reaction (Fig. 3). The percentages of Th1 (CD4 positive and IFN-γ APC positive) or Th2 cells (CD4 positive and IL-4PE positive) in all lymphocytes are presented in the second quadrant of the scatter plots, corresponding to double positive cells (Fig. 3A and D). The percentages of Th2 (IL-4PE positive and IFN-γ APC negative) and Th1 cells (IFN-γ APC positive and IL-4PE negative) in the CD4 positive lymphocytes are presented in the first and third quadrant of the scatter plots, respectively (Fig. 3B and C). The spleen index in the model group increased compared with the control group. Paeonol treatment decreased significantly the spleen index compared with the model group (Fig. 3E).
Figure 3.
Paeonol redresses the Th1/Th2 cell imbalance and inhibits immune responses. (A) Ratio of Th1/Th2 subset cells in the (A) spleen and (B) CD4+ lymphocytes. Statistical analysis of the (C) spleen and (D) CD4+ lymphocyte data in (A and B), respectively. (E) Spleen index. **P<0.01 vs. the control group; #P<0.05 vs. the model group. APC, allophycocyanin; CD4, cluster of differentiation 4; FITC, fluorescein isothiocyanate; FSC, forward scatter; IFN-γ, interferon-γ; IL, interleukin; Pae-H, paeonol-high (200 mg/kg); PE, phycoerythrin; Pred, prednisolone; SSC, side scatter; Th, T helper.
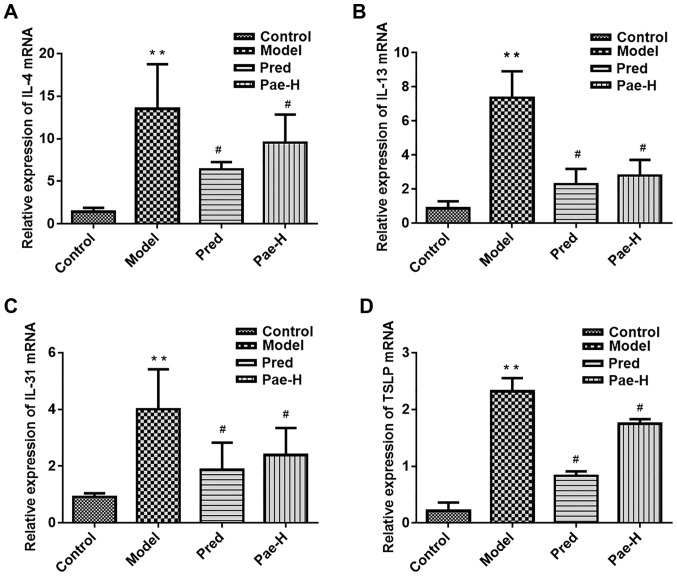
Paeonol reduces the mRNA and protein expression levels of pro-inflammatory and immune cytokines in dorsal skin lesions
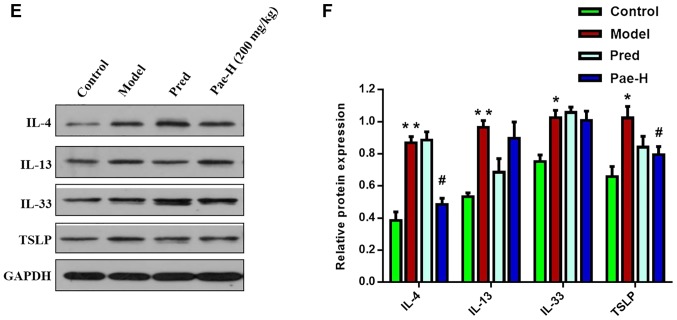
Alterations in the mRNA and protein expression levels of IL-4, IL-13, IL-31 and thymic stromal lymphopoietin (TSLP) were analyzed in order to elucidate the anti-inflammatory and immunosuppressive effects of paeonol. The mRNA expression levels of IL-4, IL-13, IL-31 and TSLP were increased in the model group compared with in the control group. Conversely, treatment with a high dose of paeonol suppressed DNCB-induced cytokine expression, and the protein expression levels of IL-4 and TSLP were also decreased in the paeonol-treated group (Fig. 4).
Figure 4.
Paeonol inhibits the mRNA and protein expression levels of cytokines that mediate inflammatory and immune reactions. mRNA expression levels of (A) IL-4, (B) IL-13, (C) IL-31 and (D) TSLP. Protein expression levels of (E) IL-4, IL-13, IL-31 and TSLP in the lesions. (F) Protein expression levels were semi-quantified. Data are presented as the means ± standard deviation (n=6). *P<0.05, **P<0.01 vs. the control group; #P<0.05 vs. the model group. IL, interleukin; Pae-H, paeonol-high (200 mg/kg); Pred, prednisolone; TSLP, thymic stromal lymphopoietin.
