Abstract
PAK6 is a Group II p21 activated kinase that unlike traditional signal transduction proteins interacts with multiple binding partners including sex-steroid receptors. PAK6 acts as a nodal checkpoint integrating multiple cellular inputs to promote distinct cellular outcomes, some of which are associated with cytoskeletal remodeling. Despite the possibility that PAK6 may couple sex-specific neuronal function and therefore serve as a valuable research, diagnostic and therapeutic target, there is currently no standardized protocol for assessing PAK6 activity in a neuronal cell line. Here, we present a protocol for assessing PAK6 levels in a commonly used neuronal cell line, PC-12. In comparison with other methodology, this approach (1) does not require ex-planted tissue to identify PAK6 in neurons and (2) unlike other protocols which require steroid depleted media for detection of PAK6 in non-neuronal cell lines, such as prostate cancer cell lines, we were easily able to detect PAK6 in PC-12 cells grown in complete, steroid-containing media. Thus the present protocol allows for the efficient detection of native PAK6 in PC-12 cells to expedite targeted basic research of the emerging importance of PAK6 function in the brain as well as to accelerate the identification and isolation of potential therapeutic targets not only in cancerous but brain disease states as well.
Keywords: Cancer research, Cell biology, Neuroscience
1. Introduction
1.1. Background
PAK6 is a Group II, p21-activated kinase of clinical importance [1, 2] in cancer progression [3, 4, 5, 6, 7] as well as brain physiology including brain development [8, 9] Parkinson's disease [10], weight regulation [11, 12] and learning and memory [11, 13].
Originally discovered in a yeast-II-hybrid screen to identify androgen receptor interacting proteins [1, 2], sequence analysis of the protein revealed a CRIB (cdc42/rac interacting binding) domain as well as homology to already classified p-21 activated kinases (PAKs) indicating that it belonged to the Group II PAK family of serine/threonine kinases and was thus labeled PAK6 [1,2]. The Group II PAKS (4–6) differ in sequence and function from Group I PAKs (1–3) notably from the effect of GTPase binding. Importantly, in the case of PAK6 it also differs from other PAKs in its ability to specifically bind sex steroid receptors.
1.2. Steroid receptor binding
Its original discovery as an androgen receptor (AR) interacting protein in prostate cancer biopsies and cell lines [1, 2] has resulted in the development of several reagents easily accessed for research of the role of PAK6 in cancer progression. However, PAK6 has been identified in several brain areas [2, 14]. Despite this, other than knock-out mice, few reagents have been specifically developed to address the molecular role of PAK6 in the brain.
Consequently, what is known of the functional role of PAK6 is primarily derived from work with readily available cancer- and not neuronal- cell lines. PAK6 interacts with the steroid receptors ERα and AR [1, 2] and while modulated by, does not appear to be activated by cdc42/rac [2] despite having a CRIB domain. Structural diversity in a non-conserved, proline rich region located between the N-terminal CRIB region and the C-terminal kinase domain [15, 16] accounts for sub-specificity of binding partners among the Group II PAKs. Indeed, PAK6 binds steroid receptor via a portion of the interdomain located 5’ to the kinase domain [17]. Because Group II PAKs are highly expressed in brain [2, 14, 18], including cortex and other sexually dimorphic brain areas and because PAK6 binds to both ERα and AR, it is possible that PAK6 may couple sex differences in the brain.
1.3. Mechanism of action
Functionally, the Group I PAKs (1–3) require binding of the Rho family of small GTPases, cdc42 or rac, to alleviate autoinhibition of the kinase domain [15,16]. However, in all Group II PAKs the autoinhibitory domain is absent [15]. Even so, constitutive kinase activity of PAK6 can be modulated by signal transduction molecules such as MAP kinase, kinase 6 (MKK6) and p38 MAP kinase [19].
Instead of serving as relievers of autoinhibition, binding of some small GTPases such as cdc42 and Chp/RhoV appear to target PAK6 to either subcellular structures [20] or the cell membrane [5, 21]. This model is generally representative of the role of cdc42 binding in the Group II PAKs [16]. Instead of activating kinase function, the interaction appears to direct intracellular localization. This in turn promotes scaffolding with other proteins likely to be potential substrates of PAK6, several of which play an important role in actin remodeling. The suggestion that a phosphoprotein may also play an essential role as a scaffolding protein, particularly in brain, has recently been supported for Calcium/calmodulin kinase II, particularly in conjunction with NMDA receptor in the hippocampus [22].
