Abstract
Background
Sepsis is among the major antecedents of lung injury characterized by mitochondrial dysfunction. The functional integrity of the cell is influenced by mitochondrial dynamics. The present investigation evaluated the protective effects of dexmedetomidine against lung injury and speculates on the possible mechanism underlying its effects on mitochondrial function.
Material/Methods
Lung injury was induced by cecal ligation and puncture (CLP) in mice treated with 0.1, 0.3, or 0.5 mg/kg intravenous dexmedetomidine after a 30-minute surgery. The effects of dexmedetomidine were determined by the oxygenation index and the wet/dry weight ratio of the lung. The expression of mitochondrial protein was assessed by western blot analyses and real-time polymerase chain reaction, to determine the effects of dexmedetomidine on mitochondrial dynamics. The histopathology of the lung tissue was determined by hematoxylin and eosin staining, and TUNEL-positive cells were counted in TUNEL assays. Activity of caspase-3, caspase-8, and caspase-9 enzymes were determined by colorimetric assay.
Results
Treatment with dexmedetomidine significantly attenuated changes in the oxygenation index and the wet/dry weight ratio in mice with CLP-induced lung injury. There was a significant decrease in pro-inflammatory mediators and markers of oxidative stress in the lung tissue of the dexmedetomidine-treated group compared to the negative control group. Moreover, treatment with dexmedetomidine attenuated the altered gene expression caused by mitochondrial fusion and fission in the lung tissue of mice with CLP-induced lung injury. The number of TUNEL-positive cells was significantly reduced in the dexmedetomidine-treated group compared to the negative control group. Moreover, dexmedetomidine ameliorated the altered activity of caspase-3, caspase-8, and caspase-9 enzyme in the lung tissues of CLP-induced lung injure mice.
Conclusions
Dexmedetomidine protected mice against CLP-induced lung injury by attenuating changes in mitochondrial fusion and fission.
MeSH Keywords: Dexmedetomidine, Lung Injury, Mitochondrial Dynamics, Oxidative Stress
Background
Sepsis is a systemic inflammation that causes multiple organ dysfunction, including lung injury [1]. The mortality rate of sepsis is high and clinical management of the condition is challenging [2]. Type I alveolar epithelial cells are destroyed by inflammation; the exact pathogenesis of this phenomenon is unknown, but the literature shows that the generation of reactive oxygen species (ROS) is enhanced in sepsis, leading to lung injury [3]. ROS are abundant in the mitochondria and their activation leads to mitochondrial damage due to oxidative stress [4].
Dysfunction of the mitochondria is a major factor contributing to lung injury. Several studies have reported that cellular function is maintained under conditions of environmental or metabolic stress by mitochondria, via delicate fission and fusion cycles [5]. Mitofusins 1 and 2 (Mfn1 and Mfn2, respectively) in the outer membrane of mitochondria, and optic atrophy 1 (OPA1) in the inner membrane, maintain fusion [6]. Fission begins with the recruitment of dynamin-related protein 1 (Drp1) from cytosol by mitochondria [7]. Cellular integrity is maintained by the dynamic behavior of mitochondria, changes lead to several pathological processes such as neuronal injury, ischemia-reperfusion, and cellular injury [8]. The mitochondrial respiratory control ratio decreases when there is mitochondrial dysfunction, resulting in sepsis-induced organ failure. Thus, maintaining an appropriate balance between fusion and fission protects against oxidative damage in cases of sepsis-induced lung injury.
Dexmedetomidine is a α2-adrenergic agent used in the intensive care unit [9]. Clinically, it is used as an analgesic and sedative, and shows cardioprotective and antioxidant activity [10–13]. Moreover, in mechanically ventilated patients, it recovers respiratory function [14]. It is also used as a pre-anesthetic antioxidant medication, reducing the concentration of cytokines in renal tissues [15], and also it attenuates the lung injury induced by lipopolysaccharide, ischemia-reperfusion, and ventilation in animal models [16]. Dexmedetomidine has also been shown to reduce oxidative stress and apoptosis injured lung tissues [17]. Moreover, it decreases pulmonary tissue fibrosis in rats after acute lung injury [18] and protects against endotoxin-induced lung injury by attenuating the acid-base imbalance [19]. We evaluated the protective effects of dexmedetomidine against sepsis-induced lung injury and investigated the molecular mechanism of action.
