Abstract
Background
Postmenopausal women are at higher risk of mental disorders. Oxidative stress has implication in the development of these disorders. Dietary total antioxidant capacity (DTAC) has been proposed as a tool for assessing dietary antioxidants intake. The relationship between DTAC with depression, anxiety and stress has not been investigated in postmenopausal women. Thus, we aimed to assess the association between DTAC and depression, stress and anxiety as well as oxidative stress biomarkers.
Methods
This cross-sectional study was carried out on 175 postmenopausal women. Data on dietary intake and mental health were collected by 147-item semi-quantitative food frequency questionnaires (FFQ) and Depression Anxiety Stress Scales (DASS-42), respectively. Dietary and serum total antioxidant capacity (TAC), malondialdehyde (MDA), oxidized-LDL, and superoxide dismutase (SOD) were measured. ANOVA test was applied to compare the mean of variables across the tertiles of DTAC. The relationship between DTAC and oxidative stress biomarkers was determined through ANCOVA method. Simple and multivariate linear regression tests were performed to measure the relationship between DTAC and mental health.
Results
Serum MDA level was significantly lower in the subjects at the highest tertiles of DTAC (P-value < 0.001). In addition, serum TAC level was significantly higher in subjects at the second tertile of DTAC (P-value = 0.04). DTAC was inversely and independently related to depression (β = − 0.16, P-value = 0.03) and anxiety scores (β = − 0.21, P-value = 0.007). There was no significant association between DTAC and stress score (β = − 0.10, P-value = 0.1).
Conclusion
An inverse relationship was found between DTAC with depression, anxiety scores and some oxidative stress biomarkers in postmenopausal women. These findings indicate DTAC may be used for developing effective dietary measures for reducing depression and anxiety in these women.
Keywords: Total antioxidant capacity, Depression, Oxidative stress, Menopause
Introduction
Mental disorders have been associated with a wide range of chronic diseases, disability, and even mortality, particularly among elderly. Depression will be the second leading cause of disease in 2020 as reported by world health organization (WHO) [1]. Postmenopausal women are often at high risk of depression due to the lower level of estrogen [2], as data from longitudinal studies and meta-analyses have noted the chance of developing the depressive symptoms substantially increases during the menopausal transition and early postmenopausal years [3, 4]. Estrogen is involved in mood regulation, and its action is mediated through modulating the receptor binding of serotonin and noradrenaline as well as availability of these neurotransmitters at the synapsis places. So, the decline of estrogen during menopause may underlie depressive mood in menopausal women [5]. Additionally, because of estrogen decline, menopausal women face various distressing symptoms such as hot flashes and sleep disturbance which may increase their vulnerability for mood disorders [6]. Considering the high prevalence of depression (44.2%) and anxiety (67%) among postmenopausal women living in Tehran, Iran [7], finding an approach to modify these symptoms among the women during menopause is of vital importance.
Menopausal women are at high risk for oxidative stress mainly due to estrogen deprivation [8]. Existing evidence suggests that oxidative stress might be linked with development of menopause-associated depression. Estradiol (E2), a form of estrogen, exerts antioxidant effect through its chemical structure containing the phenolic ring [9]. Besides, E2 can confer antioxidant activity through modulating the gene expression and function of antioxidant enzymes such as superoxide dismutase (SOD), glutathione peroxidase (GPx) and catalase [10]; thereby, reduction in estrogen level may increase the oxidative stress. It was indicated that plasma lipoperoxide (LPO) levels, a marker of oxidative damage, were increased; while total antioxidant status (TAS) and activity of antioxidant enzymes were reduced in postmenopausal women compared with premenopausal women [8, 11].
Diet is considered as the main part of lifestyle modification [12]. For decades, the association between special dietary factors, particularly fruits and vegetables and mental health has been investigated [13]. Although fruits and vegetables have abundant amounts of dietary antioxidants, they cannot adequately represent the overall ability and interactions of antioxidants in the whole diet. Thus, the dietary concept which takes the whole dietary antioxidants into account has become more prominent. Dietary total antioxidant capacity (DTAC) is an indicator of diet quality which is used to estimate the cumulative power of antioxidants in the whole diet [14]. Recently, DTAC is described as an effective tool for determining the health outcomes in middle-aged and elderly populations [15].
To date, DTAC has been associated with various chronic diseases [16–18], but little attention has been paid to DTAC in relation to mental health. We are aware of a case–control study which showed no significant difference of DTAC between depressed and normal subjects [19]. Examining the relationship between DTAC and mental health is particularly relevant for Middle East countries, where the prevalence of mental disorder is rampant and the quality of life is not desirable. Moreover, concerning the studies that linked the mental disorders to increased risk of illnesses and mortality among the elderly [1], investigating the association between DTAC and mental health in postmenopausal women can be helpful. Given the limited knowledge, we aimed to elucidate the association of DTAC with depression, stress and anxiety and some oxidative stress in postmenopausal women.
Methods
Participants and study design
The details of the study procedure have been described previously [20]. In brief, subjects were 175 postmenopausal women that postmenopausal status identified by an absence of a menstrual cycle for at least 1 year. Participants were selected randomly from municipality health houses and health centers affiliated to Tehran University of Medical Sciences. Using random sampling method, two regions from six southern regions of Tehran were randomly selected. Of these two regions, ten urban health centers affiliated to Tehran University of Medical Sciences and ten health houses affiliated with the southern municipality of Tehran were randomly selected. Finally, this study was composed of 175 postmenopausal women up to age 76 years, who enrolled through attendance to urban health centers and health houses.
