Abstract
For decades, scientists have known that carriers of the apolipoprotein E ε4 (APOE ε4) allele (homozygous/heterozygous) are at respectively higher risk for developing Alzheimer's disease (AD). Although previous research reveals that the APOE ε4 variant impacts the clearance capacity and degradation of β-amyloid from the brain, as compared with APOE ε3 (wild type with normal risk) and APOE ε2 (variant with accelerated clearance and reduced risk), little has been documented about APOE ε4's dual role in cholesterol transport, both peripheral and cerebral, and the effects of sluggish APOE ε4 cholesterol transport on cerebral metabolic rate. An understanding of the connection between brain metabolism and brain fat/cholesterol transport may unlock new prevention strategies for treating patients with a comorbidity of metabolic syndrome (MetS) with cognitive impairment. Recent findings suggest that the APOE ε4 carrier impedes the shuttling of lipids from neurons and circumvents the storage of fat within the glia lipid droplets. This sluggish transport of lipids to triglyceride droplets in the glia cells can lead to dangerous reactive oxygen species and hydroxyl-free radicals as lipids are prematurely oxidized.
This case study evaluates the effects of a 10-week clinically prescribed ketogenic diet (KD) with a 68-year-old male, heterozygous APOE ε4 carrier, with a dual diagnosis of mild AD and type 2 diabetes (T2DM). The patient was administering both long- and short-acting injectable insulin to mediate his T2DM for 15+ years. Clinical goals of the intervention included increased hypothalamic and peripheral insulin sensitivity as measured via blood ketones with the Abbott Precision Xtra Blood Ketone Meter to confirm metabolic flexibility; controlled plasma glucose as measured via Abbott Precision Xtra Blood Glucose Meter and HgA1c via venous draw; normalization of lipid panel via venous draw and improved memory with restoration of cognitive functionality measured via the Montreal Cognitive Assessment. The Montreal Cognitive Assessment is considered to be a gold standard assessment in the diagnosis of early AD. Physiological biomarkers for T2DM/MetS and cognitive functionality were assessed before/during/after intervention. These measures included HOMA-IR, triglycerides/HDL ratio, HgA1c, fasting glucose, fasting insulin, complete fasting lipid panel and the PEAK mobile application for real-time measurement of cognitive improvement. The results were statistically significant. The patient's baseline Montreal Cognitive Assessment improved from 23/30 (mild AD) to 29/30 (normal ≥ 26). His T2DM was reversed. Pre-intervention HgA1c was 7.8% (T2DM); post intervention HgA1c measured 5.5% (normal). Likewise, the patient achieved statistically significant improvements in the other aforementioned biomarkers of MetS. The results of this case study suggest that a clinically prescribed ketogenic diet has strong potential to restore systemic insulin sensitivity and metabolic flexibility in diabetic, APOE ε4 heterozygous carriers. Mechanisms of action point to normalization of homeostatic negative feedback loops resetting/restoring lipid synthesis/utilization and glucose (insulin)/fatty acid (glucagon) utilization/production in both the body and brain, resulting in increased cerebral metabolism, improved cognition, and reversal of T2DM via renewed cellular insulin sensitivity.
Keywords: APOE ε4, Alzheimer's disease (AD), Metabolic syndrome (MetS), Type 2 diabetes mellitus (T2DM), Ketogenic diet (KD), Late onset Alzheimer's disease (LOAD), Reactive oxygen species (ROS), Inverse Warburg effect
1. Introduction
The incurability of Alzheimer's disease (AD) causes feelings of hopelessness and despair for individuals at the greatest risk for the disease; patients carrying one (heterozygous) or two (homozygous) alleles for the APOE ε4 gene variant have the strongest known risk for late-onset AD [1]. The risk in these patients for eventual brain degeneration rises 30%-80% for carriers of one or two APOE ε4 allele(s), respectively. Likewise, patients with metabolic derangements such as type 2 diabetes (T2DM), insulin resistance, metabolic syndrome (MetS), or a family history of AD are at similar risk. The risk for developing AD is consequent to chronic, undetected deficits in glucose metabolism in the brain that begin 20+ years before the onset of cognitive symptoms. Cerebral hypometabolism, also referred to as type III diabetes, resembles systemic insulin resistance [2]. Inadequate nutrient absorption/availability for neuron and glial respiration sits at the forefront of the bioenergetic theories of cognitive decline [2], [3], [4]. Research indicates that the presence of an APOE ε4 allele variation compounds metabolic problems by blunting the energy-sensing capacity of hypothalamic neurons, thereby decreasing cerebral glucose metabolism [3], [5], [6], [7]. Chronic nutrient deprivation reduces neuronal oxidative phosphorylation and the synthesis of mitochondrial ATP. This deficit leads to diminished expression of oxidative enzymes necessary for mitochondrial respiration [8]. Enduring hypometabolism produces oxidative stress via the production of dangerous reactive oxygen species. Neurons lack glycolytic enzymes, so unlike peripheral cells that default to Warburg phenomenon and aerobic glycolysis under respiratory stress, starving neurons attempt to upregulate their deranged mitochondrial circuitry. This volatile “hypermetabolic” response, known as the inverse Warburg effect, permanently damages neurons via free-radical peroxidation of lipid membranes, which diminishes the structural integrity of the brain. Eventually, metabolically deranged neurons slip into a dormant, quiescent state before succumbing to senescent arrest. This study examines the powerful role of endogenous ketones to rescue quiescent neurons, thereby circumventing neurodegenerative pathology.
