Abstract
Irritable bowel syndrome (IBS) is the most prevalent functional gastrointestinal disorder, affecting approximately 14% of the global population. Symptoms of IBS are some of the most common reasons that primary care providers refer patients to gastroenterologists. IBS has a significant economic impact on the health care system and greatly reduces patients’ quality of life. The precise cause of IBS remains unknown, but likely involves a variety of factors, such as infection, inflammation, medication, and stress, in a genetically predisposed individual. Physicians can diagnose patients with IBS by obtaining a careful history and physical examination, performing limited testing, and applying the Rome IV criteria. Treating IBS symptoms can be challenging, as no medication cures the disorder. Thus, treatment focuses on improving symptoms and quality of life. Many patients report that symptoms develop from, or are exacerbated by, food. A number of physiologic and biochemical processes can occur with food ingestion that may produce heightened symptoms of IBS. Therefore, dietary interventions to improve IBS symptoms appear to be a reasonable treatment approach. This article discusses the evidence supporting dietary interventions for the treatment of IBS.
Keywords: Irritable bowel syndrome, low-FODMAP diet, elimination diet, gluten-free diet, dietary management
Irritable bowel syndrome (IBS) is the most commonly encountered functional gastrointestinal disorder, with a worldwide prevalence of approximately 14%.1 IBS is a chronic disorder for many patients and is associated with markedly elevated health care costs and a reduction in patients’ quality of life.2,3 The disorder can be diagnosed using the Rome IV criteria in combination with a careful history, physical examination, and limited diagnostic tests.2-4
Although the exact pathophysiology of IBS remains unknown and differs in extent and magnitude from patient to patient, alterations in the gut microbiome,5,6 disturbances in gastrointestinal motility, changes in the enteric nervous system, coexisting psychological distress, and visceral hypersensitivity all likely play a role.2-4 These different pathophysiologic processes lead to variations in symptom expression, making IBS a heterogeneous disorder. Targeted pharmacotherapy for IBS has been largely unfruitful due to a lack of clarity regarding local gastrointestinal nervous system and central modulation mechanisms involved in visceral hyperalgesia, as well as the multiple neurotransmitters involved in this hypersensitive state.7-9 Not surprisingly, treating IBS symptoms can very often be challenging, and no validated treatment algorithm exists.
A variety of pharmacologic therapies are available to treat IBS symptoms; however, many patients prefer to avoid medications and desire alternative approaches.10 Dietary modifications to treat IBS symptoms have received significant attention lately, in part due to the recognition that many IBS patients report that foods appear to induce or exacerbate their symptoms.11,12 Some patients believe they are able to identify the specific offending items; however, several studies show that when patients are rechallenged with the foods they perceive as triggers, they do not report the same symptoms.1,2,13,14 Although certain foods have been traditionally recognized as triggers for diarrhea, abdominal pain, gas, and bloating, no formal research existed to prove or disprove their cause-effect relationship or their therapeutic benefits until the 1940s, when reports of malabsorption of different carbohydrates, as well as their relationship with gastrointestinal symptoms, were first published.15,16
The research methodology of studies for placebo-controlled dietary interventions requires a more sophisticated design than a drug or nutrient trial, during which a similar capsule or tablet without the active ingredient can be delivered to the control group. A properly controlled diet study can be performed by developing sham diets that are comparable in feasibility and complexity both for teaching and/or instructing (to minimize investigator bias) and for following (to minimize patient bias) when compared to the studied diet.17 Placebo and nocebo effects cannot be underplayed in these trials, as the clinical effects from a diet change can be influenced by several factors, including patients’ expectations, previous responses to particular diets, taste preferences, and personal and cultural beliefs regarding the impact of food in health.17 Therefore, a rigorous design is needed for the development of the control group in dietary advice trials.18
A number of different diets are now promoted to treat IBS symptoms, and these include regimens that exclude carbohydrates, fermentable foods, gluten, and substances that might create food-related antibodies.13,19 Despite significant interest in this area from patients and providers, carefully controlled prospective studies evaluating the safety and efficacy of these diets remain limited. This article reviews the different diets available to treat IBS symptoms using the most recent data from the literature, focusing primarily on diets low in fermentable oligosaccharides, disaccharides, monosaccharides, and polyols (FODMAPs) and excluding gluten, as these 2 diets have been the most carefully studied and are commonly employed by patients.
The Low–Fermentable Oligosaccharide, Disaccharide, Monosaccharide, and Polyol Diet
The low-FODMAP diet was developed at Monash University in Australia by Dr Peter Gibson and Dr Susan Shepherd, and is now commonly used to treat IBS symptoms, based on both biologic plausibility and evidence from prospective trials showing improvement in symptoms in approximately 75% of patients.3,13,15 The low-FODMAP diet has progressively gained ground in mainstream media over the last 12 years, with growing notations in websites, blogs, tweets, and vlogs. Food companies are now even incorporating the term into their labels. As of September 2018, a search for the term FODMAP identified over 11,000 videos on the YouTube platform and 180,000 posts on Instagram. Most Twitter posts mentioning the hashtag #low-FODMAP originate from Australia, the United States, the United Kingdom, and Canada.
Fermentable Oligosaccharides, Disaccharides, Monosaccharides, and Polyols and Irritable Bowel Syndrome Symptoms
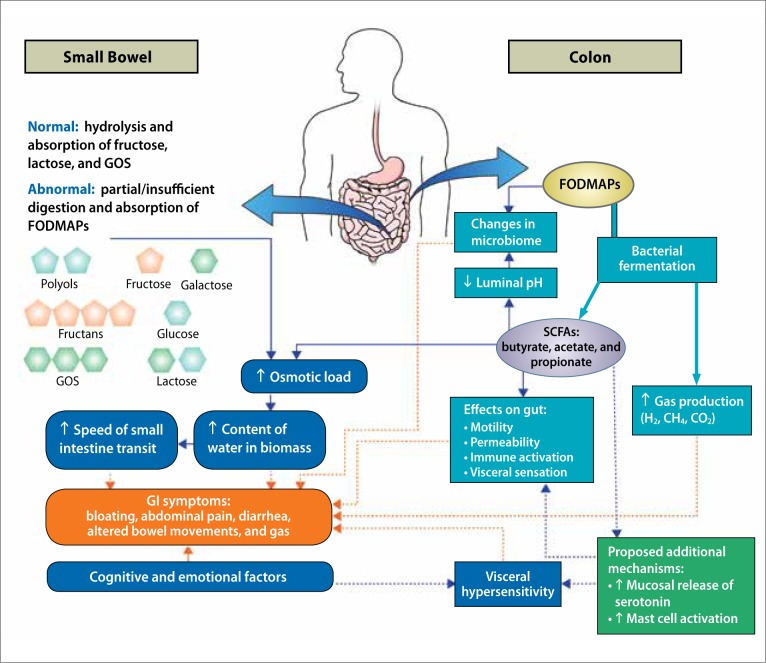
FODMAPs are short-chain carbohydrates that are characterized by limited (or minimal) small intestine absorption, intense bacterial fermentation to short-chain fatty acids (SCFAs), and high osmotic activity.5,20 Ong and colleagues demonstrated that dietary FODMAPs induced hydrogen and methane production in the intestines of patients with IBS.21 The changes in pH levels, and the probable changes in gut flora, 4-6,22 may alter colonic epithelial function and may also cause local inflammation, thereby contributing to changes in colonic function.5,20 When superimposed on baseline symptoms of abdominal pain, these changes can significantly heighten gastrointestinal symptoms in patients with IBS, especially those of excessive gas, bloating, and loose stools.23,24
Other research has proposed alternative mechanisms by which the ingestion of FODMAPs could cause symptoms; however, there are scant data to support the hypotheses. For example, elevated SCFAs could stimulate mucosal release of 5-hydroxytryptamine (serotonin) and the production of histamine, causing a localized neuro-inflammatory response involving mast cell activation. These factors could contribute to a detrimental change in intestinal secretion, sensitivity, and motility, causing or worsening IBS symptoms.25
The potential role of a diet high in FODMAPs in the development of IBS symptoms can be seen in the Figure.26,27
Phases of the Low–Fermentable Oligosaccharide, Disaccharide, Monosaccharide, and Polyol Diet
The low-FODMAP diet intervention for IBS patients consists of 3 distinct phases: the restriction or elimination phase, the reintroduction or rechallenge phase, and the maintenance or personalized phase.28,29 During the initial phase, patients eliminate FODMAPs from their diets. Importantly, the low-FODMAP diet is meant to last only 4 to 6 weeks, and it is essentially a method to determine whether symptoms are related to specific foods. It is not designed for long-term use. During the second phase, after noting symptom improvement or resolution, foods containing FODMAPs are reintroduced gradually, with the goal of identifying tolerance to individual ingredients and specific symptom triggers among fermentable carbohydrates. This phase lasts several weeks, if not longer, as foods are slowly reintroduced. After reviewing and interpreting results from the food rechallenge phase, the goals of the third phase are to continue the intake of foods that were well-tolerated and to restrict foods that produced symptoms (ie, trigger foods). As the tolerance to different FODMAPs can change over time, patients can attempt to reintroduce their trigger foods a few months after symptom control if they so desire.30
Figure.