Subjects were recruited from September 2016 to Januarys 2017. Exclusion criteria were; obesity [body mass index (BMI) ≥ 40 kg/m2], medically diagnosed of chronic diseases including cancer, diabetes, stroke, multiple sclerosis, dementia, hyper or hypothyroidism. Women were excluded if they were alcohol consumer, smoker (smoking for at least once a week), currently on hormone therapy or in the preceding 6 months or had any modifications in habitual diets. This study was approved by the Ethics Committee of Tehran University of Medical Sciences. Written informed consent was obtained from all subjects.
Dietary intake assessment
Subjects interviewed to respond to the validated semi-quantitative food frequency questionnaire (FFQ) consisted of 147 items to assess the frequency of food consumption of participants during the previous year [21]. Each subject’s dietary data were converted to the gram, and then the average daily intake of nutrients and total energy were determined according to the Nutritionist 4 software modified for Iranian foods.
DTAC was calculated by summing the individual dietary antioxidant capacities based on the oxygen radical absorbance capacity (ORAC) as described in our previous studies [20, 22]. The DTAC values of the individuals were reported as micromole of Trolox Equivalents per day (µmol TE/day).
Sociodemographic, anthropometrics and physical activity measurement
Self-reported information on general characteristics including age, educational level, marital and economic status, time since menopause, medical history, medicine and nutritional supplement use were obtained from all women. Height was measured using the wall-mounted stadiometer to the nearest 0.5 cm. Weight was measured in light clothing to the nearest 0.1 kg with a digital weighing scale (Seca725 GmbH & Co. Hamburg, Germany). Waist circumference (WC) was assessed to the nearest 0.5 cm using the flexible tape at the midpoint between the lowest rib and the iliac crest. BMI was calculated by dividing body weight (kg) by the square of body height (m2). Physical activity was measured using the short form of the International Physical Activity Questionnaire (IPAQ) [23].
Blood collection and plasma biomarker processing
12–14-h fasting blood sample was drawn and collected in a tube without ethylenediaminetetraacetic acid (EDTA) or heparin and placed on ice for 1 h. Then, it was centrifuged at 3000g for 10 min. Serum was separated, and frozen at − 80 °C for various biochemical evaluations. Serum total antioxidant capacity (TAC) was analyzed by a commercially available Kit (ZellBio GmbH, Germany) in accordance with the instructions of the manufacturer, based on the colorimetric assay of oxidation reduction at the wavelength of 490 nm. Intra- and inter-assay coefficients of variation (CVs (were less than 3.4% and 4.2%, respectively. Malondialdehyde (MDA) was measured by commercially available colorimetric assay kit (ZellBio GmbH, Germany) according to the instructions of the manufacturer. In this method, MDA reacts with thiobarbituric acid (TBA) under a high temperature, then MDA–TBA produced by this reaction is measured based on the colorimetric assay on an acidic medium and high temperature (90–100 °C) at 535 nm. Intra- and inter-assay CVs were 5.8% and 7.6%, respectively. Superoxide dismutase (SOD), were determined using commercially available kits (ZellBio GmbH, Germany) based on the colorimetric method (420 nm), with intra- and inter-assay CVs of 5.8% and 7.2%, respectively. Oxidized low-density lipoprotein (Ox-LDL) was measured using an ELISA kit (ZellBio GmbH, Germany), with intra- and inter-assay CVs less than 10% and 12%, respectively.
Mental health assessment
Depression Anxiety Stress Scales (DASS-42) was applied for measuring the mental health status of subjects. The DASS-42 consists of 42 items, 14 items per subscale: depression, anxiety, and stress. The respondent scores each item based on frequency or severity of emotional experiences over the last week on four-point scale ranged from 0 to 3. The score of 0 considered that the item “no symptoms at all” to 3 that indicates the item was “very severe”. Five categories are represented for depression including normal (sore less than 9), mild (10–13), moderate (14–20), severe (21–27) and very severe (higher than 27). Based on obtained scores for stress, the following categories are considered: normal (less than 14), mild (15–18), moderate (19–25), severe (26–33) and very severe (higher than 33). The total scores for the anxiety subscale are categorized as normal (0–7), mild (8–9), moderate (10–14), severe (15–19) and extremely severe (higher than 19) [24]. This DASS-42 scale was tested on non-clinical samples to measure its psychometric properties [25, 26]. The validity and reliability of DASS-42 questionnaire have been assessed in Iranian subjects previously, and all three subscales were found to have high Cronbach’s alpha coefficients (0.94 for the depression, 0.87 for the stress and 0.85 for the anxiety) [27].