Material and Methods
Animals
Male albino mice (weight, 20–25 g) were procured from Vital River Laboratories Ltd. (Beijing, China). Animals were maintained under standard conditions (humidity, 60±5%; temperature, 26±2°C) with a 12: 12 hour light-dark cycle. The study protocol was approved by the Ethics Committee of The First Affiliated Hospital of Fujian Medical University, Fujian, China (approval no. IACUC/FAH-FMU/2017/04). The guidelines of the Association for the Assessment and Accreditation of Laboratory Animal Care International (AAALAC) for animal experimentation were followed.
Chemicals
Dexmedetomidine was purchased from Sigma-Aldrich (St. Louis, MO, USA). Manganese superoxide dismutase (MnSOD), Ca2+-ATPase, Na+K+-ATPase, and malondialdehyde (MDA) kits were from Nanjing Jiancheng Biochemistry (Nanjing, China). Fission protein 1 (Fis1), Drp1, Mfn2, Mfn1, OPA1, and β-actin antibodies were from Santa Cruz Biotechnology (Santa Cruz, CA, USA). Enzyme-linked immunosorbent assay (ELISA) kits for interleukin (IL)-6, IL-1β, and tumor necrosis factor-alpha (TNF-α) were from Abcam (Cambridge, UK). Caspase-3, caspase-8, and caspase-9 assay kits were procured from R&D Systems, Germany.
Experimental
Lung injury was induced in all animals by sepsis via cecal ligation and puncture (CLP) as previously described [20]. All mice were fasted overnight and anesthetized using pentobarbital (30 mg/kg, intraperitoneal). The cecum was exposed to perform laparotomy and an 18-gauge needle was used to puncture the area below the ileocecal valve twice, to ligate the cecum; then the abdominal cavity was closed. In the sham-operated group, only a laparotomy was performed, without ligation of the cecum. All animals were classified into 5 groups: controls, negative control group 0.1 mg/kg intravenous dexmedetomidine group, 0.3 mg/kg intravenous dexmedetomidine group, and 0.5 mg/kg intravenous dexmedetomidine group (where dexmedetomidine was administered after the 30-minute surgery).
Arterial partial pressure of oxygen to fraction of inspired oxygen (PaO2/FiO2) ratio
All animals were anesthetized at 1 day after CLP. A 20-gauge catheter was used for endotracheal intubation and pure oxygen (7 mL/kg) was given by mechanical ventilation. PaO2 analyses of arterial blood samples were performed using a GEM Premier 3000 gas analyzer (Instrumentation Laboratory, Lexington, MA, USA) after ventilation for 15 minutes. The oxygenation index was expressed as PaO2/FiO2.
Isolation of lung
All of the animals were sacrificed by cervical dislocation and the lungs were isolated. Lobes were separated out from the isolated left lungs and sections 5 μm thick were prepared for histochemical analyses. The right lung lobes were separated out and homogenized with 0.9% saline solution for protein extraction.
Histopathological analyses
All of the animals were sacrificed; the lungs were isolated and fixed in formalin (10%) for 3 days. Slices of the pulmonary lobes were seeded in paraffin, and a microtome was used to section the lung tissues into 5 μm slices. Hematoxylin and eosin (H&E) staining was used to stain the lung tissue sections: several changes were noted such as infiltration of inflammatory cells, necrosis, interstitial edema, and hemorrhage. Each parameter was evaluated on a 0 to 3 point scale, where 0=normal (absence of pathology; <5% of maximum pathology), 1=mild pathology (<10% of maximum), 2=moderate pathology (15–20% of maximum), and 3=severe pathology (>20% of maximum).
Estimation of mitochondrial MDA content and MnSOD, Ca2+-ATPase, and Na+K+-ATPase activity
A mitochondrial fractionation kit was used for the extraction of mitochondria and a protein assay kit (Bio-Rad, Hercules, CA, USA) was used to determine the mitochondrial protein content. The MnSOD, Ca2+-ATPase, and Na+K+-ATPase activity in mitochondria in lung tissue, and the mitochondrial MDA content, were measured using a spectrophotometer. The manufacturer’s instructions were followed for all kits to determine protein expression.