1.4. Cellular localization of PAK6 varies with cell-type and milieu
Depending on the cell-type and milieu, PAK6 may be localized to the cell membrane [2, 23], in neurites [10] within cell-cell adhesions [5, 21], the cytosol or vesicular structures within the cell which then promotes interaction with a myriad of downstream effectors.
For example, in LNCaP cells co-transfection with androgen receptor translocates PAK6 interiorly to the cytosol [2], but adding AR ligand causes translocation of both AR and PAK6 to the nucleus where PAK6 represses typical androgen-induced AR transcription [23] and instead promotes cell motility [17]. In other cancer cell lines, as well, PAK6 plays a clear role in cell colony escape. For example, in HeLa cells transfected with a PAK6-GFP fusion protein, PAK6 is visualized in the cytoplasm but is more pronounced in the membrane including filopodia. In DU-145 prostate cancer and HT29 colon cancer cell lines, hepatocyte growth factor (HGF) upregulates PAK6 autophosphorylation and promotes PAK6 interaction with IQGAP1/E-cadherin at cell-cell adhesions promoting cell colony escape via phosphorylation of β-catenin [21].
On the other hand, in whole tissue mouse models utilizing adenovirus vector to introduce flag-tagged PAK6 into cortex, PAK6 is expressed in soma, dendrites and neurites [10]. In the latter case, PAK6 phosphorylates 14-3-3 which disrupts the 14-3-3/LRRK2 interaction ultimately inducing LRRK2 dephosphorylation [10, 24] to promote neurite outgrowth and in the case of double PAK5/PAK6 knockout mice there are clear deficits in cortical neuron filopodia, growth cone and neurite complexity [13]. In all cases, the role of PAK6 depends on the cell-type and milieu, but a pattern emerges suggesting that PAK6 targeting to the membrane enhances morphological changes that in the case of cancerous cell lines promotes cell motility. In brain, morphological changes may instead result in alterations in spine density, neurite outgrowth or synaptic rearrangement.
1.5. The biological relevance of PAK6
Cytoskeletal rearrangement may be the commonality that makes PAK6 critically important in both cancer progression and proper brain function. Both PAK5 [18] and PAK6 [10] are implicated in neurite outgrowth. Emerging data shows that PAK6 expression regulates morphological changes associated with the transition from migratory to post-migratory development of GABAergic interneurons in POA and cortex [9]; both areas, but particularly the POA are sexually dimorphic and differ in expression of sex steroid receptors, making the PAK6-steroid receptor interaction of critical importance.
That PAK6 overexpression can increase the pool of GABAergic neurons developmentally is particularly interesting in light of recent data showing PAK6 as a potential candidate gene in epileptic encephalopathy [25]. In less severe and more prevalent types of epilepsy, sex differences in terms of incidence rates, sequalae and the influence of sex steroids [26] implicate a putative role for the PAK6/steroid receptor interaction.
As well, mutations of a PAK6 interacting protein, LRRK2, have been implicated in Parkinson's Disease (PD) [27] and in this case, the PAK6/AR interaction may help explain the sex difference in those diagnosed with this disease. PAK6 co-localization with androgen receptor (AR) appears to be most prominent in the nigrostriatal pathway with the most extensive co-localization in the VTA/SN [14]. Perhaps, not surprisingly, then, knock-out of PAK5, PAK6 or both show behavioral phenotypes with disrupted motor function [12, 25]. Furthermore, a substrate of PAK6, the synaptic vesicle recycling protein, synpatojanin-1 [28] has been identified in some cases of early-onset Parkinson's Disease or epileptic encephalopathy [29] providing an underlying commonality for two neurological diseases that manifest very differently.
Evolutionarily, PAK6 shares sequence homology with Drosophila mushroom bodies tiny (MBT), a protein expressed in an area analogous to the hippocampus [30] and both human tissue blot [2] and Western Blot of lysate from wild-type mice [13] show highest levels of PAK6 in cortical structures, including the hippocampus. Indeed, learning and memory are impaired in PAK5/PAK6 knockout mice [11, 25]. Sex steroids have been implicated in differences in learning and memory [31,32] and estrogens have long been known to regulate energy balance [33]. Interestingly, the single PAKII isoform knockout of only PAK6 results in mice that weigh more than wild-type mice despite similar food consumption and running wheel activity; however, PAK6 null mice were found to have lower levels of ERα in whole brain samples than wild-type mice [12] which may have important implications in the study of obesity.