Statistical analysis
SPSS software version 16 (Inc. Chicago, IL) was applied to carry out all analyses. Parameters were checked for normality by the Kolmogorov–Smirnov test, and data were reported as mean ± standard deviation and median (interquartile range) for normal and non-normal distribution, respectively. DTAC was adjusted for energy intake through the residual method [28]. Comparison of participant characteristics across the tertiles of DTAC was made by ANOVA test for continuous variables and chi-square test for categorical variables. Partial correlation adjusted for energy intake was performed to assess the correlation of DTAC and serum TAC with some dietary antioxidant nutrients. Adjusted means of oxidative stress biomarkers across the tertiles of DTAC were compared using the analysis of covariance (ANCOVA). The association between oxidative stress biomarkers and DTAC was determined by multiple linear regression. In addition, multiple linear regressions were performed to evaluate the association of DTAC and log-transformed psychological factors in three sets of models: (1) unadjusted model; (2) adjusted for age and time since menopause; (3) model 2 further adjusted for education, WC, physical activity, dietary supplement use, dietary intake of fiber, energy, and coffee. These covariates have been shown to be associated with DTAC in the present and previous studies [14, 29, 30].
Results
The distribution of women in the subscale category of mental health showed that 15% of women had severe and very severe symptoms of anxiety, while this distribution for depression and stress was 7% and 5%, respectively. Furthermore, our data revealed that about 4% of the population study (seven women) had severe and very severe symptoms of all three disorders (data not shown).
As shown in Table 1, with respect to general characteristics, no significant difference was observed across the tertiles of DTAC. The subject at higher tertiles of DTAC had higher dietary supplement use; however, it was not statistically significant (P-trend = 0.08). Women included in the higher tertiles of DTAC had higher median WC than women included in the lower tertile of DTAC (106 vs. 103 cm), although the association was not statistically significant. In addition, subjects in higher tertiles of DTAC were more likely to be physically active (P-trend = 0.02). Although DTAC was not associated with intake of energy, carbohydrate, and protein, but it was inversely associated with fat intake, (P-trend = 0.01), percent of energy from fat (P-trend = 0.003) and positively associated with percent of energy from carbohydrate (P-trend < 0.001). In terms of psychological factors, our data revealed that women at the higher tertiles of DTAC had fewer symptoms of depression and anxiety (P-trend < 0.04).
Table 1.
Demographic, anthropometric, dietary and psychological characteristics of study participant across the tertiles of energy-adjusted DTAC (n = 175)
| Characteristics | Tertiles of energy-adjusted DTAC | P-trend** | ||
|---|---|---|---|---|
| T1 (n = 58) | T2 (n = 59) | T3 (n = 58) | ||
| Energy-adjusted DTAC, median (μmol/day) | 14,262.79 | 18,462.49 | 23,208.09 | |
| Sociodemographic factors | ||||
| Age (year)† | 56.0 (8.2) | 55.0 (7.0) | 57.0 (10.0) | 0.4 |
| Education (year)† | 4.0 (6.0) | 5.0 (6.0) | 5.0 (6.0) | 0.1 |
| Menopausal years (year)† | 6.5 (9.7) | 4.0 (6.0) | 8.0 (11.0) | 0.9 |
| Marital status n (%)* | ||||
| Married | 45 (25.7) | 51 (29.1) | 49 (28.0) | 0.3 |
| Single/divorced/single | 13 (7.4) | 8 (4.6) | 9 (5.1) | |
| Economic status, n (%)* | ||||
| High/average | 18 (10.3) | 22 (12.6) | 21 (12.0) | 0.5 |
| Low | 40 (22.9) | 37 (21.0) | 37 (21.0) | |
| Supplement use and medical history n (%)* | ||||
| Dietary supplement use | 33 (18.9) | 36 (20.6) | 42 (24.0) | 0.08 |
| Family history of depression | 14 (25.7) | 15 (25.7) | 15 (25.7) | 0.8 |
| CVD | 25 (14.3) | 16 (9.1) | 15 (8.6) | 0.8 |
| Metabolic disorders | 12 (14.3) | 21 (9.1) | 20 (8.6) | 0.1 |
| Anthropometric and physical activity measures | ||||
| Height (cm)† | 154.0 (6.5) | 155.0 (6.5) | 155.7 (7.6) | 0.1 |
| Weight (kg)† | 72.7 (11.8) | 74.0 (12.6) | 74.6 (12.3) | 0.3 |
| BMI (kg/m2)† | 29.6 (8.4) | 30.4 (5.5) | 30.0 (4.7) | 0.8 |
| WC (cm)† | 103.0 (13.6) | 102.5 (14.0) | 106.0 (13.6) | 0.5 |
| Physical activity (MET min/week)† | 460.0 (462.0) | 471.0 (789.0) | 585.7 (1128.0) | 0.02 |
| Dietary factors | ||||
| Energy intake (kcal/day)‡ | 2369.4 ± 397.6 | 2138.4 ± 450.6 | 2324.6 ± 511.4 | 0.5 |
| Carbohydrate (g/day)† | 331.6 (110.7) | 306.6 (103.4) | 349.8 (108.4) | 0.2 |
| Protein (g/day)‡ | 83.4 ± 17.5 | 77.2 ± 17.0 | 84.9 ± 20.7 | 0.6 |
| Fat (g/day)† | 74.6 (32.6) | 67.6 (17.6) | 67.0 (33.1) | 0.01 |
| Carbohydrate (%EI) | 57.4 ± 5.8 | 60.3 ± 5.4 | 61.5 ± 4.9 | < 0.001 |
| Protein (%EI) | 14.0 ± 1.9 | 14.5 ± 1.8 | 14.6 ± 1.8 | 0.1 |
| Fat (%EI) | 29.5 (8.7) | 27.3 (6.0) | 27.5 (6.4) | 0.003 |
| DASS-42 score | ||||
| Depression† | 8.5 (10.0) | 6.0 (8.0) | 5.5 (9.0) | 0.03 |
| Stress† | 9.0 (9.0) | 10.0 (9.0) | 9.0 (10.0) | 0.4 |
| Anxiety† | 8.0 (6.0) | 7.0 (6.0) | 5.0 (5.0) | 0.02 |
Socioeconomic status defined as: having 3 or less living items for low status, 4–6 living items for average status, and 7–9 living items at home for high status
DTAC dietary total antioxidant capacity, CVD cardiovascular disease, BMI body mass index, WC waist circumference, MET metabolic equivalents task, EI energy intake, DASS depression, anxiety and stress scale
‡Data are expressed as mean ± SD
†Data are expressed as median (IQR)
* Number of subjects having the characteristic with %
** ANOVA test used for continuous variables and chi-square test used for categorical variables
According to partial correlation adjusted for energy intake, DTAC was highly correlated with the most of the dietary antioxidant components including vitamin C, β-cryptoxanthin, β-carotene, α-tocopherol, lycopene, lutein, zinc, magnesium (P-value < 0.04), but not with selenium. While serum TAC showed no significant correlation with these antioxidants, it indicated a moderate positive correlation with selenium (r = 0.14; P-value = 0.08) (Table 2).