Real-time polymerase chain reaction (RT-PCR)
All animals were sacrificed, and lung tissues were isolated. TransZol reagent was used for isolation of total RNA from the lung tissue and a cDNA synthesis kit (Beyotime, Shanghai, China) was used to synthesize the first-strand cDNA, as per the manufacturer’s instructions. The RT-PCR was performed to determine the messenger RNA (mRNA) level of fission- and fusion-related genes, using a SYBR Premix Ex Taq kit (Takara Bio Inc., Shiga, Japan). An iCycler iQ Real-Time PCR Detection System (Bio-Rad) was used for PCR amplification with 20 μL reaction solution containing cDNA (1 μL), reverse and forward primers (1 μL), SYBR Premix Ex Taq (10 μL), and ddH2O (7 μL). Thermal cycling, including denaturation, was performed for 30 seconds at 95°C, and thereafter for 20 seconds at 94°C (40 cycles). Fis1, Drp1, OPA1, and actin were assessed at 55°C, and Mfn1 and Mfn2 were evaluated at 60°C for 20 seconds. The mRNA signal was estimated for quantification of the relative expression of the target genes, with actin used as an internal control. In all samples, the target gene copy number/actin mRNA ratio was determined.
Gene
Primer were as follows:
Drp1: forward primer 5′-CGTAGTGGGAACTCAGAGCA-3′;
products: reverse primer 5′-TGGACCAGCTGCAGAATAAG-3′.
Fis1: forward primer 5′-AAATGATGCTACGCAGGCTT-3′;
products: reverse primer 5′-CCTGGACCATGACCAAGTTT-3′.
OPA1: forward primer 5′-CAGCTGGCAGAAGATCTCAAG-3′;
products: reverse primer 5′-CATGAGCAGGATTTTGACACC-3′.
Actin: forward primer 5′-CCTCTATGCCAACACAGTGC-3′;
products: reverse primer 5′-ATACTCCTGCTTGCTGATCC-3′.
Western blot
Proteins from the mitochondria of lung tissue were extracted as per the manufacturer’s instructions using a mitochondrial protein extraction kit (Beyotime). The Bradford protein assay was performed to determine the protein levels of mitochondrial Fis1, Drp1, Mfn2, Mfn1, OPA1, and β-actin. Polyclonal antibodies such as Fis1 (dilution ratio, 1: 50), Drp1 (dilution ratio, 1: 50), Mfn2 (dilution ratio, 1: 100), Mfn1 (dilution ratio, 1: 100), OPA1 (dilution ratio, 1: 50), and β-actin (dilution ratio, 1: 1000) were incubated overnight at 4°C with phosphate-buffered saline and bovine serum albumin. Further secondary antibodies were incubated at room temperature for 90 minutes. The Odyssey Infrared Imaging System (LI-COR Biosciences, Lincoln, NE, USA) was used to quantify band density.
Determination of pro-inflammatory mediators
An ELISA assay was used to measure serum levels of IL-6, IL-1β, and TNF-α, as per the manufacturer’s instructions. A nitrite/nitrate colorimetric assay kit (Cayman Chemical Inc., Ann Arbor, MI, USA) was used to estimate the level of nitrite in the serum of the mice.
Determination of activity of caspase-3, caspase-8, and caspase-9 enzyme
Colorimetric assay was done for the estimation of activity of caspase-3, caspase-8, and caspase-9 in the lung tissue homogenate by using Colorimetric Activity assay kit. CaspACE assay system was used for the determination of activity of caspase-3, caspase-8, and caspase-9 enzymes as per the directions given by the manufacturer of kits.
TUNEL assay
A DeadEnd Fluorometric TUNEL System (Promega, Madison, WI, USA) was used to perform the TUNEL assay on the isolated lung tissue, following the manufacturer’s instructions. Nuclear staining of lung tissue was performed using Hoechst 33258 dye and a confocal laser fluorescence microscope. TUNEL-positive cells were observed by 2 blinded pathologists.
Statistical analyses
All data are expressed as means ±SEMs (N=10). One-way ANOVA was performed using GraphPad Prism software (ver. 6.1; GraphPad Software Inc., San Diego, CA, USA). Post hoc comparisons of means were carried out using Dunnett’s post hoc test. The level of statistical significance was set at P<0.05.
Results
Dexmedetomidine reduced the oxygenation index
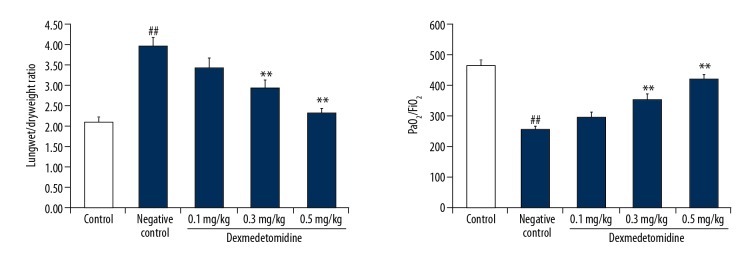
In mice with CLP-induced lung injury, the wet/dry weight ratio was significantly enhanced, and the oxygenation index was significantly reduced in negative controls compared to controls (Figure 1). However, treatment with dexmedetomidine significantly attenuated changes in the oxygenation index and the wet/dry weight ratio.
