Abstract
Background:
With the advent of more sensitive culture and molecular diagnostic testing modalities, Bartonella spp. infections have been documented in blood and/or cerebrospinal fluid specimens from patients with diverse neurological symptoms. Pediatric acute-onset neuropsychiatric syndrome (PANS) is characterized by an unusually abrupt onset of cognitive, behavioral, or neurological symptoms. Between October 2015 and January 2017, a 14-year-old boy underwent evaluation by multiple specialists for sudden-onset psychotic behavior (hallucinations, delusions, suicidal and homicidal ideation).
Methods:
In March 2017, Bartonella spp. serology (indirect fluorescent antibody assays) and polymerase chain reaction (PCR) amplification, DNA sequencing, and Bartonella enrichment blood culture were used on a research basis to assess Bartonella spp. exposure and bloodstream infection, respectively. PCR assays targeting other vector-borne infections were performed to assess potential co-infections.
Results:
For 18 months, the boy remained psychotic despite 4 hospitalizations, therapeutic trials involving multiple psychiatric medication combinations, and immunosuppressive treatment for autoimmune encephalitis. Neurobartonellosis was diagnosed after cutaneous lesions developed. Subsequently, despite nearly 2 consecutive months of doxycycline administration, Bartonella henselae DNA was PCR amplified and sequenced from the patient’s blood, and from Bartonella alphaproteobacteria growth medium enrichment blood cultures. B henselae serology was negative. During treatment with combination antimicrobial chemotherapy, he experienced a gradual progressive decrease in neuropsychiatric symptoms, cessation of psychiatric drugs, resolution of Bartonella-associated cutaneous lesions, and a return to all pre-illness activities.
Conclusions:
This case report suggests that B henselae bloodstream infection may contribute to progressive, recalcitrant neuropsychiatric symptoms consistent with PANS in a subset of patients.
Keywords: Bacteria, psychosis, transmission, stretch marks, Bartonella, schizophrenia
Bartonella spp. constitute emerging, vector-borne, intravascular pathogens that produce long-lasting bloodstream infections in reservoir-adapted (defined as natural host or passive carrier of a microorganism) or, at times, relapsing opportunistic infections in non-reservoir animals and human hosts.1,2 Recent observations support the possibility that this genus of bacteria can cause a spectrum of neurological symptoms, diverse disease manifestations, and multi-organ system pathology that varies in severity and diagnostic complexity over a protracted time course, perhaps ranging from months to decades.1-3 With the advent of more sensitive and specific diagnostic tests, there is evolving microbiological evidence to support bloodstream and cerebrospinal fluid (CSF) infections with 1 or more Bartonella spp. in patients with neurological, neurocognitive, and neuropsychiatric symptoms.4-6
A clinical syndrome in youth, defined by the sudden onset of neuropsychiatric symptoms including obsessions/compulsions or food restriction, has been designated pediatric acute-onset neuropsychiatric syndrome (PANS).7 Depression, irritability, anxiety, and decline in school performance often accompany PANS symptoms. PANS can be triggered by infection (Group A streptococcal infections), metabolic disturbances, and other inflammatory reactions.7 This report documents Bartonella henselae bloodstream infection in a boy diagnosed with schizophrenia, who, after failing psychiatric drug combinations and an immunosuppressive agent for presumptive autoimmune encephalitis, experienced symptom resolution in association with antimicrobial therapies.
Case Report
A 14-year-old boy was in his usual state of good health until October 2015, when he developed psychiatric symptoms including feeling overwhelmed, confused, depressed, and agitated. He said that he was an “evil, damned son of the devil” and wanted to kill himself because he was afraid of his new-onset homicidal thoughts toward those he cared about. In October 2015, he was admitted for emergency psychiatric hospitalization at a local hospital and was placed on aripiprazole for major depressive disorder (MDD) with psychotic features. He was discharged after 1 week, still somewhat psychotic but no longer suicidal or homicidal.
His early history was unremarkable except for an episode of depression in the 3rd grade (9 years of age) that the parents attributed to his feeling unchallenged in school and being bullied by peers, as he was very bright, but socially awkward. Placement on sertraline 25 mg for 1 year plus transfer to a small school for gifted children resulted in complete remission. The evaluating psychiatrist noted the possibility of Asperger’s syndrome as a secondary diagnosis. In April 2014, he was treated with antibiotics for Mycoplasma pneumoniae with a recurrence and retreatment in December 2014.
In June 2015, he was treated with topical steroids for poison ivy and an oral cephalosporin for multiple bug bites, obtained while visiting a farm in Missouri. The family history was positive for depression and alcohol abuse on both sides of the family tree, possible bipolar disorder, compulsive gambling, and possible attention deficit hyperactivity disorder (ADHD): maternal side—ulcerative colitis, fibromyalgia in 2 relatives, and rheumatoid arthritis; paternal side—multiple sclerosis and adult-onset diabetes mellitus.
