Abstract
Background:
There is a significant number of military personnel with a history of mild traumatic brain injury (mTBI) who suffer from comorbid posttraumatic stress symptoms (PTS). Although there is evidence of disruptions of the default mode network (DMN) associated with PTS and mTBI, previous studies have only studied static connectivity while ignoring temporal variability of connectivity.
Objective:
To assess DMN disrupted or dysregulated neurocircuitry, cognitive functioning, and psychological health of active-duty military with mTBI and PTS.
Method:
U.S. Army soldiers with PTS (n = 14), mTBI + PTS (n = 25), and healthy controls (n = 21) voluntarily completed a cognitive and symptom battery. In addition, participants had magnetic resonance imaging (MRI) to assess both static functional connectivity (SFC) and variance of dynamic functional connectivity (vDFC) of the DMN.
Results:
Both the PTS and mTBI + PTS groups had significant symptoms, but only the comorbid group had significant decrements in cognitive functioning. Both groups showed less stable and disrupted neural signatures of the DMN, mainly constituting the cingulate-frontal-temporal-parietal attention network. Specifically, the PTS group showed a combination of both reduced contralateral strength and reduced unilateral variability of frontal-cingulate-temporal connectivities, as well as increased variability of frontal-parietal connectivities. The mTBI + PTS group had fewer abnormal connectives than the PTS group, all of which included reduced strength of frontal-temporal regions and reduced variability frontal-cingulate-temporal regions. Greater SFC and vDFC connectivity of the left dorsolateral prefrontal cortex (dlPFC) ↔ precuneus was associated with higher cognitive scores and lower symptom scores.
Conclusions:
Findings suggest that individuals with PTS and mTBI + PTS have a propensity for accentuated generation of thoughts, feelings, sensations, and/or images while in a resting state. Compared with controls, only the PTS group was associated with accentuated variability of the frontal-parietal attention network. While there were no significant differences in DMN connectivity strength between the mTBI + PTS and PTS groups, variability of connectivity was able to distinguish them.
Keywords: Functional magnetic resonance imaging, functional connectivity, posttraumatic stress disorder, mild traumatic brain injury, default mode network, military
Introduction
Posttraumatic stress disorder (PTSD) is associated with significant symptoms that severely impact one’s quality of life.1 These posttraumatic symptoms (PTS) include perseverative thinking (rumination of traumatic events), which is linked with the development and maintenance of other symptoms.2 Furthermore, contributing to the chronicity of symptoms, PTS is associated with compromised cognitive functioning3 and emotional processes.4,5 Similar to individuals with PTS, a small but significant percentage of individuals with a history of mild traumatic brain injury (mTBI) report chronic symptoms that impact their quality of life,6 show cognitive decrements, and have compromised ability to regulate emotions.5,7 To complicate matters, there is a high comorbidity of PTS in military personnel who sustain an mTBI while deployed8 due to the trauma experienced when sustaining a head injury (eg, casualties occurring from an improvised explosive device [IED]). Evidence indicates that individuals with PTS and comorbid mTBI have worse clinical outcomes compared with PTS alone based on symptom severity5 and treatment response.9 Characterizing the neural functional correlates of both PTS and mTBI using magnetic resonance imaging (MRI) has shown to have some utility.10,11 By characterizing different default mode network (DMN), neural signatures may improve the process of teasing apart the etiology of cases with potential co-morbidities with overlapping symptomatology. Furthermore, this approach might provide insight of potential mechanistic properties underlying pathophysiologic-related functional impairments.
Task-based functional neuroimaging reveals disruptions in the neural circuitry associated with PTS, specifically the hippocampus, amygdala, insula, superior and middle temporal gyrus, cingulate gyrus, and medial frontal gyrus.5,7 Individuals with chronic mTBI show increased activation of regions implicated in cognitive-affective process7 such as the insula and temporal lobe. Recent studies reveal compromised neural connectivity in PTS and mTBI, implicating the middle frontal gyrus (MFG) or the dorsolateral prefrontal cortex (dlPFC), insula, amygdala, and hippocampus, with the MFG being the pivotal source of network disruption.10,11 Overall, both PTS and mTBI with co-occurring PTS may have disrupted neural circuitry with top-down origins necessary for regulation of affective processes that modulate symptom expression.
The DMN consists of interacting brain regions (eg, the medial prefrontal cortex, posterior cingulate cortex (PCC), precuneus, inferior parietal lobules, and temporal lobe regions) that are active when an individual is not focusing on an exogenous stimulus.12 Power et al13 identified 58 functionally homogeneous brain regions of the DMN. The DMN reflects internally focused thought that occurs when individuals are left undisturbed.14 While undisturbed, individuals are prone to mind-wandering, which results in thinking about one’s self, remembering events, or envisaging the future.14
Resting state connectivity of the DMN is disrupted in individuals with PTS.15 Evidence shows disrupted connectivity between the PCC and hippocampus,16 the anterior cingulate cortex (ACC) and parahippocampus,15,17 precuneus,17 and ventromedial prefrontal cortex (vmPFC).18 This disruption is posited to underpin ruminating thought processes. In active-duty military populations with a high prevalence of mTBI and subsequent comorbid PTS, exploring potential neural connectivity signatures may be beneficial for differentiating the mechanisms underpinning these combat-related neuropsychiatric conditions as well as improving individualized, targeted treatments.
A prominent limitation in the literature is that studies most often report only “static” functional connectivity (SFC) between regions and fail to assess temporal variation of the connectivity, or in other terms, dynamic FC (DFC). Static functional connectivity only measures a time-compressed snapshot of connectivity as it represents average connectivity over the entire duration of the scan, ignoring the changes in connectivity that occur naturally over the course of several minutes of the scan. Lower temporal variability (ie, vDFC) is associated with both neurologic and psychiatric conditions,10,16,19–22 including PTSD and with and without mTBI,11 suggesting a lack of cognitive flexibility (CF) associated with these neuropsychiatric conditions. Together, strength (SFC) and temporal variability (vDFC) of connectivity better explain the relationship between neural regions and ultimately better characterize these combat-related neuropsychiatric conditions.
In this study, we assessed both SFC and vDFC as objective measures of disrupted or dysregulated neurocircuitry and measures of cognitive functioning and psychological health, in active-duty Army soldiers who were categorized into one of the 3 groups: healthy controls versus a group screened positive for elevated PTS versus a group with mTBI and comorbid PTS (mTBI + PTS). Given that intrusive thoughts, dissociation, and avoidance behaviors are hallmark symptoms of PTSD, it is likely dysregulation of the DMN neurocircuitry will be observed in both the PTS and mTBI + PTS groups compared with the control group. Therefore, we hypothesized that the mTBI + PTS group will have a greater number reduced strength (SFC) and variance (vDFC) of connectivities within the DMN than the PTS alone group. Furthermore, we predict that severity of deficits in central executive functioning (EF) and greater psychological health symptoms in both diagnostic groups are also likely to be associated with dysregulated neurocircuitry.
Materials and Methods
Recruitment
This study’s protocol was reviewed and approved by the U.S. Army Medical Research and Materiel Command Institutional Review Board (MRMC IRB) and the Auburn University IRB. In all, 78 active-duty U.S. Army soldiers, with prior deployment(s) to Iraq or Afghanistan as part of Operation Iraqi Freedom (OIF) and Operation Enduring Freedom (OEF), were recruited from Fort Rucker, AL and Fort Benning, GA to participate in this study. In addition to word of mouth, recruitment flyers and posters were placed at behavioral health clinics and other instillation facilities frequented by soldiers. Interested soldiers contacted investigators via phone or email provided on the flyers/posters. Candidates were screened for MRI contraindication, PTSD, and traumatic brain injury history. Eligible volunteers provided written informed consent. A study physician reviewed soldiers’ electronic medical records for exclusionary medical conditions (eg, MRI contraindications such as evidence of shrapnel). If cleared by the study physician, the soldier was contacted and scheduled for testing at the Auburn University MRI Research Center, Auburn, AL. Participants were re-consented and further screened on arrival at the testing site.