Proposed mechanisms of FODMAP ingestion and symptoms in IBS.
FODMAP, fermentable oligosaccharide, disaccharide, monosaccharide, and polyol; GI, gastrointestinal; GOS, galactooligosaccharides; IBS, irritable bowel syndrome; SCFA, short-chain fatty acid.
Study Results
The results from all studies thus far, including observational case-control studies and randomized, controlled trials, generally support the use of a low-FODMAP diet for patients with IBS, as 50% to 80% of patients report some benefits compared to using a regular or habitual diet (Table 1).3,13,26,31,32 Two studies were conducted comparing the low-FODMAP diet to commonly recommended IBS diets (National Institute for Health and Care Excellence [NICE] or modified NICE guidelines).33,34 NICE, modified NICE, and low-FODMAP diets were reported to be effective33,34; however, one study showed significantly better results in the low-FODMAP diet group, particularly with regard to pain and bloating.34 A randomized study compared the low-FODMAP diet to a moderate-FODMAP Australian diet, finding better outcomes for IBS patients who followed the low-FODMAP diet.35
Table 1.
Studies Comparing the Low-FODMAP Diet to Other Dietary Interventions for IBS Patients
| Study | Comparison | Summary and/or Comments |
|---|---|---|
| Staudacher et al53 | Low-FODMAP diet vs NICE guidelines diet |
|
| Halmos et al35 | Low-FODMAP diet vs modified-FODMAP diet | The low-FODMAP diet improved overall symptom scores as well as flatulence, abdominal pain, and bloating. |
| Bohn et al33 | Low-FODMAP diet vs traditional IBS diet | Similar results for both diets; no significant statistical difference was found |
| Eswaran et al34 | Low-FODMAP diet vs modified NICE guidelines diet |
|
| Hustoft et al76 | Low-FODMAP diet vs high-FODMAP diet | Three weeks of a low-FODMAP diet improved IBS symptoms, decreased serum levels of proinflammatory IL-6 and IL-8, and decreased levels of fecal bacteria and total SCFAs. |
| McIntosh et al77 | Low-FODMAP diet vs high-FODMAP diet |
|
| Staudacher et al78 |
|
|
| Zahedi et al79 | Low-FODMAP diet vs standard dietary advice for diarrhea-predominant IBS | Although both groups had improvement in symptoms, the low-FODMAP diet group had better results for symptom scores, abdominal pain, bloating, stool frequency, and consistency. |
FODMAP, fermentable oligosaccharide, disaccharide, monosaccharide, and polyol; IBS, irritable bowel syndrome; IL, interleukin; NICE, National Institute for Health and Care Excellence; SCFA, short-chain fatty acid.
The low-FODMAP diet has also been compared to nondietary interventions, including hypnotherapy and yoga. Gut-directed hypnotherapy has been shown to be comparably effective to a low-FODMAP diet approach; however, the combination of both interventions did not add any significant therapeutic benefits.36,37 Hatha yoga also appears to be beneficial to IBS patients and had a positive impact on patients’ symptoms, with similar results to a low-FODMAP diet.38,39
Advantages of a Low–Fermentable Oligosaccharide, Disaccharide, Monosaccharide, and Polyol Diet
The main advantage of the low-FODMAP diet is in the positive results of several studies performed throughout the world, as a medication or intervention rarely elicits positive symptom control in over half of treated patients. The interest and benefits appear to extend beyond Western societies and English-speaking countries, with recent publications analyzing local diets and exploring the applicability of a low-FODMAP diet in South, East, and Southeast Asia.40,41 A well-conducted study from Colombia of 50 adult IBS patients showed improvement in both symptoms and quality of life.42 The results observed thus far in different trials show a consistent pattern and rate of response in patients with IBS treated with a low-FODMAP diet. However, a comprehensive meta-analysis by Dionne and colleagues analyzed 7 different low-FODMAP studies in IBS patients and concluded that the overall quality of the data was very low (using Grading of Recommendations Assessment, Development, and Evaluation criteria).32
Data on the long-term use of a low-FODMAP diet are limited. In a retrospective study by Maagaard and colleagues, some clinical benefit was observed in 57% to 74% of patients at 14 to 16 months follow-up.43
Challenges of a Low–Fermentable Oligosaccharide, Disaccharide, Monosaccharide, and Polyol Diet
Well-known disadvantages of the low-FODMAP diet are its complexity (difficult to teach, difficult to follow, and labor-intensive), expense, and potential nutritional deficiencies.26,44 The first dietary management counseling appointment is estimated to last approximately 1 hour,44 which is extremely difficult for a busy primary care provider to perform in the current medical climate. This time commitment leads to some reluctance from physicians in recommending this diet to patients. Incomplete education may then lead to partial or complete noncompliance in clinical practice. In addition, patients need to devote time to planning and shopping for a low-FODMAP diet, which can also reduce compliance.