Historically, prior to psychiatric symptom onset, the boy was socially, athletically, and academically active, as evidenced by participation in national geography and history competitions, and a lead actor in a school play, winning an award in fencing and achieving excellent course grades. In the context of animal and arthropod exposures, the family resided in a Midwestern state, in suburban housing, and had multiple pets: cats, a dog, a spotted gecko, and a giant African millipede. Two cats were adopted as strays in 2010, 1 of which had an open wound over his back requiring treatment. A visiting dog infested with fleas entered the household in 2011. The boy had sustained cat bites and scratches prior to illness onset. Outdoor activities consisted of gardening and hiking. In addition to fleas and ticks, his parents reported exposure to mosquitoes and spiders. He had traveled in northeastern, southeastern, and western states. International travel was limited to 2 trips to Mexico in June 2013 (family vacation) and July 2015 (mission trip to impoverished areas).
Weeks after initial hospitalization in October, he became more dysfunctional; school was not possible; he developed progressively severe psychiatric symptoms including obsessional intrusive thoughts, phobias, irrational fears, emotional lability, unpredictable rage outbursts, and increased psychotic thinking. He believed that he had special powers and that a family cat wanted to kill him. By December 2015, his illness had progressed in severity, causing his mother to quit her job to provide full-time supervision. In addition to persecutory delusions related to his pets, he developed auditory, visual, and tactile hallucinations and began refusing to leave the house. A second psychiatric hospitalization occurred in December 2015.
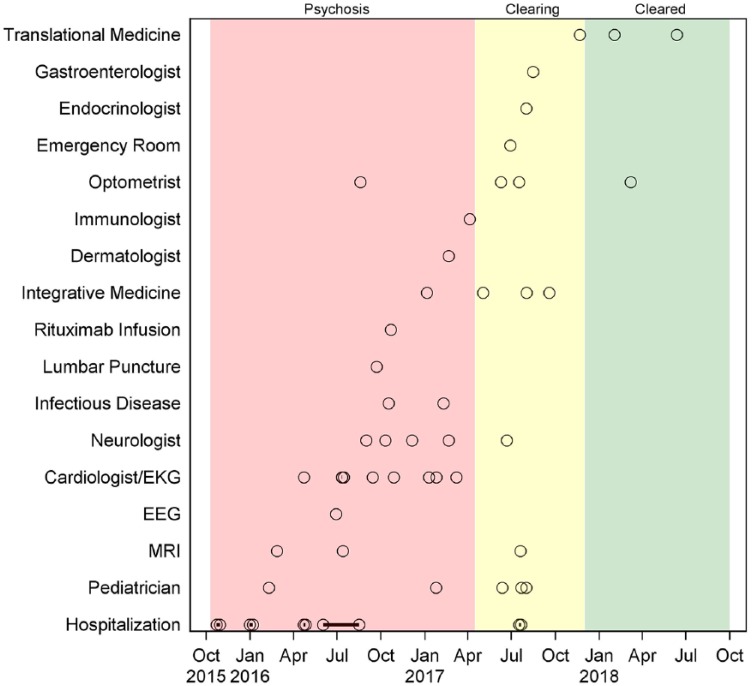
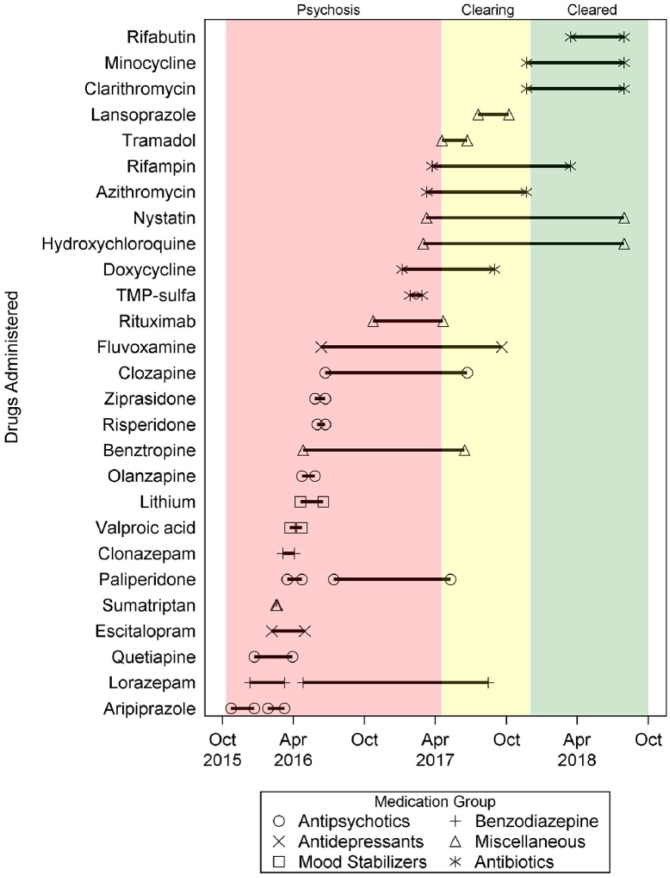
In January 2016, following discharge after a week-long hospitalization, he developed non-specific somatic symptoms, including excessive fatigue, daily headaches, chest pains, shortness of breath (possible panic anxiety), and urinary frequency. Figure 1 depicts a timeline including major medical interventions, physician consultations, and hospitalizations, during which he was treated with multiple different psychotropic medications including antipsychotics, mood stabilizers, antidepressants, and benzodiazepines, often in combination with other pharmacologic agents (Figure 2). Two local psychiatrists diagnosed schizophrenia.