Figure 1.
Dexmedetomidine attenuates changes in the oxygenation index and the wet/dry weight ratio in mice with cecal ligation and puncture (CLP)-induced lung injury. Data are means ±SEMs (N=10); ## P<0.01 versus control group; * P<0.05, ** P<0.01 versus negative control group.
Histopathology of lung tissue: effects of dexmedetomidine
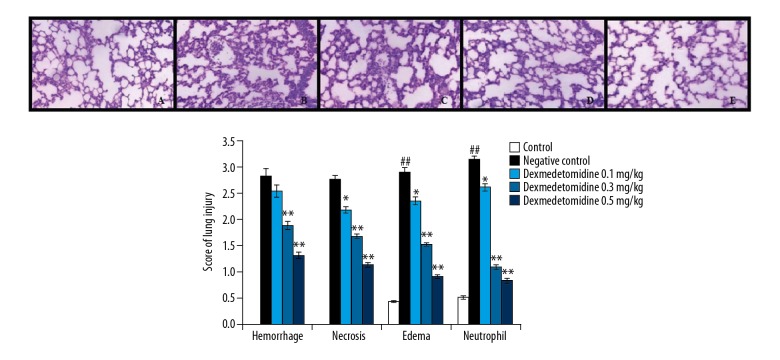
The effects of dexmedetomidine on the histopathological findings of mice with CLP-induced lung tissue injury are shown in Figure 2. The lung injury scores, encompassing hemorrhage, necrosis, edema, and neutrophil count, were significantly higher in negative controls than in controls. There was also a significant decrease in the lung injury score of the dexmedetomidine-treated group compared to negative controls.
Figure 2.
Dexmedetomidine attenuates altered lung histopathology in mice with CLP-induced lung injury. I. Histopathology of lung tissue: results of hematoxylin and eosin staining (100×): A) control, B) negative control, C) dexmedetomidine 0.1 mg/kg, D) dexmedetomidine 0.3 mg/kg, E) dexmedetomidine 0.5 mg/kg. II. Lung injury scores. Data are means ±SEMs (N=10); ## P<0.01 versus control group; P<0.05, ** P<0.01 versus negative control group.
Dexmedetomidine attenuated mitochondrial MDA expression and MnSOD, Ca2+-ATPase, and Na+K+-ATPase activity
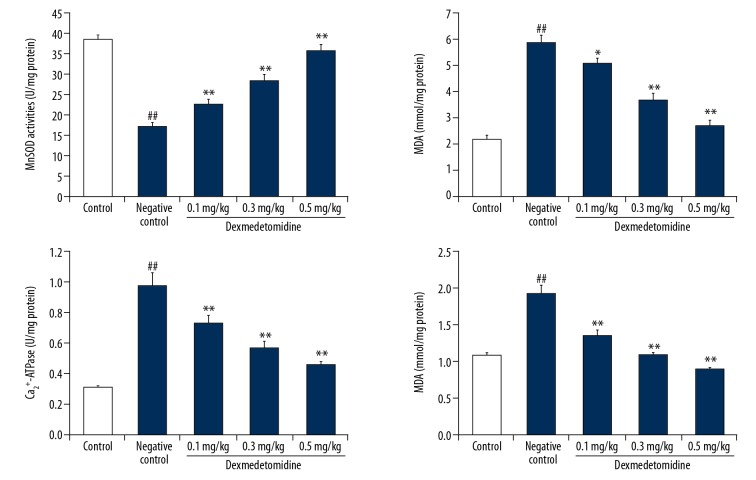
Figure 3 shows the effects of dexmedetomidine on mitochondrial MDA expression and MnSOD, Ca2+-ATPase, and Na+K+-ATPase activity in the lung tissue of mice with CLP-induced lung injury mice. There was a significant increase in the concentration of mitochondrial MDA in negative controls compared to controls. Moreover, the MnSOD activity was significantly reduced (P<0.01), and the activity of Ca2+-ATPase and Na+K+-ATPase were significantly enhanced in the lung tissue of negative controls compared to controls. However, treatment with dexmedetomidine attenuated changes in mitochondrial MDA expression and MnSOD, Ca2+-ATPase, and Na+K+-ATPase activity.
Figure 3.