Any elimination diet generates concern about potential nutritional deficiencies, inappropriate calorie intake resulting in weight loss, and deleterious consequences in body composition.25 Farida and colleagues studied the micronutrient intake during the first phase of the low-FODMAP diet, and found a higher daily intake of vita-min B6, but a significantly lower daily intake of calcium, retinol, riboflavin, thiamin, and transfatty acids.45
A pilot study analyzed 26 patients with IBS before and after an 8 week–long low-FODMAP diet with regard to nutritional status and body composition, which were evaluated using bioelectrical impedance vector analysis, anthropometric data, and laboratorial serum studies.46 Although statistically significant changes were observed in serum albumin and lipids after the introduction of a low-FODMAP diet, the differences were very small and the laboratory results remained within normal range. Overall, this study did not show detrimental effects on body composition or nutritional status in a small group of IBS patients treated with a low-FODMAP diet.46
Teaching a Low–Fermentable Oligosaccharide, Disaccharide, Monosaccharide, and Polyol Diet to Patients
In all of the studies published to date, including both prospective and retrospective studies, registered dietitians provided diet education to patients.47,48 Unfortunately, not all patients have access to dietitians, and most insurance companies will not pay for a nutrition consult to discuss the implementation of a low-FODMAP diet. Therefore, alternative methods of teaching IBS patients how to follow a low-FODMAP diet are required. Nurse-led dietary counseling has been attempted in 2 different studies, with neither producing conclusive or promising results.49,50 Another study focused on a dietitian-led, group education intervention, comparing it to one-onone education, with promising results: the group-led program was clinically effective and reasonable with regard to costs.51 Kinrade and colleagues also found that 82% of patients had symptom improvement after receiving low-FODMAP education via group sessions.52 However, the challenge with group-led discussions for the low-FODMAP diet remains the lack of insurance coverage.
Given the paucity of proven similarly efficacious teaching methods, current guidelines still recommend that low-FODMAP dietary guidance can only be given by a health care professional with expertise in dietary management.53,54 No studies have compared dietitian-led interventions to other methods of dietary management, nor have any studies evaluated an educational program delivered by a dietitian in a one-on-one session vs a group session.
The Gluten-Free Diet
The elimination of gluten from the diet of IBS patients has demonstrated efficacy beyond patients with celiac disease.13,55-57 A number of studies published within the last 6 years have investigated the role of gluten in patients with IBS. Biesiekierski and colleagues55 enrolled 34 patients meeting Rome III criteria for IBS into a double-blind, placebo-controlled, rechallenge dietary study. Patients had noted previous improvement in IBS symptoms with a gluten-free diet, and were randomized to receive a high-gluten diet (16 g/day) or a gluten-free diet during a 6-week period. For the high-gluten group, carbohydrate-depleted wheat gluten was added to the same gluten-free base mix used for the gluten-free group. The majority of patients exposed to gluten (68%) reported uncontrolled symptoms compared with patients exposed to placebo (40%; P<.001). No differences between rates of celiac serology, fecal lactoferrin, or C-reactive protein levels, or measures of intestinal permeability were found between the groups.
Vazquez-Roque and colleagues57 performed a 4-week, prospective, randomized, controlled trial evaluating 45 patients with diarrhea-predominant IBS who did not have celiac disease, and found a reduction in patient-reported stool frequency (P=.04) with a gluten-free diet, with the most pronounced effect in those patients who were HLA-DQ2– or HLA-DQ8–positive (P=.019). Biesiekierski and colleagues followed up their 2011 study55 with a double-blind, crossover study of 37 IBS patients of all subtypes without celiac disease who had previously reported improvement with a gluten-free diet for at least 6 weeks before study enrollment.56 Patients were prescribed a low-FODMAP diet, and, following a 2-week run-in, were randomized to a high-gluten diet (16 g/day), a low-gluten diet (2 g/day), or placebo (no gluten). After 1 week, patients were randomized to the second arm, and then the third arm. The 2-week low-FODMAP run-in delivered an improvement in gastrointestinal symptoms (P<.001), whereas during the 1-week diet study period, symptoms worsened in all patients (P<.001) irrespective of their diet. These findings highlight the likely role that factors other than gluten play in patients using a gluten-free diet for IBS symptoms.
Elli and colleagues58 and Zanwar and colleagues59 conducted double-blind, placebo-controlled, gluten rechallenge trials in patients with IBS and negative celiac testing for 3 and 4 weeks, respectively. The first study58 implemented a 7-day crossover using gluten capsules, with 18 of 53 patients (34%) developing worse symptoms with gluten exposure. However, a substantial number of patients (14/48; 29.2%) also noted symptoms in the placebo challenge. The second study59 showed an increase in gastrointestinal symptoms with a wheat bread challenge compared to gluten-free bread (55.7% vs 33.3%; P<.05). It has been suggested that the presence of additional components in both the gluten capsules and the wheat bread could be responsible for at least part of the effect.60
Challenges of a Gluten-Free Diet
The main limitations of the current literature on gluten-free diets for IBS lie in small study sample sizes and concern for contamination of the vehicle of gluten exposure. To this point, a large meta-analysis reviewing 1726 studies evaluating the efficacy of a gluten-free diet on the management of IBS recently found insufficient evidence to recommend this diet for IBS symptoms, as findings were not statistically significant.32
Isolation of gluten from the diet without also removing other potential symptom-driving substances is both difficult to study and nebulous for IBS patients. It is possible that many IBS patients improve on a gluten-free diet, as it also reduces fructan intake, a significant component of modern wheat products.61 Skodje and colleagues performed a double-blind, placebo-controlled, crossover challenge to discover the effect of gluten (without fructan) and the effect of fructan (without gluten) in patients with self-reported gluten sensitivity.61 Their results weaken the role of gluten and strengthen the symptom-inducing effect of fructans in patients with self-reported sensitivity to rye, wheat, and barley.61
Additional Diets for the Management of Irritable Bowel Syndrome
Given that 70% to 89% of patients with IBS report exacerbation of symptoms with specific foods,12,26,62 it is not surprising that patients would attempt to reduce or eliminate symptom-producing foods from their diets. One of the limitations of exclusion diets is that, thus far, clinicians have been unable to identify (and, therefore, unable to develop validated diagnostic testing for) the specific mechanisms by which individual foods cause gastrointestinal symptoms. If such tests existed, a more efficient approach would be possible: clinicians would be able to immediately recommend the elimination of individual foods rather than going through the process of elimination/restriction followed by reintroduction/personalization. There are insufficient data to recommend panel allergy testing with immunoglobulin (Ig) G for patients who meet the criteria for IBS, although this testing is a common request from patients in clinical practice. Furthermore, panel blood tests can cost up to $1000.63 Based on current guidelines, food-specific serum IgG4 indicates only repeated exposure to food components, and does not represent allergy, intolerance, or hypersensitivity.64,65
Prior to the growing popularity of the low-FODMAP diet, a traditional IBS diet had been routinely recommended in clinical practice in the United Kingdom, and dietary guidelines were published and updated by NICE.28 Contrary to the low-FODMAP diet, the traditional IBS diet focuses on the number of meals, and when, how, and how much to eat, rather than the content of the diet itself.33 General recommendations for the traditional IBS diet are summarized in Table 2. Although the NICE or modified NICE recommendations have not been compared to placebo, there are 3 trials comparing them to the low-FODMAP diet, all showing similar positive results for the studied diets.33,34,53
Table 2.
| Encouraged Habits and Behaviors | Limited Intake | Other Considerations |
|---|---|---|
| Eating regular meals and limiting the volume/amount of food per meal | The intake of fat, spicy foods, and sorbitol (an artificial sweetener found in diabetic/ slimming/sugar-free drinks and sweets) should be minimized in patients with diarrhea-predominant IBS or mixed IBS. | If increasing the fiber intake is advised, the option should be for soluble instead of insoluble fiber, as insoluble fiber is discouraged for patients with IBS. |
| Eating slowly and chewing thoroughly | Daily fruit intake should be limited to 3 portions. | Although adding probiotics is safe in patients with IBS, they do not seem to provide benefit regarding symptom control and, therefore, are not recommended. |
| No long gaps between meals or skipping meals | The intake of coffee, tea, alcohol, and carbonated beverages should be reduced. |
IBS, irritable bowel syndrome.