Taken together, the biological importance of PAK6 in neuronal function and its subsequent effect on physiology in the normal and disease state, during development or in the adult merit further investigation.
1.6. Necessity of an efficient cell model to assess the PAK6/Steroid receptor interaction in a neuronal cell line
PAK6 has come to be seen as a nodal checkpoint in signal transduction due to its many interacting partners-which are still being discovered; its range of substrates-which are also still being identified, and its subsequent subcellular localization. It is clear that diverse cellular events merge at PAK6, including differential function depending on presence of steroid receptor and ligand, which make it an important target in both cancer progression and brain function, and particularly sex-specific brain differences.
Despite the clear importance of PAK6 in neuronal function and its exceptional ability to integrate multiple intracellular events, including the presence of sex steroid hormone, there is no published protocol for assessing PAK6 function at the neuronal level. A Pubmed search for “PAK6 and neuronal cell line,” as well as Pubmed searches with specific neuronal cell lines such as “PAK6 and HCN-2” or “PAK6 and PC-12” currently yields no results.
To address this deficiency, we chose to assess the presence of PAK6 in a commonly available neuronal cell line, PC-12 cells. Emerging data supports a role for PAK6 in cytoskeletal rearrangement which in some cases may impact neurite outgrowth and in others vesicular trafficking and in furthering these investigations PC-12 cells which have long been utilized in studies of neurosecretion [34] and neurite outgrowth [35] are an ideal model. And, while a role for PAK6 in neurological disorders such as Parkinson's Disease is rather more recent, dopamine has long been implicated in neurodevelopmental disorders such as schizophrenia and neurodegenerative disorders such as Parkinson's Disease. Thus, it is worth noting that in addition to being well-established as a neuronal cell model for investigations of neurosecretion [34] and neurite outgrowth [35], PC-12 cells are also dopaminergic (https://www.atcc.org/products/all/CRL-1721.aspx#characteristics) making PC-12 cells and ideal neuronal cell line to study PAK6 effects on neuronal function.
In transferring to neurons one approach may be to adapt the readily available protocols from cancer cells [19]. However, there is an important difference here and in detection of PAK6 in general. PAK6 is minimally detectable in LNCaP cells, unless grown in androgen-depleted media supplemented with charcoal stripped-FBS instead of common FBS [3]. On the other hand, here, we report that we were able to detect PAK6 in PC-12 cells grown in media with commonly available FBS. This is an important distinction in technique and expense as charcoal stripped-FBS is twice the cost of FBS. While AR is expressed in LNCaP cells, ARs are down-regulated in NGF differentiated PC-12 cells [36]. Mechanistically, it's likely that the distinction generalizes to pursing two distinct strategies depending on the presence or absence of known ligand-dependent, PAK-6 interacting proteins such as AR or ERα.
We show here, that PAK6 can be detected in PC-12 cells, a readily accessible neuronal cell line. Furthermore, we show that PAK6 is readily detectable in PC-12 cells grown in complete media eliminating both the need to generate constructs for transfection or to grow cells in costly steroid depleted (i.e. charcoal-stripped) media. This makes PC-12 cells an excellent candidate to study PAK6 function.
2. Methods
2.1. Cells and reagents
LNCaP cells, a human prostate cancer cell line, and PC12 cells, a pheochromocytoma cell line derived from rat adrenal medulla were both obtained from American Tissue Culture Collection (ATCC, Washington DC; LNCaP, CRL-1740 and PC-12, CRL-1721). Cell culture media including fetal bovine serum (FBS), and other supplements were from Invitrogen (Grand Island, NY). Antibodies for Western Blot and markers were obtained from Santa Cruz Biotechnology and DMSO, protein standards and 1X Bradford reagent were from Bio-Rad (Hercules, CA). See Fig. 1.
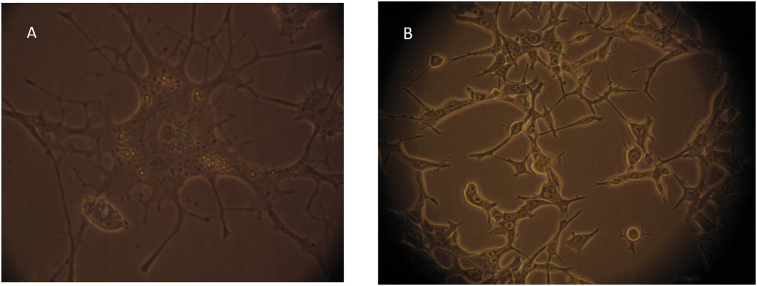
Fig. 1.