Figure 1.
Hospitalization, major medical interventions, and physician consultation timeline for the boy in this report. Open circles represent specific examinations, testing time points, or treatment interventions. Circles with a dot in the center reflect short-duration hospitalization (days to a week). The circles connected by a solid line represent an extended 11-week hospitalization period (see case report). From October 2015 through April 2017, 24-hour psychosis remained unchanged despite the administration of various neuropsychiatric drug combinations (see Figure 2). Beginning in January 2017, antibiotics were administered, after which psychoses decreased in frequency (yellow panel). By November 2017, no psychoses were reported (green panel).
EEG, electroencephalography; EKG, electrocardiogram; MRI, magnetic resonance imaging.
Figure 2.
Drug administration timeline for the boy in this report. Medications were categorized by therapeutic indication. Solid lines between each medication symbol reflect the duration of drug administration. The pink, yellow, and green panels reflect 24-hour psychosis, isolated psychotic episodes, and resolution of the psychosis, respectively.
In the summer of 2016, he was hospitalized for 11 weeks at a large out-of-state psychiatric teaching hospital where extensive testing, including inflammation markers (erythrocyte sedimentation rate [ESR], C-reactive protein [CRP]), a Mayo Clinic Encephalopathy Autoimmune Evaluation Panel, voltage-gated potassium channel antibody testing, an electroencephalogram (EEG), and a brain magnetic resonance imaging (MRI) were performed, all of which were considered unremarkable. Provisional discharge diagnoses were schizophrenia and obsessive-compulsive disorder.
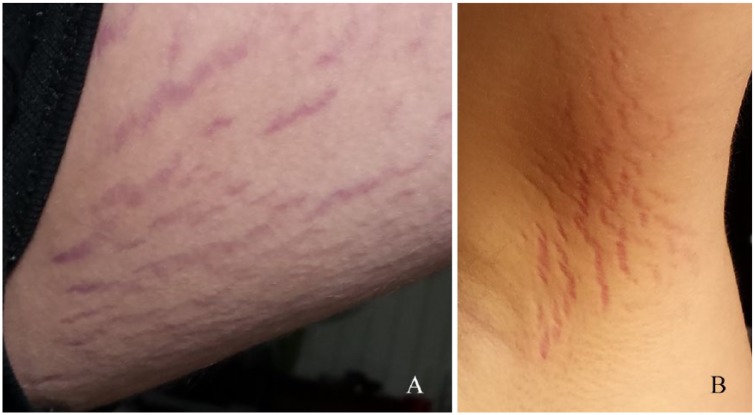
After returning home in August 2016, the parents noted cutaneous “stretch marks.” Although he remained quite ill, his parents refused psychiatric re-hospitalization in favor of in-home treatment. In October 2016, a consulting neurologist proposed the diagnosis of autoimmune encephalitis based on a positive antinuclear antibody (ANA) titer (1:320), increasing CRP levels = 1.03 (Nl < 0.30 mg/dL) on August 31 and 1.96 on October 11, as well as increased CSF gamma interferon level = 6.0 (Nl < 4 pg/mL). His CSF neopterin value was 2.0 nmol/L (NI < 16.5 nmol/L).
An outpatient lumbar puncture was otherwise unrevealing. He was treated with rituximab infusion for autoimmune encephalitis, which decreased the duration and frequency of psychotic episodes; however, daily behavioral disruptions continued.
In January 2017, PANS was diagnosed based on historical symptoms (Table 1). Testing for Group A streptococcal infection, including antistreptolysin O titer, anti-DNase B titer, and other infections including M pneumoniae Immunoglobulin class G (IgG) and Immunoglobulin class M (IgM) antibodies, a Lyme enzyme-linked immunosorbent assay (ELISA) and Western blot IgG and IgM and viral titers were negative or equivocal. ANAs were not detected. When the evaluating physician observed the cutaneous “stretch mark-like” lesions on the boy’s thighs and armpit (Figure 3), neurobartonellosis was proposed as a differential diagnostic consideration. Subsequent consultation with a dermatologist confirmed that cutaneous lesions were not oriented along the skin tension lines and the location and color were not consistent with striae and fluctuations in body weight. Histological examination of a 3-mm punch biopsy revealed sparse lymphohistiocytic dermal infiltration. Doxycycline monohydrate (100 mg BID) was administered. Preantibiotic Bartonella spp. diagnostic testing was not pursued.