A study by Lenhart and colleagues found that the majority of gastroenterologists practicing in the United States recommend dietary changes to more than 75% of their IBS patients, but very few (21%) refer them to a dietitian.66 The diets most commonly recommended by gastroenterologists for IBS patients are low-FODMAP, lactose-free, high-fiber, gluten-free, and low-fat diets.
Besides the previously discussed low-FODMAP and gluten-free diets, over half of IBS patients decide to self-manage and follow different dietary interventions to improve their symptoms prior to seeking advice from a gastrointestinal physician.66 The Paleolithic diet, very low–carbohydrate diet (or ketogenic diet), and IgG-based avoidance diet are commonly recommended in clinical practice and on blogs and different social media channels. However, there is a lack of substantial evidence for the majority of these specialized diets, as summarized in Table 3.
Table 3.
Evidence Behind Other Diets Used to Treat IBS13
| Type of Diet | Description of Diet | Supporting Evidence |
|---|---|---|
| Lactose-free diet | No lactose-containing products, except if products are treated with lactase (ie, lactose-free cow’s milk, lactose-free yogurt) | Only helpful for patients with lactose intolerance; not efficacious for patients with IBS without lactose intolerance81,82 |
| Low-fructose/fructan diet | Avoid foods high in fructose and fructans. | |
| Paleolithic diet | Only foods available during the Paleolithic era: seafood, lean meat, fruits, vegetables, nuts, and seeds; no processed food, dairy, added salt, added sugar, grains, legumes, or alcohol83 | No studies available |
| Specific carbohydrate diet | Reduce ingestion of disaccharides and polysaccharides. | Trend toward improvement in symptoms, but not statistically significant; inferior results compared to the low-FODMAP diet84 |
| IgG-based avoidance diet | Exclude foods to which patients have increased serum IgG antibodies. |
|
| Very low–carbohydrate diet or ketogenic diet |
|
|
| Fiber supplementation | Psyllium supplement |
|
| Low-fat diet | Less than 27 g of fat per day, considering a diet of 2000 kcal/day89 | |
| Low-fiber diet | Less than 10-15 g of fiber/day | No evidence available; common practice is to recommend decreasing fiber for patients with diarrhea-predominant IBS to increase transit time |
| Low-histamine diet |
|
FODMAP, fermentable oligosaccharide, disaccharide, monosaccharide, and polyol; IBS, irritable bowel syndrome; Ig, immunoglobulin; RCT, randomized, controlled trial.
Patients as Consumers
The notion from Hippocrates of food as medicine seems to be regaining popularity, especially among millennials, who are interested in a holistic approach both for disease prevention and for treatment, and prefer nonpharmaco-logic interventions, if available.31 Driven by consumers’ desire for foods that optimize health and/or prevent chronic illnesses, the market for functional foods has been one of the fastest-growing existing food sectors over the last decade.31,67 The general trend of consumer commitment to gluten-free diets has sparked a dramatic growth in this market, increasing 136% from 2013 to 2015, and, thus, creating an $11.6 billion annual industry as of 2015.68
As previously mentioned, the complexity of the low-FODMAP diet and its different phases is a limiting factor for its use and compliance. In an effort to assist patients, Dr Gibson’s group at Monash University created a certification program to assist consumers to easily identify low-FODMAP foods.30 The online application is available for smartphones, and is designed to help patients choose the dishes and ingredients that are appropriate for the different phases of their diet.30
Considerations Regarding Dietary Interventions in Irritable Bowel Syndrome Patients
The concern about orthorexia, or orthorexia nervosa, is another important factor when recommending an elimination or restrictive diet. The term orthorexia was first introduced in the literature in 1998 to describe an obsession with healthy eating,69,70 and, although not formally recognized as a disease by the most recent Diagnostic and Statistical Manual of Mental Disorders, the number of publications and social media references to the term is on the rise.71 Signs and symptoms described in the literature include compulsive checking of nutritional labels; avoidance of a high number of food groups; inability to eat anything not deemed healthy, clean, or pure; high levels of stress in relation to eating; and unhealthy time investment in planning or worrying about future meals.72,73
There are no studies currently linking IBS and orthorexia, nor orthorexia and a low-FODMAP diet or a gluten-free diet. However, orthorexia may innocently start as a simple desire to improve one’s eating habits or health,74 such as with a recommended IBS diet, and then slowly evolve into toxic and anxiety-generating behaviors.
Another eating disorder that could be of concern when treating IBS patients with dietary modifications is avoidant/restrictive food intake disorder (ARFID).75 This is characterized by an avoidant and/or restrictive eating behavior that negatively impacts the intake of macro- or micronutrients, potentially causing calorie and/or protein malnutrition. ARFID can be distinguished from anorexia nervosa by the lack of worry about one’s weight.75 For this reason, when following IBS patients undergoing dietary changes, clinicians should be vigilant to the development of extreme and unnecessary dietary restrictions and avoidant behaviors that are not objectively beneficial to the patients and could cause nutritional deficiencies in the long term.
Summary
The role of diets for the treatment of IBS symptoms is complex and remains poorly defined. Investigations into the relationship between diet and IBS symptoms have been limited by small sample sizes, placebo effects, and the lack of specificity of symptoms. More complicated diets are difficult for patients, and patient recall of diet is often poor. Cheap, effective, point-of-care testing for food intolerances are lacking, and cross-contamination of specific dietary IBS triggers is likely prevalent. Initial management of IBS with dietary adjustment involves either single-food elimination for common culprits such as lactose and fructose, or potentially a larger elimination diet (eg, low-FODMAP) with targeted reintroduction after 4 weeks, under the guidance of a registered dietitian. Expensive and unproven commercial food-specific allergy testing should be avoided. Future larger studies likely requiring multicenter designs are needed to further define the efficacy of specific dietary options for IBS patients.