PC-12 and LNCaP cells in culture. (A) PC-12 cells, at 1000×, in 10% FBS-DMEM, supplemented with NGF. (B) LNCaP, at 200×, cells grown in 10% FBS-DMEM.
2.2. Cell culture maintenance
Cells were grown in Dulbecco's modified Eagle's medium (DMEM) with 10% FBS supplemented with 0.5 mg/mL penicillin/streptomycin/glutamine. PC-12 cells were further supplemented with nerve growth factor (NGF), but otherwise treated exactly as the LNCaP cells. Cells were incubated at 37 °C in 5% CO2. When necessary, approximately twice weekly, cells were passaged with 500 μL of trypsin in DMEM then centrifuged at 5000 rpm for 10 minutes. The supernatant would then be re-suspended in 4 ml DMEM, and cells transferred to two separate plates with 8 mL of fresh 10% FBS-DMEM added to each. To maintain cells, between splitting, they were washed briefly with 1X PBS and then fresh media would be added to the cells. Importantly, both PC-12 and LNCaP cells were maintained in this manner.
2.3. Total protein staining
Cells were collected in PBS by scraping from culture flasks and then washed twice with cold PBS. The slurry was centrifuged and lysis buffer consisting of 1.0 ml NP40 supplemented with protease inhibitor cocktail and 1 mM phenylmethylsulfonyl fluoride (PMSF) was added to the pellet, then incubated for 30 minutes on ice with vortexing every 10 minutes. The cell extract was then transferred to a microfuge tube and centrifuged at 13,000 rpm for 10 minutes at 4 °C. Clear lysates were then aliquoted into clean microfuge tubes and stored at 4 °C.
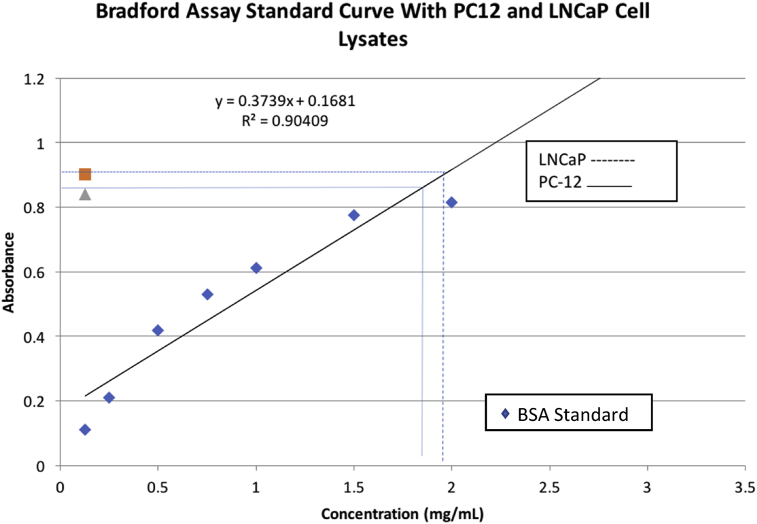
To determine total protein, a Bradford Assay was carried out. However, we modified the manufacturer's instructions by reducing the volume of protein standard, containing BSA, (obtained from Biorad) from 50 μl to 5 μl per well. PC-12 and LNCaP lysates were added and dH2O served as a control. To compensate for the decreased volume, 250 μl of Bradford reagent was added to each well with a multi-channel pipettor. Standards, control and lysates were run in triplicate. The plates were placed on a shaker for 10 minutes then read at an absorbance of 595 nm. See Fig. 2.
Fig. 2.
Protein totals in each lysate via Bradford Assay.
2.4. Western Blot
Next, we prepared samples of LNCaP and PC-12 were prepared at 15 μl, 21 μl and 27 μl. Samples were heated at 70C for 5 minutes, loaded and then electrophoresed at 200 V for 60 minutes on NuPAGE gel. Proteins were transferred electrophoretically to nitrocellulose membrane in 1X NuPAGE Transfer Buffer at 30 V for 60 minutes. The membrane was blocked with blocking buffer (Invitrogen) for 30 min at room temperature then incubated with primary antibodies in 5%BSA-PBS for 1hr with shaking. After incubation with primary antibodies, the membrane was washed and then incubated with secondary antibodies for 1hr with shaking. Primary antibodies were: goat polyclonal anti-PAK6 (1:2000; Santa Cruz Biotechnology, Santa Cruz, CA, sc-25976) and mouse monoclonal anti-actin (1:2000: Santa Cruz Biotechnology, Santa Cruz, CA, sc-376421).