Participants
Active-duty U.S. Army soldiers (aged between 18 and 50 years) were recruited from Fort Rucker, AL, USA and Fort Benning, GA, USA to voluntarily participate in the study. The study was conducted in accordance with the Declaration of Helsinki. The procedures were approved by Auburn University’s Institutional Review Board (IRB) and the Headquarters U.S. Army Medical Research and Materiel Command, IRB (HQ USAMRMC IRB).
A total of 75 participants were enrolled in the study, all of which had prior deployment(s) in Afghanistan (OEF) and/or Iraq (OIF). Participants were 73% Caucasian, 12% African-American, and 8.5% Hispanic (non-white), with all other racial groups making up the remaining 6.5%. Subjects were grouped based on PTSD symptom severity using the PTSD Checklist-523 (PCL5) score, clinician referral, and medical history. Eligibility required the absence of any history of a moderate-to-severe TBI and no history of a Diagnostic and Statistical Manual of Mental Disorders (4th ed.; Text Revision; DSM-IV-TR) or Diagnostic and Statistical Manual of Mental Disorders (5th ed.; DSM-V) diagnosis of a psychotic disorder (eg, schizophrenia) or substance dependency disorder. After removing 11 women from the sample due to a significant group disparity in the ratio of women to men, and 4 additional participants due to acquisition error, our sample included data from 60 participants who fell into one of the 3 groups:
(1) A combat control group (n = 21) was made up of participants with a score <20 on the PCL5 with no reported mTBI within the last 5 years. (2) A posttraumatic stress (PTS) group (n = 14) consisted of participants with no reported history of mTBI in the last 5 years and total score ⩾20 on the PCL5. (3) A comorbid mTBI + PTS group (n = 25) consisted of participants with a history of medically documented mTBI within the last 5 years and scores ⩾20 on the PCL5 and actively experiencing postconcussive symptoms as assessed via the Neurobehavioral Symptom Inventory (NSI).
In addition, we also tested effort to improve the validity of our assessment data. To this end, we administered the Test of Memory Malingering (TOMM),24 which consists of 2 learning trials and a retention trial that uses pictures of common, everyday objects (eg, chair, pencil). A cut-off score (<45 correct) was used to determine eligibility for participation in the study. All participants passed the TOMM on the first trial.
Measures
Clinical symptoms
Clinical symptoms were assessed using a battery of common measures including the PTSD Checklist-5,23 NSI,25 Zung Depression Scale (ZDS) and Zung Anxiety Scale (ZAS),26,27 Epworth Sleepiness Scale28 (ESS), Perceived Stress Scale29 (PSS), AUDIT,30 and Pittsburgh Sleep Quality Index31 (PSQI).
Neurocognitive function
For neurocognitive assessment, we administered the Central Nervous System-Vital Signs®32 (CNS-VS). This study used 5 computerized CNS-VS subtests (verbal memory [VM], symbol digit coding, Stroop test, continuous performance test, and the shifting attention test). The domain scores calculated were VM, complex attention (CA), reaction time (RT), processing speed (PS), CF, and EF. All domain scores are presented as index scores, with a mean of 100 and standard deviation of 15.
More information about the clinical and neurocognitive measures is provided in the supplemental document (Appendix).
Procedures
Participants arriving at the research lab for their scheduled testing appointment were re-screened for eligibility, thoroughly screened for MRI contraindications, and re-consented to ensure full comprehension of the study’s procedures, benefits and their rights.
Functional magnetic resonance imaging. Participants were scanned in a 3T MAGNETOM Verio scanner (Siemens Healthcare, Erlangen, Germany) using T2*-weighted multiband echo planar imaging (EPI) sequence in resting state (participants were required to keep their eyes open and not think of anything specific and fixated on a white cross displayed in dark background on the screen using an Avotec projection system), with TR = 600 ms, TE = 30 ms, voxel size = 3 × 3 × 5 mm3, FA = 55°, multiband factor = 2, 1000 volumes. Brain coverage was limited to cerebral cortex, subcortex, and midbrain (cerebellum was excluded).
Data analysis
Non-imaging measures
Mean and standard deviation were calculated for clinical symptoms and neurocognitive measures. Ordinal data were analyzed using Kendall Tau B (τb) test. Independent multivariate analyses of variance (MANOVAs), with pairwise comparisons and Bonferroni corrections to control for inflation in familywise error rate, were used for (1) demographic and descriptive variables—age, education, lifetime mTBIs, CES, LEC, CE, and AUDIT; (2) clinical symptoms—PCL-5, NSI, ESS, PSIQ, ZAS, ZDS, and PSS; and (3) neurocognitive scores—RT, CA, PS, CF, VM, and EF. Scatterplots showed there was reasonable normality.
Functional magnetic resonance imaging data pre-processing
Standard resting-state functional magnetic resonance imaging (fMRI) pre-processing steps were carried out, including realignment, normalization to Montreal Neurological Institute (MNI) space, and detrending and regressing covariates (six head-motion parameters, white matter, and cerebrospinal fluid signal). Pre-processing was carried out using Data Processing Assistant for Resting-State fMRI33 (DPARSF, v1.7), which is based on Statistical Parametric Mapping34 (SPM8) and Resting-State fMRI Data Analysis Toolkit (REST).35
The data were temporally normalized, rendering each timeseries with zero mean and unit variance. These data were then entered into a blind deconvolution algorithm36 to reduce non-neural confound of the hemodynamic response function (HRF), thus estimating latent neuronal timeseries. This deconvolution is blind given that both HRF and the underlying latent neural timeseries are estimated from only the observed fMRI data. Specifically, we employed the method proposed by Wu et al,36 which has gained wide usability and acceptance owing to its simplicity, interpretability, validity, and robustness.11,37–39 Briefly, the method models resting-state fMRI data as event related with randomly occurring events using point processes, then estimates the best-fit voxel-specific HRFs, and then obtains latent neural time series using Weiner deconvolution.
Deconvolution was performed as confounds emerging from inter-subject and spatial variability of the HRF could give rise to a scenario wherein 2 fMRI timeseries have high directional connectivity while the underlying neural variables do not and vice versa.10 Given the high dimensionality of whole-brain fMRI data, mean deconvolved fMRI timeseries were obtained from 58 functionally homogeneous brain regions of the DMN as defined in Power et al.13
fMRI post-processing analysis
Analysis of SFC and variance of dynamic functional connectivity (vDFC) was performed using custom MATLAB® modules. Static functional connectivity was obtained as the Pearson correlation between pairs of time series, with it being obtained for all the time series pairs in the DMN. Dynamic functional connectivity was obtained using the sliding window method, with the window lengths determined based on time series stationarity using the augmented Dickey-Fuller (ADF) test, as described in Rangaprakash et al.21 The vDFC was obtained by taking the variance of the DFC time series for each connection. A multivariate analysis of variance (MANOVA) followed by pairwise t-tests (P < .05, false discovery rate (FDR) corrected) was used to compare differences in SFC and vDFC of the 58 region(s) of interest (ROI) timeseries between the control group versus PTSD group versus mTBI + PTS group (Figure 1). Common neuroanatomy nomenclature was used in Tables 4 and 5 to describe significant group differences in connectivity, which varies from that in Figures 2 and 3. This change was done to increase reader generalizability and utility of the study’s findings.