References
- 1.Lovell RM, Ford AC. Global prevalence of and risk factors for irritable bowel syndrome: a meta-analysis. Clin Gastroenterol Hepatol. 2012;10(7):712–721.e4. doi: 10.1016/j.cgh.2012.02.029. [DOI] [PubMed] [Google Scholar]
- 2.Mearin F, Lacy BE, Chang L Bowel disorders [published online February 18, 2016]. Gastroenterologydoi:10.1053/j.gastro.2016.02.031
- 3.Ford AC, Lacy BE, Talley NJ. Irritable bowel syndrome. N Engl J Med. 2017;376(26):2566–2578. doi: 10.1056/NEJMra1607547. [DOI] [PubMed] [Google Scholar]
- 4.Ford AC, Moayyedi P, Chey WD et al. ACG Task Force on Management of Irritable Bowel Syndrome. American College of Gastroenterology monograph on management of irritable bowel syndrome. Am J Gastroenterol. 2018;113(2):1–18. doi: 10.1038/s41395-018-0084-x. Suppl. [DOI] [PubMed] [Google Scholar]
- 5.Halmos EP, Christophersen CT, Bird AR, Shepherd SJ, Gibson PR, Muir JG. Diets that differ in their FODMAP content alter the colonic luminal microenvironment. Gut. 2015;64(1):93–100. doi: 10.1136/gutjnl-2014-307264. [DOI] [PubMed] [Google Scholar]
- 6.Halmos EP, Christophersen CT, Bird AR, Shepherd SJ, Muir JG, Gibson PR. Consistent prebiotic effect on gut microbiota with altered FODMAP intake in patients with Crohn’s disease: a randomised, controlled cross-over trial of well-defined diets. Clin Transl Gastroen. 2016;7:e164. doi: 10.1038/ctg.2016.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Buéno L, Fioramonti J, Garcia-Villar R. Pathobiology of visceral pain: molecular mechanisms and therapeutic implications. III. Visceral afferent pathways: a source of new therapeutic targets for abdominal pain. Am J Physiol Gastrointest Liver Physiol. 2000;278(5):G670–G676. doi: 10.1152/ajpgi.2000.278.5.G670. [DOI] [PubMed] [Google Scholar]
- 8.Mertz H, Morgan V, Tanner G et al. Regional cerebral activation in irritable bowel syndrome and control subjects with painful and nonpainful rectal distention. Gastroenterology. 2000;118(5):842–848. doi: 10.1016/s0016-5085(00)70170-3. [DOI] [PubMed] [Google Scholar]
- 9.Silverman DH, Munakata JA, Ennes H, Mandelkern MA, Hoh CK, Mayer EA. Regional cerebral activity in normal and pathological perception of visceral pain. Gastroenterology. 1997;112(1):64–72. doi: 10.1016/s0016-5085(97)70220-8. [DOI] [PubMed] [Google Scholar]
- 10.Lahner E, Bellentani S, Bastiani RD et al. A survey of pharmacological and nonpharmacological treatment of functional gastrointestinal disorders. United European Gastroenterol J. 2013;1(5):385–393. doi: 10.1177/2050640613499567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lacy BE, Weiser K, Noddin L et al. Irritable bowel syndrome: patients’ attitudes, concerns and level of knowledge. Aliment Pharmacol Ther. 2007;25(11):1329–1341. doi: 10.1111/j.1365-2036.2007.03328.x. [DOI] [PubMed] [Google Scholar]
- 12.Böhn L, Störsrud S, Törnblom H, Bengtsson U, Simrén M. Self-reported food-related gastrointestinal symptoms in IBS are common and associated with more severe symptoms and reduced quality of life. Am J Gastroenterol. 2013;108(5):634–641. doi: 10.1038/ajg.2013.105. [DOI] [PubMed] [Google Scholar]
- 13.Lacy BE. The science, evidence, and practice of dietary interventions in irritable bowel syndrome. Clin Gastroenterol Hepatol. 2015;13(11):1899–1906. doi: 10.1016/j.cgh.2015.02.043. [DOI] [PubMed] [Google Scholar]
- 14.Nanda R, James R, Smith H, Dudley CR, Jewell DP. Food intolerance and the irritable bowel syndrome. Gut. 1989;30(8):1099–1104. doi: 10.1136/gut.30.8.1099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gibson PR. History of the low FODMAP diet. J Gastroenterol Hepatol. 2017;32(1):5–7. doi: 10.1111/jgh.13685. Suppl. [DOI] [PubMed] [Google Scholar]
- 16.Shepherd SJ, Parker FC, Muir JG, Gibson PR. Dietary triggers of abdominal symptoms in patients with irritable bowel syndrome: randomized placebo-controlled evidence. Clin Gastroenterol Hepatol. 2008;6(7):765–771. doi: 10.1016/j.cgh.2008.02.058. [DOI] [PubMed] [Google Scholar]
- 17.Staudacher HM, Irving PM, Lomer MCE, Whelans K. The challenges of control groups, placebos and blinding in clinical trials of dietary interventions. Proc Nutr Soc. 2017;76(4):628. doi: 10.1017/S0029665117002816. [DOI] [PubMed] [Google Scholar]
- 18.Yao CK, Gibson PR, Shepherd SJ. Design of clinical trials evaluating dietary interventions in patients with functional gastrointestinal disorders. Am J Gastroenterol. 2013;108(5):748–758. doi: 10.1038/ajg.2013.77. [DOI] [PubMed] [Google Scholar]
- 19.Moayyedi P, Quigley EM, Lacy BE et al. The effect of dietary intervention on irritable bowel syndrome: a systematic review. Clin Transl Gastroenterol. 2015;6:e107. doi: 10.1038/ctg.2015.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Thomas A, Quigley EM. Diet and irritable bowel syndrome. Curr Opin Gastroenterol. 2015;31(2):166–171. doi: 10.1097/MOG.0000000000000158. [DOI] [PubMed] [Google Scholar]
- 21.Ong DK, Mitchell SB, Barrett JS et al. Manipulation of dietary short chain carbohydrates alters the pattern of gas production and genesis of symptoms in irritable bowel syndrome. J Gastroenterol Hepatol. 2010;25(8):1366–1373. doi: 10.1111/j.1440-1746.2010.06370.x. [DOI] [PubMed] [Google Scholar]
- 22.Valeur J, Røseth AG, Knudsen T et al. Fecal fermentation in irritable bowel syndrome: influence of dietary restriction of fermentable oligosaccharides, disaccharides, monosaccharides and polyols. Digestion. 2016;94(1):50–56. doi: 10.1159/000448280. [DOI] [PubMed] [Google Scholar]
- 23.Hookway C, Buckner S, Crosland P, Longson D. Irritable bowel syndrome in adults in primary care: summary of updated NICE guidance. BMJ. 2015;350:h701. doi: 10.1136/bmj.h701. [DOI] [PubMed] [Google Scholar]
- 24.Murray K, Wilkinson-Smith V, Hoad C et al. Differential effects of FODMAPs (fermentable oligo-, di-, mono-saccharides and polyols) on small and large intestinal contents in healthy subjects shown by MRI. Am J Gastroenterol. 2014;109(1):110–119. doi: 10.1038/ajg.2013.386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bellini M, Rossi A. Is a low FODMAP diet dangerous? Tech Coloproctol. 2018;22(8):569–571. doi: 10.1007/s10151-018-1835-9. [DOI] [PubMed] [Google Scholar]
- 26.Staudacher HM, Whelan K. The low FODMAP diet: recent advances in understanding its mechanisms and efficacy in IBS. Gut. 2017;66(8):1517–1527. doi: 10.1136/gutjnl-2017-313750. [DOI] [PubMed] [Google Scholar]
- 27.Spencer M, Chey WD, Eswaran S. Dietary renaissance in IBS: has food replaced medications as a primary treatment strategy? Curr Treat Options Gastroenterol. 2014;12(4):424–440. doi: 10.1007/s11938-014-0031-x. [DOI] [PubMed] [Google Scholar]
- 28.McKenzie YA, Alder A, Anderson W et al. Gastroenterology Specialist Group of the British Dietetic Association. British Dietetic Association evidence-based guidelines for the dietary management of irritable bowel syndrome in adults. J Hum Nutr Diet. 2012;25(3):260–274. doi: 10.1111/j.1365-277X.2012.01242.x. [DOI] [PubMed] [Google Scholar]
- 29.Chey WD. Diet and irritable bowel syndrome. Gastroenterol Hepatol (N Y). 2018;14(5):309–312. [PMC free article] [PubMed] [Google Scholar]
- 30.Dwyer E. Monash University. The 3 steps of the FODMAP diet. https://www.monashfodmap.com/blog/3-phases-low-fodmap-diet/ Published January 15, 2018. Accessed December 17, 2018.
