Abstract
5-Fluorouracil (5-FU) is used in the treatment of head and neck cancer patients. However, adverse effects experienced such as mucositis and poor appetite may lead to interruption in chemotherapy. The aim of this study is to evaluate the efficacy of GMI, one fungal immunomodulatory protein found in Ganoderma microsporum, for mucositis induced by 5-FU in a mouse model. Mice were administered 5-FU intraperitoneally for 4 days per cycle for a total of 2 chemotherapy cycles. In addition, mice were pretreated with GMI or phosphate-buffered saline 3 days before 5-FU intraperitoneal injection and daily until day 14. On histological analysis, GMI prevented 5-FU-induced damage to the intestinal mucosa and tongue epithelium. We also demonstrated that GMI enhanced the cytotoxicity of 5-FU in 2 oral cancer cell lines, while GMI could not promote this effect in an oral normal cell. In conclusion, GMI alleviates 5-FU-induced damage and decelerates cell death in normal alimentary tract tissue.
Keywords: 5-fluorouracil, immunomodulatory protein, mucositis
Introduction
5-Fluorouracil (5-FU) is an antimetabolite cancer agent that is widely used in the treatment of a variety of malignant tumors, including breast, colorectal, esophageal, stomach, pancreatic, and head and neck cancer. It is an analogue of uracil and can be converted into various active metabolites in the liver. The mechanism of 5-FU is to inhibit thymidylate synthase and to incorporate its metabolites into DNA and RNA to block replication and transcription.1 Despite its efficacy, serious side effects such as diarrhea and mucositis frequently cause discontinuation of treatment or reduction of drug dose, thereby compromising the success of the cancer chemotherapy.2-5 Intestinal mucositis (characterized by the shortening of villi and disruption of crypt cell homeostasis) and oral mucositis (characterized by the severe oral erythema and ulceration) are considered to be a consequence of excessive inflammation and epithelial ablation and apoptosis, together with cellular hypoproliferation and decrease in mucosa thickness.6,7
The therapeutic effects of lingzhi are attributed to triterpenoids, polysaccharides, and fungal immunomodulatory proteins (FIPs).8-10 For induced mucosal damage from chemotherapeutic agents, one study showed Ganoderma lucidum polysaccharides could reduce methotrexate-induced damage in mice through induction of epithelial proliferation and migration,11 while another study demonstrated that FIPs from Flammulina velutipes (FIP-fve) and Ganoderma tsugae (FIP-gts) could protect intestinal villi via anti-inflammatory and immunomodulatory activities.12 In previous studies, FIPs have been discovered with anticancer abilities and may act as chemopreventative agents.13 Ling-Zhi-8 (LZ-8) extracted from the fungus Ganoderma lucidum was the first FIP found in mushroom. LZ-8 has immunomodulatory function decreasing antibody production induced by Arthus type 3 hypersensitivity reaction in mice14 and activate dendritic cells by the NF-κB and MAPK pathways.15 Another fungal immunomodulatory protein, Ganoderma microsporum immunomodulatory protein (GMI), extracted from Ganoderma microsporum, was shown to have amino acid sequences highly homologous to those of LZ-8 (83%).16
In this study, we examined the effects of GMI in the prevention and treatment of chemotherapy-induced mucositis in mice and whether it can inhibit the apoptosis response and protect oral cavity and gastrointestinal tract mucosa from mucositis.
Materials and Methods
Preparation of 5-Fluorouracil and GMI
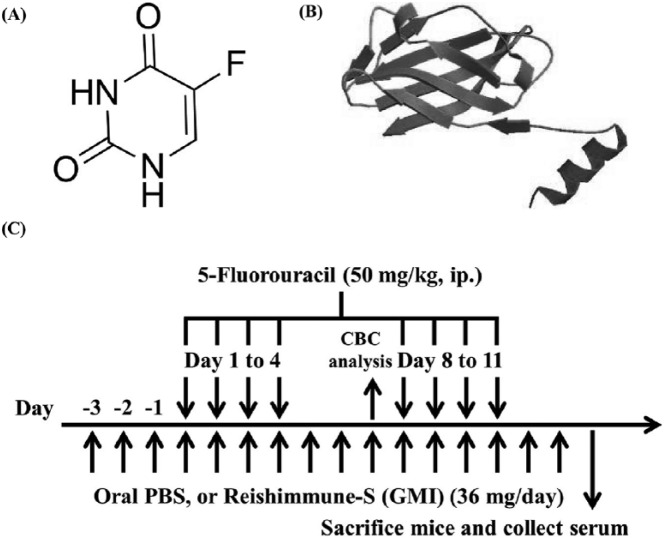
5-FU was purchased from Nang Kuang Pharmaceutical Co, Ltd. The structure of 5-FU is shown in Figure 1A. The original concentration of 5-FU was 50 mg/mL and was diluted to 10 mg/mL with phosphate-buffered saline (PBS). For animal experiments, Reishimmune-S was used, purchased from Mycomagic Biotechnology Co, Ltd (Taipei). GMI is the main ingredient in the oral mushroom nutritional supplement Reishimmune-S. The protein structure of GMI is shown in Figure 1B. A slice of Reishimmune-S had 72 mg (contained about 320 µg GMI) and was dissolved in 800 µL PBS. For in vitro experiments, GMI, manufactured by Mycomagic Biotechnology Co, Ltd, was generated from Ganoderma microsporum. The detailed extraction methods of GMI were described in our previous study.17
Figure 1.
