Abstract
Background: National health surveys indicate that chronic kidney disease (CKD) is an increasingly prevalent condition in Australia, placing a significant burden on the health budget and on the affected individuals themselves. Yet, there are relatively limited data on the prevalence of CKD within Australian general practice patients. In part, this could be due to variation in the terminology used by general practitioners (GPs) to identify and document a diagnosis of CKD. This project sought to investigate the variation in terms used when recording a diagnosis of CKD in general practice. Methods: A search of routinely collected de-identified Australian general practice patient data (NPS MedicineWise MedicineInsight from January 1, 2013, to June 1, 2016; collected from 329 general practices) was conducted to determine the terms used. Manual searches were conducted on coded and on “free-text” or narrative information in the medical history, reason for encounter, and reason for prescription data fields. Results: From this data set, 61 102 patients were potentially diagnosable with CKD on the basis of pathology results, but only 14 172 (23.2%) of these had a term representing CKD in their electronic record. Younger patients with pathology evidence of CKD were more likely to have documented CKD compared with older patients. There were a total of 2090 unique recorded documentation terms used by the GPs for CKD. The most commonly used terms tended to be those included as “pick-list” options within the various general practice software packages’ standard “classifications,” accounting for 84% of use. Conclusions: A diagnosis of CKD was often not documented and, when recorded, it was in a variety of ways. While recording CKD with various terms and in free-text fields may allow GPs to flexibly document disease qualifiers and enter patient specific information, it might inadvertently decrease the quality of data collected from general practice records for clinical audit or research purposes.
Keywords: chronic kidney disease, general practice, electronic health records, documentation, terminology, classification, coding, epidemiology
Introduction
Chronic kidney disease (CKD) is a growing worldwide health problem and affects approximately 10% of adult Australians.1 The increasing burden of CKD reinforces the importance of general practitioners (GPs) identifying CKD early and implementing appropriate guideline-based management to prevent or slow disease progression.2
There is relatively limited data on the prevalence of CKD within Australian general practice patients. In addition, there is currently no reported data on the terms used by GPs to record and identify a diagnosis of CKD. Potentially, there could be significant variation in the nomenclature used; one possible consequence would then be limited utility of general practice records to estimate the community prevalence of CKD. This study sought to learn more about this variation.
Methods
MedicineInsight, developed and managed by NPS MedicineWise with funding support from the Australian Government Department of Health, is a large-scale national data program in Australia to extract and collate longitudinal, whole-of-practice data from the clinical information systems of consenting general practices.3,4 MedicineInsight collects de-identified patient data, including patient demographics, clinical encounters (defined as any professional interchange between a patient and a GP or practice nurse, excluding for administrative reasons), diagnoses, prescriptions, and pathology tests. The extraction tool collects incremental data regularly, allowing the development of a longitudinal database in which patients within sites can be tracked over time. We used MedicineInsight data from January 1, 2013, to June 01, 2016, collected from 329 general practices. Regular patients (defined by the Royal Australian College of General Practitioners as those with 3 or more encounters in 2 years) were included if at the time of data extraction (July 2016) they were aged at least 18 years. Patients were determined as “diagnosable” with CKD if they had 2 or more estimated glomerular filtration rate (eGFR) recorded values less than 60 mL/min/1.73 m2, and/or 2 urinary albumin/creatinine ratios ≥3.5 mg/mmol in females or ≥2.5 mg/mmol in males, at least 90 days apart.2 Comorbidities were recorded based on “condition flags” provided by MedicineInsight, using an algorithm that analyses coded and free-text patient information.
CKD is one of the specific conditions flagged by MedicineInsight within its database, based on codes used by the medical coding vocabularies supplied with the general practice clinical software packages from which MedicineInsight extracts data (most of the terms are shown in Table 1). Searches were first made for CKD in the active diagnoses list of each patient. Manual searches were also conducted on free-text or narrative information in the medical history (active diagnoses), reason for encounter and reason for prescription fields. Searches included the same codes used by the clinical software packages’ medical coding vocabularies. The main search terms used were variations of “chronic kidney/renal disease/impairment/failure,” and also included “renal insufficiency,” “MDRD,” “Cockcroft-Gault,” and “end-stage kidney/renal disease.” The full list of search terms was collated based on discussions with nephrologists and GPs, and using the Shrimp tool,5 a multiversion clinical terminology browser that includes SNOMED Clinical Terms (SNOMED CT). Spelling mistakes and synonyms were deliberately included to maximize the identification of patients with a recorded diagnosis of CKD. Deliberate spelling mistakes included “failier,” “failiure,” and “faulier,” for failure, and “impairement” and “imparement” for impairment. Search terms excluded were terms that included “acute” only (eg, “acute kidney disease”). Terms that had both “acute” and “chronic” were included (ie, “acute on chronic renal failure”). If a term did not definitively identify CKD, it was excluded from this dataset. This included synonyms of likely, possible, probable and episodic, as well as terms that included question marks (ie, “?CKD,” “Chronic Renal Failure?”). In addition, terms that mentioned a patient’s family history (eg, “familial CKD,” “mother had CKD”) were excluded.