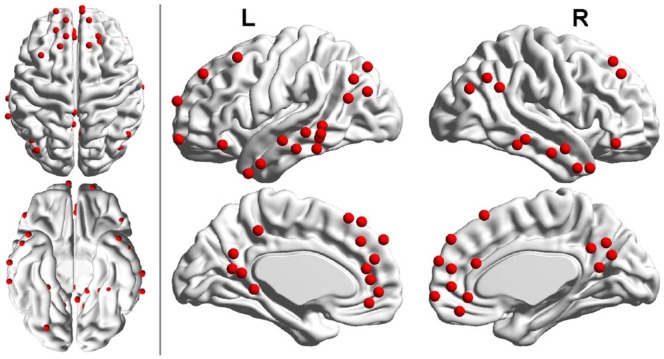
Figure 1.
Regions of interest (ROIs) of the default mode network (DMN) as proposed in Power et al. These 58 ROIs were used in this study.
Table 4.
Static functional connectivity (SFC) findings.
| Group contrast | Connectivity between
ROIs |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ROI | Region | x | y | z | ROI | Region | x | y | z | t | F | |
| Control > PTS | ||||||||||||
| 15 | L. posterior cingulate | −11 | −56 | 16 | 25 | L. dorsolateral PFC | −35 | 20 | 51 | 5.077 | 15.568 | |
| 17 | R. middle cingulate | 8 | −48 | 31 | 45 | L. middle temporal gyrus | −68 | −41 | −5 | 4.914 | 13.948 | |
| 41 | R. middle temporal gyrus | 65 | −12 | −19 | 45 | L. middle temporal gyrus | −68 | −41 | −5 | 5.014 | 12.721 | |
| PTS > Control | — | — | — | |||||||||
| Control > mTBI + PTS | ||||||||||||
| 6 | L. middle temporal gyrus | −46 | −61 | 21 | 35 | R. orbitomedial PFC | 8 | 42 | −5 | 3.907 | 12.192 | |
| mTBI + PTS > Control | — | — | — | |||||||||
| PTS >mTBI + PTS | — | — | — | |||||||||
| mTBI + PTS > PTS | — | — | — | |||||||||
Abbreviations: mTBI, mild traumatic brain injury; PFC, prefrontal cortex; PTS, posttraumatic stress symptoms; ROIs: regions of interest.
Table 5.
Variance of dynamic functional connectivity (vDFC) findings.
| Group contrast | Connectivity between
ROIs |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ROI | Region | x | y | z | ROI | Region | x | y | z | t | F | |
| Control > PTS | ||||||||||||
| 10 | L. middle temporal gyrus | −68 | −23 | −16 | 17 | R. middle cingulate | 8 | −48 | 31 | 4.256 | 8.940 | |
| 16 | L. posterior cingulate | −3 | −49 | 13 | 29 | L. dorsolateral PFC | −20 | 45 | 39 | 4.462 | 11.533 | |
| 8 | L. superior temporal gyrus | −44 | 12 | −34 | 38 | L. anterior cingulate | −3 | 42 | 16 | 4.143 | 9.611 | |
| 5 | L. ventromedial PFC | −18 | 63 | −9 | 57 | L. ventrolateral PFC | −46 | 31 | −13 | 4.046 | 9.836 | |
| PTS > Control | ||||||||||||
| 2 | R. orbitomedial PFC | 6 | 67 | −4 | 18 | R. precuneus | 15 | −63 | 26 | 4.601 | 10.656 | |
| 18 | R. precuneus | 15 | −63 | 26 | 37 | L. dorsomedial PFC | −2 | 38 | 36 | 4.231 | 11.552 | |
| 18 | R. precuneus | 15 | −63 | 26 | 40 | L. dorsomedial PFC | −8 | 48 | 23 | 4.257 | 7.987 | |
| 23 | L. dorsomedial PFC | −10 | 39 | 52 | 58 | R. ventrolateral PFC | 49 | 35 | −12 | 3.935 | 9.806 | |
| 42 | L. middle temporal gyrus | −56 | −13 | −10 | 58 | R. ventrolateral PFC | 49 | 35 | −12 | 4.310 | 10.926 | |
| Control > mTBI + PTS | ||||||||||||
| 8 | L. superior temporal gyrus | −44 | 12 | −34 | 17 | R. middle cingulate | 8 | −48 | 31 | 4.888 | 13.266 | |
| 23 | L. dorsomedial PFC | −10 | 39 | 52 | 32 | L. anterior cingulate | −7 | 51 | −1 | 5.645 | 15.953 | |
| mTBI + PTS > Control | ||||||||||||
| — | — | |||||||||||
| PTS > mTBI + PTS | x | y | z | x | y | z | ||||||
| 10 | L. middle temporal gyrus | −68 | −23 | −16 | 14 | R. precuneus | 6 | −59 | 35 | 5.085 | 14.383 | |
| 2 | R. orbitomedial PFC | 6 | 67 | −4 | 18 | R. occipital/precuneus | 15 | −63 | 26 | 3.848 | 10.656 | |
| 3 | R. ventromedial PFC | 8 | 48 | −15 | 18 | R. occipital/precuneus | 15 | −63 | 26 | 3.977 | 7.980 | |
| 2 | R. orbitomedial PFC | 6 | 67 | −4 | 19 | L. middle cingulate | −2 | −37 | 44 | 3.842 | 6.299 | |
| 4 | L. precuneus | −13 | −40 | 1 | 26 | R. dorsolateral PFC | 22 | 39 | 39 | 3.722 | 10.956 | |
| 23 | L. dorsomedial PFC | −10 | 39 | 52 | 32 | L. anterior cingulate | −7 | 51 | −1 | 4.528 | 15.953 | |
| 23 | L. dorsomedial PFC | −10 | 39 | 52 | 35 | R. orbitomedial PFC | 8 | 42 | −5 | 4.163 | 11.720 | |
| 18 | R. occipital/precuneus | 15 | −63 | 26 | 37 | L. dorsomedial PFC | −2 | 38 | 36 | 3.751 | 11.552 | |
| 23 | L. dorsomedial PFC | −10 | 39 | 52 | 37 | L. dorsomedial PFC | −2 | 38 | 36 | 3.716 | 8.390 | |
| 25 | L. dorsolateral PFC | −35 | 20 | 51 | 39 | L. frontopolar | −20 | 64 | 19 | 3.938 | 8.222 | |
| 25 | L. dorsolateral PFC | −35 | 20 | 51 | 41 | R. middle temporal gyrus | 65 | −12 | −19 | 3.747 | 8.963 | |
| 42 | L. middle temporal gyrus | −56 | −13 | −10 | 48 | R. middle temporal gyrus | 52 | −2 | −16 | 3.709 | 6.014 | |
| 18 | R. occipital/precuneus | 15 | −63 | 26 | 52 | R. cerebellum | 28 | −77 | −32 | 4.113 | 7.995 | |
| 24 | L. dorsolateral PFC | −16 | 29 | 53 | 57 | L. ventrolateral PFC | −46 | 31 | −13 | 4.124 | 6.094 | |
| 25 | L. dorsolateral PFC | −35 | 20 | 51 | 57 | L. ventrolateral PFC | −46 | 31 | −13 | 4.059 | 6.729 | |
| 35 | R. orbitomedial PFC | 8 | 42 | −5 | 57 | L. ventrolateral PFC | −46 | 31 | −13 | 4.510 | 8.304 | |
| 23 | L. dorsomedial PFC | −10 | 39 | 52 | 58 | R. ventrolateral PFC | 49 | 35 | −12 | 4.012 | 9.806 | |
| 30 | R. dorsomedial PFC | 6 | 54 | 16 | 58 | R. ventrolateral PFC | 49 | 35 | −12 | 3.639 | 8.671 | |
| mTBI + PTS > PTS | ||||||||||||
| 6 | L. middle temporal gyrus | −46 | −61 | 21 | 28 | L. dorsolateral PFC | −10 | 55 | 39 | 3.913 | 7.667 | |
| 9 | R. middle temporal pole | 46 | 16 | −30 | 28 | L. dorsolateral PFC | −10 | 55 | 39 | 3.596 | 6.028 | |
| 14 | R. precuneus | 6 | −59 | 35 | 42 | L. middle temporal gyrus | −56 | −13 | −10 | 4.129 | 10.637 | |
Abbreviations: mTBI, mild traumatic brain injury; PFC, prefrontal cortex; PTS, posttraumatic stress symptoms; ROIs: regions of interest.