The schematics of experimental procedure and the chemical structure of 5-fluorouracil (5-FU) and GMI (Ganoderma microsporum immunomodulatory protein). (A) The structure of 5-FU. (B) The protein structure of GMI. (C) Six-week-old mice were divided into 3 groups and treated as follows: (1) phosphate-buffered saline (PBS) as control, (2) 5-FU + PBS, (3) 5-FU + GMI. The 5-FU-treated mice were pretreated with Reishimmune-S (GMI; 36 mg/day) for 3 days before 5-FU injection. 5-FU was injected on day 1 to 4 and day 8 to 11. At day 7, blood was collected for complete blood count analysis. At day 14 after the final injection of 5-FU, mice were sacrificed and the blood serum were collected.
Animal Experiments
All animal experimentation procedures were conducted according to the Affidavit of Approval of Animal Use Protocol, Chung Shan Medical University Experimental Animal Center, Taichung (Approval Number 1375). Five-week-old male BALB/c mice (body weight [BW] = 18-20 g) were purchased from the National Laboratory Breeding Research Centre. Mice were housed under pathogen-free conditions with a 12-hour light/12-hour dark cycle and fed an autoclaved diet with ad libitum access to standard rodent chow (LabDiet, 5001) during the study. After 1 week, animals were randomly divided into 3 groups consisting of 5 animals each: (1) Control group: from day −3, 200 µL PBS were administered by gavage twice daily; (2) 5-FU group: 5-FU were dissolved in PBS and administered by a single intraperitoneal injection (50 mg/kg/day) to induce oral and intestinal mucositis at days 1 to 4 and 8 to 11. The accumulated dose of 5-FU was similar to those reported in previous studies.18 (3) 5-FU + GMI group: Reishimmune-S (GMI) 72 mg was dissolved in 800 µL PBS and administered by gavage at 18 mg per mouse twice daily (contained about 160 µg GMI daily). Three days before the beginning of 5-FU administration, Reishimmune-S was continuously fed for 3 days (day −3 to −1). The day 0 was not counted in the protocol. The schematic of the treatment regimen is shown in Figure 1C. All animals were sacrificed on day 14. Blood samples were obtained with some of the blood rinsed with ethylenediaminetetraacetic acid to prevent coagulation and used to determine complete blood count (CBC) on a Hemavet automated cell counter (Sysmex KX-21). The remaining blood was centrifuged at 4°C, and the plasma was frozen at −80°C until analysis. A 10-cm section of the proximal jejunum was collected and gently flushed with saline. The contents of the jejunum and tongue were immediately removed and fixed in 10% buffered formalin for 24 hours for histological analysis and scoring.
Histological Analysis
For the assessment of pathological changes in jejunum and tongue, these organs were fixed in 10% formaldehyde solution and embedded in paraffin. Five-micrometer sections were stained with hematoxylin and eosin. The method for producing histological samples was performed as described in our previous study.12
Cell Line
SCC9, a human tongue squamous cell carcinoma cell line, and SAS, a human oral squamous cell carcinoma cell line, were cultured in Dulbecco’s modified Eagle’s medium supplemented with an equal volume of a nutrient mixture, F-12 Ham’s medium (Life Technologies), 10% fetal bovine serum (Hyclone Laboratories), 2 mM glutamine, 100 U/mL penicillin, and 100 µg/mL streptomycin. SG, immortalized human gingival keratinocytes, were cultured in Dulbecco’s modified Eagle’s medium supplement with aforementioned compounds without F-12 Ham’s medium. The medium for SCC-9 also contains 400 ng/mL hydrocortisone and 1% nonessential amino acids. All cell cultures were maintained at 37°C in a humidified atmosphere of 5% CO2.
Cell Viability
Tongue squamous carcinoma cells SAS and SCC9 (2 × 104) were seeded onto 96-well plates containing 100 µL of culture medium. After 24-hour incubation, the medium was carefully removed, and 100 µL of fresh medium with different concentrations of 5-FU (0, 1, 10, 100 µM) and different concentration of GMI (0, 0.3, 0.6, 1.2 µM) were added. Cells were pretreated with GMI for 1 hour before 5-FU was added. Cells were treated with 5-FU and GMI for 24 and 48 hours. At the end of this process, the medium was carefully removed and 100 µL of fresh medium containing 0.5 mg/mL MTT (thiazolyl blue tetrazolium bromide; Sigma) were added to the wells. The intensity is measured colorimetrically at a wavelength of 570 nm. Absorbance values are presented as the mean ± standard error of 3 replicates for each treatment. Cells in controls and compound controls were included. Absorbance of untreated cells was considered 100%.
Western Blot Analysis
Cells were lysed with RIPA buffer containing protease and phosphatase inhibitor cocktail (Roche), and protein concentration was assayed with Bio-Rad Protein Assay Kit (Bio-Rad). Equal amounts of proteins from each sample were separated by sodium dodecyl sulfate polyacrylamide gel electrophoresis and transferred to polyvinylidene difluoride membrane (Amersham). A 0.4-µm membrane was blocked for 1 hour in TBS containing 5% nonfat milk and 0.2% Tween 20. For the detection of Bax, Bcl-2, PARP, cleaved caspase 7 and β-actin, monoclonal anti-Bax (Cell Signaling), monoclonal-Bcl-2 (Cell Signaling, 4223), monoclonal anti-PARP (Cell Signaling), monoclonal cleaved caspase 7 (Cell signaling), and monoclonal anti-β-actin (Sigma), the various compounds were incubated with membranes at 4°C overnight. Membranes were subsequently washed for 3 to 5 minutes in 0.2% TBS-Tween 20, incubated in horseradish peroxidase–conjugated secondary antibody for 1 hour, washed again, and visualized with enhanced luminol reagent for chemiluminescence (PerkinElmer).