Table 2.
Correlation of DTAC and serum TAC value with intakes of some dietary antioxidant component
| Dietary antioxidant intake | Partial correlations (n = 175)† | |||
|---|---|---|---|---|
| DTAC | Serum TAC | |||
| r | P | r | P | |
| Vitamin C (mg/day) | 0.53 | < 0.001 | 0.03 | 0.6 |
| β-Cryptoxanthin (μg/day) | 0.43 | < 0.001 | 0.02 | 0.7 |
| β-Carotene (μg/day) | 0.31 | < 0.001 | 0.01 | 0.8 |
| α-Tocopherol (mg/day) | 0.18 | 0.01 | 0.07 | 0.3 |
| Lycopene (μg/day) | 0.15 | 0.04 | 0.04 | 0.5 |
| Lutein (μg/day) | 0.20 | 0.007 | 0.08 | 0.2 |
| Zinc (mg/day) | 0.16 | 0.03 | 0.01 | 0.8 |
| Selenium (μg/day) | 0.04 | 0.5 | 0.14 | 0.06 |
| Magnesium (mg/day) | 0.41 | 0.001 | 0.11 | 0.1 |
DTAC dietary total antioxidant capacity, TAC total antioxidant capacity
† Partial correlations between DTAC and serum TAC. Dietary antioxidants intakes were adjusted for energy
As indicated in Table 3, after controlling for the potential confounders including age, time since menopause, education (year), WC (cm), physical activity (MET-min/week), dietary supplement use (yes/no), dietary intake of fiber (g/day), energy (Kcal/day) and coffee (g/day) in multivariable linear regressions, DTAC was inversely associated with depression (β = − 0.16, P-value = 0.03), anxiety (β = − 0.21, P-value = 0.007), but not stress (β = − 0.10, P-value = 0.1). We ran the same multiple regression for the women with family history of depression (n = 44), and found no significant association between DTAC and depression, stress and anxiety (data not shown).
Table 3.
Multivariate linear regression analyses of the energy-adjusted DTAC and depression, stress, and anxiety scores (n = 175)
| DTAC (μmol/day)† | Depression score | Stress score | Anxiety score | |||
|---|---|---|---|---|---|---|
| Coefficient standard β | P | Coefficient standard β | P | Coefficient standard β | P | |
| Unadjusted model | − 0.14 | 0.053 | − 0.05 | 0.3 | − 0.19 | 0.008 |
| Model 1 | − 0.14 | 0.06 | − 0.06 | 0.3 | − 0.19 | 0.01 |
| Model 2 | − 0.16 | 0.03 | − 0.10 | 0.1 | − 0.21 | 0.007 |
Depression, anxiety, stress scores were log10 transformed
DTAC dietary total antioxidant capacity
Model 1, adjustment for age (year) and years since menopause (year)
Model 2, further adjustment for education (year), waist circumference (cm), physical activity (MET min/week), dietary supplement use (yes/no), dietary intake of fiber (g/day), energy (kcal/day) and coffee (g/day)
†DTAC was adjusted for energy intake by residual method
Multiple linear regression with the same covariates presented in Table 3 showed no association between serum TAC and depression (β = 0.04, P-value = 0.5), stress (β = 0.07, P-value = 0.3) and anxiety score (β = 0.08, P-value = 0.3). MDA level also had no relationship with depression (β = 0.05, P-value = 0.4), stress (β = − 0.008, P-value = 0.9) and anxiety score (β = 0.04, P-value = 0.5). There was no significant association between SOD and depression (β = − 0.03, P-value = 0.6), stress (β = − 0.06, P-value = 0.4) and anxiety (β = − 0.002, P-value = 0.9). Ox-LDL also failed to exhibit any association with depression (β = − 0.03, P-value = 0.6), stress (β = 0.06, P-value = 0.4) and anxiety score (β = 0.03, P-value = 0.6) (data not shown).