- 31.Zannini E, Arendt EK. Low FODMAPs and gluten-free foods for irritable bowel syndrome treatment: lights and shadows. Food Res Int. 2018;110:33–41. doi: 10.1016/j.foodres.2017.04.001. [DOI] [PubMed] [Google Scholar]
- 32.Dionne J, Ford AC, Yuan Y et al. A systematic review and meta-analysis evaluating the efficacy of a gluten-free diet and a low FODMAPs diet in treating symptoms of irritable bowel syndrome. Am J Gastroenterol. 2018;113(9):1290–1300. doi: 10.1038/s41395-018-0195-4. [DOI] [PubMed] [Google Scholar]
- 33.Bohn L, Störsrud S, Liljebo T et al. Diet low in FODMAPs reduces symptoms of irritable bowel syndrome as well as traditional dietary advice: a randomized controlled trial. Gastroenterology. 2015;149(6):1399–1407.e2. doi: 10.1053/j.gastro.2015.07.054. [DOI] [PubMed] [Google Scholar]
- 34.Eswaran SL, Chey WD, Han-Markey T, Ball S, Jackson K. A randomized controlled trial comparing the low FODMAP diet vs. modified NICE guidelines in US adults with IBS-D. Am J Gastroenterol. 2016;111(12):1824–1832. doi: 10.1038/ajg.2016.434. [DOI] [PubMed] [Google Scholar]
- 35.Halmos EP, Power VA, Shepherd SJ, Gibson PR, Muir JG. A diet low in FODMAPs reduces symptoms of irritable bowel syndrome. Gastroenterology. 2014;146(1):67–75.e5. doi: 10.1053/j.gastro.2013.09.046. [DOI] [PubMed] [Google Scholar]
- 36.Peters SL, Yao CK, Philpott H, Yelland GW, Muir JG, Gibson PR. Randomised clinical trial: the efficacy of gut-directed hypnotherapy is similar to that of the low FODMAP diet for the treatment of irritable bowel syndrome. Aliment Pharmacol Ther. 2016;44(5):447–459. doi: 10.1111/apt.13706. [DOI] [PubMed] [Google Scholar]
- 37.Peters SL, Muir JG, Gibson PR. Review article: gut-directed hypnotherapy in the management of irritable bowel syndrome and inflammatory bowel disease. Aliment Pharmacol Ther. 2015;41(11):1104–1115. doi: 10.1111/apt.13202. [DOI] [PubMed] [Google Scholar]
- 38.Schumann D, Anheyer D, Lauche R, Dobos G, Langhorst J, Cramer H. Effect of yoga in the therapy of irritable bowel syndrome: a systematic review. Clin Gastroenterol Hepatol. 2016;14(12):1720–1731. doi: 10.1016/j.cgh.2016.04.026. [DOI] [PubMed] [Google Scholar]
- 39.Schumann D, Langhorst J, Dobos G, Cramer H. Randomised clinical trial: yoga vs a low-FODMAP diet in patients with irritable bowel syndrome. Aliment Pharmacol Ther. 2018;47(2):203–211. doi: 10.1111/apt.14400. [DOI] [PubMed] [Google Scholar]
- 40.Hewawasam SP, Iacovou M, Muir JG, Gibson PR. Dietary practices and FODMAPs in South Asia: applicability of the low FODMAP diet to patients with irritable bowel syndrome. J Gastroenterol Hepatol. 2018;33(2):365–374. doi: 10.1111/jgh.13885. [DOI] [PubMed] [Google Scholar]
- 41.Iacovou M, Tan V, Muir JG, Gibson PR. The low FODMAP diet and its application in East and Southeast Asia. J Neurogastroenterol Motil. 2015;21(4):459–470. doi: 10.5056/jnm15111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yepes IJ, Múnera MN, Martelo C. Diet low in fermentable oligosaccharides, disaccharides, monosaccharides and polyols, and quality of life in patients with irritable bowel syndrome in Colombia [in Spanish] Biomedica. 2018;38(0):61–68. doi: 10.7705/biomedica.v38i0.3443. [DOI] [PubMed] [Google Scholar]
- 43.Maagaard L, Ankersen DV, Végh Z et al. Follow-up of patients with functional bowel symptoms treated with a low FODMAP diet. World J Gastroenterol. 2016;22(15):4009–4019. doi: 10.3748/wjg.v22.i15.4009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hill P, Muir JG, Gibson PR. Controversies and recent developments of the low-FODMAP diet. Gastroenterol Hepatol (N Y). 2017;13(1):36–45. [PMC free article] [PubMed] [Google Scholar]
- 45.Farida JP, Shah ED, Ball S, Chey WD, Eswaran S. Micronutrient intake changes with the low FODMAP and mNICE diets. Presented at: World Congress of Gastroenterology at ACG2017; October 13-18, 2017; Orlando, FL. Abstract P2011
- 46.Bellini M, Gambaccini D, Bazzichi L et al. Bioelectrical impedance vector analysis in patients with irritable bowel syndrome on a low FODMAP diet: a pilot study. Tech Coloproctol. 2017;21(6):451–459. doi: 10.1007/s10151-017-1639-3. [DOI] [PubMed] [Google Scholar]
- 47.de Roest RH, Dobbs BR, Chapman BA et al. The low FODMAP diet improves gastrointestinal symptoms in patients with irritable bowel syndrome: a prospective study. Int J Clin Pract. 2013;67(9):895–903. doi: 10.1111/ijcp.12128. [DOI] [PubMed] [Google Scholar]
- 48.O’Keeffe M, Lomer MCE. Who should deliver the low FODMAP diet and what educational methods are optimal: a review. J Gastroenterol Hepatol. 2017;32(01):23–26. doi: 10.1111/jgh.13690. suppl. [DOI] [PubMed] [Google Scholar]
- 49.Mazzawi T, Hausken T, Gundersen D, El-Salhy M. Effects of dietary guidance on the symptoms, quality of life and habitual dietary intake of patients with irritable bowel syndrome. Mol Med Rep. 2013;8(3):845–852. doi: 10.3892/mmr.2013.1565. [DOI] [PubMed] [Google Scholar]
- 50.Ostgaard H, Hausken T, Gundersen D, El-Salhy M. Diet and effects of diet management on quality of life and symptoms in patients with irritable bowel syndrome. Mol Med Rep. 2012;5(6):1382–1390. doi: 10.3892/mmr.2012.843. [DOI] [PubMed] [Google Scholar]
- 51.Whigham L, Joyce T, Harper G et al. Clinical effectiveness and economic costs of group versus one-to-one education for short-chain fermentable carbohydrate restriction (low FODMAP diet) in the management of irritable bowel syndrome. J Hum Nutr Diet. 2015;28(6):687–696. doi: 10.1111/jhn.12318. [DOI] [PubMed] [Google Scholar]