Table 1.
PANS criteria assessment by the pediatrician in January 2017.
| 1. Abrupt, dramatic onset of obsessive-compulsive disorder or
severely restricted food intake. ✓ Went from highly functional (gifted school) to dysfunctional in a very brief timeframe with a rapid onset of OCD symptoms of intrusive thoughts, phobias, unfounded fears, and repetitive behaviors. Severely restricted food intake was not noted. 2. Concurrent presence of additional neuropsychiatric symptoms, with similarly severe and acute onset, from at least 2 of the following 7 categories: ✓ Anxiety (severe); ✓ Emotional lability and/or depression; ✓ Irritability, aggression, and/or severely oppositional behaviors; ✓ Behavioral (developmental) regression; ✓ Deterioration in school performance (could not attend school during illness); ✓ Sensory or motor abnormalities; ✓ Somatic signs and symptoms, including sleep disturbances, enuresis, or urinary frequency. 3. Symptoms are not better explained by a known neurologic or medical disorder, such as Sydenham chorea, systemic lupus erythematosus, Tourette disorder, or others. ✓ No known neurological or medical disorder to explain symptoms. Plus persistent psychosis and visual, auditory, and tactile hallucinations. |
OCD, obsessive-compulsive disorder; PANS, pediatric acute-onset neuropsychiatric syndrome.
The check marks reflect specific symptoms reported in the patient that supported the criteria.
Figure 3.
Photographs taken by the parents in February 2017, approximately 7 months after cutaneous lesions were first observed. These cutaneous lesions prompted the attending physician to suspect neurobartonellosis as the cause of pediatric acute-onset neuropsychiatric syndrome in this patient. (A) Cutaneous lesions on the left medial aspect of the thigh. (B) Cutaneous lesions located in the medial aspect of the right axilla.
In February, the parents requested that their son be entered into a research study approved by North Carolina State University’s Institutional Review Board (Protocol No. 1960, Detection of Bartonella Species in the Blood of Healthy and Sick People).
Research Methods
In collaboration with the boy’s attending physicians, sequential serology, polymerase chain reaction (PCR), enrichment blood culture, and Bartonella sp. DNA sequencing results were generated at the Comparative Medicine Institute, College of Veterinary Medicine, North Carolina State University.
Bartonella spp. serology
Serum samples were tested by indirect fluorescent antibody (IFA) assays using a panel of Bartonella spp. antigens, including B henselae Houston 1 (Bh H1), B henselae strain San Antonio 2 (BhSA2), Bartonella vinsonii subspecies berkhoffii genotype I (Bvb I), B vinsonii subspecies berkhoffii genotype II (Bvb II), B vinsonii subspecies berkhoffii genotype III (Bvb III), and Bartonella koehlerae (Bk), as previously described.5,6 Seroreactivity was defined as endpoint titers ⩾ 1:64 using a twofold dilution scale of 1:16 to 1:8192.
Bartonella alphaproteobacteria growth medium enrichment blood culture/PCR
Beginning in March 2017, 3 blood sample sets (triple draw) were obtained over a 5-day period to increase the sensitivity of Bartonella spp. detection by PCR amplification and DNA sequencing.8 Bartonella testing was performed using Bartonella alphaproteobacteria growth medium (BAPGM) as described previously.9,10 Blood, collected aseptically into ethylenediaminetetraacetic acid (EDTA)-anticoagulated and serum separator tubes, was shipped to our research laboratory for BAPGM enrichment blood culture/PCR and IFA serology testing.
PCR amplicon identity was confirmed by DNA sequencing in a commercial laboratory (7030 Kit Creek Rd Suite 120; GENEWIZ Inc, Research Triangle Park, NC). The bacterial species and strain were defined by comparing similarities with other sequences deposited in the GenBank database using the Basic Local Alignment Search Tool (Blast v. 2.0) and an in-house curated database.
Research test results were communicated to the attending physician, after which Bartonella spp. IFA serology and BAPGM enrichment blood culture/PCR were sequentially followed during antimicrobial drug administration. Immunofluorescent antibody testing for Borrelia burgdorferi IgM and IgG using an ELISA and Western immunoblot assays were repeated in a commercial laboratory (7030 Kit Creek Rd Suite 120; Galaxy Diagnostics Inc, Research Triangle Park, NC).
Research Results
Despite nearly 2 consecutive months of doxycycline administration, B henselae DNA was PCR amplified from the boy’s blood, serum, and from 2 of 3 7-day BAPGM enrichment blood cultures obtained 4 days apart. All negative controls (DNA extraction, molecular grade water, and 7-, 14-, and 21-day un-inoculated BAPGM ePCR controls) were PCR negative. DNA sequencing of the Bartonella spp. 16S-23S intergenic transcribed spacer (ITS) region and the rpoB gene PCR amplicons (2 Bartonella spp. gene targets) confirmed infection with B henselae. The 16S-23S ITS DNA sequences were 99.3% (137/138 base pairs [bp]) similar to GenBank Bh H1 (NC005956) and 100% similar to GenBank B henselae California 1 strain (CAL1; AF369527). The rpoB DNA sequence was 99.8% (612/613 bp) similar to Bh H1 (GenBank B henselae NC005956).