Adjusted mean values of serum oxidative stress biomarkers across the tertiles of DTAC are shown in Table 4. There was a significant difference between the adjusted mean of serum level of MDA (P-value < 0.001) and TAC (P-value = 0.04) across the tertiles of DTAC. The lowest adjusted mean MDA was observed at the third tertile of DTAC, while the women at second tertile of DTAC had the highest adjusted mean of serum TAC. No significant relationship was found for ox-LDL and SOD.
Table 4.
Adjusted mean values for serum biomarkers of oxidative stress across tertiles of energy-adjusted DTAC (n = 175)
| Variables | Tertiles of energy-adjusted DTAC | |||||||
|---|---|---|---|---|---|---|---|---|
| T1 (n = 58) | T2 (n = 59) | T3 (n = 58) | P-value* | Effect size of ANCOVA test | ||||
| Mean | 95% CI | Mean | 95% CI | Mean | 95% CI | |||
| TAC (mM) | 0.44 | 0.42–0.45 | 0.46 | 0.44–0.47 | 0.44 | 0.42–0.45 | 0.04 | 0.03 |
| MDA (µM) | 5.3 | 4.7–6.0 | 4.8 | 4.2–5.4 | 3.4 | 3.0–3.8 | < 0.001 | 0.13 |
| Ox-LDL (ng/l) | 1548.8 | 1339.6–1794.7 | 1655.7 | 1432.1–1918.6 | 1489.3 | 1285.2–1725.8 | 0.6 | 0.03 |
| SOD (U/ml) | 36.0 | 34.1–38.1 | 33.8 | 32.0–35.8 | 33.9 | 31.9–35.7 | 0.1 | 0.02 |
Values are geometric means and 95% CI
DTAC dietary total antioxidant capacity, TAC total antioxidant capacity, MDA malondialdehyde, Ox-LDL oxidized low-density lipoprotein, SOD superoxide dismutase
* ANCOVA test, adjusted for age, time since menopause, education (year), waist circumference (cm), physical activity (MET min/week), dietary supplement use (yes/no), dietary intake of fiber (g/day), energy (kcal/day), and coffee (g/day)
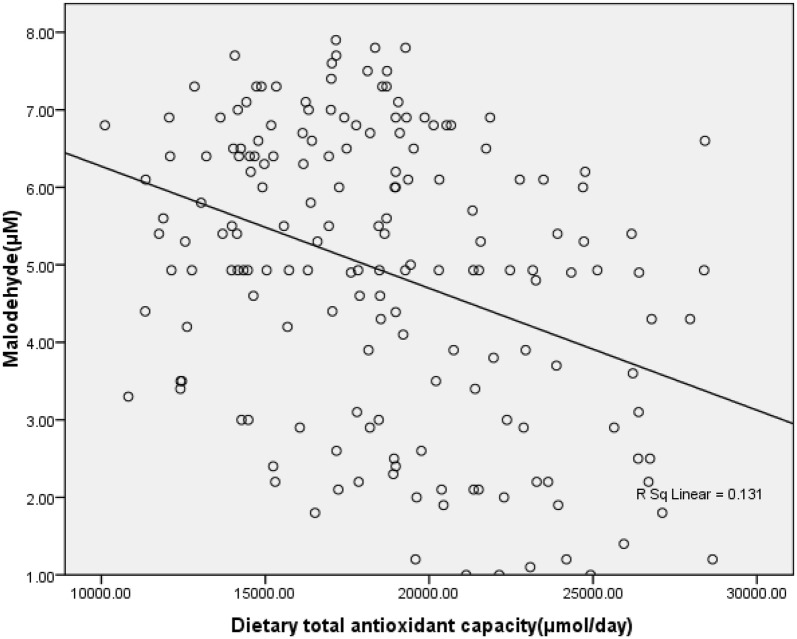
Serum MDA showed an inverse correlation with DTAC (Fig. 1), whereas there was no correlation between DTAC and serum TAC, ox-LDL and SOD (data not shown). Multiple linear regression showed that the association between DTAC and serum TAC (β = 0.006, P-value = 0.9), ox-LDL (β = − 0.04, P-value = 0.5) and SOD (β = − 0.13, P-value = 0.07) was insignificant; whereas DTAC was inversely associated with serum MAD level (β = − 0.37, P-value < 0.001) as shown in Table 5.
Fig. 1.
Correlation between dietary total antioxidant capacity (DTAC) and malondialdehyde (MDA) (r = − 0.36, P-value < 0.001)
Table 5.
Multivariate linear regression analyses of the energy-adjusted DTAC and serum biomarkers of oxidative stress (n = 175)
| DTAC (μmol/day)† | Coefficient standard β | P-value |
|---|---|---|
| TAC (mM) | 0.006 | 0.9 |
| MDA (µM) | − 0.37 | < 0.001 |
| Ox-LDL (ng/l) | − 0.04 | 0.5 |
| SOD (U/ml) | − 0.13 | 0.07 |
Adjusted for age (year) and years since menopause (year) education (year), waist circumference (cm), physical activity (MET min/week), dietary supplement use (yes/no), dietary intake of fiber (g/day), energy (kcal/day) and coffee (g/day)
Serum biomarkers of oxidative stress were log10 transformed
DTAC dietary total antioxidant capacity, TAC total antioxidant capacity, MDA malondialdehyde, Ox-LDL oxidized low-density lipoprotein, SOD superoxide dismutase
†DTAC was adjusted for energy intake by residual method
Discussion
To the best of our knowledge, the present study is among the few studies investigating the association between DTAC and mental disorders, and is the first study to examine this association among the postmenopausal women. After adjusting for multiple variables, we found an inverse association between DTAC and depression and anxiety scores. However, no association was detected between DTAC and stress score. In respect of oxidative status, serum MDA level was inversely associated with DTAC. Although, there was no linear relationship between serum TAC and DATC, subjects in the second tertile of DTAC had higher serum TAC level compared with the subjects in the first tertile. No association was observed between DTAC and serum ox-LDL and SOD concentration.