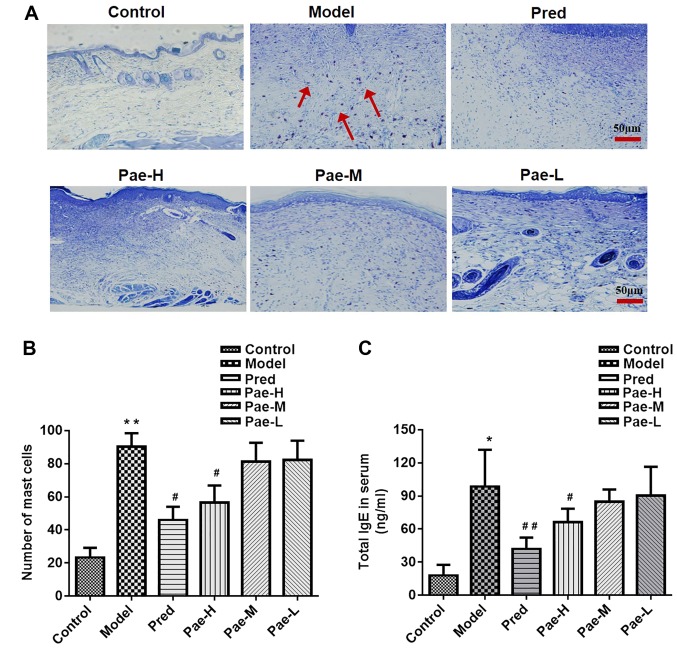
Paeonol downregulates mast cell infiltration in the dermis, and IgE and inflammatory cytokine levels in the serum
The DNCB-induced skin lesions exhibited increased infiltration of mast cells compared with in the control group, whereas administration of a high dose of paeonol markedly reduced the number of mast cells compared with in the model group. Treatment with the medium and low doses of paeonol had no significant effect (Fig. 5A and B).
Figure 5.
Paeonol reduces the number of mast cells in the dermis, and the concentration of IgE and inflammatory cytokines in the serum. (A) Mast cell infiltration in the dorsal skin was determined using toluidine blue staining (magnification, ×200; scale bar, 50 µm). Red arrows indicate the stained mast cells. (B) Mast cell counts in the different groups. Serum levels of (C) IgE, and the inflammatory cytokines (D) IL-4 and (E) histamine in the different groups, as measured using an ELISA. Data are presented as the means ± standard deviation (n=8). *P<0.05, **P<0.01 vs. the control group; #P<0.05, ##P<0.01 vs. the model group. IgE, immunoglobulin E; IL-4, interleukin-4; Pae-H, paeonol-high (200 mg/kg); Pae-L, paeonol-low (50 mg/kg); Pae-M, paeonol-medium (100 mg/kg); Pred, prednisolone.
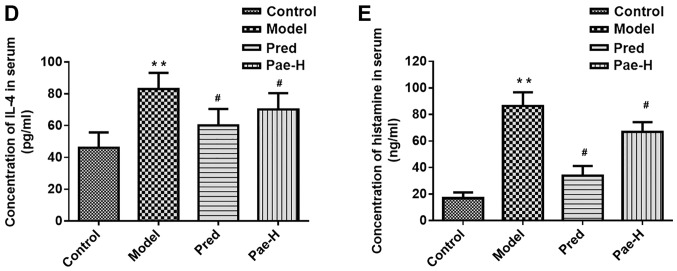
High levels of serum IgE were detected in the DNCB-treated group, and high-dose paeonol reduced these levels (Fig. 5C). The serum levels of the inflammatory factors IL-4 and histamine were higher in the model group than in the control group. High-dose paeonol markedly reduced the levels of the inflammatory cytokines compared with in the model group (Fig. 5D and E).