Flow Cytometry
SAS and SCC9 cells (8 × 105 cells/60 mm dish) were first pretreated with GMI (0.3 or 0.6 µM) for 1 hour and then co-treated with GMI and 5-FU (10 µM) for 48 hours. Cells were then washed twice with precooled PBS, trypsinized, and incubated with a binding buffer containing annexin V–fluorescein isothiocyanate and propidium iodide (BioVision). Flow cytometry analysis was performed using FACScalibur Flow Cytometer (BD Biosciences). A minimum of 10 000 cells were analyzed per sample and illustrated as dots plot using CellQuest Pro software.
Statistical Analysis
All data are presented as mean ± standard deviation (SD). In our animal study, statistical comparisons of the different treatment groups were carried out by Student’s t test or Tukey post hoc test in analysis of variance. P < .05 was considered statistically significant.
Results
Effects of GMI on Body Weight
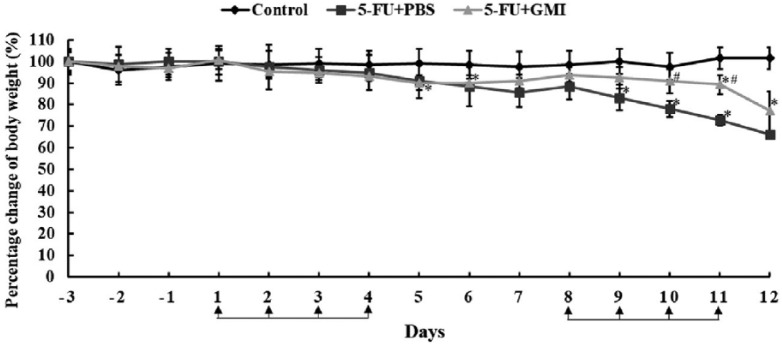
Body weight loss and anorexia are common adverse effects after treatment with 5-FU. To figure out whether these adverse effects are associated with mucositis, the weight was first measured daily and the results of all groups were compared. The mice in the 5-FU group had higher BW loss compared with those in the control group, and there was significant loss at days 9 to 11 (Figure 2). However, the BW loss of the mice in the 5-FU + GMI group was significantly less than those in the 5-FU group on the second week. However, because too many mice in the 5-FU group were lost and the remaining number was inadequate to do statistical analysis at days 13 and 14, our data only present the percentage change of BW from day −3 to 12.
Figure 2.
The effects of GMI (Ganoderma microsporum immunomodulatory protein) on mice body weight. Body weights of mice were measured daily before GMI was introduced. The percentage of body weight of every mice was calculated and compared with the control group at day −3. The mean body weight of every group at day −3 was defined as 100%. Means ± SD are showed. *P < .05 when it was compared with the control group. #P < .05 when it was compared with the 5-fluorouracil (5-FU) + phosphate-buffered saline (PBS) group.
Effects of GMI on Complete Blood Counts
Chemotherapy, including 5-FU, may cause severe leukopenia. To examine whether GMI could prevent mice from 5-FU-induced leukopenia, we analyzed the CBCs. The data are shown in Table 1. We observed significant decreases in white blood cell (WBC) counts in 5-FU-treated mice compared with controls (P < .01). GMI administration induced a slight increase in the WBC counts, but there was no significant difference between the 5-FU alone group and the 5-FU + GMI group. In addition, mean corpuscular hemoglobin content and mean corpuscular hemoglobin concentration significantly rose in the 5-FU + GMI group compared with the control group (P < .05).
Table 1.
The Effect of 5-FU and GMI on Complete Blood Counts.
| Group | WBC (×103/µL) | RBC (×106/µL) | HGB (g/dL) | HCT (%) | MCV (fL) | MCH (pg) | MCHC (g/dL) | PLT (×103/µL) |
|---|---|---|---|---|---|---|---|---|
| Control | 9.53 ± 1.066 | 10.27 ± 1.21 | 15.45 ± 2.03 | 51.78 ± 7.51 | 50.3 ± 1.67 | 15.05 ± 0.98 | 29.93 ± 1.70 | 942.5 ± 252.6 |
| 5-FU | 3.42 ± 1.00** | 9.03 ± 0.51 | 14.58 ± 0.68 | 45 ± 2.70 | 49.82 ± 0.36 | 16.16 ± 0.61 | 32.44 ± 1.24 | 416.6 ± 54.2 |
| 5-FU + GMI | 3.7 ± 0.53** | 9.48 ± 2.20 | 15.675 ± 2.53 | 47.05 ± 11.46 | 49.6 ± 0.88 | 16.6 ± 2.04* | 33.5 ± 4.88* | 379.5 ± 138.1 |
Abbreviations: 5-FU, 5-fluorouracil; GMI, Ganoderma microsporum immunomodulatory protein; WBC, white blood cell; RBC, red blood cell; HGB, hemoglobin; HCT, hematocrit; MCV, mean corpuscular volume; MCH, mean corpuscular hemoglobin content; MCHC, mean corpuscular hemoglobin; PLT, platelets.
P < .01, **P < .01 versus the control group. Data presented are means ± SD.