In line with our findings, a study on postmenopausal women revealed a strong negative link between the whole plant dietary pattern (consisting of whole grains, fruits, and vegetables) and depression [31]. Several studies also showed that adherence to healthy dietary patterns such as Mediterranean-type diet and Alternative Healthy Eating Index (AHEI) was associated with reduced risk of depression [32, 33] and anxiety [34]. However, a Brazilian study reported no association between DTAC and depression and anxiety [35]. These inconsistencies could be explained by various food processing and cooking in different cultures, which can affect the availability and content of dietary antioxidants [36].
In our study, no significant association was found between DTAC and depression, anxiety and stress in women with family history of depression. This may suggest that high TAC diets may not be as protective in individuals with genetic components of depression as it is in women without family history, although it needs to be confirmed in a larger study.
In the current study, no association was detected between DTAC and stress score. However, the result of other studies reported Mediterranean diet or appropriate consumption of fruits and vegetables (five servings/day) were associated with reduced risk of psychological distress [34, 37]. Further interventions are required to elucidate the effect of DTAC in relation to stress symptoms.
We found that women with higher DTAC had lower serum MDA level. Moreover, participants in the second tertile of DTAC had higher serum TAC level, while the level did not change in the third tertile, suggesting the existence of threshold levels associated with DTAC. However, serum TAC had no association with DTAC in multivariable model. These findings may suggest that high TAC diets may prevent lipid peroxidation, independent of serum TAC in postmenopausal women. So far, studies on the association between DTAC and blood antioxidant status among postmenopausal women and geriatric populations are scare and controversial [38–40]. A study in overweight and obese postmenopausal women reported that plasma TAC was positively associated with DTAC and dietary intake of antioxidant vitamins such as flavones and proanthocyanidins [38]. Proanthocyanidins and catechins are among the main compounds responsible for the increase of plasma TAC [41]. Notably, tea as the major food source for these compounds [42] was accounted for about 36% of total DTAC estimated in our study, followed by fruit (30%) and vegetable (14%) (data not shown); therefore, consuming more tea and fruits in the subjects with higher DTAC might have resulted in somehow elevated serum TAC and reduced MDA level. However, no association was found between DTAC with plasma TAC, MDA, ox-LDL level and antioxidant enzymes such as SOD in overweight and obese postmenopausal women [40], and elderly subjects [43]. Similarly, consumption of a high TAC diet for 2 weeks did not significantly alter the plasma TAC, ox-LDL, and protein carbonyls compared to low TAC diet in older people, although serum MDA reduced during low TAC diet. The short duration of intervention and compensatory response by homeostatic mechanisms was described for the lack of association [39]. Nevertheless, DTAC was negatively linked with plasma ox-LDL in young adults [30], suggesting that habitual high TAC diet might not be efficient in the substantial modification in ox-LDL in postmenopausal women. These discrepancies could be explained by many factors such as various food processing and cooking which can affect the availability and content of dietary antioxidants, as we mentioned before [36]. In addition, differences in bioavailability or absorption of antioxidants may affect blood TAC level [43]. Furthermore, it is possible that other components of blood antioxidants such as carotenoids may show better association with DTAC [44]. Some experimental studies found no significant increase in TAC level after intake of antioxidant-rich foods, while single carotenoids significantly increased [45, 46]. However, this information was not collected in the present study. Future studies are needed to clarify the effect of DTAC on various blood antioxidants in the menopause age.
The anti-oxidative and anti-inflammatory essence of the antioxidant-rich diets might explain the inverse association between DTAC and depression and anxiety. It is documented that depressive mood is highly related with impaired antioxidant defense and inflammatory status [47, 48]. Additionally, age-related reduction in the level of antioxidants has been shown in the frontal lobe of brain that is vital in mental functions [49, 50].
In our study, serum biomarkers were not associated with psychological factors. Plasma TAC and some other markers of oxidative stress were not associated with anxiety and depression [35]. However, a study in university male students found a negative correlation between serum TAC level and depression score [19]. Measurements of a few oxidative stress biomarkers may not truly reflect the in vivo antioxidant status and investigating various biomarkers has been suggested [51].
The present study has strengths and limitations. First, this study is the first study that explored association between DTAC and mental health in postmenopausal women. Second, to achieve the more accurate DTAC estimation, we assessed the intake of spices and herbs with high TAC value that are frequently used in Iranian diet. The under/overestimation in DTAC assessment due to cultivation procedures, storage, and cooking or some unmeasured confounding factors could have mediated the observed association [36]. Finally, cross-sectional design of study precluded us from concluding any cause–effect relationship.