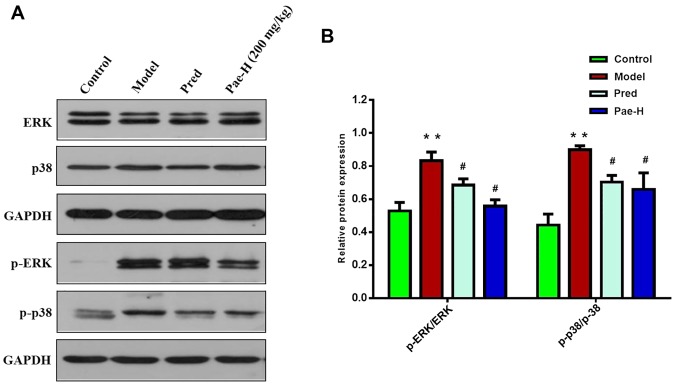
Paeonol decreases p-p38 and p-ERK proteins in a mouse model of AD
The present study further explored how paeonol suppresses mast cells and investigated the effects of paeonol on MAPK signaling in skin lesions. The results revealed that the protein expression levels of p-p38 and p-ERK were reduced in the paeonol-high dose group compared with in the model group (Fig. 6A and B).
Figure 6.
Paeonol decreases p-p38 and p-ERK protein expression in skin lesions of an acute dermatitis-like mouse model. (A) Protein expression levels of p-p38 and p-ERK were assessed by western blotting and (B) the levels of each were semi-quantified. **P<0.01 vs. the control group; #P<0.05 vs. the model group. ERK, extracellular signal-regulated kinase; p, phosphorylated; Pae-H, paeonol-high (200 mg/kg); Pred, prednisolone.
Paeonol reduces the levels of histamine and TNF-α secreted by P815 cells
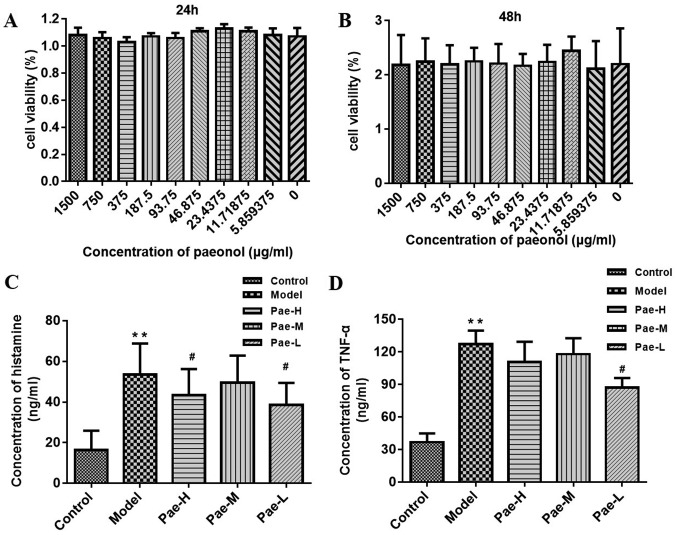
This study investigated whether paeonol affected the viability of P815 cells using the CCK-8 assay; the results demonstrated that paeonol did not exert a cytotoxic effect on P815 cells (Fig. 7A and B). Safe concentrations were used in subsequent experiments. Compared with the levels in untreated P815 cells, the concentrations of histamine and TNF-α in the C48/80-treated groups were markedly increased. There was a marked reduction in histamine and TNF-α in the paeonol-pretreated groups compared with in the model group. The inhibitory effect in the low-dose group was significant for both histamine and TNF-α (Fig. 7C and D).
Figure 7.
Paeonol reduces the levels of histamine and inflammatory cytokines secreted by P815 cells. (A and B) Effects of paeonol on cell viability, as measured using a CCK-8 assay. Detection of (C) histamine and (D) TNF-α release using ELISA. **P<0.01 vs. the control group; #P<0.05 vs. the model group. CCK-8, Cell Counting kit-8; Pae-H, paeonol-high (1,500 µg/ml); Pae-L, paeonol-low (375 µg/ml) Pae-M, paeonol-medium (750 µg/ml); TNF-α, tumor necrosis factor-α.
Paeonol inhibits protein expression levels of p-p38 and p-ERK in activated P815 cells
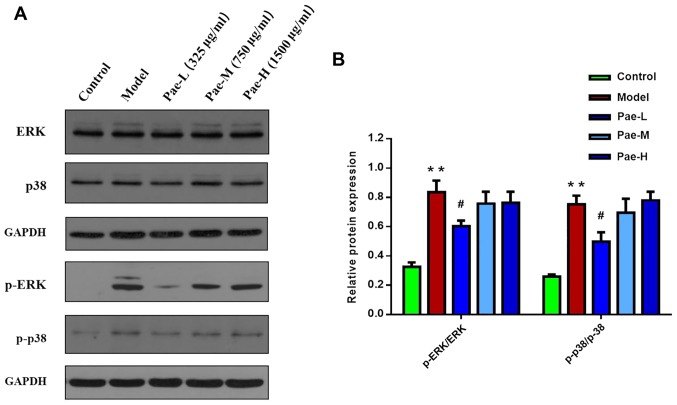
Western blot analysis was performed to investigate the effects of paeonol on MAPK signaling in mast cells. The results demonstrated that the protein expression levels of p-p38 and p-ERK were reduced in the paeonol-treated groups compared with in the C48/80-treated group. Consistent with the results of the ELISA, a low dose of paeonol induced a more powerful effect than the other two doses. These results indicated that paeonol may exert its therapeutic action by blocking the p38/ERK/MAPK signaling pathway (Fig. 8).