Ketones provide the starving brain with an alternative fuel source via the cerebral monocarboxylate transporter pathway [9]. Two forms of ketones, acetoacetate and beta-hydroxybutyrate, bypass deficiencies in GLUT transport and supply needed energy directly to the brain [8], [9], [10], [11], [12], [13]. Cerebral metabolic rate, emission tomography, and F-fluorodeoxyglucose studies reveal that AD patients with impaired glucose transport are able to fully utilize ketone bodies in the brain as an alternative fuel [4], [9].
2. Methods
A 68-year-old male with comorbid mild AD and a 15+ year history of insulin-dependent T2DM completed a 10-week lifestyle intervention, which incorporated a clinically prescribed ketogenic diet (KD) designed to ameliorate cognitive function, restore memory loss, and functionally reverse T2DM via homeostatic restoration of metabolic fuel flux in the cells of the brain and body. The prescribed nutritional protocol utilized a clinically prescribed KD with moderate protein (based on lean mass and activity level) designed to reduce fasting insulin levels, thereby sustain hepatic synthesis of endogenous blood ketones (beta-hydroxybutyrate) as measured by the Precision Extra Abbott Blood Ketone Meter (≥0.5 mmol/L). Intermittent fasting utilizing an 8 hour time-restricted feeding window together with moderate intensity exercise was integrated into the lifestyle intervention plan approximately 3 days per week. The patient was monitored in real time by licensed health care providers as well as the student researcher. PEAK brain training games were completed/scored and recorded 5 days per week on a mobile device. The electronic application challenges memory, problem solving, language, mental agility, focus, and mental coordination; the PEAK domains correlate with executive function in the four lobes of the cerebral cortex: frontal, parietal, occipital, and temporal lobes. While the PEAK application was designed to improve functionality in the cerebral lobes and sharpen action of the prefrontal cortex and hippocampal region [14], recent longitudinal studies demonstrated that stand-alone, cognitive exercises, such as PEAK, fail to increase neuroplasticity [15]. In this case analysis, the PEAK application was utilized to measure and quantify improvements in cerebral cortex activity resultant of increased metabolic flux in ketone oxidation; ketones offered the patient an alternative fuel thereby rescuing the starving brain. The conclusions of this study support the previously documented bioenergetic hypothesis of cognitive degeneration; because ATP is rate limiting, the mediation of cerebral hypometabolism via restoration of metabolic flexibility in the neurons is a first-order change occurring before neuroplasticity.
Biomarkers of MetS were measured and tracked before/during/after intervention via venous blood draws. These biomarkers included HOMA-IR, triglycerides/HDL ratio, HgA1c, fasting glucose, fasting insulin, and comprehensive lipid panels. Memory function was assessed daily via the PEAK brain application; cognitive function was quantified using a pre- and post Montreal Cognitive Assessment (MoCA) test, administered by a licensed professional clinical counselor. Weekly point of care blood ketone readings were obtained via the Abbott Precision Xtra Blood Ketone Meter; the patient's ketone levels were assessed/recorded and tracked by a licensed health care professional. Blood ketones served as a biological proxy to determine the patient's compliance with the intervention protocol. In addition, moderate intensity exercise was implemented 3 days per week for 30-minute durations; the exercise protocol was monitored and supervised by the student researcher in the wellness center of the university.