Dexmedetomidine attenuates mitochondrial malondialdehyde (MDA) and manganese superoxide dismutase (MnSOD), Ca2+-ATPase, and Na+K+-ATPase activity in lung tissues of mice with CLP-induced lung injury. Data are means ±SEMs (N=10); ## P<0.01 versus control group; * P<0.05; ** P<0.01 versus negative control group.
Dexmedetomidine reduced mRNA expression of Fis1, Drp1, OPA1, Mfn1, and Mfn2
Figure 4 shows the effects of dexmedetomidine on the mRNA expression levels of Fis1, Drp1, OPA1, Mfn1, and Mfn2 in lung tissue of mice with CLP-induced lung injury, as revealed by RT-PCR. There was a significant decrease in the mRNA expression levels of Fis1, Mfn1, and Mfn2 (P<0.01), and an increase in the mRNA expression of OPA1 and Drp1 in lung tissues in negative controls compared to controls. However, treatment with dexmedetomidine significantly attenuated changes in the expression of Fis1, Drp1, OPA1, Mfn1, and Mfn2.
Figure 4.
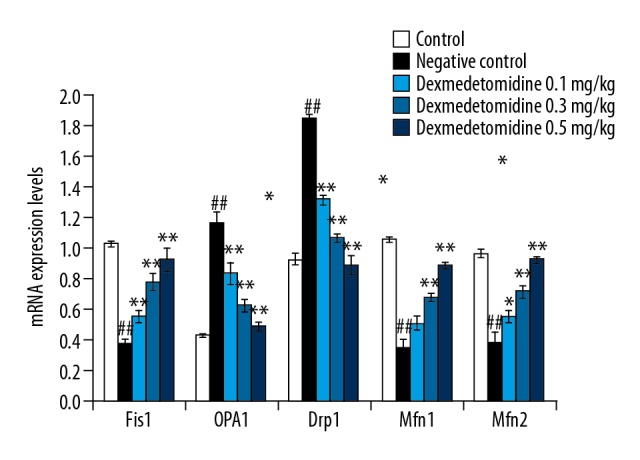
Dexmedetomidine attenuates messenger RNA (mRNA) expression levels of fission protein 1 (Fis1), dynamin-related protein-1 (Drp1), optic atrophy 1 (OPA1), mitofusin 1 (Mfn1), and mitofusin 2 (Mfn2) in lung tissues of mice with CLP-induced lung injury: Results of real-time polymerase chain reaction (RT-PCR). Data are means ±SEMs (N=10); ## P<0.01 versus control group; * P<0.05; ** P<0.01 versus negative control group.
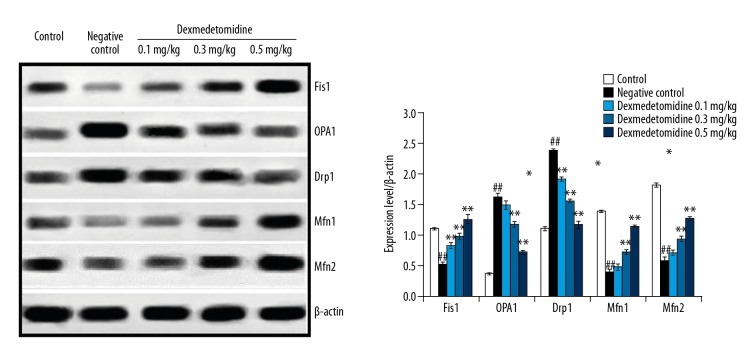
Dexmedetomidine reduced the expression of Fis1, Drp1, OPA1, Mfn1, and Mfn2
Figure 5 shows the effects of dexmedetomidine on the mRNA expression levels of Fis1, Drp1, OPA1, Mfn1, and Mfn2 in lung tissue of mice with CLP-induced lung injury as revealed by western blot assays. Treatment with dexmedetomidine attenuated the expression of Fis1, Drp1, OPA1, Mfn1, and Mfn2 in lung tissues of mice with CLP-induced lung injury.
Figure 5.
Dexmedetomidine attenuates the expression of Fis1, Drp1, OPA1, Mfn1, and Mfn2 in lung tissues of mice with CLP-induced lung injury: Results of western blot assay. Data are means ±SEMs (N=10); ## P<0.01 versus control group; * P<0.05; ** P<0.01 versus negative control group.