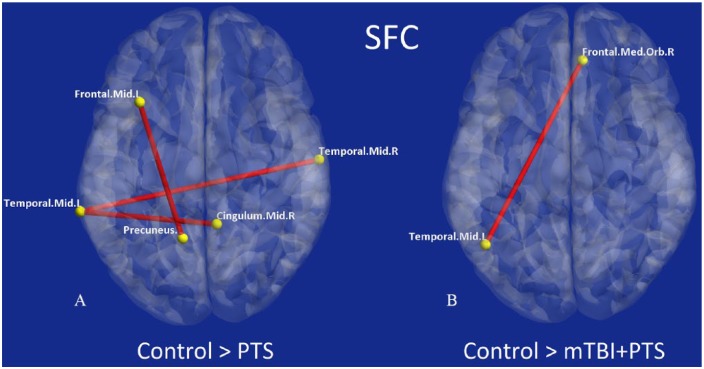
Figure 2.
Connections exhibiting significantly different SFC for pairwise comparisons between groups: connections significantly different for (A) control > PTS comparison and (B) control > mTBI + PTS comparison. There were no significant connections for other pairwise comparisons (PTS > control, mTBI + PTSD > control, PTSD > mTBI + PTS, or mTBI + PTS > PTS). mTBI indicates mild traumatic brain injury; PTS, posttraumatic stress symptoms; PTSD, posttraumatic stress disorder; SFC, static functional connectivity.
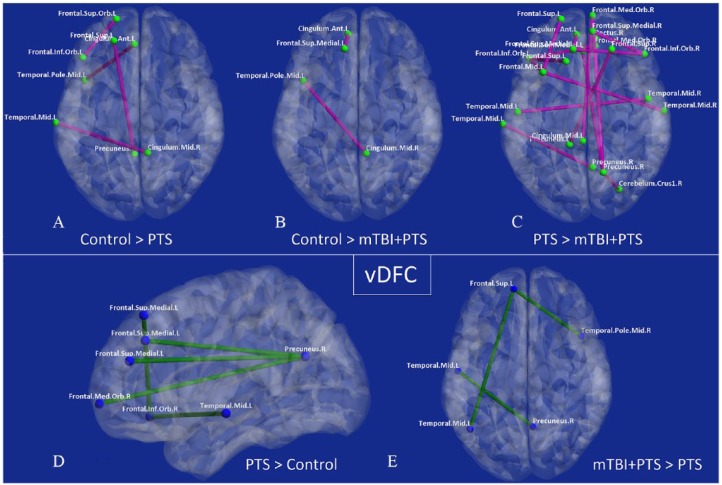
Figure 3.
Connections exhibiting significantly different vDFC for pairwise comparisons between groups: connections significantly different for (A) control > PTS comparison, (B) control > mTBI + PTS comparison, (C) PTS > mTBI + PTS comparison, (D) PTS > control, and (E) mTBI + PTS > PTS. There were no significant connections for the mTBI + PTS > control comparison. Connections that were higher in control group or less severe group (A-C) are shown in magenta with yellow nodes, while connections that were higher in disease group or more severe group (D and E) are shown in green with blue nodes. mTBI indicates mild traumatic brain injury; PTS, posttraumatic stress symptoms; PTSD, posttraumatic stress disorder; vDFC, variance of dynamic functional connectivity.
Connectivity associations
Associations were tested between 47 connectivity features (7 SFC and 40 vDFC connectivities identified in the MANOVA) and measures of neurocognitive functioning and clinical symptoms. Independent partial least squares (PLS) regression analyses21 were performed to assess the aggregate association of significant connectivities found in this study with psychological health symptoms and neurocognitive functioning. We assessed this separately for combined features within each of these 2 groups of measures—(1) clinical symptom severity scores (PCL-5, NSI, AUDIT, PSS, PSQI, ESS, ZAS, and ZDS) and (2) neurocognitive functioning (CNS-VS subtest scores).
Results
Demographics and descriptives
The MANOVA for specific demographic variables and descriptives was significant, F(7,14) = 6.2, P < .001, (Wilk Lambda), indicating there were significant group differences. Although the groups were matched in age and education, P > .05 (and racial composition, τb = .004, P = .970), there were significant group differences in scores on the Life Events Checklist, with both the PTS (M = 48.1; SD = 9.7) and mTBI + PTS (M = 42.8; SD = 9.6) groups having a greater number of lifetime experiences to traumatic events than the control group (M = 26.1; SD = 11.4), P < .05. The difference between the PTS and mTBI + PTS was nonsignificant, P = .441. Although there were significant group differences in lifetime traumatic experiences, childhood familial experiences was not significantly different, P > .05, between the groups as assessed using the Childhood Environment Scale.40 In addition, there were significant group differences in scores on the Combat Exposure Scale41 between all 3 groups in the order of mTBI + PTS (M = 28.8; SD = 9.0) > PTS (M = 20.8; SD = 8.8) > control (M = 8.0; SD = 10.4), P < .05. There was a significant difference between the groups in the number of reported lifetime mTBIs, specifically between the mTBI + PTS group (M = 1.6; SD = 0.8) and both the control group (M = 0.4; SD = 0.7), P < .001, and PTS group (M = 0.8; SD = 1.0), P = .031.
There were significant group differences in reported use of prescribed antidepressants, τb = 2.8, P = .004, and benzodiazepines, τb = 2.5, P = .013, medication, with the comorbid group having the highest frequency of use (antidepressant: Control = 5% vs PTS = 14% vs mTBI + PTS = 36%; benzodiazepines: Control = 0% vs PTS = 0% vs mTBI + PTS = 25%).
Clinical symptoms and neurocognitive function
The results of the MANOVA for clinical symptom measures were significant, F(7,14) = 6.7, P < .001, (Pillai Trace), indicating there were significant group differences on a number of different clinical scales (Table 1). All P values remained significant after corrections for multiple comparisons, with the mTBI + PTS group having the highest scores out of the 3 groups on these respective measures.
Table 1.
Clinical symptoms and neurocognitive measures for the 3 groups.
| Clinical symptoms | Control | PTS | mTBI + PTS | ||||
|---|---|---|---|---|---|---|---|
| Posttraumatic symptoms | M | 3.85 | 32.50 | 52.52 | |||
| SD | 4.57 | 10.68 | 15.48 | ||||
| d | 3.49* | 1.51* | 4.26a | ||||
| Post-concussive symptoms | M | 7.15 | 25.00 | 43.68 | |||
| SD | 4.93 | 16.17 | 16.74 | ||||
| d | 1.49* | 1.14* | 2.96a | ||||
| Depression | M | 32.20 | 40.83 | 52.44 | |||
| SD | 7.30 | 11.90 | 10.15 | ||||
| d | 0.87 | 1.45* | 2.29a | ||||
| Anxiety | M | 29.80 | 38.50 | 51.08 | |||
| SD | 5.56 | 12.77 | 9.95 | ||||
| d | 0.88* | 1.10* | 2.64a | ||||
| Sleepiness | M | 8.25 | 8.00 | 13.32 | |||
| SD | 3.19 | 3.59 | 5.41 | ||||
| d | 0.01 | 1.16* | 1.14a | ||||
| Perceived stress | M | 15.40 | 25.08 | 32.16 | |||
| SD | 6.95 | 8.61 | 9.06 | ||||
| d | 1.24* | 0.80 | 2.08a | ||||
| Sleep quality | M | 23.65 | 22.50 | 46.60 | |||
| SD | 17.63 | 15.15 | 25.29 | ||||
| d | 0.07 | 1.16* | 1.05a | ||||
| Neurocognitive | |||||||
| Reaction time | M | 98.60 | 96.43 | 86.68 | |||
| SD | 20.23 | 12.86 | 29.62 | ||||
| d | 0.13 | 0.43 | 0.47 | ||||
| Complex attention | M | 94.25 | 87.00 | 73.24 | |||
| SD | 19.76 | 18.95 | 21.81 | ||||
| d | 0.37 | 0.67 | 1.01a | ||||
| Cognitive flexibility | M | 102.55 | 100.64 | 80.72 | |||
| SD | 17.45 | 13.89 | 22.55 | ||||
| d | 0.12 | 1.06* | 1.08a | ||||
| Processing speed | M | 104.35 | 102.07 | 92.04 | |||
| SD | 23.07 | 12.53 | 15.95 | ||||
| d | 0.12 | 0.67 | 0.62 | ||||
| Executive functioning | M | 105.55 | 103.00 | 83.92 | |||
| SD | 14.22 | 12.23 | 21.18 | ||||
| d | 0.19 | 1.10* | 1.20a | ||||
| Verbal memory | M | 95.55 | 100.29 | 87.04 | |||
| SD | 22.96 | 19.86 | 22.78 | ||||
| d | 0.22 | 0.62 | 0.37 | ||||
Abbreviations: d, Cohen d effect size; M, mean; mTBI, mild traumatic brain injury; PTS, posttraumatic stress symptoms; SD, standard deviation.