3. Case report
The patient, a 68-year-old morbidly obese male, is a heterozygous APOE ε4 (ε4/ε3) carrier; he presents with comorbid mild-AD and T2DM. The patient is a retired businessman who engages in low-impact cardio and resistance training approximately 2 days/week and plays golf; he self-reports using a motorized cart, so the golf offers minimal cardiovascular benefits. The patient appeared highly motivated to participate in the KD nutritional intervention for weight loss, reduction of medication (insulin) load/expense, and to prevent further cognitive decline. Foggy thinking, forgetfulness, and impaired verbal fluency were the chief cerebral complaints. The patient had an extensive history of obesity, uncontrolled appetite regulation with weight fluctuation, degradation of lean mass, accumulation of truncated visceral fat, chronic fatigue, lethargy, and insomnia. The clustered symptomatology substantially impacted his quality of life; the patient appeared intrinsically motivated to implement both diet and lifestyle changes, despite a history of repetitive failure at weight loss. The patient reported a 15+ year history of insulin-dependent T2DM and tested positive for a heterozygous APOE ε4 variation (ε4/ε3) via cheek swab genome assessment; the subject presented with no family history for diagnosed AD, but strong histology of obesity, dyslipidemia, cardiovascular disease, and T2DM.
4. Results
Diabetic male with a 15+ year history of insulin-dependent T2DM was prescribed a 10-week KD nutritional intervention protocol purposed at endogenous hepatic production of blood ketones to restore peripheral and cerebral metabolic flexibility. Metabolic syndrome biomarkers and cognitive assessments were measured/recorded before/during/after intervention. The results of the intervention were statistically significant. The patient normalized his HgA1c, fasting glucose, fasting insulin, and blood lipids; he achieved remarkable restoration in cellular insulin sensitivity and dramatically reduced his cardiovascular risk as measured by an 88% reduction in HOMA-IR and 55% drop in Tri/HDL ratio. Likewise, the patient's HDL increased by 35% during the 10-week KD intervention. Despite insulin injections, the subject's fasting insulin level plummeted from 97mU/L to 14mU/L, reflecting an 85% reduction. Memory, cognition, and verbal fluency also reflected clinically significant change; the patient's MoCA score improved from the baseline pre-screening value of mild AD (23/30); his post intervention MoCA screening was normative (29/30).
5. Data
Significant results were recorded and are shown below for biomarkers of MetS and MoCA assessments. Improvements in the MetS biomarkers are displayed in Table 1. The improvement in MoCA screening is shown in Table 2.
Table 1.
Biomarkers for MetS before/during/after intervention
| Results | Pre-intervention | Mid-intervention | Post-intervention | Percent change |
|---|---|---|---|---|
| HOMA-IR (<1.0) | 31.0 | 6.3 | 3.5 | −88.8% |
| Tri/HDL ratio (<2.0) | 4.9 | 3.3 | 2.2 | −55.5% |
| WHtR (<0.5) | 0.62 | 0.61 | .58 | −6.45% |
| Fasting insulin mU/L (3-5) | 97.2 | 21.3 | 14.3 | −85.3% |
| Fasting glucose mg/dL (70-90) | 129 | 119 | 98 | −24.0% |
| HgA1c (%) | 7.8% (diabetic) | 5.6% | 5.5% (normal) | −29.5% |
| Triglycerides mg/dL (<150) | 137 | 96 | 83 | −39.4% |
| HDL mg/dL (>50) | 28 | 29 | 38 | +35.7% |
| LDL mg/dL (<100) | 74 | 65 | 61 | −17.6% |
| VLDL mg/dL (9-13) | 27.4 | 19.2 | 16.6 | −39.4% |
| Weight | 257.2 | 250.4 | 243.0 | −5.5% |
| Body fat % (<30%) | 39.8% | 37.4% | 36.8% | −3% |
Table 2.
MoCA scores before/after intervention
| Memory assessment | Pre-intervention | Post-intervention | Percent change |
|---|---|---|---|
| MoCA (>26) | 23 | 29 | +26.1% |
Abbreviation: MoCA, Montreal Cognitive Assessment.