Subculture from the 7-, 14-, and 21-day liquid BAPGM flasks did not result in bacterial isolation. By indirect antibody (IFA) testing, the boy’s B henselae, B vinsonii subsp. berkhoffii genotypes I, II, and III, B koehlerae, and Bartonella quintana antibody titers were <1:16, <1:16, 1:128, <1:16, 1:32, and 1:32, respectively (titers < 1:64 non-seroreactive). Anaplasma spp., Babesia spp., B burgdorferi sensu lato, Ehrlichia spp., and hemotropic Mycoplasma spp. blood PCR assays were negative.
Azithromycin (250 mg BID), hydroxychloroquine (200 mg BID), and nystatin (500 000 units BID) were added to the doxycycline treatment regimen with rifampin (300 mg BID) added 2 weeks later. Due to severe migrating pain (suspected Jarisch-Herxheimer-like reaction associated with initial rifampin administration), 3 weeks of increasing titration of rifampin was required to achieve consistent twice-daily dosing. During antimicrobial administration, there was a gradual and progressive decrease in neuropsychiatric symptoms, a reduction and eventual elimination of the cutaneous lesions, and a gradual withdrawal of all antipsychotic drugs (Figure 2). In June 2017, following the abrupt withdrawal of clozapine and tramadol (for migratory pain), he experienced severe anorexia, nausea, and vomiting, which resulted in hospitalization and a 20.5-kg weight loss over 40 days. Reflux esophagitis was diagnosed endoscopically. Proton pump inhibitor therapy resulted in symptom resolution and weight gain.
When retested in June 2017, Bartonella spp. antibodies were not detected by IFA testing. BAPGM enrichment blood culture ITS quantitative polymerase chain reaction (qPCR) results were negative (9 independent DNA extractions) and no subculture isolates were obtained. However, 2 of the 3 triple draw blood samples were PCR+ (ie, each yielded Bartonella amplicons), potentially representing dead or non-viable bacteria that failed to grow in culture. Confirmation of these amplicons by DNA sequencing was not successful, potentially due to an inadequate quantity of bacterial DNA for sequencing. Based on potential B henselae persistence, antimicrobial therapy was continued.
Repeat Bartonella spp. IFA and blood, serum, and BAPGM enrichment blood culture/PCR results in October 2017 were negative. After missing nearly 2 years of school, the boy resumed pre-illness level of academic functioning by attaining A’s in all fall semester 2017 and spring semester 2018 classes. As of September 2018, the boy has resumed all pre-illness activities, interacts frequently with peers, maintains a part-time job as a restaurant server, and according to his parent’s assessment has fully recovered.
Discussion
Although causation cannot be established by case reports, microbiological documentation of B henselae bloodstream infection in conjunction with symptomatic improvement during antimicrobial therapy and failure to identify other causes of PANS supports a potential role for neurobartonellosis in this boy’s neuropsychiatric illness. Based on the extended family medical history, the boy may have been genetically predisposed to develop autoimmune or neuropsychiatric symptoms in association with B henselae infection. Once B henselae DNA was amplified from the boy’s blood and enrichment blood cultures (reflecting the growth of live bacteria despite 2 months of doxycycline administration), antimicrobial therapy was accompanied by sequential removal of all antipsychotic drugs, a progressive decline in neuropsychiatric symptoms, resolution of what we are referring to as Bartonella-associated cutaneous lesions, return to school, and a gradual return to pre-illness activities. Prior use of psychotropics and immunosuppression with rituximab for possible autoimmune encephalitis was of limited therapeutic benefit. The boy and his family paid a very high emotional, social, and financial price during his 2-year illness. In addition to daily stress induced by his illness among all family members, the mother quit her job to provide homecare, and pets were removed from the home (due to the boy’s delusions). Based on insurance claims and bills paid by the parents, the cost of the various medical interventions prior to documentation of B henselae bloodstream infection exceeded $400,000.