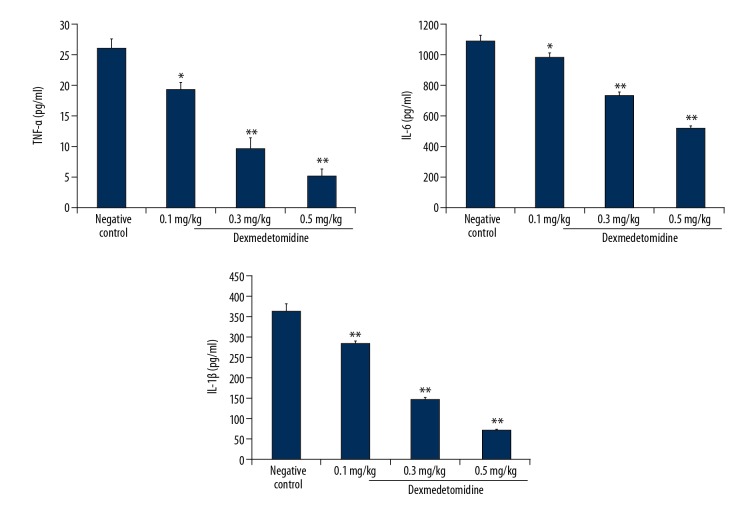
Dexmedetomidine reduced the expression of pro-inflammatory mediators
Figure 6 shows the effects of dexmedetomidine on TNF-α, IL-6, and IL-1β concentrations in lung tissues of mice with CLP-induced lung injury. The expression levels of TNF-α, IL-6, and IL-1β were significantly higher in negative controls than in controls. There was a significant decrease in the concentrations of TNF-α, IL-6, and IL-1β in lung tissues of dexmedetomidine-treated animals compared to negative controls (P<0.01).
Figure 6.
Dexmedetomidine attenuates tumor necrosis factor-alpha (TNF-α), interleukin (IL)-6, and IL-1β expression in lung tissues of mice with CLP-induced lung injury. Data are means ±SEMs (N=10); ## P<0.01 versus control group; * P<0.05; ** P<0.01 versus negative control group.
Dexmedetomidine attenuated TUNEL-positive cells
Figure 7 shows the effects of dexmedetomidine on the number of TUNEL-positive cells in mice with CLP-induced lung injury, as revealed by TUNEL assays. The number of TUNEL-positive cells was significantly increased in lung tissues of negative controls compared to controls (P<0.01). However, treatment with dexmedetomidine significantly decreased the number of TUNEL-positive cells compared to negative controls (P<0.01).
Figure 7.
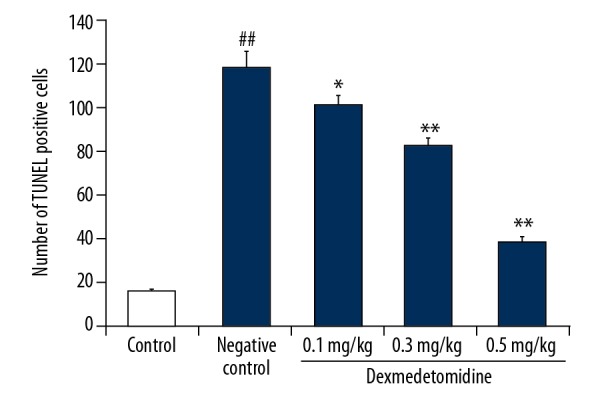
Dexmedetomidine attenuates TUNEL-positive cells in lung tissues of mice with CLP-induced lung injury: Results of the TUNEL assay. Data are means ±SEMs (N=10); ## P<0.01 versus control group; * P<0.05; ** P<0.01 versus negative control group.
Dexmedetomidine attenuated the altered activity of caspase-3, caspase-8, and caspase-9 enzymes
Effect of dexmedetomidine on the activity of caspase-3, caspase-8, and caspase-9 enzymes in the lung tissues homogenate of CLP-induced lung injured mice was shown in Figure 8. There was significant enhancement in the activity of caspase-3, caspase-8, and caspase-9 enzymes in the lung tissues of negative control group than control group of mice. However, treatment with dexmedetomidine ameliorates the altered activity of caspase-3, caspase-8, and caspase-9 enzymes than negative control group of mice.
Figure 8.
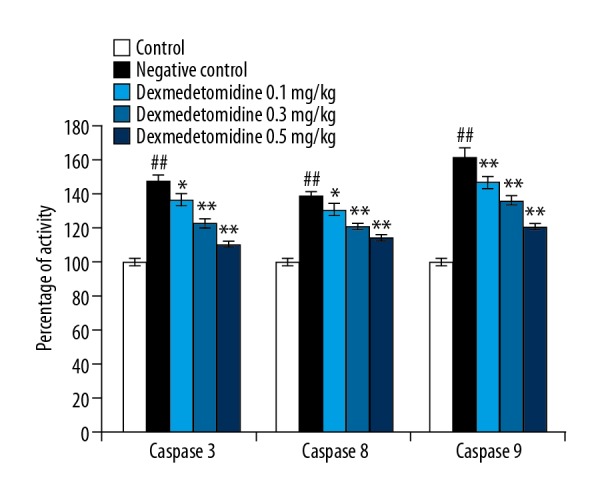
Dexmedetomidine attenuates the activity of caspase-3, caspase-8, and caspase-9 enzyme in lung tissues of mice with CLP-induced lung injury. Data are means ±SEMs (N=10); ## P<0.01 versus control group; * P<0.05; ** P<0.01 versus negative control group.