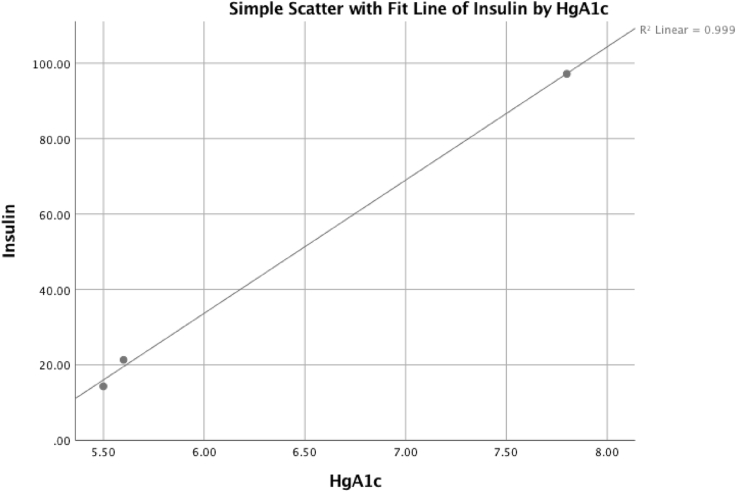
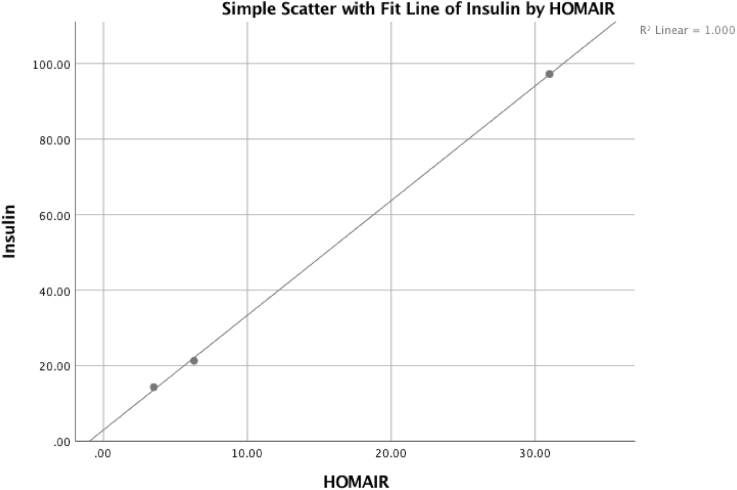
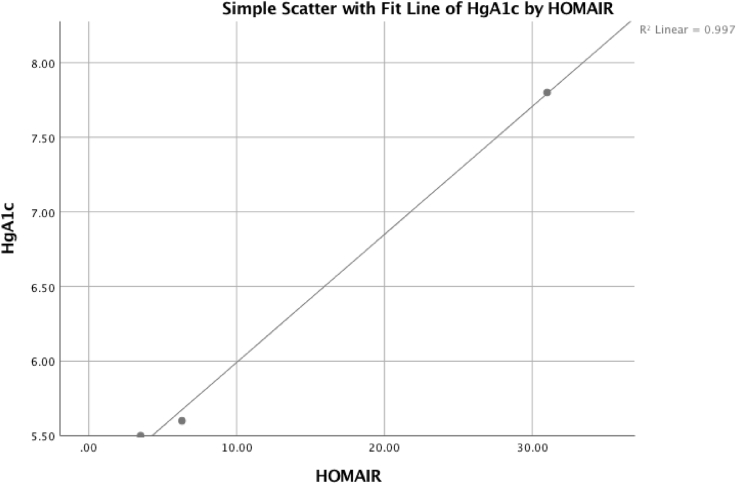
Fig. 1, Fig. 2, Fig. 3 reflect multiple regression analyses examining the correlated influence of insulin, HgA1c, and HOMA-IR. The significant linear regression among the variables demonstrates increased cerebral insulin sensitivity corresponds with reductions in brain hypometabolism.
Fig. 1.
The patient's HgA1c was positively correlated with reduced fasting insulin levels and reflects statistical significance, adjusted R2 = 0.997, P = .024.
Fig. 2.
The patient's HOMA-IR was positively correlated with reduced fasting insulin levels and reflects statistical significance, adjusted R2 = 0.999, P = .010.
Fig. 3.
The patient's HOMA-IR was positively correlated with reduced HgA1c and reflects statistical significance, adjusted R2 = 0.994, P = .034.