In the context of differential diagnostic considerations, typical indicators of bacteremia are often lacking or only intermittently positive in patients with Bartonella bloodstream infections. Also, as illustrated by this case report, autoimmune tests can be positive, supporting treatment with immunosuppressive drugs. Antineutrophil cytopathic antibodies (ANCAs) have been reported in Bartonella-infected humans11 and, on a comparative medicine basis, statistical associations between ANA and ANCA seroreactivity and Bartonella spp. antibodies have been reported in dogs.12,13 There are several mechanisms employed by Bartonella spp. that downregulate immune responses.1-3 Although not studied in a clinical context, B quintana lipopolysaccharide (LPS) is an anti-inflammatory, mechanistically inhibiting pattern recognition receptor TLR4.14 In addition, the ability of Bartonella spp. to invade numerous cell types, including microglia, and to modify surface proteins most likely contributes to evasion of immune recognition.15 For these, and potentially other reasons, Bartonella spp. bloodstream infections do not consistently elicit neutrophilia, increases in erythrocyte sedimentation rate, and CRP or CSF pleocytosis. In addition, B henselae appears to suppress immunoglobulin production and natural killer cell function in some patients.6 Thus, patients with neurobartonellosis can develop autoantibodies (ANCA and ANA), lack routine clinicopathologic and immunologic indicators of bacteremia, and can be concurrently immunosuppressed secondary to drug administration or the long-standing Bartonella spp. infection. Collectively, documentation of autoantibodies in conjunction with negative biomarkers for inflammation would decrease clinical suspicion for an infectious cause in patients with neuropsychiatric symptoms.3,15,16 In addition, administration of rituximab to this patient could have contributed to the documented decrease in ANA reactivity and potentially the lack of diagnostic antibodies to B henselae and the other organisms tested.
As described in case reports,4-6 case series,2,8-10 and reviews,1,3,15,16 the difficulty in identifying Bartonella spp. as potential pathogens in patients with neurobartonellosis is further complicated by poor diagnostic sensitivity for bacterial isolation, serology, and PCR. Although the BAPGM enrichment PCR blood culture approach used in this case improves sensitivity,8 only 1 of the boy’s blood and 2 out of 3 alternate day BAPGM enrichment blood cultures were B henselae PCR+. Unfortunately, agar plate isolation of B henselae from patient specimens remains highly insensitive in both research-intensive and clinical microbiological settings. In this case, 2 months of doxycycline treatment may have further compromised the possibility for successful subculture isolation. By IFA testing, the boy was not seroreactive to either Bh H1 or BhSA2 strain type. This result is consistent with previous studies indicating that a subset of Bartonella spp.-infected individuals remain IFA seronegative, despite PCR documentation of chronic bloodstream infection.9,10,17,18 The reason(s) for the lack of Bartonella spp. seroreactivity remain unclear, but immunologic anergy is a suspected mechanism.
Bartonella-associated cutaneous lesions, including vasoproliferative lesions (bacillary angiomatosis), nodular panniculitis, multifocal erythema (erythema multiforme), rashes, and linear dermal lesions, may be more prevalent dermatological manifestations of bartonellosis than is currently appreciated.1-3,17,18 We have previously amplified B henselae DNA from a linear dermal lesion in another young man, whose infection was further confirmed by scanning confocal immunohistochemical visualization of the bacteria within the lesional biopsy.18 Histologically, the cutaneous lesions in both of these boys were characterized by a mild lymphocytic/plasmacytic perivascular inflammatory response. The potential role of Bartonella spp. as a cause of linear cutaneous lesions in a subset of patients with neurological symptoms deserves future research consideration.
In general, medical knowledge about neurobartonellosis is limited to case reports and case series. Physicians are familiar with cat scratch disease (CSD), a benign self-limiting infection that generally resolves spontaneously without the need for antibiotic administration.19 In contrast to classical CSD, recent evidence indicates that B henselae, the primary or sole cause of CSD, can be isolated from the blood of healthy blood donors,20 at-risk animal health workers,9,10 and patients with rheumatologic21-23 and neuropsychiatric symptoms.4,5 Proving disease causation for stealth pathogens, such as Bartonella spp., is challenging24 and a One Health approach16 has been proposed to more effectively characterize the comparative medical importance of this genus. The response following the initiation of antimicrobial therapy in this case and in another patient with suspected neurobartonellosis25 indirectly supports a causative or cofactor role for these bacteria. Although in vitro sensitivity data indicate therapeutic efficacy for a large number of antibiotics, including doxycycline, it is noteworthy that in vivo patient responses to single-antibiotic regimens have resulted in treatment failures.26-28 Due to the severity of the boy’s psychoses and the fear of bacterial reactivation if treatment was not curative, a very long duration of antimicrobial therapy was administered. With the important and ongoing concerns over antibiotic resistance, the administration of antibiotics for a protracted period can only be justified if patient outcomes support the efficacy of the regimen. Phylogenetically, the genus Brucella is the closest bacteria relative to the genus Bartonella, a factor that may ultimately guide the development of effective treatment protocols. Of note, 3 complimentary modes of intervention were recently recommended for the treatment of PANS29: (1) treat neuropsychiatric symptoms, (2) remove the source of inflammation with antimicrobial interventions, and (3) treat autoimmune, immune-mediated, or immunosuppression as indicated, all of which were addressed to varying degrees by the physicians managing this patient.