Discussion
This study evaluated the effects of dexmedetomidine on lung injury and investigated the possible molecular mechanism of action. Lung injury was induced by CLP and the effects of dexmedetomidine on lung injury were estimated by reference to the oxygenation index and the wet/dry weight ratio of the lung. The expression of mitochondrial protein was determined by western blot assays, and RT-PCR was used to assess the effects of dexmedetomidine on mitochondrial dynamics. Histopathological analyses of lung tissue were performed using H&E staining and TUNEL-positive cells were counted in TUNEL assays.
Mitochondria are normally subject to fusion and fission, where both of these processes are mediated by guanosine triphosphatases. In sepsis-induced lung injury, the balance between mitochondrial fusion and fission is disturbed, which results in the induction of cellular apoptosis. Several factors, such as oxidative stress and inflammation, contribute to an imbalance in mitochondrial function. The present study revealed that treatment with dexmedetomidine ameliorated CLP-induced lung injury.
Sepsis is among the causes of lung injury and has a high mortality rate. Meanwhile, inflammation and oxidative stress can induce organ injury due to sepsis [21]. Pro-inflammatory mediators such as IL-6, IL-1β, TNF-α, and oxidative stress promote apoptosis by enhancing the activity of caspase-3 in lung tissues [22]. We found that the concentrations of pro-inflammatory mediators as well as markers of oxidative stress such as mitochondrial MDA and MnSOD activity were significantly attenuated in lung tissues of dexmedetomidine-treated mice with CLP-induced lung injury.
Impairment of mitochondrial function in sepsis-induced organ damage has been reported both clinically and experimentally [23]. Mitochondria are important for numerous cellular functions, such as apoptosis, calcium signaling, metabolism, and energy production [24]. Mitochondrial dysfunction results in injury to all tissues. In a previous study, mitochondrial fusion promoted the expression of OPA1 in the inner membrane, and Mfn1 and MFn2 [25]. Treatment with dexmedetomidine significantly attenuated OPA1, Mfn1, and Mfn2 expression in lung tissues of mice with CLP-induced lung injury.
Conclusions
Dexmedetomidine protects against CLP-induced lung injury in mice by attenuating mitochondrial damage. Specifically, it attenuates mitochondrial fusion and fission by decreasing the level of proinflammatory mediators and oxidative stress in lung tissue.
Footnotes
Source of support: Education and Research Project for Middle-Aged and Young Teacher of the Education Department of Fujian Province (grant no: JAT170233)
Conflicts of interest
None.
References
- 1.Fujishima S. Organ dysfunction as a new standard for defining sepsis. Inflamm Regen. 2016;36:24. doi: 10.1186/s41232-016-0029-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Martin-Loeches I, Levy MM, Artigas A. Management of severe sepsis: Advances, challenges, and current status. Drug Des Devel Ther. 2015;9:2079–88. doi: 10.2147/DDDT.S78757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mittal M, Siddiqui MR, Tran K, et al. Reactive oxygen species inflammation and tissue injury. Antioxid Redox Signal. 2014;20(7):1126–67. doi: 10.1089/ars.2012.5149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zorov DB, Juhaszova M, Sollott SJ. Mitochondrial reactive oxygen species (ROS) and ROS-induced ROS release. Physiol Rev. 2014;94(3):909–50. doi: 10.1152/physrev.00026.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bartolák-Suki E, Imsirovic J, Nishibori Y, et al. Regulation of mitochondrial structure and dynamics by the cytoskeleton and mechanical factors. Int J Mol Sci. 2017;18(8) doi: 10.3390/ijms18081812. pii: E1812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Song Z, Ghochani M, McCaffery JM, et al. Mitofusins and OPA1 mediate sequential steps in mitochondrial membrane fusion. Mol Biol Cell. 2009;20(15):3525–32. doi: 10.1091/mbc.E09-03-0252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Strack S, Cribbs JT. Allosteric modulation of Drp1 assembly and mitochondrial fission by the variable domain. J Biol Chem. 2012;287(14):10990–1001. doi: 10.1074/jbc.M112.342105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Paradis S, Charles A-L, Meyer A, et al. Chronology of mitochondrial and cellular events during skeletal muscle ischemia-reperfusion. Am J Physiol Cell Physiol. 