6. Discussion
The hallmark of AD is progressive neurodegeneration consequent of prolonged deficits in the rate of cerebral glucose metabolism [2], [3], [4], [5], [6], [7], [16]. Chronic fuel deprivation will eventually exhaust paracrine-specific metabolic coupling of lactate from astrocytes to neurons. Shortages in cerebral fuel triggers a paradoxical, “hypermetabolic” response in the oxidative machinery of starving neurons to mitigate starvation; the deranged oxidation produces damaging free radicals and reactive oxygen species in its wake [4], [9]. This stress-driven increase of oxidative phosphorylation is referred to as the inverse Warburg effect and results in permanent damage to the neuronal mitochondrion. In an attempt to meet the increasing need for lactate in the deranged neurons, astrocytes upregulate aerobic glycolysis via the Warburg effect. Recent studies suggest that deficits in cerebral fuel supply and the compensatory Warburg and inverse Warburg phenomenon may be at the core of brain atrophy disorders [1], [2], [8], [11]. Research also supports a strong connection between AD and T2DM rooted in cellular metabolic inflexibility [4], [11], [12]. The desensitization of systemic insulin receptors and blunting of hypothalamic energy sensing is common to both T2DM and AD [2]. Likewise, the APOE ε4 variant is known to exacerbate insulin resistance and interfere with glucose homeostasis as well as cholesterol synthesis and transport [3], [5], [6], [7], [9]. Fortunately, hungry neurons in the Alzheimer's brain retain their oxidative capacity to burn ketones for ATP synthesis; ATP awakens dormant, quiescent cells, thereby restoring functionality to the lobes of the cerebral cortex [4], [9], [11], [12], [13], [16].
This case examined the potential of a clinically prescribed KD to reverse early-stage memory loss (mild AD) and improve biomarkers of MetS by supplying the brain with an alternative fuel source. The results of the case support the outcomes of the aforementioned studies and clinical trials. The data and significant findings demonstrate the dire need to explore metabolic dysregulation as an initiating factor in the development of MetS, mild cognitive impairment, and AD. In addition, genome testing for the APOE ε4 variant among patients with MetS could offer a mode of AD prevention.
Limitations of this study include the small sample size, short intervention period and gender exclusiveness. In future studies, it would be advantageous to include both male and female participants of various ages, ethnic heritage, and include carriers of both heterozygous and homozygous APOE variants. Longitude research with periodic follow-up and subsequent data collection would validate the long-term efficacy of the KD to ameliorate metabolic flexibility and cerebral metabolism.
7. Conclusion
Ketogenic protocols seem to exert powerful modulatory effects on the most treatment-resistant conditions including obese carriers of the APOE ε4 variant who suffer from comorbid mild cognitive impairment and T2DM. Previous clinical trials have demonstrated the neurological efficacy of the KD. The significant improvements in memory, executive function, MetS pathologies, and cerebral metabolic rate demonstrated in this case study are reproducible and could be easily translated to a general population of MetS patients with self-reported impairments in cognition. The novel approach of nutritional modulation to halt brain atrophy and restore cognitive functionality warrants further investigation.
Research in context.
-
1.
Systematic review: The authors reviewed the literature using traditional (e.g., Google Scholar) sources. Although the role of a ketogenic diet applied to Alzheimer's disease and APOE ε4 has not yet been widely studied as other aspects of AD physiology, there have been several recent publications describing the clinical aspects of a ketogenic diet and ketone supplementation. These relevant citations are appropriately cited.
-
2.
Interpretation: Our findings led to an integrated hypothesis describing the role of a ketogenic diet and insulin levels, MetS biomarkers, and memory function. The hypothesis is consistent with clinical findings currently in the public domain.
-
3.
Future directions: The article proposes a framework for the generation of new hypotheses and the conduct of additional studies regarding this area of study. Examples include further understanding (A) the neuroprotective properties of monocarboxylate transporter (ketone) oils and their defense against mild cognitive impairment and AD; (B) universal routine insulin level screenings to identify cerebral hypometabolism before the detrimental neurodegenerative cascade begins.
Acknowledgments
No funding was required for this study.
Authors' contributions: All persons who meet the authorship criteria are listed as authors, and all authors certify that they have participated sufficiently in the work to take public responsibility for the content, including participation in the concept.
Footnotes
Statement of Ethics: This study was approved by an ethics committee. All the participants gave their written informed consent before taking part of the study.
Conflict of Interest: The authors declare that there is no conflict of interest.