Figure 8.
Paeonol inhibits compound 48/80-mediated p-p38 and p-ERK expression in P815 cells. The expression levels of p38, ERK, p-p38 and p-ERK were assessed following treatment with paeonol using (A) western blotting and (B) the levels of each were semi-quantified. **P<0.01 vs. the control group; #P<0.05 vs. the model group. ERK, extracellular signal-regulated kinase; p, phosphorylated; Pae-H, paeonol-high (1,500 µg/ml); Pae-L, paeonol-low (375 µg/ml) Pae-M, paeonol-medium (750 µg/ml)
Discussion
Paeonol is an active component extracted from the TCM Cortex Moutan, which is widely used and has high clinical development prospects (20,21). Numerous pharmacological and clinical studies have confirmed the biological properties of paeonol, including anti-inflammatory, anti-bacterial and antitumor effects (22,23). Our previous study demonstrated that oral administration of paeonol results in anti-inflammatory and immunoregulatory effects in BALB/c mice with psoriasis-like lesions (16). In order to further investigate the pharmacological activities of paeonol, a series of experiments were performed on damaged skin.
AD is an allergic and inflammatory skin disorder that results in itching and recurrent eczematous lesions, and seriously affects quality of life (24). In addition to genetic and environmental factors, immunological disarrangement appears to serve a significant role in the pathogenesis of AD (25). A previous study demonstrated that increased infiltration of Th cells and related cytokines contributes to the release of high levels of histamine and numerous pro-inflammatory factors, thus resulting in itching, dry skin and eczema (26). Topical therapy is common practice to alleviate clinical symptoms; however, the side effects of this treatment include thinner and more sensitive skin. On the basis of previous studies, it was hypothesized that oral administration of paeonol may be an effective treatment for allergic disease, and in vivo and in vitro experiments were designed to investigate this.
In the present study, an AD-like mouse model was established via topical application of DNCB, which is a sensitizer that is used worldwide for chemically inducing contact dermatitis. The animals that were subjected to repeated DNCB challenge exhibited clinical and immunological presentations that were similar to human AD. The irritated skin and ears of the animals progressively developed into clear allergic reactions with various symptoms including dryness, scales and pruritus, followed by erythema, swelling and erosion. Subsequently, experiments to investigate the anti-atopic effect of paeonol were conducted. Paeonol markedly improved the skin lesions, with a reduction in the SCORAD scores and frequency of scratching. Histological examination of the skin revealed a thicker epidermis and increased inflammatory infiltration compared with in the control group, whereas these pathological alterations were significantly ameliorated by oral administration of paeonol in a dose-dependent manner. Ear thickness was also measured, in order to confirm the effectiveness of paeonol. The H&E staining results revealed a thicker ear dermis in the model group, whereas the paeonol-treated groups exhibited a significant reduction in thickness compared with in the model group. These results demonstrated that DNCB may induce damage to the epidermis and dermis, whereas paeonol exhibited clear anti-atopic activity, and was involved in regulating the abnormal condition of the skin.
The immune dysfunction that results from a disturbance in the Th1/Th2 balance serves a role in the progression of allergic inflammation (27). Therefore, the proportion of Th1 and Th2 cells in the spleen and lymphocytes of the animals in the present study was detected. The results revealed that the proportion of Th1 cells was markedly reduced following exposure to DNCB. Paeonol significantly regulated this effect by inhibiting the Th2 immune response.