In summary, neurobartonellosis should be considered in patients with PANS and treatment-resistant neuropsychiatric symptoms. To enhance diagnostic sensitivity, microbiological confirmation of neurobartonellosis should be pursued prior to antibiotic administration, and therapeutic immunosuppression should potentially be used with caution in these patients. Clearly, there is a need for greater delineation of the role of Bartonella spp. in neuropsychiatric disease causation, improvements to current serological and molecular diagnostic testing modalities, determination of antibiotic treatment duration, and critical evaluation of the efficacy of various treatment regimens. Prospective cohort studies and longitudinal case-controlled studies are needed to determine if, how often, or to what extent Bartonella spp. infections contribute to the development of neuropsychiatric symptoms, PANS, and Bartonella-associated cutaneous lesions. Specifically, to further delineate the potential contribution of B henselae as a cause or contributor to neuropsychiatric symptoms, serial Bartonella spp. serology, enrichment blood and CSF culture/PCR with DNA sequence confirmation of amplicons to establish species and genotype, directed antimicrobial therapy, and sequential follow-up would be recommended, in conjunction with other standard neuropsychiatric testing modalities.
Acknowledgments
The authors thank the many physicians who cared for this patient and the parents for their detailed communications and contributions that facilitated generation of this case report. The authors also thank Sancta Familia Center for Integrative Medicine’s Nicole Snyder, CNP for helping with the urgent initial evaluation and Anita Cochran, RN for guiding the family through the very difficult journey of treatment and recovery.
Footnotes
Funding:The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This case study was supported in part by the State of North Carolina and donations to the Bartonella/Vector Borne Disease Research Fund, North Carolina State University Veterinary Medical Foundation.
Declaration of Conflicting Interests:The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: In conjunction with Dr Sushama Sontakke and North Carolina State University, EBB, DVM holds US Patent No. 7115385; Media and Methods for cultivation of microorganisms, which was issued on October 3, 2006. He is a co-founder, shareholder, and chief scientific officer for Galaxy Diagnostics, a company that provides advanced diagnostic testing for the detection of Bartonella species infections. Dr RGM is the chief technical officer for Galaxy Diagnostics and Dr BRM is the chief medical officer. All the other authors have no potential conflicts of interest to report.
Author Contributions: EBB and RG coordinated communications with the family and other physicians and drafted the manuscript. RGM and JMB performed BAPGM enrichment PCR and Bartonella spp. serology, respectively, contributed to the interpretation of results, helped draft the manuscript, and reviewed the manuscript content. BRM and AL managed the medical treatment of neurobartonellosis and provided data interpretation for the case report. All authors reviewed the final manuscript.
Availability of Data and Materials: To assure patient confidentiality, please contact EBB relative to data and materials.
Consent for Publication: The boy’s parents provided written permission for the publication of the information reported in this case report.
Ethical Approval: Ethics approval for testing described in this case report was obtained from the North Carolina State University’s Institutional Review Board for a research study entitled Detection of Bartonella Species in the Blood of Healthy and Sick People (Protocol No. 1960).
ORCID iD: Edward B Breitschwerdt  https://orcid.org/0000-0002-3506-0279
https://orcid.org/0000-0002-3506-0279
References
- 1. Pulliainen AT, Dehio C. Persistence of Bartonella spp. stealth pathogens: from subclinical infections to vasoproliferative tumor formation. FEMS Microbiol Rev. 2012;36:563–599. [DOI] [PubMed] [Google Scholar]
- 2. Vayssier-Taussat M, Moutailler S, Féménia F, et al. Identification of novel zoonotic activity of Bartonella spp., France. Emerg Infect Dis. 2016;22:457–462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Breitschwerdt EB. Bartonellosis: one health perspectives for an emerging infectious disease. ILAR J. 2014;55:46–58. [DOI] [PubMed] [Google Scholar]
- 4. Breitschwerdt EB, Maggi RG, Nicholson WL, Cherry NA, Woods CW. Bartonella spp. bacteremia in patients with neurological and neuro-cognitive dysfunction. J Clin Microbiol. 2008;46:2856–2861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Breitschwerdt EBPE, Mascarelli LA, Schweickert RG, et al. Hallucinations, sensory neuropathy, and peripheral visual deficits in a young woman infected with Bartonella koehlerae. J Clin Microbiol. 2011;49:3415–3417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Kaufman DL, Kogelnik AM, Mozayeni RB, Cherry NA, Breitschwerdt EB. Neurological and immunological dysfunction in two patients with Bartonella henselae bacteremia. Clin Case Rep. 2017;5:931–935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Swedo SE, Seidlitz J, Kovacevic M, et al. Clinical presentation of pediatric autoimmune neuropsychiatric disorders associated with streptococcal infections in research and community settings. J Child Adolesc Psychopharmacol. 2015;25:26–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Pultorak EL, Maggi RG, Mascarelli PE, Breitschwerdt EB. Serial testing from a 3-day collection period by use of the Bartonella alphaproteobacteria growth medium platform may enhance the sensitivity of Bartonella species detection in bacteremic human patients. J Clin Microbiol. 2013;51:1673–1677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Maggi RG, Mascarelli PE, Pultorak EL, et al. Bartonella spp. bacteremia in high-risk immunocompetent patients. Diagn Microbiol Infect Dis. 2011;71:430–437. [DOI] [PubMed] [Google Scholar]