P < .05 pairwise comparisons (Bonferroni corrected) of control vs mTBI + PTS group.
P < .05 pairwise comparisons (Bonferroni corrected).
The results of the MANOVA for neurocognitive scores, approached significance, F(6,12) = 1.8, P = .055, (Pillai Trace). Pairwise comparisons revealed the control group had significantly better scores than the mTBI + PTS group on CA, P = .004, CF, P = .001, and EF, P < .001, but not on RT, PS, and VM, P > .05. The mTBI + PTS group also had significantly lower scores in CF, P = .009, and EF, P = .005, compared with the PTS group. The findings suggest that both the PTS and mTBI + PTS groups have mild decrements in cognition compared with controls, but also the comorbid group has greater decrements than the PTS group (Table 1).
Strength and variance of ROI connectivity
The results of the MANOVAs for strength of connectivity (SFC; Table 2) and variability of connectivity (vDFC; Table 3) revealed significant differences across the 3 groups. Both PTS and mTBI + PTS groups were markedly different compared with controls (Tables 2 and 3).
Table 2.
MANOVAs (control vs PTS vs mTBI + PTS): static functional connectivity.
| Connection | F |
|---|---|
| L. precuneus ↔ R. precuneus | 12.85 |
| L. precuneus ↔ L. middle frontal gyrus | 15.57 |
| L. middle temporal gyrus ↔ R. medial frontal gyrus | 12.19 |
| L. precuneus ↔ L. middle temporal gyrus | 13.02 |
| R. middle cingulate ↔ L. middle frontal gyrus | 13.95 |
| L. middle cingulate ↔ L. middle frontal gyrus | 12.37 |
| R. middle frontal gyrus ↔ L. middle frontal gyrus | 12.72 |
Abbreviations: MANOVAs, multivariate analyses of variance; mTBI, mild traumatic brain injury; PTS, posttraumatic stress symptoms.
Table 3.
MANOVAs (control vs PTS vs mTBI + PTS): variance of dynamic functional connectivity.
| Connection | F |
|---|---|
| L. dorsomedial PFC ↔ L. anterior cingulate | 15.95 |
| L. middle frontal gyrus ↔ R. precuneus | 14.38 |
| L. middle temporal pole ↔ R. middle cingulate | 13.27 |
| L. dorsomedial PFC ↔ R. medial orbitofrontal | 11.72 |
| R. precuneus ↔ L. dorsomedial PFC | 11.55 |
| L. precuneus ↔ L. dorsomedial PFC | 11.53 |
| L. precuneus ↔ R. dorsomedial PFC | 10.96 |
| L. middle frontal gyrus ↔ R. ventrolateral PFC | 10.93 |
| L. dorsomedial PFC ↔ L. dorsomedial PFC | 10.84 |
| L. anterior cingulate ↔ R. angular gyrus | 10.71 |
| R. medial orbitofrontal ↔ R. precuneus | 10.66 |
| R. precuneus ↔ L. middle frontal gyrus | 10.64 |
| L. ventromedial PFC ↔ L. ventrolateral PFC | 9.84 |
| L. dorsomedial PFC ↔ R. ventrolateral PFC | 9.81 |
| L. middle temporal pole ↔ L. anterior cingulate | 9.61 |
| R. medial orbitofrontal ↔ R. cerebellum crus 1 | 9.59 |
| R. rectus gyrus ↔ L. anterior cingulate | 9.28 |
| L. dorsomedial PFC ↔ R. cerebellum crus 1 | 9.20 |
| L. middle frontal gyrus ↔ R. middle frontal gyrus | 8.96 |
| L. middle frontal gyrus ↔ R. middle cingulate | 8.94 |
| R. middle frontal gyrus ↔ L. ventrolateral PFC | 8.85 |
| R. dorsomedial PFC ↔ R. ventrolateral PFC | 8.67 |
| L. middle occipital ↔ R. middle frontal gyrus | 8.47 |
| R. dorsomedial PFC ↔ R. ventrolateral PFC | 8.46 |
| L. dorsomedial PFC ↔ L. dorsomedial PFC | 8.40 |
| L. dorsomedial PFC ↔ L. dorsomedial PFC | 8.39 |
| R. medial orbitofrontal ↔ L. ventrolateral PFC | 8.30 |
| L. middle frontal gyrus ↔ L. dorsomedial PFC | 8.22 |
| R. precuneus ↔ R. cerebellum crus 1 | 8.00 |
| R. precuneus ↔ L. dorsomedial PFC | 7.99 |
| R. rectus gyrus ↔ R. precuneus | 7.98 |
| L. precuneus ↔ L. anterior cingulate | 7.93 |
| L. precuneus ↔ L. dorsomedial PFC | 7.90 |
| R. angular gyrus ↔ L. dorsomedial PFC | 7.84 |
| R. angular gyrus ↔ R. dorsomedial PFC | 7.77 |
| L. middle frontal gyrus ↔ L. dorsomedial PFC | 7.67 |
| L. dorsomedial PFC ↔ L. ventrolateral PFC | 7.62 |
| R. dorsomedial PFC ↔ L. medial orbitofrontal | 7.41 |
| R. precuneus ↔ R. dorsomedial PFC | 7.31 |
| L. anterior cingulate ↔ R. ventrolateral PFC | 7.14 |
Abbreviations: MANOVAs, multivariate analyses of variance; mTBI, mild traumatic brain injury; PFC, prefrontal cortex; PTS, posttraumatic stress symptoms.
As observed in Table 4, there was reduced connectivity strength between ROIs (SFC) in the PTS group (Figure 2A), which were specific to regions that included the prefrontal ↔ posterior cingulate, cingulate ↔ temporal, and contralateral temporal ↔ temporal regions. In contrast, the mTBI + PTS group showed significantly reduced connectivity strength between only the right dorsomedial prefrontal cortex (dmPFC) and left medial temporal lobe (MTL; Figure 2B). Compared with the mTBI + PTS group, the PTS group showed a greater number of connectivity abnormalities in SFC (3/1 = 67%). Contrary to our hypothesis that the comorbid group would have reduced strength in a greater number of connections, there were no significant differences between PTS and mTBI + PTS groups in SFC, implying that the strength of connectivity is compromised in both of these disorders and somewhat different among themselves.