Various inflammatory cytokines are involved in regulating and directing the nature of AD, including IL-4, IL-13, IL-31 and TSLP, and they are predominantly Th2-derived cytokines (28,29). IL-4 and IL-13, which act as the key drivers for isotype switching to IgE, generation of inflammatory factors and receptor expression on the surface of mast cells, commonly activate IL-4 receptor (IL-4R) and subsequently downregulate skin barrier proteins, thus impairing the skin barrier (30,31). Therapies that target IL-4R and lead to the inhibition of the IL-4 and IL-13 signaling pathways are key treatment targets in the complex pathological mechanism of AD (32,33). TSLP, which is capable of eliciting a powerful immune response, is secreted by the epithelial cells of damaged skin. Released TSLP results in priming of resident dendritic cells, which induces susceptibility to AD and Th2 immune deviation (29). IL-31 is thought to serve a critical role in the pathogenesis of AD, particularly in mediating skin pruritus by transmitting the itch sensation to the central nervous system (34,35). Consistent with previous studies, increased mRNA expression levels of IL-4, IL-13, IL-31 and TSLP were detected in AD-like mouse skin in the present study (36,37). Furthermore, there was a reduction in IL-4, IL-13, IL-31 and TSLP mRNA expression following paeonol treatment. These findings provide further evidence to suggest that paeonol may downregulate the Th2 immune response.
Localized mast cells serve a key role in the development of allergic diseases and the activated state of mast cells may be responsible for signs of dermatitis (38). Toluidine blue staining in the present study revealed an increased number of mast cells in the skin lesions of the model group, whereas paeonol treatment significantly reduced the number in a dose-dependent manner. Increased serum IgE levels are the hallmark of allergic disease and trigger the activation of mast cells (39). Enhanced expression levels of IgE and IL-4 were observed in the serum following chemical stimulation in the present study, further validating the feasibility of the animal model. Furthermore, the anti-inflammatory and anti-allergy activities of paeonol were determined. Therefore, the underlying mechanism was subsequently investigated, and the protein expression levels of p-p38 and p-ERK were detected in vivo; the expression levels of these proteins were significantly reduced in the high-dose paeonol-treated group.
Allergic reactions are characterized by activation of mast cells and they are a key cause of the condition worsening (40). To the best of our knowledge, there are no studies regarding the effects of paeonol on mast cells or the allergic response. Following the results obtained from the animal experiments of the present study, the effects on inflammatory effector cells were investigated. Activated mast cells express and release pro-inflammatory cytokines, including TNF-α (41). The in vitro experiments demonstrated that pretreatment with paeonol inhibited the expression of TNF-α with no cytotoxic effect. Histamine is a biogenic amine that is primarily stored by mast cells and is released when they are activated, leading to immediate allergic symptoms (42). Paeonol diminished the histamine content in the supernatant of P815 cells. Since the MAPK signaling cascade is an important signaling pathway in immune responses (43), the proteins associated with this transduction pathway were investigated in the present study. Application of paeonol attenuated the phosphorylation of p38 and ERK, inhibiting p38 and ERK activation in the C48/80-stimulated P815 cells, which is essential for the degranulation of mast cells. Therefore, it was hypothesized that prevention of mast cell activation by inhibiting the MAPK/ERK/p38 signaling pathway was one critical characteristic of the anti-allergic activity of paeonol. In vitro, the effects of paeonol were not revealed to be dose-dependent, and numerous factors may be responsible for this effect, including the solubility of paeonol. The present results suggested the molecular mechanism underlying the effects of paeonol on mast cells. Our future studies aim to explore the effect of lower dosages within safe levels, and determine the potential mechanism underlying the effects of paeonol on C48/80-induced P815 cells. Further studies are required to determine whether paeonol can inhibit AD via immunoregulation of T and mast cells.
Numerous studies have identified various biological effects of paeonol, including inhibition of the proliferation of SGC-7901 cells, induction of tumor cell apoptosis in breast cancer, and anti-inflammatory, cardioprotective and neuroprotective effects (44,45). Our previous study (16) revealed that paeonol inhibits inflammation and blocks the progression of psoriasis. To the best of our knowledge, the present study is the first to determine the effects of paeonol on epidermal thickness, mast cell activation and proliferation, and the balance of Th1/Th2 cells in allergic disease.
In conclusion, to the best of the authors' knowledge, the present study is the first to demonstrate that paeonol may be used to treat AD. The application of paeonol may correct the proportional imbalance of the immune system in order to treat inflammatory skin disease. The precise mechanism of action of paeonol on target proteins requires further investigation, including that which utilizes modern biotechnology, such as molecular docking.
Acknowledgements
Not applicable.
Funding
The present study was supported by the National Science Foundation Project (grant nos. 81403410 and 81673989) and the Beijing Municipal Project (grant no. PXM2017_026273_000001).
Availability of data and materials
All datasets used or analysed during the current study are available from the corresponding author on reasonable request.