- 52.Kinrade SK, Twamley RM, Fell L, Heald L, Healy A. An audit to assess feasibility and efficacy of group education for irritable bowel syndrome (IBS) patients in the delivery of low FODMAP (fermentable oligosaccharides, disaccharides, monosaccharides and polyols) dietary advice. Gut. 2014;63(1):A238. Suppl. [Google Scholar]
- 53.Staudacher HM, Whelan K, Irving PM, Lomer MC. Comparison of symptom response following advice for a diet low in fermentable carbohydrates (FODMAPs) versus standard dietary advice in patients with irritable bowel syndrome. J Hum Nutr Diet. 2011;24(5):487–495. doi: 10.1111/j.1365-277X.2011.01162.x. [DOI] [PubMed] [Google Scholar]
- 54.Dalrymple J, Bullock I. Diagnosis and management of irritable bowel syndrome in adults in primary care: summary of NICE guidance. BMJ. 2008;336(7643):556–558. doi: 10.1136/bmj.39484.712616.AD. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Biesiekierski JR, Newnham ED, Irving PM et al. Gluten causes gastrointestinal symptoms in subjects without celiac disease: a double-blind randomized placebo-controlled trial. Am J Gastroenterol. 2011;106(3):508–514. doi: 10.1038/ajg.2010.487. [DOI] [PubMed] [Google Scholar]
- 56.Biesiekierski JR, Peters SL, Newnham ED, Rosella O, Muir JG, Gibson PR. No effects of gluten in patients with self-reported non-celiac gluten sensitivity after dietary reduction of fermentable, poorly absorbed, short-chain carbohydrates. Gastroenterology. 2013;145(2):320–328.e1-e3. doi: 10.1053/j.gastro.2013.04.051. [DOI] [PubMed] [Google Scholar]
- 57.Vazquez-Roque MI, Camilleri M, Smyrk T et al. A controlled trial of gluten-free diet in patients with irritable bowel syndrome-diarrhea: effects on bowel frequency and intestinal function. Gastroenterology. 2013;144(5):903–911.e3. doi: 10.1053/j.gastro.2013.01.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Elli L, Tomba C, Branchi F et al. Evidence for the presence of non-celiac gluten sensitivity in patients with functional gastrointestinal symptoms: results from a multicenter randomized double-blind placebo-controlled gluten challenge. Nutrients. 2016;8(2):84. doi: 10.3390/nu8020084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Zanwar VG, Pawar SV, Gambhire PA et al. Symptomatic improvement with gluten restriction in irritable bowel syndrome: a prospective, randomized, double blinded placebo controlled trial. Intest Res. 2016;14(4):343–350. doi: 10.5217/ir.2016.14.4.343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Dimidi E, Rossi M, Whelan K. Irritable bowel syndrome and diet: where are we in 2018? Curr Opin Clin Nutr Metab Care. 2017;20(6):456–463. doi: 10.1097/MCO.0000000000000416. [DOI] [PubMed] [Google Scholar]
- 61.Skodje GI, Sarna VK, Minelle IH et al. Fructan, rather than gluten, induces symptoms in patients with self-reported non-celiac gluten sensitivity. Gastroenterology. 2018;154(3):529–539.e2. doi: 10.1053/j.gastro.2017.10.040. [DOI] [PubMed] [Google Scholar]
- 62.Monsbakken KW, Vandvik PO, Farup PG. Perceived food intolerance in subjects with irritable bowel syndrome—etiology, prevalence and consequences. Eur J Clin Nutr. 2006;60(5):667–672. doi: 10.1038/sj.ejcn.1602367. [DOI] [PubMed] [Google Scholar]
- 63.Choosing Wisely. Allergy tests: when you need them and when you don’t. http://www.choosingwisely.org/patient-resources/allergy-tests/ Published July 2012. Accessed December 17, 2018.
- 64.Stapel SO, Asero R, Ballmer-Weber BK et al. EAACI Task Force. Testing for IgG4 against foods is not recommended as a diagnostic tool: EAACI Task Force Report. Allergy. 2008;63(7):793–796. doi: 10.1111/j.1398-9995.2008.01705.x. [DOI] [PubMed] [Google Scholar]
- 65.Gocki J, Bartuzi Z. Role of immunoglobulin G antibodies in diagnosis of food allergy. Postepy Dermatol Alergol. 2016;33(4):253–256. doi: 10.5114/ada.2016.61600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Lenhart A, Ferch C, Shaw M, Chey WD. Use of dietary management for irritable bowel syndrome: results of a survey of over 1,500 members of the American College of Gastroenterology. J Neurogastroenterol Motil. 2018;24(3):437–451. doi: 10.5056/jnm17116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Kraus A. Factors influencing the decisions to buy and consume functional food. Br Food J. 2015;117(6):1622–1636. [Google Scholar]
- 68.Mintel. Half of Americans think gluten-free diets are a fad while 25% eat gluten-free foods. http://www.mintel.com/press-centre/food-and-drink/half-of-americans-think-gluten-free-diets-are-a-fad-while-25-eat-gluten-free-foods Published December 4, 2015. Accessed December 17, 2018.
- 69.Håman L, Barker-Ruchti N, Patriksson G, Lindgren EC. Orthorexia nervosa: an integrative literature review of a lifestyle syndrome. Int J Qual Stud Health Well-being. 2015;10:26799. doi: 10.3402/qhw.v10.26799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Bratman S. Health food junkie. http://www.beyondveg.com/bratman-s/hfj/hf-junkie-1a.shtml Accessed December 17, 2018.
- 71.Varga M, Dukay-Szabó S, Túry F, van Furth EF. Evidence and gaps in the literature on orthorexia nervosa. Eat Weight Disord. 2013;18(2):103–111. doi: 10.1007/s40519-013-0026-y. [DOI] [PubMed] [Google Scholar]
- 72.National Eating Disorders Association. Orthorexia. https://www.nationaleatingdisorders.org/learn/by-eating-disorder/other/orthorexia Accessed December 17, 2018.