Variability of connectivity (vDFC) showed more differences across the groups (Table 5). Both the PTS and mTBI + PTS groups showed reduced variability of temporal ↔ cingulate and prefrontal ↔ cingulate connectivities compared with controls (Figure 3A and B). However, only the PTS group showed increased vDFC, which primarily occurred between prefrontal ↔ parietal and prefrontal ↔ temporal regions (Figure 3D).
As hypothesized, when comparing the mTBI + PTS group to the PTS group, the mTBI + PTS group showed a higher number of prefrontal ↔ parietal and prefrontal ↔ prefrontal connectivities (Figure 3C) with reduced variability (18/3 = 83%). In contrast, the PTS group had reduced variability in just 3 connectivities constituting prefrontal ↔ temporal and parietal ↔ temporal regions compared with the mTBI + PTS group in vDFC (9/2 = 78%; Figure 3E).
Overall, the findings reveal a pattern that suggests reduced strength and variance of connectivities that included prefrontal, temporal, and cingulate regions in the mTBI + PTS group, which suggests compromised neural efficiency.11,21 This might contribute to the severity of the clinical symptoms and cognitive decrements observed in this comorbid group.
These findings suggest compromised DMN connectivity in PTS and mTBI + PTS, with increased variability of connectivity in one part of the DMN and reduced variability in another. While there were no significant differences in connectivity strength between the mTBI + PTS and PTS groups, variability of connectivity was able to distinguish them, which is an interesting and important finding because most studies ignore variability of connectivity.
Connectivity predictors of clinical symptoms and neurocognitive function
In accord with our predictions that deficits in central EF and greater psychological health symptoms are also likely to be associated with dysregulated neurocircuitry, the results of the PLS regression analyses showed that the 47 connectivities, which significantly varied across the 3 groups, were significantly associated with both scores of clinical symptom severity (R = 0.51, R2 = 0.26, P = 3.9 × 10−9) and neurocognitive functioning (R = 0.51, R2 = 0.26, P = 2.4 × 10−9), explaining 26% variance in each of them. These findings suggest that a large percentage of the variance in both clinical symptoms and neurocognitive function can be attributed to compromised efficiency of the neural network underpinning the DMN in soldiers who experienced psychological and neurologic trauma.
More specifically, greater SFC and vDFC connectivity of the left dlPFC ↔ precuneus was associated with higher neurocognitive scores and lower clinical symptom scores. In other words, weaker and less variable connections between these ROIs were linked with poorer overall neuropsychological outcomes. Connectivity between these ROIs has been implicated in self-referential processing. In addition, the fact that both regions are from the left hemisphere is interesting given that the left hemisphere predominantly processes negative emotions compared with the right.
Discussion
This study revealed significant group differences between active-duty Army soldiers with PTS and mTBI with comorbid PTS compared with matched controls on a number of different symptom, neurocognitive, and neuroimaging measures. Overall, lower performance on neurocognitive tests and clinical symptoms were on the order of severity from mTBI + PTS > PTS > Controls. There were differences between the PTS and mTBI + PTS groups in terms of reduced connectivity strength compared with controls. While the PTS group showed reduced strength in frontal-cingulate-temporal connectivities, the mTBI + PTS had reduced strength in only one connection (omPFC ↔ middle temporal gyrus [MTG]) constituting part of the frontal-temporal network. Both groups showed decreased variability of frontal-cingulate-temporal regions of the attention network. Interestingly, the PTS group, which was less severe than the comorbid group in terms of clinical symptoms, showed a greater number of abnormal connectivities compared with the mTBI + PTS group (9/2 = 78%). Another key finding not observed in the more severe mTBI + PTS group was the PTS group’s increased variability of the frontal-parietal attention network, which has been implicated in achieving and maintaining an alert state. Although the bulk of the abnormal connectivities accounted for a significant percentage of the variance in clinical and neurocognitive measures for the groups when combined, both strength (SFC) and variability (vDFC) of connectivity of the left dlPFC ↔ precuneus were associated with higher neurocognitive scores and lower clinical symptoms scores.
The dlPFC has been implicated in a number of neuropsychological processes such as regulating attention and executive functions such as planning of future actions, taking initiative, and working memory and attention.42,43 Connections between the left dlPFC and precuneus have been implicated in cognitive processes such as attentional shifting during mental sets or rules.44 Cognitive impairments are commonly associated with PTSD,4 including compromised performance on emotion-based tasks.3,7 Evidence suggests that cognitive impairments in PTSD may be due to accentuated affective processes and attenuated dlPFC-related processes and compromised regulatory network.7,45 Although our PTS group did not have significantly lower neurocognitive scores than the control group, the mTBI + PTS group showed significant decrements in CA, CF, and EF. Our findings that the PTS and mTBI + PTS groups had weaker and less stable connections may have contributed to these individuals having difficulty in regulating attention to intrinsic thoughts, feelings, sensations, and/or images while in a resting state. The dlPFC has been implicated in language processes.46 Increased variability in connectivity between the dlPFC and cingulate cortex for both the PTS and mTBI + PTS groups compared with controls might suggest the propensity for perseverative self-talk.
The precuneus, the medial part of the superior parietal lobe appears to have a prominent role in the DMN.17,47 The region has been implicated in mental imagery concerning the self48 and episodic memory.49 Furthermore, decreased connectivity of the precuneus has been observed in war veterans with PTSD.50 Our findings suggest that abnormal connectivity between the precuneus and other regions (especially the dmPFC) might reflect disrupted functional connections within the neural network associated with forming mental representations.46 Although hypothetical, this might be explained by the PTS group being more engaged in mental imagery compared with controls while in a resting state.
It is easy to assume that the total number of abnormal connectivities would be a direct proxy of clinical severity. However, this is not supported by our findings in that the mTBI + PTS was more severe than the PTS alone group as assessed via clinical symptoms and cognitive decrements, but had fewer abnormal connectivities overall when compared with controls. As such, it is likely that the combination of ROIs that constitute the disrupted connectivities has a greater impact on functioning than the mere number of disrupted connectivities.
Connectivity and neurobehavioral measures
Only one connection, which included connectivity strength (SFC) and variability (vDFC) between the dlPFC and precuneus, exhibited significant associations with the different neurobehavioral measures. Interestingly, stronger and more variable connectivity was associated with higher neurocognitive scores and lower clinical symptom scores. As such, flexibility, or lack thereof, of this frontal ↔ posterior neurocircuitry seems to be implicated in both cognitive and affective processes that have a direct impact on behavior and symptoms. Greater strength and flexibility contribute to more efficiency in neurocognitive functioning, whereas weaker and reduced flexibility or rigidity results in compromised functioning.11,21 These top-down processes are necessary for efficiency of regulatory processes (eg, emotional regulation), and therefore, when compromised may negatively impact one’s ability to recover and/or cope with stressors (ie, intrinsic and extrinsic). In essence, the integrity of top-down functions implemented in regulation of emotion could contribute to the development and maintenance of trauma-related symptoms, whether this is of psychological or neurological etiology.
Limitations
There were a few limitations that should be highlighted. First, reproducibility of our findings is necessary to show reliability. Without reliability, it cannot be guaranteed that our sample of subjects is representative of the clinical population in the Army, other branches of the Armed Forces, or general population to include civilians. The lack of robust abnormal connectivities in the mTBI + PTS group may reflect a generalized dampening of the DMN, associated with either medication use or injury pathophysiology. Indeed, many of our subjects were using prescribed medications for treatment of their conditions. Future studies should attempt to recruit subjects who are not being treated with psychotropic medication, as well as explore the impact of specific medications on the DMN. Furthermore, we did not have a pure mTBI group (ie, absent of PTSD symptoms) or true PTSD group to assess whether the DMN findings were due to the combination of mTBI and PTS or mTBI alone. Prior studies have shown that PTSD is a partial mediator of mTBI symptomatology.51 However, we did not run mediation analyses or control for PTSD symptoms as a covariate. Future studies should attempt to compare PTSD versus mTBI alone to better understand the impact of mTBI on DMN neurocircuitry. Finally, unbeknownst to us, there is evidence that hearing loss is associated with altered network connectivity.52 Although military service members who were exposed to blast-related injuries are at risk for hearing loss,53 we did not attain these data for this study. As such, it is important that future studies on mTBI in military populations acquire the necessary data to address this confound.