Authors' contributions
YM, YW and PL conceived and supervised the study. YM, ZL and CZ performed the animal experiments. YM, LuZ and XX performed physiological test and the pathological experiments. YM, TD and JZ performed cell experiments. YM, NW, YL and LeZ performed the data analysis. YM drafted the manuscript and made substantial contributions to the manuscript. YM interpreted the data. All authors revised and approved the present manuscript.
Ethics approval and consent to participate
All experimental procedures were performed in accordance with the National Guidelines on Laboratory Research and were approved by the Animal Ethics Committee of Capital Medical University.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Sidbury R, Khorsand K. Evolving concepts in atopic dermatitis. Curr Allergy Asthma Rep. 2017;17:42. doi: 10.1007/s11882-017-0710-5. [DOI] [PubMed] [Google Scholar]
- 2.Griffiths CE, van de Kerkhof P, Czarnecka-Operacz M. Psoriasis and atopic dermatitis. Dermatol Ther (Heidelb) 2017;7:31–41. doi: 10.1007/s13555-016-0167-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Genuneit J, Seibold AM, Apfelbacher CJ, Konstantinou GN, Koplin JJ, La Grutta S, Logan K, Perkin MR, Flohr C, Task Force ‘Overview of Systematic Reviews in Allergy Epidemiology (OSRAE)’ of the EAACI Interest Group on Epidemiology. Overview of systematic reviews in allergy epidemiology. Allergy. 2017;72:849–856. doi: 10.1111/all.13123. [DOI] [PubMed] [Google Scholar]
- 4.Guttman-Yassky E, Krueger JG, Lebwohl MG. Systemic immune mechanisms in atopic dermatitis and psoriasis with implications for treatment. Exp Dermatol. 2018;27:409–417. doi: 10.1111/exd.13336. [DOI] [PubMed] [Google Scholar]
- 5.Hello M, Aubert H, Bernier C, Néel A, Barbarot S. Atopic dermatitis of the adult. Rev Med Interne. 2016;37:91–99. doi: 10.1016/j.revmed.2015.10.345. [DOI] [PubMed] [Google Scholar]
- 6.Liour SS, Tom A, Chan YH, Chang TW. Treating IgE-mediated diseases via targeting IgE-expressing B cells using an anti-CεmX antibody. Pediatr Allergy Immunol. 2016;27:446–451. doi: 10.1111/pai.12584. [DOI] [PubMed] [Google Scholar]
- 7.Stokes J. Anti-IgE Treatment for disorders other than asthma. Front Med (Lausanne) 2017;4:152. doi: 10.3389/fmed.2017.00152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.De Kouchkovsky DA, Ghosh S, Rothlin CV. Negative regulation of type 2 Immunity. Trends Immunol. 2017;38:154–167. doi: 10.1016/j.it.2016.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Osinka K, Dumycz K, Kwiek B, Feleszko W. Novel therapeutic approaches to atopic dermatitis. Arch Immunol Ther Exp (Warsz) 2018;66:171–181. doi: 10.1007/s00005-017-0487-1. [DOI] [PubMed] [Google Scholar]
- 10.Saluja R, Khan M, Church M, Maurer M. The role of IL-33 and mast cells in allergy and inflammation. Clin Transl Allergy. 2015;5:33. doi: 10.1186/s13601-015-0076-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Malik K, Heitmiller KD, Czarnowicki T. An update on the pathophysiology of atopic dermatitis. Dermatol Clin. 2017;35:317–326. doi: 10.1016/j.det.2017.02.006. [DOI] [PubMed] [Google Scholar]
- 12.Jin X, Wang J, Xia ZM, Shang CH, Chao QL, Liu YR, Fan HY, Chen DQ, Qiu F, Zhao F. Antiinflammatory and anti-oxidative activities of paeonol and its metabolites through blocking MAPK/ERK/p38 signaling pathway. Inflammation. 2016;39:434446. doi: 10.1007/s10753-015-0265-3. [DOI] [PubMed] [Google Scholar]
- 13.Liu CM, Yang HX, Ma JQ, Yang W, Feng ZJ, Sun JM, Cheng C, Li J, Jiang H. Role of AMPK pathway in lead-induced endoplasmic reticulum stress in kidney and in paeonol-induced protection in mice. Food Chem Toxicol Oct. 2018;122:87–94. doi: 10.1016/j.fct.2018.10.024. [DOI] [PubMed] [Google Scholar]