Effects of GMI on Oral and Intestinal Histology in 5-FU-Treated Mice
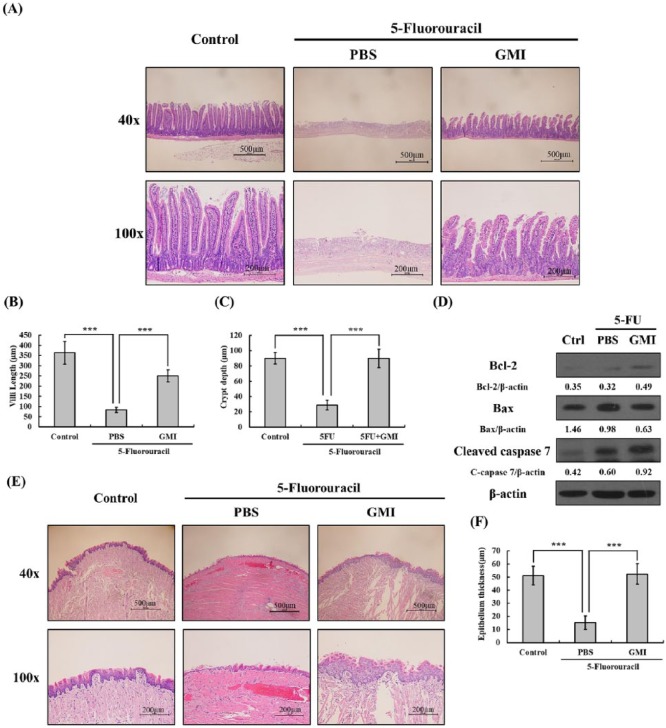
Leukocyte infiltration is an important process of inflammation and tissue healing following 5-FU-induced mucositis. To examine whether GMI can prevent damage to intestinal mucosa induced by 5-FU and whether GMI can alleviate leukocyte infiltration, the histology of jejunum samples was examined by hematoxylin and eosin staining. Repeated administration of 5-FU (50 mg/kg) caused substantial changes in the intestinal mucosal layer including flattened epithelial layer, shortened villi, and thinning lamina propria with inflammatory cell infiltration (Figure 3A). The mucosa in 5-FU group underwent necrosis, and the villi were virtually undetectable. Intestinal villus length and crypt of Lieberkühn depth was determined on NIS-Elements D 3.2 imaging system. Mice treated with GMI prior to 5-FU showed significant reductions in structural damage to the mucosal layer and shortening of intestinal villi length compared with mice treated with 5-FU alone (Figure 3B and C).
Figure 3.
Histological changes in the intestinal villi and tongue were determined using hematoxylin and eosin (H&E) staining. (A) The upper 3 pictures showed the longitudinal section of jejunum on 40× field, while the lower 3 pictures showed the structure on 100× field. (B and C) The villi length and crypt depth of intestinal villi were randomly measured in different parts of jejunum on the same group of samples. ***P < 0.001. (D) Cell lysates of intestinal samples were extracted, and the level of Bcl-2, Bax, and cleaved caspase 7 were analyzed by Western blot. Beta-actin was used as an internal control. (E) The upper 3 pictures showed the cross section of tongue on 40× field, while the lower 3 pictures showed the structure on 100× field. (F) The epithelial thickness of the tip tongue was measured. ***P < .001. Data presented are means ± SD.
5-FU administration led to a significantly thinner tongue mucosa compared with the control group (Figure 3E). In addition, 5-FU also damaged the filiform papilla on the mucosal layer and caused a reduction in the total number compared with the control group. However, GMI administration could protect the tongue epithelium from damage. After GMI treatment, the thickness of tongue mucosa was shown to have recovered to a degree similar to the control group (Figure 3F), and the filiform papilla could be observed more frequently than in the 5-FU group.
GMI Does Not Protect Intestine From Apoptosis After 5-FU Treatment
5-FU might inhibit proliferation of mouse intestine crypt and increase the number of cleaved caspase 3- and caspase 8-positive cells.19 This suggests that 5-FU induces apoptosis of intestinal epithelial cells. Therefore, to examine whether GMI can attenuate apoptosis of enterocytes in small intestine caused by 5-FU, proteins of mouse jejunum tissue were extracted and the expression of various apoptosis markers were analyzed by Western blot. The data are shown in Figure 3D. The expression of Bcl-2 and cleaved caspase 7 mildly increased in the 5-FU + GMI group when it was compared with either the control or the 5-FU + PBS group. However, there were no other obvious differences between these 3 groups.