Our findings suggest that higher DTAC is associated with lower depression and anxiety scores in postmenopausal women. In addition, higher DTAC is associated with lower serum MDA; hence postmenopausal women who are more prone to oxidative stress can reap the benefits of high TAC diets to improve their psychological disturbances and reduce the oxidative damage in their body.
Authors’ contributions
FS and GS conceived and developed the idea for the paper and revised the manuscript; ZA, MS and ME contributed to data collection and MA and HM wrote numerous drafts; MQ and FK contributed to data analysis and interpretation of the data. All authors read and approved the final manuscript.
Acknowledgements
We would like to express our gratitude to the participants of the current study.
Competing interests
The authors declare that they have no competing interests.
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Consent for publication
Not applicable.
Ethics approval and consent to participate
The study protocol was approved by the Ethics Committee of Tehran University of Medical Sciences.
Funding
This research has been supported by Tehran University of Medical Sciences (TUMS) (Grant No. 95-02-161-32419) as well as by Students’ Scientific Research Center, Tehran University of Medical Sciences, Tehran, Iran (Grant No 96-01-61-33952).
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Fereydoun Siassi, Phone: 09121938512, Email: siassif@tums.ac.ir.
Gity Sotoudeh, Phone: 09123906617, Email: gsotodeh@tums.ac.ir.
References
- 1.Organization W.H. Depression and other common mental disorders: global health estimates. Geneva: WHO; 2017. [Google Scholar]
- 2.Unsal A, Tozun M, Ayranci U. Prevalence of depression among postmenopausal women and related characteristics. Climacteric. 2011;14(2):244–251. doi: 10.3109/13697137.2010.510912. [DOI] [PubMed] [Google Scholar]
- 3.Bromberger JT, Matthews KA, Schott LL, Brockwell S, Avis NE, Kravitz HM, et al. Depressive symptoms during the menopausal transition: the Study of Women’s Health Across the Nation (SWAN) J Affect Disord. 2007;103(1–3):267–272. doi: 10.1016/j.jad.2007.01.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Woods NF, Smith-DiJulio K, Percival DB, Tao EY, Mariella A, Mitchell ES. Depressed mood during the menopausal transition and early postmenopause: observations from the Seattle Midlife Women’s Health Study. Menopause (New York, NY). 2008;15(2):223–232. doi: 10.1097/gme.0b013e3181450fc2. [DOI] [PubMed] [Google Scholar]
- 5.Schmidt PJ, Rubinow DR. Sex hormones and mood in the perimenopause. Ann N Y Acad Sci. 2009;1179(1):70–85. doi: 10.1111/j.1749-6632.2009.04982.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Joffe H, Crawford SL, Freeman MP, White DP, Bianchi MT, Kim S, et al. Independent contributions of nocturnal hot flashes and sleep disturbance to depression in estrogen-deprived women. J Clin Endocrinol Metab. 2016;101(10):3847–3855. doi: 10.1210/jc.2016-2348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Abdi N, Solhi M. Quality of life in postmenopausal women in Tehran. Iran J Health Educ Health Promot Owner. 2014;2(2):87–96. [Google Scholar]
- 8.Sánchez-Rodríguez MA, et al. Menopause as risk factor for oxidative stress. Menopause. 2012;19(3):361–367. doi: 10.1097/gme.0b013e318229977d. [DOI] [PubMed] [Google Scholar]
- 9.Subbiah MR. Mechanisms of cardioprotection by estrogens. Proc Soc Exp Biol Med. 1998;217(1):23–29. doi: 10.3181/00379727-217-44201. [DOI] [PubMed] [Google Scholar]
- 10.Miller AA, De Silva TM, Jackman KA, Sobey CG. Effect of gender and sex hormones on vascular oxidative stress. Clin Exp Pharmacol Physiol. 2007;34(10):1037–1043. doi: 10.1111/j.1440-1681.2007.04732.x. [DOI] [PubMed] [Google Scholar]
- 11.Sánchez-Rodríguez MA, Castrejón-Delgado L, Zacarías-Flores M, Arronte-Rosales A, Mendoza-Núñez VM. Quality of life among post-menopausal women due to oxidative stress boosted by dysthymia and anxiety. BMC women’s Health. 2017;17(1):1. doi: 10.1186/s12905-016-0358-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hartley M. Lifestyle modification as first line of treatment for chronic disease. J Diabetes Metab Disord Control. 2014;1:00009. [Google Scholar]
- 13.McMartin SE, Jacka FN, Colman I. The association between fruit and vegetable consumption and mental health disorders: evidence from five waves of a national survey of Canadians. Prev Med. 2013;56(3–4):225–230. doi: 10.1016/j.ypmed.2012.12.016. [DOI] [PubMed] [Google Scholar]
- 14.Puchau B, et al. Dietary total antioxidant capacity: a novel indicator of diet quality in healthy young adults. J Am Coll Nutr. 2009;28(6):648–656. doi: 10.1080/07315724.2009.10719797. [DOI] [PubMed] [Google Scholar]