Secondary antibodies conjugated to horseradish peroxidase (HRP) were donkey anti-goat for visualization of PAK6 (1:5000; Santa Cruz Biotechnology, Santa Cruz, CA, sc-2020) and chicken anti-mouse for the actin control (1:5000; Santa Cruz Biotechnology, sc-2954). After incubation in the secondary antibodies, the membrane was washed in PBS-Tris and prepared for detection with CN-DAB in stable peroxidase substrate buffer for Western Blot (ThermoFisher Scientific, #3400) according to the manufacturer's instructions.
3. Results
PC-12 cells and LNCaP cells were grown in similar standard cell culture conditions except that NGF was added to the PC-12 neuronal cell line.
The Bradford Assay indicated total protein in LNCaP lysate was 1.70 mg/ml and in PC12 cells was 1.95 mg/ml, Fig. 2. Subsequent Commaisse blue staining verified protein visualization in 10 μl of lysate for each cell-type.
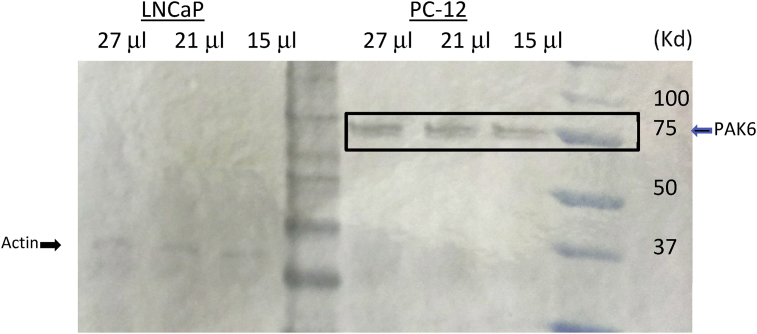
Next, we carried out a Western Blot and as can be seen in Fig. 3, PAK6 is easily visualized in PC-12 cells, but not LNCaP cells grown in FBS-DMEM. Thus, given typical cell culture conditions PC-12 cells are an excellent candidate to investigate neuronal PAK6.
Fig. 3.
PAK6 in PC-12, but not LNCaP cells grown in FBS-DMEM.
4. Discussion
PAK6, like all Group II PAKs is highly expressed in brain, particularly in cortex including hippocampus and the striatum [2, 14]. Its interaction with small GTPases alters function either by stimulating additional kinase activity or by targeting PAK6 to various intracellular structures or the membrane. However, PAK6 is the only Group II PAK to interact with the sex steroid receptors. As such, PAK6 is an excellent candidate to couple sex-steroid specific brain function and the PC-12 cell model lends itself well to both investigations of basic research, such as mechanisms of sex differences in learning and memory in the brain, as well as clinically focused investigations into potential novel targets for PD.
PAK6 is highly expressed in hippocampus, has been implicated behaviorally in learning and memory and co-localizes with both AR and ERα. Given its differential function depending on presence of ligand, it is possible that PAK6 expression contributes to activational sex differences in learning and memory. Because of this and its role in actin rearrangement and membrane morphology, it is tempting to speculate further whether PAK6 might mediate estrogen induced changes in dendritic spine density [37] and again, an efficient way to test this would be in a neuronal cell line already demonstrated to express PAK6, as we present here.
From an oncological perspective the group II PAKs, including PAK6, are emerging as novel drug targets themselves or as part of a targeted pathway, particularly for cancers that do not respond to current treatments [3, 4, 5, 7, 21,38,39]. It is tantalizing to consider how these novel drugs might also be used to further our understanding of the role of PAK6 in the brain. Clinically, PAK6 might be a novel target for PD treatment and it would be expeditious to use the same “inhibitors” in a neuronal cell line to determine and compare relevant downstream effects. Such investigations would increase our understanding of the role of PAK6 in brain function.
5. Conclusion
The research presented here demonstrates that (1) PAK6 can be detected via Western Blot in a commonly used neuronal cell line, PC-12 cells which makes it an excellent neuronal cell model to study PAK6 function and (2) unlike other cell lines, detection of PAK6 is possible even in complete media containing steroid receptor ligands.