- 10. Lantos PM, Maggi RG, Ferguson B, et al. Detection of Bartonella species in the blood of veterinarians and veterinary technicians: a newly recognized occupational hazard? Vector Borne Zoonotic Dis. 2015;14:563–570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Raybould JE, Raybould AL, Morales MK, et al. Bartonella endocarditis and pauci-immune glomerulonephritis: a case report and review of the literature. Infect Dis Clin Pract (Baltim Md). 2016;24:254–260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Smith BE, Tompkins MB, Breitschwerdt EB. Antinuclear antibodies can be detected in dog sera reactive to Bartonella vinsonii subsp. berkhoffii, Ehrlichia canis, or Leishmania infantum antigens. J Vet Intern Med. 2004;18:47–51. [DOI] [PubMed] [Google Scholar]
- 13. Karagianni AE, Solano-Gallego L, Breitschwerdt EB, et al. Perinuclear antineutrophil cytoplasmic autoantibodies in dogs infected with various vector borne pathogens and in dogs with immune mediated haemolytic anemia. Am J Vet Res. 2012;73:1403–1409. [DOI] [PubMed] [Google Scholar]
- 14. Malgorzata-Miller G, Heinbockel L, Brandenburg K, van der Meer JW, Netea MG, Joosten LA. Bartonella quintana lipopolysaccharide (LPS): structure and characteristics of a potent TLR4 antagonist for in-vitro and in-vivo applications. Sci Rep. 2016;6:34221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Harms A, Dehio C. Intruders below the radar: molecular pathogenesis of Bartonella spp. Clin Microbiol Rev. 2012;25:42–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Regier YO, Rourke F, Kempf VA. Bartonella spp.—a chance to establish One Health concepts in veterinary and human medicine. Parasit Vectors. 2016;9:261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Schattner A, Uliel L, Dubin I. The cat did it: erythema nodosum and additional atypical presentations of Bartonella henselae infection in immunocompetent hosts [published online ahead of print February 16, 2018]. BMJ Case Rep. doi: 10.1136/bcr-2017-222511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Maggi RG, Ericson M, Mascarelli PE, Bradley JM, Breitschwerdt EB. Bartonella henselae bacteremia in a mother and son exposed to ticks in the Netherlands. Parasit Vectors. 2013;6:101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Shin OR, Kim YR, Ban TH, et al. A case report of seronegative cat scratch disease, emphasizing the histopathologic point of view. Diagn Pathol. 2014;9:62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Pitassi LH, de Paiva Diniz PP, Scorpio DG, et al. Bartonella spp. bacteremia in blood donors from Campinas, Brazil. PLoS Negl Trop Dis. 2015;9:e0003467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Giladi M, Maman E, Paran D, et al. Cat-scratch disease-associated arthropathy. Arthritis Rheum. 2005;52:3611–3617. [DOI] [PubMed] [Google Scholar]
- 22. Maggi RG, Mozayeni BR, Pultorak EL, et al. Bartonella spp. bacteremia and rheumatic symptoms in patients from Lyme disease-endemic region. Emerg Infect Dis. 2012;18:783–791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Ericson M, Balakrishnan N, Mozayeni BR, et al. Culture, PCR, DNA sequencing, and second harmonic generation (SHG) visualization of Bartonella henselae from a surgically excised human femoral head. Clin Rheumatol. 2017;36:1669–1675. [DOI] [PubMed] [Google Scholar]
- 24. Breitschwerdt EB, Linder KL, Day MJ, Maggi RG, Chomel BB, Kempf VAJ. Koch’s postulates and the pathogenesis of comparative infectious disease causation associated with Bartonella species. J Comp Pathol. 2013;148:115–125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Greenberg R. The role of infection and immune responsiveness in a case of treatment-resistant pediatric bipolar disorder. Front Psychiatry. 2017;8:78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Sykes JE, Lindsay LL, Maggi RG, Breitschwerdt EB. Human coinfection with Bartonella henselae and two hemotropic mycoplasma variants resembling Mycoplasma ovis. J Clin Microbiol. 2010;48:3782–3785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Maggi RG, Mascarelli PE, Havenga LN, NAidoo V, Breitschwerdt EB. Co-infection with Anaplasma platys, Bartonella henselae and Candidatus Mycoplasma haematoparvum in a veterinarian. Parasites and Vectors. 2013;6:106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Breitschwerdt EB, Sontakke S, Hopkins S. Neurological manifestations of bartonellosis in immunocompetent patients: a composite of reports from 2005-2012. J Neuroparasitol. 2012;3:1–15. [Google Scholar]
- 29. Swedo SE, Frankovich J, Murphy TK. Overview of treatment of pediatric acute-onset neuropsychiatric syndrome. J Child Adolesc Psychopharmacol. 2017;7:566–573. [DOI] [PMC free article] [PubMed] [Google Scholar]