GMI Enhances the Cytotoxic Effects of 5-FU Against Oral Cancer Cells
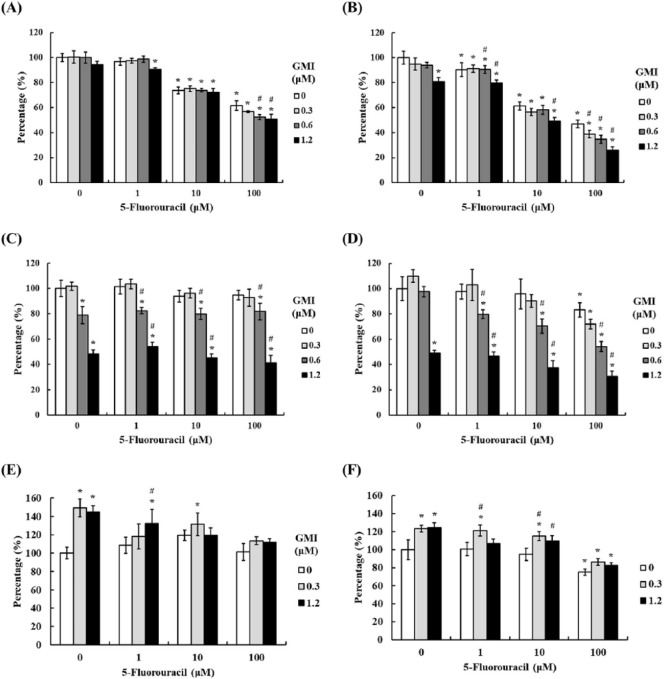
To examine whether GMI may interfere with the therapeutic effect of 5-FU on head and neck cancer, we assessed the cell viability of 2 oral cancer cell lines and 1 oral keratinocyte cell line. Cells were first pretreated with GMI in 4 doses (0, 0.3, 0.6, and 1.2 µM) for 1 hour and then treated with 5-FU in different doses (0, 1, 10, 100 µM) for 24 and 48 hours. Finally, MTT assay were used to analyze the cell viability. The results showed that 5-FU induced oral cancer cell death in a concentration-dependent manner, and there were significant concentration-dependent differences found in SCC9 cells. Compared with the untreated cells, cell death of 38% and 6% at 24 hours (Figure 4A and C) and 53% and 17% at 48 hours (Figure 4B and D) were observed for SAS and SCC9 cells treated with 5-FU at 100 µM. All data have significance except SAS cells at 24 hours. For treatment with lower dose of GMI (0.3 µM), GMI only could significantly enhance the cell death of SAS cells with the highest dose of 5-FU (100 µM) at 48 hours while co-treating with different doses of 5-FU with the highest dose of GMI (1.2 µM) could induce higher cell death. The combination effect was more obvious in SCC9. However, there was little influence on cell death when SCC9 cells were treated with different doses of GMI alone. As for the results of SG, the oral keratinocyte, significant cell death was only observed when cells were treated at 100-µM 5-FU at 48 hours while there were no enhancing effects observed in co-treatment of 5-FU and GMI (Figure 4E and F). For 2 cell models, we did not observe a higher cell viability when cells were pretreated with GMI, which suggests that GMI would not interfere with the effect of 5-FU in oral cancer but may enhance the cytotoxicity. Moreover, this enhancing effect was only observed in oral cancer cells but not in normal cells.
Figure 4.
The effects of 5-fluorouracil (5-FU) and GMI (Ganoderma microsporum immunomodulatory protein) on cell viability of SAS and SCC9 cells. (A and B) SAS, (C and D) SCC9 cells, and (E and F) SG cells (1 × 104 cells/well of 96-well plate) were treated with various concentrations of GMI (0, 0.3, 0.6, and 1.2 µM) and various concentration of 5-FU (0, 1, 10, and 100 µM) for 24 and 48 hours. Cell viability was analyzed by the MTT assay. The untreated group was presented 100%, and the percentages of other groups were calculated versus the untreated group. Means ± SD are shown. *P < .05 versus the untreated group, while #P < .05 versus the same concentration of 5-FU + no GMI group.
GMI Enhances Apoptosis of Oral Cancer Cells Induced by 5-FU
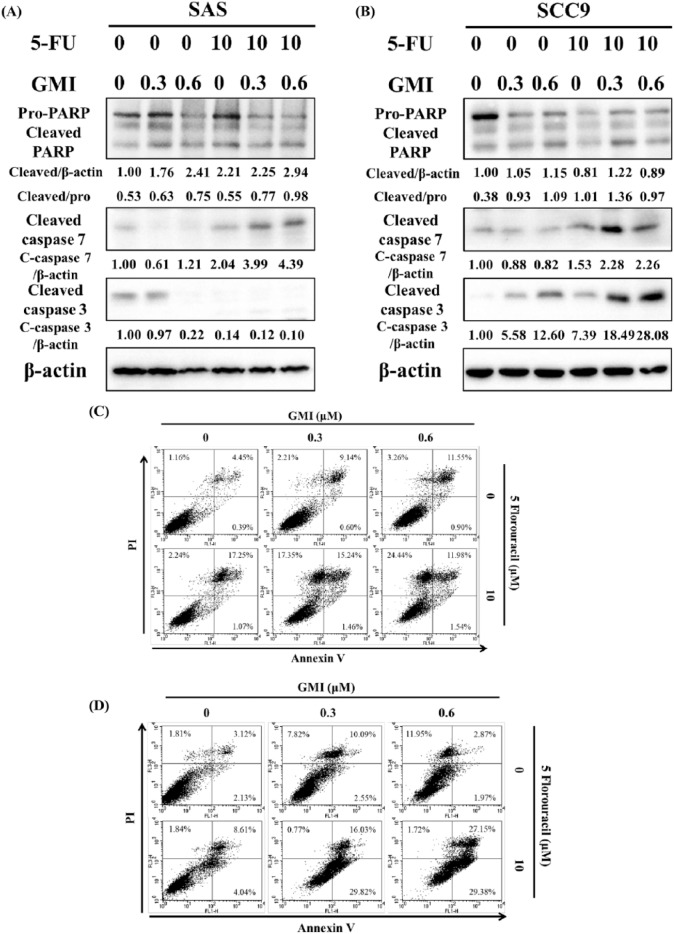
The cell viability study investigated whether GMI could affect the 5-FU-induced apoptosis on oral cancer. Cells were pretreated with 0, 0.3, or 0.6 µM of GMI for 1 hour and then co-treated with 0 or 10 µM of 5-FU for another 48 hours. Western blot and flow cytometry were used to analyze apoptosis. Western blot showed that in SAS cells, the higher expression of cleaved caspase 7 and the ratio of cleaved PARP to pro-form PARP were found in the 2 combination treatments of 5-FU and GMI groups (Figure 5A). There was no higher expression of cleaved caspase 3 in most of the treatment groups. In SCC9 cells, the combination groups, the 5-FU + 0.3-µM GMI and the 5-FU + 0.6-µM GMI group, presented higher expression of these 3 protein markers (Figure 5B). Furthermore, annexin-V and propidium iodide staining were utilized to confirm apoptotic changes in SAS and SCC9 cells treated with 5-FU and GMI (Figure 5C and D). The results revealed that both cell lines treated with 5-FU for 48 hours exhibited late apoptosis (17.25% and 8.61% for SAS and SCC9 cells in late apoptosis stage, respectively). SAS cells showed a dose-dependent apoptosis after treatment with GMI, while obvious apoptosis was observed in SCC9 cells treated with the middle dose of GMI. For combined treatment, both cells exhibited a good response of either apoptosis or necrosis. More cells in necrosis appeared in SAS cells, while more cells in late apoptosis appeared in SCC9 cells. Both cells demonstrated a dose-dependent response after co-treatment of 5-FU and GMI for 48 hours when cells in late apoptosis and necrosis phases were both accounted.