References
- 1.Sonntag K., Ryu W., Amirault K.M., Healy R.A., Siegel A.J., Mcphie D.L. Late-onset Alzheimer's disease is associated with inherent changes in bioenergetics profiles. Scientific Rep. 2017;7 doi: 10.1038/s41598-017-14420-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ferreira L.S.S., Fernandes C.S., Vieira M.N.N., De Felice F.G. Insulin Resistance in Alzheimer's Disease. Front Neurosci. 2018;12:830. doi: 10.3389/fnins.2018.00830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lane-Donovan C., Philips G.T., Herz J. More than cholesterol transporters: lipoprotein receptors in CNS function and neurodegeneration. Neuron. 2014;83:771–787. doi: 10.1016/j.neuron.2014.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gibas K.J. The starving brain: overfed meets undernourished in the pathology of mild cognitive impairment (MCI) and Alzheimer's disease (AD) Neurochem Int. 2017;110:57–68. doi: 10.1016/j.neuint.2017.09.004. [DOI] [PubMed] [Google Scholar]
- 5.Chan E.S., Shetty M.S., Sajikumar S., Chen C., Soong T.W., Wong B.-S. ApoE4 expression accelerates hippocampus-dependent cognitive deficits by enhancing A impairment of insulin signaling in an Alzheimer's disease mouse model. Sci Rep. 2016;6:26119. doi: 10.1038/srep26119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhao N., Liu C.-C., Ingelgom A.J.V., Martens Y.A., Linares C., Knight J.A. Apolipoprotein E4 impairs neuronal insulin signaling by trapping insulin receptor in the endosomes. Neuron. 2017;96:115–129.e5. doi: 10.1016/j.neuron.2017.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hertz L., Chen Y., Waagepetersen H.S. Effects of ketone bodies in Alzheimer's disease in relation to neural hypometabolism, b-amyloid toxicity, and astrocyte function. J Neurochem. 2015;134:7–20. doi: 10.1111/jnc.13107. [DOI] [PubMed] [Google Scholar]
- 8.Demetrius L.A., Magistretti P.J., Pellerin L. Alzheimer's disease: The amyloid hypothesis and the Inverse Warburg effect. Front Physiol. 2015;5 doi: 10.3389/fphys.2014.00522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cunnane S.C., Courchesne-Loyer A., St-Pierre V., Vandenberghe C., Pierotti T., Fortier M. Can ketones compensate for deteriorating brain glucose uptake during aging? Implications for the risk and treatment of Alzheimer's disease. Ann New York Acad Sci. 2016;1367:12–20. doi: 10.1111/nyas.12999. [DOI] [PubMed] [Google Scholar]
- 10.Freitas S., Simões M.R., Alves L., Santana I. Montreal Cognitive Assessment. Alzheimer Dis Associated Disord. 2013;27:37–43. doi: 10.1097/WAD.0b013e3182420bfe. [DOI] [PubMed] [Google Scholar]
- 11.Kashiwaya Y., Takeshima T., Mori N., Nakashima K., Clarke K., Veech R.L. D-Beta -hydroxybutyrate protects neurons in models of Alzheimer's and Parkinson's disease. Proc Natl Acad Sci U S A. 2000;97:5440–5444. doi: 10.1073/pnas.97.10.5440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gibas M.K., Gibas K.J. Induced and controlled dietary ketosis as a regulator of obesity and metabolic syndrome pathologies. Diabetes Metab Syndr Clin Res Rev. 2017;11 doi: 10.1016/j.dsx.2017.03.022. [DOI] [PubMed] [Google Scholar]
- 13.Henderson S.T., Vogel J.L., Barr L.J., Garvin F., Jones J.J., Costantini L.C. Study of the ketogenic agent AC-1202 in mild to moderate Alzheimer's disease: A randomized, double-blind, placebo-controlled, multicenter trial. Nutr Metab. 2009;6:31. doi: 10.1186/1743-7075-6-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Whiteford K.M. Southern Illinois University Carbondale; Illinois: 2014. Testing the validity of the PEAK relational training system in assessing language & cognition after brain injury. [Unpublished doctoral dissertation] [Google Scholar]
- 15.Staff R.T., Hogan M.J., Williams D.S., Whalley L.J. Intellectual engagement and cognitive ability in later life (the “use it or lose it” conjecture): longitudinal, prospective study. BMJ. 2018;363:k4925. doi: 10.1136/bmj.k4925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lange K.W., Lange K.M., Makulska-Gertruda E., Nakamura Y., Reissmann A., Kanaya S. Ketogenic diets and Alzheimer's disease. Food Sci Hum Wellness. 2017;6:1–9. [Google Scholar]