- 14.Choy KW, Mustafa MR, Lau YS, Liu J, Murugan D, Lau CW, Wang L, Zhao L, Huang Y. Paeonol protects against endoplasmic reticulum stress-induced endothelial dysfunction via AMPK/PPAR delta signaling pathway. Biochem Pharmacol. 2016;116:51–62. doi: 10.1016/j.bcp.2016.07.013. [DOI] [PubMed] [Google Scholar]
- 15.Liu MH, Lin AH, Lee HF, Ko HK, Lee TS, Kou YR. Paeonol attenuates cigarette smoke-induced lung inflammation by inhibiting ROS-sensitive inflammatory signaling. Mediators Inflamm. 2014;2014:651890. doi: 10.1155/2014/651890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Meng Y, Wang M, Xie X, Di T, Zhao J, Lin Y, Xu X, Li N, Zhai Y, Wang Y, Li P. Paeonol ameliorates imiquimod-induced psoriasis-like skin lesions in BALB/c mice by inhibiting the maturation and activation of dendritic cells. Int J Mol Med. 2017;39:1101–1110. doi: 10.3892/ijmm.2017.2930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Choi JH, Kim HG, Jin SW, Han EH, Khanal T, Do MT, Hwang YP, Choi JM, Chun SS, Chung YC, et al. Topical application of Pleurotus eryngii extracts inhibits 2,4-dinitrochlorobenzene-induced atopic dermatitis in NC/Nga mice by the regulation of Th1/Th2 balance. Food Chem Toxicol. 2013;53:38–45. doi: 10.1016/j.fct.2012.11.025. [DOI] [PubMed] [Google Scholar]
- 18.Charlton E. Committee on ethical issues of the international association for the study of pain. Pain. 1995;63:277–278. doi: 10.1016/0304-3959(95)90040-3. [DOI] [PubMed] [Google Scholar]
- 19.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 20.Liu M, Zhong S, Kong R, Shao H, Wang C, Piao H, Lv W, Chu X, Zhao Y. Paeonol alleviates interleukin-1beta-induced inflammatory responses in chondrocytes during osteoarthritis. Biomed Pharmacother. 2017;95:914–921. doi: 10.1016/j.biopha.2017.09.011. [DOI] [PubMed] [Google Scholar]
- 21.Kim JH, Seo CS, Shin HK. Development of validated determination of the eleven marker compounds in Gyejibokryeong-hwan for the quality assessment using HPLC analysis. Arch Pharm Res. 2015;38:52–62. doi: 10.1007/s12272-014-0363-z. [DOI] [PubMed] [Google Scholar]
- 22.Zhu TH, Cao SW, Yu YY. Synthesis, characterization and biological evaluation of paeonol thiosemicarbazone analogues as mushroom tyrosinase inhibitors. Int J Biol Macromol. 2013;62:589–595. doi: 10.1016/j.ijbiomac.2013.09.056. [DOI] [PubMed] [Google Scholar]
- 23.Scheerer C, Eyerich K. Pathogenesis of atopic dermatitis. Hautarzt. 2018;69:191–196. doi: 10.1007/s00105-018-4127-4. [DOI] [PubMed] [Google Scholar]
- 24.Köberle M, Biedermann T. Microbiome, atopic eczema and blockade of type 2 immunity. Hautarzt. 2018;69:197–203. doi: 10.1007/s00105-018-4129-2. [DOI] [PubMed] [Google Scholar]
- 25.Soumelis V. Molecular and cellular discoveries in inflammatory dermatoses. J Eur Acad Dermatol Venereol. 2017;31:3–7. doi: 10.1111/jdv.14373. [DOI] [PubMed] [Google Scholar]
- 26.Hashimoto Y, Arai I, Nakanishi Y, Sakurai T, Nakamura A, Nakaike S. Scratching of their skin by NC/Nga mice leads to development of dermatitis. Life Sci. 2004;76:783–794. doi: 10.1016/j.lfs.2004.07.022. [DOI] [PubMed] [Google Scholar]
- 27.Eyerich K, Novak N. Immunology of atopic eczema: Overcoming the Th1/Th2 paradigm. Allergy. 2013;68:974–982. doi: 10.1111/all.12184. [DOI] [PubMed] [Google Scholar]
- 28.Manti S, Chimenz R, Salpietro A, Colavita L, Pennisi P, Pidone C, Sturiale M, Arrigo T, Miraglia Del Giudice M, Salpietro C, Cuppari C. Atopic Dermatitis: Expression of Immunological Imbalance. J Biol Regul Homeost Agents. 2015;29:13–7. [PubMed] [Google Scholar]
- 29.Lawrence MG, Steinke JW, Borish L. Cytokine-targeting biologics for allergic diseases. Ann Allergy Asthma Immunol. 2018;120:376–381. doi: 10.1016/j.anai.2018.01.009. [DOI] [PubMed] [Google Scholar]
- 30.Lee DE, Clark AK, Tran KA, Shi VY. New and emerging targeted systemic therapies: A new era for atopic dermatitis. J Dermatolog Treat. 2018;29:364–374. doi: 10.1080/09546634.2017.1373736. [DOI] [PubMed] [Google Scholar]