Figure 5.
The effects of 5-fluorouracil (5-FU) and GMI (Ganoderma microsporum immunomodulatory protein) on the apoptosis of SAS and SCC9 cells. Apoptosis-related proteins were detected by Western blot after (A) SAS and (B) SCC9 cells (8 × 105 cells/60 mm dish) were treated with various concentrations of GMI and 5-FU for 48 hours. GMI was pretreated for 1 hour before 5-FU. Beta-actin was used as an internal control. (C) SAS and (D) SCC9 cells (8 × 105 cells/60 mm dish) were treated the same way as (A) and (B). The number of apoptotic cells were assessed by annexin-V and propidium iodide staining through flow cytometry.
Discussion
Oral and intestinal mucositis, characterized by inflammation and cell damage, is one of the most common side effects induced by chemotherapy and radiotherapy. Patients who received chemotherapy or radiotherapy may present with anorexia, nausea, vomiting, abdominal pain, and diarrhea. According to the model of Sonis, the pathophysiology of mucositis may can be divided into 5 phases.3 (1) Reactive oxygen species form in the initiation stage while DNA damage may or may not occur. (2) NF-κB is activated to upregulate many genes with regard to inflammation and apoptosis. The level of pro-inflammatory cytokines, including tumor necrosis factor (TNF)-α, interleukin (IL)-1β, and IL-6, increase in the mucosa during this phase. Metalloproteinases may also be secreted by fibroblasts in the submucosa to break down the epithelial basement membrane. (3) The damage and inflammation is amplified through signal transduction induced by these cytokines. (4) Apoptosis occurs in many epithelial cells during the ulceration phase, and patients will feel pain during this phase. (5) In most patients, mucositis will spontaneously heal after the cancer therapy ends. Therefore, inflammation plays an important role in the mechanism of mucositis. Many previous studies exhibited that inhibition of several pro-inflammatory cytokines, such as TNF-α, IL-1, IL-6, and interferon-γ, could alleviate chemotherapy-induced mucositis in animal models.19,20 The commensal intestinal microbiota is also known to modulate the inflammation in the intestine and decrease the activation of NF-κB, and thus a high diversity of microbiota could protect the enterocytes from some harmful stimuli, such as chemotherapeutic agents.21
Despite various available treatment options, there is still a need for effective therapies to alleviate or even prevent chemotherapy-induced mucositis. In our previous study, we found FIP-gts and FIP-fve were resistant to simulated gastric and intestinal fluid, which suggest they may be suitable for oral supplementation.22 This digestive fluid-resistant effect may be due to many hydrophobic amino acids appearing in their amino acid sequences. The hydrophobic interactions increase the stability of FIPs. Because of the high similarity of amino sequences between GMI and FIP-fve, GMI may also be suitable as an oral supplement and remain active in the intestine to elicit its effects.
The results of the current study suggest that GMI reduced 5-FU-induced damage in the tongue and jejunal samples. The villi length of jejunum and the thickness of tongue epithelium are able to spontaneously recover significantly. However, the intestinal tissue was so severely damaged that leukocyte infiltration could not be observed; and therefore, there are no obvious differences of leukocyte infiltration between the 5-FU group and the 5-FU + GMI groups. Yet, the Western blot examination of jejunum samples showed that only the expression of cleaved caspase 7 increased in the 5-FU + PBS group while it mildly further upregulated in the 5-FU + GMI group. The results may indicate that 5-FU induces intestinal cell death, which may be apoptosis-independent. Further investigation such as analyzing of cleaved caspase 3 or 8 should be done to confirm the results.