- 73.Dunn TM, Bratman S. On orthorexia nervosa: a review of the literature and proposed diagnostic criteria. Eat Behav. 2016;21:11–17. doi: 10.1016/j.eatbeh.2015.12.006. [DOI] [PubMed] [Google Scholar]
- 74.Bratman S, Knight D. New York, NY: Random House; 2000. Health Food Junkies: Orthorexia Nervosa: Overcoming the Obsession With Healthful Eating. [Google Scholar]
- 75.Kohn JB. What Is ARFID? J Acad Nutr Diet. 2016;116(11):1872. doi: 10.1016/j.jand.2016.08.016. [DOI] [PubMed] [Google Scholar]
- 76.Hustoft TN, Hausken T, Ystad SO et al. Effects of varying dietary content of fermentable short-chain carbohydrates on symptoms, fecal microenvironment, and cytokine profiles in patients with irritable bowel syndrome. Neurogastroenterol Motil. 2017;29:4. doi: 10.1111/nmo.12969. [DOI] [PubMed] [Google Scholar]
- 77.McIntosh K, Reed DE, Schneider T et al. FODMAPs alter symptoms and the metabolome of patients with IBS: a randomised controlled trial. Gut. 2017;66(7):1241–1251. doi: 10.1136/gutjnl-2015-311339. [DOI] [PubMed] [Google Scholar]
- 78.Staudacher HM, Lomer MCE, Farquharson FM et al. A diet low in FODMAPs reduces symptoms in patients with irritable bowel syndrome and a probiotic restores Bifidobacterium species: a randomized controlled trial. Gastroenterology. 2017;153(4):936–947. doi: 10.1053/j.gastro.2017.06.010. [DOI] [PubMed] [Google Scholar]
- 79.Zahedi MJ, Behrouz V, Azimi M. Low fermentable oligo-di-mono-saccharides and polyols diet versus general dietary advice in patients with diarrhea-predominant irritable bowel syndrome: a randomized controlled trial. J Gastroenterol Hepatol. 2018;33(6):1192–1199. doi: 10.1111/jgh.14051. [DOI] [PubMed] [Google Scholar]
- 80.McKenzie YA, Thompson J, Gulia P, Lomer MC. IBS Dietetic Guideline Review Group on behalf of Gastroenterology Specialist Group of the British Dietetic Association. British Dietetic Association systematic review of systematic reviews and evidence-based practice guidelines for the use of probiotics in the management of irritable bowel syndrome in adults (2016 update) J Hum Nutr Diet. 2016;29(5):576–592. doi: 10.1111/jhn.12386. [DOI] [PubMed] [Google Scholar]
- 81.Böhmer CJ, Tuynman HA. The effect of a lactose-restricted diet in patients with a positive lactose tolerance test, earlier diagnosed as irritable bowel syndrome: a 5-year follow-up study. Eur J Gastroenterol Hepatol. 2001;13(8):941–944. doi: 10.1097/00042737-200108000-00011. [DOI] [PubMed] [Google Scholar]
- 82.Vernia P, Ricciardi MR, Frandina C, Bilotta T, Frieri G. Lactose malabsorption and irritable bowel syndrome. Effect of a long-term lactose-free diet. Ital J Gastroenterol. 1995;27(3):117–121. [PubMed] [Google Scholar]
- 83.Gibson PR, Varney J, Malakar S, Muir JG. Food components and irritable bowel syndrome. Gastroenterology. 2015;148(6):1158–1174.e4. doi: 10.1053/j.gastro.2015.02.005. [DOI] [PubMed] [Google Scholar]
- 84.Vincenzi M, Del Ciondolo I, Pasquini E, Gennai K, Paolini B. Effects of a low FODMAP diet and specific carbohydrate diet on symptoms and nutritional adequacy of patients with irritable bowel syndrome: preliminary results of a single-blinded randomized trial. J Transl Int Med. 2017;5(2):120–126. doi: 10.1515/jtim-2017-0004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Zuo XL, Li YQ, Li WJ et al. Alterations of food antigen-specific serum immunoglobulins G and E antibodies in patients with irritable bowel syndrome and functional dyspepsia. Clin Exp Allergy. 2007;37(6):823–830. doi: 10.1111/j.1365-2222.2007.02727.x. [DOI] [PubMed] [Google Scholar]
- 86.Atkinson W, Sheldon TA, Shaath N, Whorwell PJ. Food elimination based on IgG antibodies in irritable bowel syndrome: a randomised controlled trial. Gut. 2004;53(10):1459–1464. doi: 10.1136/gut.2003.037697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Austin GL, Dalton CB, Hu Y et al. A very low-carbohydrate diet improves symptoms and quality of life in diarrhea-predominant irritable bowel syndrome. Clin Gastroenterol Hepatol. 2009;7(6):706–708.e1. doi: 10.1016/j.cgh.2009.02.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Moayyedi P, Quigley EM, Lacy BE et al. The effect of fiber supplementation on irritable bowel syndrome: a systematic review and meta-analysis. Am J Gastroenterol. 2014;109(9):1367–1374. doi: 10.1038/ajg.2014.195. [DOI] [PubMed] [Google Scholar]
- 89.U.S. Department of Health and Human Services; U.S. Department of Agriculture. Dietary guidelines for Americans 2015-2020, eighth edition. https://health.gov/dietaryguidelines/2015/resources/2015-2020_Dietary_Guidelines.pdf Published December 2015. Accessed December 28, 2018.
- 90.Faresjö A, Johansson S, Faresjö T, Roos S, Hallert C. Sex differences in dietary coping with gastrointestinal symptoms. Eur J Gastroenterol Hepatol. 2010;22(3):327–333. doi: 10.1097/MEG.0b013e32832b9c53. [DOI] [PubMed] [Google Scholar]
- 91.Hayes P, Corish C, O’Mahony E, Quigley EM. A dietary survey of patients with irritable bowel syndrome. J Hum Nutr Diet. 2014;27(2):36–47. doi: 10.1111/jhn.12114. suppl. [DOI] [PubMed] [Google Scholar]
- 92.Simrén M, Månsson A, Langkilde AM et al. Food-related gastrointestinal symptoms in the irritable bowel syndrome. Digestion. 2001;63(2):108–115. doi: 10.1159/000051878. [DOI] [PubMed] [Google Scholar]
- 93.Serra J, Salvioli B, Azpiroz F, Malagelada JR. Lipid-induced intestinal gas retention in irritable bowel syndrome. Gastroenterology. 2002;123(3):700–706. doi: 10.1053/gast.2002.35394. [DOI] [PubMed] [Google Scholar]
- 94.Simrén M, Abrahamsson H, Björnsson ES. Lipid-induced colonic hypersensitivity in the irritable bowel syndrome: the role of bowel habit, sex, and psychologic factors. Clin Gastroenterol Hepatol. 2007;5(2):201–208. doi: 10.1016/j.cgh.2006.09.032. [DOI] [PubMed] [Google Scholar]
- 95.Caldarella MP, Milano A, Laterza F et al. Visceral sensitivity and symptoms in patients with constipation- or diarrhea-predominant irritable bowel syndrome (IBS): effect of a low-fat intraduodenal infusion. Am J Gastroenterol. 2005;100(2):383–389. doi: 10.1111/j.1572-0241.2005.40100.x. [DOI] [PubMed] [Google Scholar]
- 96.Rosell-Camps A, Zibetti S, Pérez-Esteban G, Vila-Vidal M, Ferrés-Ramis L, García-Teresa-García E. Histamine intolerance as a cause of chronic digestive complaints in pediatric patients. Rev Esp Enferm Dig. 2013;105(4):201–206. doi: 10.4321/s1130-01082013000400004. [DOI] [PubMed] [Google Scholar]
- 97.Son JH, Chung BY, Kim HO, Park CW. A histamine-free diet is helpful for treatment of adult patients with chronic spontaneous urticaria. Ann Dermatol. 2018;30(2):164–172. doi: 10.5021/ad.2018.30.2.164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Chung BY, Cho SI, Ahn IS et al. Treatment of atopic dermatitis with a lowhistamine diet. Ann Dermatol. 2011;23(1):S91–S95. doi: 10.5021/ad.2011.23.S1.S91. Suppl. [DOI] [PMC free article] [PubMed] [Google Scholar]