Table 1.
Most Common 20 Terms Used to Record a Diagnosis of Chronic Kidney Disease.
| Term | n (% of total terms used) |
|---|---|
| RENAL IMPAIRMENT | 8248 (27) |
| CHRONIC RENAL FAILURE | 2567 (8) |
| RENAL FAILURE | 2373 (8) |
| CHRONIC KIDNEY DISEASE, STAGE 3A | 1726 (6) |
| CKD (CHRONIC KIDNEY DISEASE) STAGE 3 | 1551 (5) |
| CHRONIC KIDNEY DISEASE | 1544 (5) |
| CHRONIC KIDNEY DISEASE, STAGE 3 | 1075 (4) |
| RENAL FAILURE, CHRONIC | 1020 (3) |
| CHRONIC KIDNEY DISEASE, STAGE 3B | 965 (3) |
| CHRONIC KIDNEY DISEASE - STAGE 3 | 740 (2) |
| CHRONIC KIDNEY DISEASE, STAGE 2 | 628 (2) |
| CHRONIC KIDNEY DISEASE, STAGE 4 | 598 (2) |
| CKD (CHRONIC KIDNEY DISEASE) STAGE 4 | 412 (1) |
| CKD (CHRONIC KIDNEY DISEASE) STAGE 2 | 374 (1) |
| RENAL INSUFFICIENCY - CHRONIC | 361 (1) |
| CHRONIC KIDNEY DISEASE, STAGE 1 | 358 (1) |
| CKD (CHRONIC KIDNEY DISEASE) STAGE 1 | 324 (1) |
| CHRONIC KIDNEY DISEASE - STAGE 2 | 268 (1) |
| MILD RENAL IMPAIRMENT | 257 (1) |
| ANAEMIA - CHRONIC RENAL FAILURE | 224 (1) |
The Tasmanian Health and Medical Human Research Ethics Committee approved the study (H0015651).
Results
From this data set, 61 102 patients were potentially diagnosable with CKD on the basis of eGFR or urinary albumin/creatinine ratio results, but only 14 172 (23.2%) of these had a term representing CKD in their list of diagnoses or problems, reasons for encounter or reasons for prescription. Younger patients with pathology evidence of CKD were more likely to have documented CKD compared to older patients: 30.8% of patients under 60 years compared with 22.7% of patients 60 years and older (P < .0001; Figure 1). Patients with pathology evidence of CKD who had a recorded comorbidity of either hypertension, diabetes, cardiovascular disease, or atrial fibrillation were also more likely to have documented CKD compared with patients without these comorbidities (all P < .001 by chi-square test). The documentation rate increased with the severity of CKD (eg, 16% in patients with pathology evidence of stage 3a, 33% for stage 3b, 52% for stage 4 and 65% for stage 5; P < .0001 by chi-square test).
Figure 1.