To evaluate any immunomodulatory effect, a cytokine array was performed to analyze various cytokines in mouse plasma. However, several pro-inflammatory cytokines, including IL-1, IL-6, TNF-α, and interferon-γ, did not increase after 5-FU treatment. Although we did not observe the same results as similar studies with regard to 5-FU-induced mucositis,20 we still observed that there were higher levels of some chemokines including MCP-1, MIP-1α/β, and RANTES in the 5-FU group. However, due to the sample size being only 1 or 2 in each group, these results were not included. More detailed investigations need to be conducted to confirm whether GMI can inhibit cell death of enterocytes by preventing chemotaxis.
Leukopenia is a common adverse effect caused by chemotherapy including 5-FU. If leukopenia occurs, treatment outcomes may be compromised and cancer patients may experience a higher mortality rate due to infection.23 At the same time, mucositis may also be prolonged. These adverse effects lead to poor prognosis of patients who are receiving chemotherapy due to dose reduction or treatment cessation. From our previous study, FIP-fve and FIP-gts could recover the WBCs and alleviate the myelosuppression induced by docetaxel in mice.12 Because GMI is one kind of FIP, whose amino acid sequence is similar to FIP-fve or FIP-gts and has the ability to modulate the immune system, we also examined whether GMI would affect the CBCs and reverse leukopenia. However, GMI did not recover 5-FU-induced leukopenia. This result may be due to lower dose of GMI used in our study when it is compared with the dose of GMI used to administrate nude mice in our past study.24
From our previous study, we found GMI could induce apoptosis via activation of calcium-dependent autophagy in vitro and in vivo.24 It elicits autophagy through the PKB (Akt)/mammalian target of rapamycin signaling pathways and lysosome inhibitors bafilomycin-A1 and chloroquine increased GMI-mediated autophagic cell death.25 In addition, GMI potentiates cisplatin-mediated apoptosis in lung cancer cells.26 Co-treatment of GMI and cisplatin can induce the formation of autophagosomes and apoptotic nuclei. For this present study, we not only combined 5-FU and GMI to examine whether GMI could protect mice from damage by 5-FU in oral and small intestine but also tested the effects of 5-FU and GMI on oral normal and cancer cells. As 5-FU is the first-line chemotherapy used on the head and neck squamous cell carcinoma, including oral cancer,27 we used 3 oral cells to examine the effects of GMI as an in vitro model. We found that GMI could enhance 5-FU-mediated cytotoxicity in both cancer cell models, especially in SCC9. Moreover, GMI did not potentiate the cytotoxicity in normal keratinocytes. In addition, co-treatment of 5-FU and GMI could induce more apoptotic cells in SCC9 cells compared with single treatment, while 5-FU induced apoptosis-independent cell death and GMI caused apoptosis in SAS cells. Although the highest expression of apoptosis-related proteins was found in the 5-FU + middle dose of GMI group rather than the 5-FU + high dose of GMI group, the synergistic effect was more apparently observed in SCC9 cells, which indicates that SCC9 may be more sensitive to the combination treatment. Therefore, the results of flow cytometry may explain the more consistent expression trend of apoptosis-related proteins that was observed in SCC9 cells (even though the highest expression of these proteins were not found in the 5-FU + high dose of GMI) when doing Western blot analysis. From our previous study mentioned above, GMI may also lead to autophagy-mediated cell death. However, whether this mechanism is also happening in the combination treatment of 5-FU and GMI needs to be confirmed by further evidence. Briefly, our results suggest that GMI may enhance the cytotoxicity effects of 5-FU in oral cancer cells.
Conclusions
In conclusion, our data provide evidence that GMI could potentially attenuate 5-FU-induced damage on mice tongue and small intestine while enhancing the anticancer effects of 5-FU in oral cancer. The effects of GMI suggest it may be a suitable candidate as an adjuvant medicine in the attenuation of chemotherapy-induced oral and intestinal mucositis.
Acknowledgments
Flow cytometry and use of microplate reader were performed at the Instrument Center of Chung-Shan Medical University.
Footnotes
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by the Ministry of Science and Technology, Taiwan (MOST 104-2311-B-040-001, MOST 106-2632-B-040-002, MOST 106-2314-B-040-017, and MOST 107-2314-B-040-016-MY2) and Chung Shan Medical University Hospital, Taiwan (CSH-2017-C-015 and CSH-2019-D-008).