Declarations
Author contribution statement
Sharon Ramos Goyette: Conceived and designed the experiments; Performed the experiments; Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data; Wrote the paper.
Eric Schott: Performed the experiments; Analyzed and interpreted the data. Astopheline Uwimana, David W. Nelson, Jacob Boganski: Performed the experiments.
Funding statement
This work was supported by funds made available through Stonehill College.
Competing interest statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
References
- 1.Yang F., Li X., Sharma M., Zarnegar M., Lim B., Sun Z. Androgen receptor specifically interacts with a novel p21-activated kinase, PAK6. J. Biol. Chem. 2001 doi: 10.1074/jbc.M010311200. [DOI] [PubMed] [Google Scholar]
- 2.Lee S.R., Ramos S.M., Ko A., Masiello D., Swanson K.D., Lu M.L., Balk S.P. AR and ER interaction with a p21-activated kinase (PAK6) Mol. Endocrinol. 2002 doi: 10.1210/mend.16.1.0753. [DOI] [PubMed] [Google Scholar]
- 3.Kaur R., Yuan X., Lu M.L., Balk S.P. Increased PAK6 expression in prostate cancer and identification of PAK6 associated proteins. Prostate. 2008 doi: 10.1002/pros.20787. [DOI] [PubMed] [Google Scholar]
- 4.Jernström S., Hongisto V., Leivonen S.K., Due E.U., Tadele D.S., Edgren H., Kallioniemi O., Perälä M., Mælandsmo G.M., Sahlberg K.K. Drug-screening and genomic analyses of HER2-positive breast cancer cell lines reveal predictors for treatment response. Breast Cancer. 2017;9:185–198. doi: 10.2147/BCTT.S115600. Dove Med. Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Morse E.M., Sun X., Olberding J.R., Ha B.H., Boggon T.J., Calderwood D.A. PAK6 targets to cell-cell adhesions through its N-terminus in a Cdc42-dependent manner to drive epithelial colony escape. J. Cell Sci. 2016;129:380–393. doi: 10.1242/jcs.177493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chen J., Lu H., Yan D., Cui F., Wang X., Yu F., Xue Y., Feng X., Wang J., Wang X., Jiang T., Zhang M., Zhao S., Yu Y., Tang H., Peng Z. PAK6 increase chemoresistance and is a prognostic marker for stage II and III colon cancer patients undergoing 5-FU based chemotherapy. Oncotarget. 2015;6:355–367. doi: 10.18632/oncotarget.2803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.King H., Nicholas N.S., Wells C.M. Role of p-21-activated kinases in cancer progression. Int. Rev. Cell Mol. Biol. 2014;309:347–387. doi: 10.1016/B978-0-12-800255-1.00007-7. [DOI] [PubMed] [Google Scholar]
- 8.Pensold D., Zimmer G. Single-cell transcriptomics reveals regulators of neuronal migration and maturation during brain development. J. Exp. Neurosci. 2018;12 doi: 10.1177/1179069518760783. 1179069518760783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pensold D., Symmank J., Hahn A., Lingner T., Salinas-Riester G., Downie B.R., Ludewig F., Rotzsch A., Haag N., Andreas N., Schubert K., Hübner C.A., Pieler T., Zimmer G. The DNA methyltransferase 1 (DNMT1) controls the shape and dynamics of migrating POA-derived interneurons fated for the murine cerebral cortex. Cerebr. Cortex. 2017;27:5696–5714. doi: 10.1093/cercor/bhw341. [DOI] [PubMed] [Google Scholar]
- 10.Civiero L., Cirnaru M.D., Beilina A., Rodella U., Russo I., Belluzzi E., Lobbestael E., Reyniers L., Hondhamuni G., Lewis P.A., Van den Haute C., Baekelandt V., Bandopadhyay R., Bubacco L., Piccoli G., Cookson M.R., Taymans J.M., Greggio E. Leucine-rich repeat kinase 2 interacts with p21-activated kinase 6 to control neurite complexity in mammalian brain. J. Neurochem. 2015;135:1242–1256. doi: 10.1111/jnc.13369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Furnari M.A., Jobes M.L., Nekrasova T., Minden A., Wagner G.C. Functional deficits in Pak5, Pak6 and Pak5/Pak6 knockout mice. PLoS One. 2013;8 doi: 10.1371/journal.pone.0061321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Furnari M.A., Jobes M.L., Nekrasova T., Minden A., Wagner G.C. Differential sensitivity of Pak5, Pak6, and Pak5/Pak6 double-knockout mice to the stimulant effects of amphetamine and exercise-induced alterations in body weight. Nutr. Neurosci. 