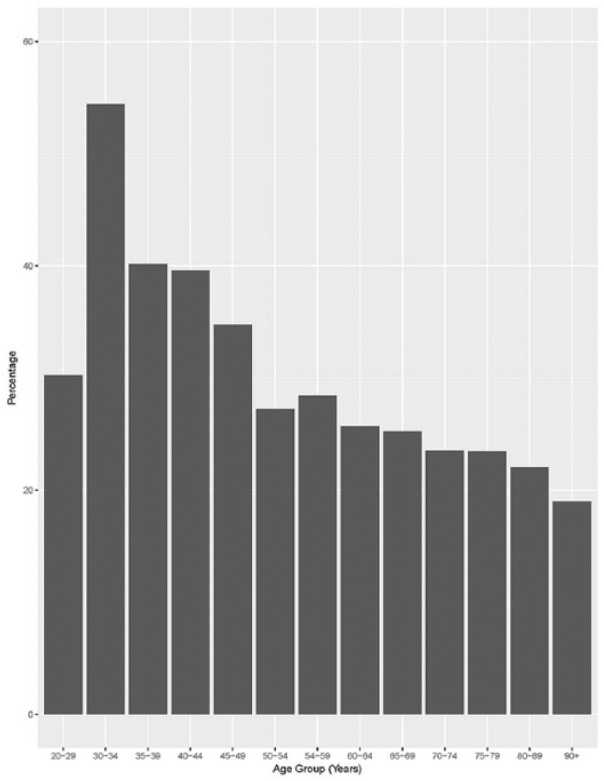
Rate of documentation of chronic kidney disease (CKD) in patients with pathology evidence of CKD, by age.
In total, when including 6078 patients without eGFR or urinary albumin/creatinine ratio results indicating CKD, the search found 20 250 patients with CKD documented across their medical history (active diagnosis), encounter, and reason for prescription data. The majority of these patients (16 953; 83.6%) were identified from the medical history, while the remainder were identified via the free-text fields “reason for prescription” and “reason for encounter.”
There were a total of 2090 unique recorded documentation terms used by the GPs for CKD; these were used 30 676 times across the 20 250 patients. The most commonly used terms tended to be those included as pick-list options within the various clinical software packages’ standard classifications, accounting for 84% of use (Table 1). The majority (>90%) of the list of 2090 different terms had been entered as free-text. These included spelling mistakes, nonstandard abbreviations and codes, as well as including combinations of diagnoses into the one recording (eg, “UTI/CRF/CCF/IDDM/CELLULITIS”).
Discussion
Despite the desirability of using a standard clinical nomenclature for CKD,6 there was wide variation in recording diagnosable CKD in general practice—ranging from an absence of recording (in more than three-quarters of patients) through to the use of multiple clinical terms. Variation related to both differing and numerous terms within clinical software packages and the use of free-text by GPs. With the various clinical information system available in Australian general practice, GPs can use medical coding vocabularies to register diagnosis, reason for encounter, and reason for prescription into their systems. Although GPs are required to complete all these fields every time they see a patient, the use of the codes is not mandatory and clinicians can enter medical terms as free-text.3,4
There was an association between age of the patient and having a recorded diagnosis of CKD, most likely reflecting reluctance by GPs to label older patients with a formal diagnosis of CKD.7 Still, more than 50% of those with CKD documented were aged at least 75 years.
The challenges in the use of electronic medical records, intended to assist in the delivery and documentation of care in clinical practice, when applied for quality improvement and research purposes have been well characterized in Australia and internationally. Symptom lists may be incomplete, diagnoses may vary in their accuracy, and accessing data can be difficult when outcomes or conditions are recorded without a standard nomenclature or when details are entered in progress notes or other text fields not readily accessible for data queries.8-16 This is not only a research problem but also a clinical issue. Clinical information systems generally provide users with add-on clinical audit tools. However, the algorithms in these clinical audit tools are based on GPs using drop-down options when recording a patient diagnosis. So, if a GP uses a free-text term or misspells their term, a clinical audit tool would not “find” this term/patient.
If GPs only used the pick-list options within the various clinical software packages’ standard classifications, the variation between the packages would not be an issue if all the packages’ codes were collected. However, having too many codes does make it hard to extract, and still misses instances of CKD. More sophisticated extraction procedures across multiple data fields in addition to searching free-text entries, as in this study, can minimize some of these limitations.
Acknowledgments
The authors thank NPS MedicineWise for providing the data.
Author Biographies
Alex Kitsos is a statistician and health analyst, School of Medicine and Wicking Dementia Research and Education Centre, University of Tasmania.
Gregory M. Peterson is professor of Pharmacy, School of Medicine, University of Tasmania.
Matthew D. Jose is professor of Medicine, University of Tasmania and Consultant Nephrologist, Royal Hobart Hospital.
Masuma Akter Khanam is post-doctoral research fellow, School of Health Sciences, University of Tasmania.
Ronald L. Castelino is senior lecturer in Pharmacology & Clinical Pharmacy at Sydney Nursing School, University of Sydney and Adjunct Senior Lecturer, University of Tasmania.