ORCID iD: Yu-Ping Hsiao  https://orcid.org/0000-0002-8482-4108
https://orcid.org/0000-0002-8482-4108
References
- 1. Longley DB, Harkin DP, Johnston PG. 5-fluorouracil: mechanisms of action and clinical strategies. Nat Rev Cancer. 2003;3:330-338. [DOI] [PubMed] [Google Scholar]
- 2. Nde MM, Silveira RC, dos Reis PE. Prophylaxis for mucositis induced by ambulatory chemotherapy: systematic review. J Adv Nurs. 2016;72:735-746. [DOI] [PubMed] [Google Scholar]
- 3. Sonis ST. The pathobiology of mucositis. Nat Rev Cancer. 2004;4:277-284. [DOI] [PubMed] [Google Scholar]
- 4. Benson AB, 3rd, Ajani JA, Catalano RB, et al. Recommended guidelines for the treatment of cancer treatment-induced diarrhea. J Clin Oncol. 2004;22:2918-2926. [DOI] [PubMed] [Google Scholar]
- 5. Katona C, Kralovánszky J, Rosta A, et al. Putative role of dihydropyrimidine dehydrogenase in the toxic side effect of 5-fluorouracil in colorectal cancer patients. Oncology. 1998;55:468-474. [DOI] [PubMed] [Google Scholar]
- 6. Campos MI, Campos CN, Aarestrup FM, Aarestrup BJ. Oral mucositis in cancer treatment: Natural history, prevention and treatment. Mol Clin Oncol. 2014;2:337-340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Duncan M, Grant G. Oral and intestinal mucositis—causes and possible treatments. Aliment Pharmacol Ther. 2003;18:853-874. [DOI] [PubMed] [Google Scholar]
- 8. Boh B, Berovic M, Zhang J, Zhi-Bin L. Ganoderma lucidum and its pharmaceutically active compounds. Biotechnol Annu Rev. 2007;13:265-301. [DOI] [PubMed] [Google Scholar]
- 9. El Enshasy HA, Hatti-Kaul R. Mushroom immunomodulators: unique molecules with unlimited applications. Trends Biotechnol. 2013;31:668-677. [DOI] [PubMed] [Google Scholar]
- 10. Jinn TR, Wu CM, Tu WC, Ko JL, Tzen JT. Functional expression of FIP-gts, a fungal immunomodulatory protein from Ganoderma tsugae in Sf21 insect cells. Biosci Biotechnol Biochem. 2006;70:2627-2634. [DOI] [PubMed] [Google Scholar]
- 11. Chen LH, Lin ZB, Li WD. Ganoderma lucidum polysaccharides reduce methotrexate-induced small intestinal damage in mice via induction of epithelial cell proliferation and migration. Acta Pharmacol Sin. 2011;32:1505-1512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Ou CC, Hsiao YM, Hou TY, Wu MF, Ko JL. Fungal immunomodulatory proteins alleviate docetaxel-induced adverse effects. J Funct Foods. 2015;19:451-463. [Google Scholar]
- 13. Liao CH, Hsiao YM, Lin CH, et al. Induction of premature senescence in human lung cancer by fungal immunomodulatory protein from Ganoderma tsugae. Food Chem Toxicol. 2008;46:1851-1859. [DOI] [PubMed] [Google Scholar]
- 14. Lull C, Wichers HJ, Savelkoul HF. Antiinflammatory and immunomodulating properties of fungal metabolites. Mediators Inflamm. 2005;2005:63-80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Lin YL, Liang YC, Tseng YS, et al. An immunomodulatory protein, Ling Zhi-8, induced activation and maturation of human monocyte-derived dendritic cells by the NF-kappaB and MAPK pathways. J Leukoc Biol. 2009;86:877-889. [DOI] [PubMed] [Google Scholar]
- 16. Li S, Jiang Z, Sun L, et al. Characterization of a new fungal immunomodulatory protein, FIP-dsq2 from Dichomitus squalens. J Biotechnol. 2017;246:45-51. [DOI] [PubMed] [Google Scholar]
- 17. Lin CH, Sheu GT, Lin YL, et al. A new immunomodulatory protein from Ganoderma microsporum inhibits epidermal growth factor mediated migration and invasion in A549 lung cancer cells. Process Biochem. 2010;45:1537-1542. [Google Scholar]
- 18. Kissow H, Hartmann B, Holst JJ, Poulsen SS. Glucagon-like peptide-1 as a treatment for chemotherapy-induced mucositis. Gut. 2013;62:1724-1733. [DOI] [PubMed] [Google Scholar]
- 19. Yasuda M, Kato S, Yamanaka N, et al. 5-HT3 receptor antagonists ameliorate 5-fluorouracil-induced intestinal mucositis by suppression of apoptosis in murine intestinal crypt cells. Br J Pharmacol. 2013;168:1388-1400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Yeung CY, Chan WT, Jiang CB, et al. Amelioration of chemotherapy-induced intestinal mucositis by orally administered probiotics in a mouse model. PLoS One. 2015;10:e0138746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. van Vliet MJ, Harmsen HJ, de Bont ES, Tissing WJ. The role of intestinal microbiota in the development and severity of chemotherapy-induced mucositis. PLoS Pathog. 2010;6:e1000879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Ou CC, Hsiao YM, Wang WH, Ko JL, Lin MY. Stability of fungal immunomodulatory protein, FIP-gts and FIP-fve, in IFN-γ production. Food Agric Immunol. 2009;20:319-332. [Google Scholar]
- 23. Crawford J, Dale DC, Lyman GH. Chemotherapy-induced neutropenia: risks, consequences, and new directions for its management. Cancer. 2004;100:228-237. [DOI] [PubMed] [Google Scholar]
- 24. Hsin IL, Ou CC, Wu TC, et al. GMI, an immunomodulatory protein from Ganoderma microsporum, induces autophagy in non-small cell lung cancer cells. Autophagy. 2011;7:873-882. [DOI] [PubMed] [Google Scholar]
- 25. Hsin IL, Sheu GT, Jan MS, et al. Inhibition of lysosome degradation on autophagosome formation and responses to GMI, an immunomodulatory protein from Ganoderma microsporum. Br J Pharmacol. 2012;167:1287-1300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Hsin IL, Ou CC, Wu MF, et al. GMI, an immunomodulatory protein from Ganoderma microsporum, potentiates cisplatin-induced apoptosis via autophagy in lung cancer cells. Mol Pharm. 2015;12:1534-1543. [DOI] [PubMed] [Google Scholar]
- 27. Wilken R, Veena MS, Wang MB, Srivatsan ES. Curcumin: a review of anti-cancer properties and therapeutic activity in head and neck squamous cell carcinoma. Mol Cancer. 2011;10:12. [DOI] [PMC free article] [PubMed] [Google Scholar]