2014 doi: 10.1179/1476830513Y.0000000072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nekrasova T., Jobes M.L., Ting J.H., Wagner G.C., Minden A. Targeted disruption of the Pak5 and Pak6 genes in mice leads to deficits in learning and locomotion. Dev. Biol. 2008;322:95–108. doi: 10.1016/j.ydbio.2008.07.006. [DOI] [PubMed] [Google Scholar]
- 14.Mahfouz A., Lelieveldt B.P., Grefhorst A., van Weert L.T., Mol I.M., Sips H.C., van den Heuvel J.K., Datson N.A., Visser J.A., Reinders M.J., Meijer O.C. Genome-wide coexpression of steroid receptors in the mouse brain: identifying signaling pathways and functionally coordinated regions. Proc. Natl. Acad. Sci. U. S. A. 2016;113:2738–2743. doi: 10.1073/pnas.1520376113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jaffer Z.M., Chernoff J. p21-Activated kinases: three more join the Pak. Int. J. Biochem. Cell Biol. 2002;34:713–717. doi: 10.1016/s1357-2725(01)00158-3. [DOI] [PubMed] [Google Scholar]
- 16.Ha B.H., Morse E.M., Turk B.E., Boggon T.J. Signaling, regulation, and specificity of the type II p21-activated kinases. J. Biol. Chem. 2015;290:12975–12983. doi: 10.1074/jbc.R115.650416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Liu X., Busby J., John C., Wei J., Yuan X., Lu M.L. Direct interaction between AR and PAK6 in androgen-stimulated PAK6 activation. PLoS One. 2013;8 doi: 10.1371/journal.pone.0077367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dan C., Nath N., Liberto M., Minden A. PAK5, a new brain-specific kinase, promotes neurite outgrowth in N1E-115 cells. Mol. Cell Biol. 2002;22:567–577. doi: 10.1128/MCB.22.2.567-577.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kaur R., Liu X., Gjoerup O., Zhang A., Yuan X., Balk S.P., Schneider M.C., Lu M.L. Activation of p21-activated kinase 6 by MAP kinase kinase 6 and p38 MAP kinase. J. Biol. Chem. 2005;280:3323–3330. doi: 10.1074/jbc.M406701200. [DOI] [PubMed] [Google Scholar]
- 20.Shepelev M.V., Korobko I.V. Pak6 protein kinase is a novel effector of an atypical Rho family GTPase Chp/RhoV. Biochem. Biokhimi͡ia. 2012;77:26–32. doi: 10.1134/S0006297912010038. [DOI] [PubMed] [Google Scholar]
- 21.Fram S., King H., Sacks D.B., Wells C.M. A PAK6-IQGAP1 complex promotes disassembly of cell-cell adhesions. Cell. Mol. Life Sci. 2014;71:2759–2773. doi: 10.1007/s00018-013-1528-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Incontro S., Díaz-Alonso J., Iafrati J., Vieira M., Asensio C.S., Sohal V.S., Roche K.W., Bender K.J., Nicoll R.A. The CaMKII/NMDA receptor complex controls hippocampal synaptic transmission by kinase-dependent and independent mechanisms. Nat. Commun. 2018;9 doi: 10.1038/s41467-018-04439-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schrantz N., da Silva Correia J., Fowler B., Ge Q., Sun Z., Bokoch G.M. Mechanism of p21-activated kinase 6 (PAK6) - mediated inhibition of androgen receptor signaling. J. Biol. Chem. 2003 doi: 10.1074/jbc.M311145200. [DOI] [PubMed] [Google Scholar]
- 24.Civiero L., Cogo S., Kiekens A., Morganti C., Tessari I., Lobbestael E., Baekelandt V., Taymans J.M., Chartier-Harlin M.C., Franchin C., Arrigoni G., Lewis P.A., Piccoli G., Bubacco L., Cookson M.R., Pinton P., Greggio E. PAK6 phosphorylates 14-3-3gamma to regulate steady state phosphorylation of LRRK2. Front. Mol. Neurosci. 2017;10:417. doi: 10.3389/fnmol.2017.00417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Oliver K.L., Lukic V., Freytag S., Scheffer I.E., Berkovic S.F., Bahlo M. In silico prioritization based on coexpression can aid epileptic encephalopathy gene discovery. Neurol. Genet. 2016;2:e51. doi: 10.1212/NXG.0000000000000051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Reddy D.S. The neuroendocrine basis of sex differences in epilepsy. Pharmacol. Biochem. Behav. 2017;152:97–104. doi: 10.1016/j.pbb.2016.07.002. [DOI] [PubMed] [Google Scholar]