Conclusions
Overall, we interpret the findings to suggest that PTS, with and without mTBI, is associated with less stable and disrupted neural signature during resting state. However, the number of disrupted connectivities does not appear to reflect clinical severity in that the PTS group, although having lower symptom scores, had a greater number of abnormal connectivities than the mTBI + PTS group. Furthermore, weaker and less variable connectivity of the dlPFC and precuneus was associated with worse neuropsychological outcomes. In other words, weaker and less variable connections between these ROIs were linked with poorer overall neuropsychological outcomes. Being that the dlPFC is associated with attention and executive functions and the precuneus with self-related mental representations during rest,52 these regions potentially have a functional role in that underlies unprovoked generation of thoughts, feelings, sensations, and/or images in service members with PTS and mTBI + PTS. While there were no significant differences in DMN connectivity strength between the mTBI + PTS and PTS groups, variability of connectivity was able to distinguish them, which is an interesting and important finding because most studies ignore variability of connectivity.
Supplemental Material
Supplemental material, APPENDIX for Strength and Temporal Variance of the Default Mode Network to Investigate Chronic Mild Traumatic Brain Injury in Service Members with Psychological Trauma by Michael N Dretsch, D Rangaprakash, Jeffrey S Katz, Thomas A Daniel, Adam M Goodman, Thomas S Denney and Gopikrishna Deshpande in Journal of Experimental Neuroscience
Acknowledgments
We would like to thank the staff and leadership at the USAARL and Auburn University for their support that made this study possible.
Footnotes
Funding:The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This project was funded by the U.S. Army Medical Research and Materiel Command (USAMRMC) and Military Operational Medicine Research Program and was supported in part by an appointment to the Internship/Research Participation Program for the USAMRMC, administered by the Oak Ridge Institute for Science and Education (ORISE) through an agreement between the U.S. Department of Energy and the USAMRMC. Dr Dretsch was the principal investigator (PI) of the study who secured funding, developed the protocol, analyzed the psychological health data, and was the primary author of the manuscript.
Declaration of conflicting interests:The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Author Contributions: MND was the overall PI who orchestrated the development of the protocol and oversight of the study, performed statistical analyses, and was the lead author for this article. JSK, GD, and TSD(Co-PI) assisted with the development of the protocol, oversight of data collection, and provided feedback/editing of the manuscript. DR was instrumental in the analysis of MRI data. TAD and AMG assisted with data collection and analysis.
Disclaimer: Material has been reviewed by the Walter Reed Army Institute of Research. There is no objection to its presentation and/or publication. The opinions or assertions contained herein are the private views of the author, and are not to be construed as official, or as reflecting true views of the Department of the Army or the Department of Defense. The investigators have adhered to the policies for protection of human subjects as prescribed in AR 70 -25.
Supplemental material: Supplemental material for this article is available online.
ORCID iD: Michael N Dretsch  https://orcid.org/0000-0001-8773-6376
https://orcid.org/0000-0001-8773-6376
References
- 1. Thorp SR, Stein MB. Posttraumatic stress disorder and functioning. PTSD Res Q. 2005;16:1–7. [Google Scholar]
- 2. Valdez CE, Lilly MM. Posttraumatic rumination: content, correlates, and processes. J Clin Psychol. 2017;73:707–721. [DOI] [PubMed] [Google Scholar]
- 3. Dretsch MN, Thiel KJ, Athy JR, Irvin CR, Sirmon-Fjordbak B, Salvatore A. Mood symptoms contribute to working memory decrement in active-duty soldiers being treated for posttraumatic stress disorder. Brain Behav. 2012;2:357–364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Dretsch MN, Thiel KJ, Athy JR, Born S, Prue-Owens K. Posttraumatic stress disorder in the U.S. Warfighter: sensitivity to punishment and antidepressant use contribute to decision-making performance. Traumatology. 2013;19:118–125. [Google Scholar]
- 5. Dretsch MN, Wood KH, Daniel TA, et al. Exploring the neurocircuitry underpinning predictability of threat in soldiers with PTSD compared to deployment exposed controls. Open Neuroimaging J. 2016;10:111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Dretsch M, Bleiberg J, Williams K, et al. Three scoring approaches to the neurobehavioral symptom inventory for measuring clinical change in service members receiving intensive treatment for combat-related mTBI. J Head Trauma Rehabil. 2016;31:23–29. [DOI] [PubMed] [Google Scholar]
- 7. Dretsch MN, Daniel TA, Goodman AM, et al. Differential neural activation when voluntarily regulating emotions in service members with chronic mild traumatic brain injury [published online ahead of print September 19, 2017]. Appl Neuropsychol Adult. doi: 10.1080/23279095.2017.1362406. [DOI] [PubMed] [Google Scholar]
- 8. Holdeman TC. Invisible wounds of war: psychological and cognitive injuries, their consequences, and services to assist recovery. Psychiatr Serv. 2009;60:273. [Google Scholar]
- 9. King NS. Perseveration of traumatic re-experiencing in PTSD: a cautionary note regarding exposure based psychological treatments for PTSD when head injury and dysexecutive impairment are also present. Brain Inj. 2002;16:65–74. [DOI] [PubMed] [Google Scholar]
- 10. Rangaprakash D, Dretsch MN, Yan W, Katz JS, Denney TS, Jr, Deshpande G. Hemodynamic variability in soldiers with trauma: implications for functional MRI connectivity studies. Neuroimage Clin. 2017;16:409–417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Rangaprakash D, Dretsch MN, Venkataraman A, Katz JS, Denney TS, Jr, Deshpande G. Identifying disease foci from static and dynamic effective connectivity networks: illustration in soldiers with trauma. Hum Brain Mapp. 2018;39:264–287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Raichle ME. The brain’s default mode network. Annu Rev Neurosci. 2015;38:433–447. [DOI] [PubMed] [Google Scholar]
- 13. Power JD, Cohen AL, Nelson SM, et al. Functional network organization of the human brain. Neuron. 2011;72:665–678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Andrews-Hanna JR. The brain’s default network and its adaptive role in internal mentation. Neuroscientist. 2012;18:251–270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Koch SB, van Zuiden M, Nawijn L, Frijling JL, Veltman DJ, Olff M. Aberrant resting-state brain activity in posttraumatic stress disorder: a meta-analysis and systematic review. Depress Anxiety. 2016;33:592–605. [DOI] [PubMed] [Google Scholar]
- 16. Miller DR, Hayes SM, Hayes JP, Spielberg JM, Lafleche G, Verfaellie M. Default mode network subsystems are differentially disrupted in posttraumatic stress disorder. Biol Psychiatry Cogn Neurosci Neuroimaging. 2017;2:363–371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Nathan DE, Bellgowan JAF, French LM, et al. Assessing the impact of post-traumatic stress symptoms on the resting-state default mode network in a military chronic mild traumatic brain injury sample. Brain Connect. 2017;7:236–249. [DOI] [PubMed] [Google Scholar]
- 18. Sripada RK, King AP, Welsh RC, et al. Neural dysregulation in posttraumatic stress disorder: evidence for disrupted equilibrium between salience and default mode brain networks. Psychosom Med. 2012;74:904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Garrett DD, Samanez-Larkin GR, MacDonald SWS, Lindenberger U, McIntosh AR, Grady CL. Moment-to-moment brain signal variability: a next frontier in human brain mapping? Neurosci Biobehav Rev. 2013;37:610–624. doi: 10.1016/j.neubiorev.2013.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Jia H, Hu X, Deshpande G. Behavioral relevance of the dynamics of the functional brain connectome. Brain Connect. 2014;4:741–759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Rangaprakash D, Deshpande G, Daniel TA, et al. Compromised hippocampus-striatum pathway as a potential imaging biomarker of mild-traumatic brain injury and posttraumatic stress disorder. Hum Brain Mapp. 2017;38:2843–2864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Rashid B, Arbabshirani MR, Damaraju E, et al. Classification of schizophrenia and bipolar patients using static and dynamic resting-state fMRI brain connectivity. Neuroimage. 2016;134:645–657. doi: 10.1016/j.neuroimage.2016.04.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Weathers FW, Litz BT, Keane TM, Palmieri PA, Marx BP, Schnurr PP. The PTSD checklist for DSM-5 (PCL-5). www.ptsd.va.gov. Up-dated 2013.