Jan C. Radford is associate professor of General Practice, Launceston Clinical School, School of Medicine, University of Tasmania.
Footnotes
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
ORCID iDs: Gregory M. Peterson  https://orcid.org/0000-0002-6764-3882
https://orcid.org/0000-0002-6764-3882
Jan C. Radford  https://orcid.org/0000-0002-5751-0488
https://orcid.org/0000-0002-5751-0488
References
- 1. Australian Institute for Health and Welfare. Chronic Kidney Disease. Canberra, Australia: Australian Institute for Health and Welfare; 2017. http://www.aihw.gov.au/chronic-kidney-disease. Accessed February 1, 2019. [Google Scholar]
- 2. Kidney Health Australia. Chronic Kidney Disease (CKD) Management in General Practice. 3rd ed. Melbourne, Australia: Kidney Health Australia; 2015. https://kidney.org.au/cms_uploads/docs/ckd-management-in-gp-handbook-3rd-edition.pdf. Accessed February 1, 2019. [Google Scholar]
- 3. Gonzalez-Chica DA, Vanlint S, Hoon E, et al. Epidemiology of arthritis, chronic back pain, gout, osteoporosis, spondyloarthropathies and rheumatoid arthritis among 1.5 million patients in Australian general practice: NPS MedicineWise MedicineInsight dataset. BMC Musculoskelet Disord. 2018;19:20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. NPS MedicineWise. Using MedicineInsight data. https://www.nps.org.au/medicine-insight/using-medicineinsight-data. Accessed February 1, 2019.
- 5. SNOMED International. SNOMED CT browsers—online. https://confluence.ihtsdotools.org/display/DOC/SNOMED+CT+Browsers+-+Online. Accessed February 1, 2019.
- 6. National Kidney Foundation. K/DOQI clinical practice guidelines for chronic kidney disease: evaluation, classification, and stratification. Am J Kidney Dis. 2002;39(2 suppl 1):S1-S266. [PubMed] [Google Scholar]
- 7. Ellam T, Twohig H, Khwaja A. Chronic kidney disease in elderly people: disease or disease label? BMJ. 2016;352:h6559. [DOI] [PubMed] [Google Scholar]
- 8. Barnett S, Henderson J, Hodgkins A, et al. A valuable approach to the use of electronic medical data in primary care research: panning for gold. Health Inf Manag. 2017;46:51-57. [DOI] [PubMed] [Google Scholar]
- 9. Verheij RA, Curcin V, Delaney BC, McGilchrist MM. Possible sources of bias in primary care electronic health record data use and reuse. J Med Internet Res. 2018;20:e185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Cohen DJ, Dorr DA, Knierim K, et al. Primary care practices’ abilities and challenges in using electronic health record data for quality improvement. Health Aff (Millwood). 2018;37:635-643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Liaw ST, Taggart J, Yu H, de Lusignan S. Data extraction from electronic health records—existing tools may be unreliable and potentially unsafe. Aust Fam Physician. 2013;42:820-823. [PubMed] [Google Scholar]
- 12. de Lusignan S, Sadek N, Mulnier H, Tahir A, Russell-Jones D, Khunti K. Miscoding, misclassification and misdiagnosis of diabetes in primary care. Diabet Med. 2012;29:181-189. [DOI] [PubMed] [Google Scholar]
- 13. Peiris D, Agaliotis M, Patel B, Patel A. Validation of a general practice audit and data extraction tool. Aust Fam Physician. 2013;42:816-819. [PubMed] [Google Scholar]
- 14. Lin J, Jiao T, Biskupiak JE, McAdam-Marx C. Application of electronic medical record data for health outcomes research: a review of recent literature. Expert Rev Pharmacoecon Outcomes Res. 2013;13:191-200. [DOI] [PubMed] [Google Scholar]
- 15. Vickery AW, Ryan J, Pang J, Garton-Smith J, Watts GF. Increasing the detection of familial hypercholesterolaemia using general practice electronic databases. Heart Lung Circ. 2017;26:450-454. [DOI] [PubMed] [Google Scholar]
- 16. Hersh WR, Weiner MG, Embi PJ, et al. Caveats for the use of operational electronic health record data in comparative effectiveness research. Med Care. 2013;51(8 suppl 3):S30-S37. [DOI] [PMC free article] [PubMed] [Google Scholar]