- 27.Li J.-Q., Tan L., Yu J.-T. The role of the LRRK2 gene in Parkinsonism. Mol. Neurodegener. 2014;9:47. doi: 10.1186/1750-1326-9-47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Strochlic T.I., Concilio S., Viaud J., Eberwine R.A., Wong L.E., Minden A., Turk B.E., Plomann M., Peterson J.R. Identification of neuronal substrates implicates Pak5 in synaptic vesicle trafficking. Proc. Natl. Acad. Sci. U. S. A. 2012;109:4116–4121. doi: 10.1073/pnas.1116560109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hardies K., Cai Y., Jardel C., Jansen A.C., Cao M., May P., Djémié T., Hachon Le Camus C., Keymolen K., Deconinck T., Bhambhani V., Long C., Sajan S.A., Helbig K.L., AR working group of the EuroEPINOMICS RES Consortium. Suls A., Balling R., Helbig I., De Jonghe P., Depienne C., De Camilli P., Weckhuysen S. Loss of SYNJ1 dual phosphatase activity leads to early onset refractory seizures and progressive neurological decline. Brain. 2016;139:2420–2430. doi: 10.1093/brain/aww180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hofmann C., Shepelev M., Chernoff J. The genetics of Pak. J. Cell Sci. 2004;117:4343–4354. doi: 10.1242/jcs.01392. [DOI] [PubMed] [Google Scholar]
- 31.Goyette S.R., McCoy J.G., Kennedy A., Sullivan M. Sex differences on the judgment of line orientation task: a function of landmark presence and hormonal status. Physiol. Behav. 2012;105 doi: 10.1016/j.physbeh.2011.11.018. [DOI] [PubMed] [Google Scholar]
- 32.Kimura D. Sex, sexual orientation and sex hormones influence human cognitive function. Curr. Opin. Neurobiol. 1996;6:259–263. doi: 10.1016/s0959-4388(96)80081-x. [DOI] [PubMed] [Google Scholar]
- 33.López M., Tena-Sempere M. Estrogens and the control of energy homeostasis: a brain perspective. Trends Endocrinol. Metabol. 2015;26:411–421. doi: 10.1016/j.tem.2015.06.003. [DOI] [PubMed] [Google Scholar]
- 34.Westerink R.H., Ewing A.G. The PC12 cell as model for neurosecretion. Acta Physiol. (Oxf). 2008;192:273–285. doi: 10.1111/j.1748-1716.2007.01805.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fujii D.K., Massoglia S.L., Savion N., Gospodarowicz D. Neurite outgrowth and protein synthesis by PC12 cells as a function of substratum and nerve growth factor. J. Neurosci. 1982;2:1157–1175. doi: 10.1523/JNEUROSCI.02-08-01157.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Alexaki V.I., Dermitzaki E., Charalampopoulos I., Kampa M., Nifli A.P., Gravanis A., Margioris A.N., Castanas E. Neuronal differentiation of PC12 cells abolishes the expression of membrane androgen receptors. Exp. Cell Res. 2006;312:2745–2756. doi: 10.1016/j.yexcr.2006.04.023. [DOI] [PubMed] [Google Scholar]
- 37.Woolley C.S. Estrogen-mediated structural and functional synaptic plasticity in the female rat hippocampus. Horm. Behav. 1998;34:140–148. doi: 10.1006/hbeh.1998.1466. [DOI] [PubMed] [Google Scholar]
- 38.Cai S., Chen R., Li X., Cai Y., Ye Z., Li S., Li J., Huang H., Peng S., Wang J., Tao Y., Huang H., Wen X., Mo J., Deng Z., Wang J., Zhang Y., Gao X., Wen X. Downregulation of microRNA-23a suppresses prostate cancer metastasis by targeting the PAK6-LIMK1 signaling pathway. Oncotarget. 2015;6:3904–3917. doi: 10.18632/oncotarget.2880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sobhani N., Generali D., Roviello G. PAK6-Associated support vector machine classifier: a new way to evaluate response and survival of gastric cancer treated by 5-FU/oxaliplatin chemotherapy. EBioMedicine. 2017;22:18–19. doi: 10.1016/j.ebiom.2017.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]