- 31.Mc Cormick SM, Heller NM. Commentary: IL-4 and IL-13 receptors and signaling. Cytokine. 2015;75:38–50. doi: 10.1016/j.cyto.2015.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hurdayal R, Brombacher F. The role of IL-4 and IL-13 in cutaneous Leishmaniasis. Immunol Lett. 2014;16:179–183. doi: 10.1016/j.imlet.2013.12.022. [DOI] [PubMed] [Google Scholar]
- 33.Di Lernia V. Therapeutic strategies in extrinsic atopic dermatitis: Focus on inhibition of IL-4 as a new pharmacological approach. Expert Opin Ther Targets. 2015;19:87–96. doi: 10.1517/14728222.2014.965682. [DOI] [PubMed] [Google Scholar]
- 34.Danso MO, van Drongelen V, Mulder A, van Esch J, Scott H, van Smeden J, El Ghalbzouri A, Bouwstra JA. TNF-alpha and Th2 cytokines induce atopic dermatitis-like features on epidermal differentiation proteins and stratum corneum lipids in human skin equivalents. J Invest Dermatol. 2014;134:1941–1950. doi: 10.1038/jid.2014.83. [DOI] [PubMed] [Google Scholar]
- 35.Nygaard U, Hvid M, Johansen C, Buchner M, Fölster-Holst R, Deleuran M, Vestergaard C. TSLP, IL-31, IL-33 and sST2 are new biomarkers in endophenotypic profiling of adult and childhood atopic dermatitis. J Eur Acad Dermatol Venereol. 2016;30:1930–1938. doi: 10.1111/jdv.13679. [DOI] [PubMed] [Google Scholar]
- 36.Han H, Roan F, Ziegler SF. The atopic march: Current insights into skin barrier dysfunction and epithelial cell-derived cytokines. Immunol Rev. 2017;278:116–130. doi: 10.1111/imr.12546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cianferoni A, Spergel J. The importance of TSLP in allergic disease and its role as a potential therapeutic target. Expert Rev Clin Immunol. 2014;10:1463–1474. doi: 10.1586/1744666X.2014.967684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bağci IS, Ruzicka T. IL-31: A new key player in dermatology and beyond. J Allergy Clin Immunol. 2018;141:858–866. doi: 10.1016/j.jaci.2017.10.045. [DOI] [PubMed] [Google Scholar]
- 39.Otsuka A, Nomura T, Rerknimitr P, Seidel JA, Honda T, Kabashima K. The interplay between genetic and environmental factors in the pathogenesis of atopic dermatitis. Immunol Rev. 2017;278:246–262. doi: 10.1111/imr.12545. [DOI] [PubMed] [Google Scholar]
- 40.Silvestre MC, Sato MN, Reis VMSD. Innate immunity and effector and regulatory mechanisms involved in allergic contact dermatitis. An Bras Dermatol. 2018;93:242–250. doi: 10.1590/abd1806-4841.20186340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Campana R, Dzoro S, Mittermann I, Fedenko E, Elisyutina O, Khaitov M, Karaulov A, Valenta R. Molecular aspects of allergens in atopic dermatitis. Curr Opin Allergy Clin Immunol. 2017;17:269–277. doi: 10.1097/ACI.0000000000000378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Korošec P, Gibbs BF, Rijavec M, Custovic A, Turner PJ. Important and specific role for basophils in acute allergic reactions. Clin Exp Allergy. 2018;48:502–512. doi: 10.1111/cea.13117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mukai K, Tsai M, Saito H, Galli SJ. Mast cells as sources of cytokines, chemokines, and growth factors. Immunol Rev. 2018;282:121–150. doi: 10.1111/imr.12634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kawakami T, Kashiwakura J, Kawakami Y. Histamine-releasing factor and immunoglobulins in asthma and allergy. Allergy Asthma Immunol Res. 2014;6:6–12. doi: 10.4168/aair.2014.6.1.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Patterson H, Nibbs R, McInnes I, Siebert S. Protein kinase inhibitors in the treatment of inflammatory and autoimmune diseases. Clin Exp Immunol. 2014;176:1–10. doi: 10.1111/cei.12248. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All datasets used or analysed during the current study are available from the corresponding author on reasonable request.