- 24. Tombaugh TN. The Test of Memory Malingering (TOMM) in forensic psychology. J Forensic Neuropsychol. 2003;2:69–96. [Google Scholar]
- 25. Cicerone KD, Kalmar K. Persistent postconcussion syndrome: the structure of subjective complaints after mild traumatic brain injury. J Head Trauma Rehabil. 1995;10:1–17. [Google Scholar]
- 26. Zung WW. A rating instrument for anxiety disorders. Psychosomatics. 1971;12:371–379. [DOI] [PubMed] [Google Scholar]
- 27. Zung WW. Depression in the normal adult population. Psychosomatics. 1971;12:164–167. [DOI] [PubMed] [Google Scholar]
- 28. Johns MW. A new method for measuring daytime sleepiness: the Epworth sleepiness scale. Sleep. 1991;14:540–545. [DOI] [PubMed] [Google Scholar]
- 29. Cohen S, Kamarck T, Mermelstein R. Perceived stress scale. Meas Stress Guide Health Soc Sci. 1994:235–283. [PubMed] [Google Scholar]
- 30. Saunders JB, Aasland OG, Babor TF, De la Fuente JR, Grant M. Development of the alcohol use disorders identification test (AUDIT): WHO collaborative project on early detection of persons with harmful alcohol consumption-II. Addiction. 1993;88:791–804. [DOI] [PubMed] [Google Scholar]
- 31. Buysse DJ, Reynolds IIICF, Monk TH, Berman SR, Kupfer DJ. The Pittsburgh Sleep Quality Index: a new instrument for psychiatric practice and research. Psychiatry Res. 1989;28:193–213. [DOI] [PubMed] [Google Scholar]
- 32. Gualtieri CT, Johnson LG. Reliability and validity of a computerized neurocognitive test battery, CNS Vital Signs. Arch Clin Neuropsychol. 2006;21:623–643. [DOI] [PubMed] [Google Scholar]
- 33. Chao-Gan Y. Data Processing Assistant for Resting-State fMRI (DPARSF). http://rfmri.org/DPARSF. Up-dated 2014.
- 34. Penny WD, Friston KJ, Ashburner JT, Kiebel SJ, Nichols TE. Statistical Parametric Mapping: The Analysis of Functional Brain Images. New York, NY: Elsevier; 2011. [Google Scholar]
- 35. Song XW, Long X, Zang Y. RESTing-State fMRI Data Analysis Toolkit (REST) Manual. Beijing, China: Beijing Normal University; 2008. [Google Scholar]
- 36. Wu G-R, Liao W, Stramaglia S, Ding J-R, Chen H, Marinazzo D. A blind deconvolution approach to recover effective connectivity brain networks from resting state fMRI data. Med Image Anal. 2013;17:365–374. [DOI] [PubMed] [Google Scholar]
- 37. Boly M, Sasai S, Gosseries O, et al. Stimulus set meaningfulness and neurophysiological differentiation: a functional magnetic resonance imaging study. PLoS ONE. 2015;10:e0125337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Liang P, Deshpande G, Zhao S, Liu J, Hu X, Li K. Altered directional connectivity between emotion network and motor network in Parkinson’s disease with depression. Medicine (Baltimore). 2016;95:e4222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Lamichhane B, Adhikari BM, Brosnan SF, Dhamala M. The neural basis of perceived unfairness in economic exchanges. Brain Connect. 2014;4:619–630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. King DW, King LA, Vogt DS. Manual for the Deployment Risk and Resilience Inventory (DRRI): A Collection of Measures for Studying Deployment-Related Experiences of Military Veterans. Boston, MA: National Center for PTSD; 2003. [Google Scholar]
- 41. Keane TM, Fairbank JA, Caddell JM, Zimering RT, Taylor KL, Mora CA. Clinical evaluation of a measure to assess combat exposure. Psychol Assess J Consult Clin Psychol. 1989;1:53. [Google Scholar]
- 42. Rushworth MFS, Walton ME, Kennerley SW, Bannerman DM. Action sets and decisions in the medial frontal cortex. Trends Cogn Sci. 2004;8:410–417. [DOI] [PubMed] [Google Scholar]
- 43. Li W, Qin W, Liu H, et al. Subregions of the human superior frontal gyrus and their connections. Neuroimage. 2013;78:46–58. [DOI] [PubMed] [Google Scholar]
- 44. Nagahama Y, Okada T, Katsumi Y, et al. Transient neural activity in the medial superior frontal gyrus and precuneus time locked with attention shift between object features. Neuroimage. 1999;10:193–199. [DOI] [PubMed] [Google Scholar]
- 45. Allard CB. Dorsolateral prefrontal cortex activation during emotional anticipation and neuropsychological performance in posttraumatic stress disorder. Arch Gen Psychiatry. 2012;69:360. doi: 10.1001/archgenpsychiatry.2011.1539. [DOI] [PubMed] [Google Scholar]
- 46. Willems RM, Özyürek A, Hagoort P. Differential roles for left inferior frontal and superior temporal cortex in multimodal integration of action and language. Neuroimage. 2009;47:1992–2004. [DOI] [PubMed] [Google Scholar]
- 47. Utevsky AV, Smith DV, Huettel SA. Precuneus is a functional core of the default-mode network. J Neurosci. 2014;34:932–940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Kjaer TW, Nowak M, Lou HC. Reflective self-awareness and conscious states: PET evidence for a common midline parietofrontal core. Neuroimage. 2002;17:1080–1086. doi: 10.1006/nimg.2002.1230. [DOI] [PubMed] [Google Scholar]
- 49. Lundstrom BN, Ingvar M, Petersson KM. The role of precuneus and left inferior frontal cortex during source memory episodic retrieval. Neuroimage. 2005;27:824–834. [DOI] [PubMed] [Google Scholar]
- 50. Misaki M, Phillips R, Zotev V, et al. Connectome-wide investigation of altered resting-state functional connectivity in war veterans with and without posttraumatic stress disorder. Neuroimage Clin. 2018;17:285–296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Dretsch M, Silverberg N, Iverson G. Multiple past concussions are associated with ongoing post-concussive symptoms but not cognitive impairment in active-duty army soldiers. J Neurotrauma. 2015;32:1301–1653. [DOI] [PubMed] [Google Scholar]
- 52. Zhang Y, Mao Z, Feng S, et al. Altered functional networks in long-term unilateral hearing loss: a connectome analysis. Brain Behav. 2018;8:e00912. doi: 10.1002/brb3.912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Joseph AR, Shaw JL, Clouser MC, et al. Impact of blast injury on hearing in a screened male military population. Am J Epidemiol. 2018;187:7–15. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, APPENDIX for Strength and Temporal Variance of the Default Mode Network to Investigate Chronic Mild Traumatic Brain Injury in Service Members with Psychological Trauma by Michael N Dretsch, D Rangaprakash, Jeffrey S Katz, Thomas A Daniel, Adam M Goodman, Thomas S Denney and Gopikrishna Deshpande in Journal of Experimental Neuroscience