2016;310(11):C968–8. doi: 10.1152/ajpcell.00356.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kaur M, Singh PM. Current role of dexmedetomidine in clinical anesthesia and intensive care. Anesth Essays Res. 2011;5(2):128–33. doi: 10.4103/0259-1162.94750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tang C, Xia Z. Dexmedetomidine in perioperative acute pain management: A non-opioid adjuvant analgesic. J Pain Res. 2017;10:1899–904. doi: 10.2147/JPR.S139387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gertler R, Brown HC, Mitchell DH, Silvius EN. Dexmedetomidine: A novel sedative-analgesic agent. Proc (Bayl Univ Med Cent) 2001;14(1):13–21. doi: 10.1080/08998280.2001.11927725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gong Z, Ma L, Zhong Y-L, et al. Myocardial protective effects of dexmedetomidine in patients undergoing cardiac surgery: A meta-analysis and systematic review. Exp Ther Med. 2017;13(5):2355–61. doi: 10.3892/etm.2017.4227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wang X, Zhao B, Li X. Dexmedetomidine attenuates isoflurane-induced cognitive impairment through antioxidant, anti-inflammatory and anti-apoptosis in aging rat. Int J Clin Exp Med. 2015;8(10):17281–88. [PMC free article] [PubMed] [Google Scholar]
- 14.Kaur M, Singh PM. Current role of dexmedetomidine in clinical anesthesia and intensive care. Anesth Essays Res. 2011;5(2):128–33. doi: 10.4103/0259-1162.94750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fu C, Dai X, Yang Y, et al. Dexmedetomidine attenuates lipopolysaccharide-induced acute lung injury by inhibiting oxidative stress, mitochondrial dysfunction and apoptosis in rats. Mol Med Rep. 2017;15(1):131–38. doi: 10.3892/mmr.2016.6012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Meng PZ, Liu J, Hu PS, Tong F. Protective effect of dexmedetomidine on endotoxin-induced acute lung injury in rats. Med Sci Monit. 2018;24:4869–75. doi: 10.12659/MSM.908887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fu C, Dai X, Yang Y, et al. Dexmedetomidine attenuates lipopolysaccharide induced acute lung injury by inhibiting oxidative stress, mitochondrial dysfunction and apoptosis in rats. Mol Med Rep. 2017;15(1):131–38. doi: 10.3892/mmr.2016.6012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Xie C, Li Y, Liang J, et al. [The effect of dexmedetomidine on autophagy and apoptosis intestinal ischemia reperfusion-induced lung injury]. Zhonghua Jie He He Hu Xi Za Zhi. 2015;38:761–64. [in Chinese] [PubMed] [Google Scholar]
- 19.Zhang Q, Wu D, Yang Y, et al. Effects of dexmedetomidine on the protection of hyperoxia-induced lung injury in new born rats. Int J Clin Exp Pathol. 2015;8(6):6466–73. [PMC free article] [PubMed] [Google Scholar]
- 20.Wen H. Sepsis induced by cecal ligation and puncture. Methods Mol Biol. 2013;1031:117–24. doi: 10.1007/978-1-62703-481-4_15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bosmann M, Ward PA. The inflammatory response in sepsis. Trends Immunol. 2013;34(3):129–36. doi: 10.1016/j.it.2012.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Manke A, Wang L, Rojanasakul Y. Mechanisms of nanoparticle-induced oxidative stress and toxicity. Biomed Res Int. 2013;2013 doi: 10.1155/2013/942916. 942916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Singer M. The role of mitochondrial dysfunction in sepsis-induced multi-organ failure. Virulence. 2014;5(1):66–72. doi: 10.4161/viru.26907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pinton P, Giorgi C, Siviero R, et al. Calcium and apoptosis: ER-mitochondria Ca2+ transfer in the control of apoptosis. Oncogene. 2008;27(50):6407–18. doi: 10.1038/onc.2008.308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cipolat S, de Brito OM, Dal Zilio B, Scorrano L. OPA1 requires mitofusin 1 to promote mitochondrial fusion. Proc Natl Acad Sci USA. 2004;101(45):15927–32. doi: 10.1073/pnas.0407043101. [DOI] [PMC free article] [PubMed] [Google Scholar]