- 15.Nascimento-Souza MA, Paiva PG, Martino HSD, Ribeiro AQ. Dietary total antioxidant capacity as a tool in health outcomes in middle-aged and older adults: a systematic review. Crit Rev Food Sci Nutr. 2018;58(6):905–912. doi: 10.1080/10408398.2016.1230089. [DOI] [PubMed] [Google Scholar]
- 16.Mozaffari H, et al. Dietary total antioxidant capacity and cardiovascular disease risk factors: a systematic review of observational studies. J Am Coll Nutr. 2018;37:1–13. doi: 10.1080/07315724.2018.1441079. [DOI] [PubMed] [Google Scholar]
- 17.Sotoudeh G, Abshirini M, Bagheri F, Siassi F, Koohdani F, Aslany Z. Higher dietary total antioxidant capacity is inversely related to prediabetes: a case-control study. Nutr J. 2018;46:20–25. doi: 10.1016/j.nut.2017.08.005. [DOI] [PubMed] [Google Scholar]
- 18.Pantavos A, Ruiter R, Feskens EF, de Keyser CE, Hofman A, Stricker BH, et al. Total dietary antioxidant capacity, individual antioxidant intake and breast cancer risk: the R otterdam study. Int J Cancer. 2015;136(9):2178–2186. doi: 10.1002/ijc.29249. [DOI] [PubMed] [Google Scholar]
- 19.Prohan M, et al. Total antioxidant capacity of diet and serum, dietary antioxidant vitamins intake, and serum hs-CRP levels in relation to depression scales in university male students. Redox Rep. 2014;19(3):133–139. doi: 10.1179/1351000214Y.0000000085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Abshirini M, Siassi F, Koohdani F, Qorbani M, Khosravi S, Hedayati M, et al. Dietary total antioxidant capacity is inversely related to menopausal symptoms: a cross-sectional study among Iranian postmenopausal women. Nutr J. 2018;55:161–167. doi: 10.1016/j.nut.2018.04.014. [DOI] [PubMed] [Google Scholar]
- 21.Hosseini Esfahani F, Asghari G, Mirmiran P, Azizi F. Reproducibility and relative validity of food group intake in a food frequency questionnaire developed for the Tehran Lipid and Glucose Study. Int J Epidemiol. 2010;20(2):150–158. doi: 10.2188/jea.JE20090083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Haytowitz DB, Bhagwat S. USDA database for the oxygen radical absorbance capacity (ORAC) of selected foods, release 2. Depart Agric. 2010;11:10–48. [Google Scholar]
- 23.International Physical Activity Questionnaire Research Committee. Guidelines for data processing and analysis of the International Physical Activity Questionnaire (IPAQ). http://www.ipaq.ki.se. Accessed Jan 2017.
- 24.Lovibond PF, Lovibond SH. The structure of negative emotional states: comparison of the Depression Anxiety Stress Scales (DASS) with the beck depression and anxiety inventories. Behav Res Ther. 1995;33(3):335–343. doi: 10.1016/0005-7967(94)00075-u. [DOI] [PubMed] [Google Scholar]
- 25.Brown TA, Chorpita BF, Korotitsch W, Barlow DH. Psychometric properties of the Depression Anxiety Stress Scales (DASS) in clinical samples. Behav Res Ther. 1997;35(1):79–89. doi: 10.1016/s0005-7967(96)00068-x. [DOI] [PubMed] [Google Scholar]
- 26.Crawford JR, Henry JD. The Depression Anxiety Stress Scales (DASS): normative data and latent structure in a large non-clinical sample. Br J Clin Psychol. 2003;42(2):111–131. doi: 10.1348/014466503321903544. [DOI] [PubMed] [Google Scholar]
- 27.Afzali A, Delavar A, Borjali A, Mirzamani M. Psychometric properties of DASS-42 as assessed in a sample of Kermanshah High School students. Res Behav Sci. 2007;5:81–92. [Google Scholar]
- 28.Willett WC, Howe GR, Kushi LH. Adjustment for total energy intake in epidemiologic studies. Am J Clin Nutr. 1997;65(4):1220S–1228S. doi: 10.1093/ajcn/65.4.1220S. [DOI] [PubMed] [Google Scholar]
- 29.Rautiainen S, Levitan EB, Mittleman MA, Wolk A. Total antioxidant capacity of diet and risk of heart failure: a population-based prospective cohort of women. Am J Med. 2013;126(6):494–500. doi: 10.1016/j.amjmed.2013.01.006. [DOI] [PubMed] [Google Scholar]
- 30.Hermsdorff HHM, Puchau B, Volp ACP, Barbosa KB, Bressan J, Zulet MÁ, et al. Dietary total antioxidant capacity is inversely related to central adiposity as well as to metabolic and oxidative stress markers in healthy young adults. Nutr Metab. 2011;8(1):59. doi: 10.1186/1743-7075-8-59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Liu Z, Ho SC, Xie YJ, Chen Y, Chen Y, Chen B, et al. Associations between dietary patterns and psychological factors: a cross-sectional study among Chinese postmenopausal women. Menopause. 2016;23(12):1294–1302. doi: 10.1097/GME.0000000000000701. [DOI] [PubMed] [Google Scholar]
- 32.Akbaraly TN, Sabia S, Shipley MJ, Batty GD, Kivimaki M. Adherence to healthy dietary guidelines and future depressive symptoms: evidence for sex differentials in the Whitehall II study. J Am Coll Nutr. 2013;97(2):419–427. doi: 10.3945/ajcn.112.041582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Olveira C, et al. Mediterranean diet is associated on symptoms of depression and anxiety in patients with bronchiectasis. Gen Hosp Psychiatry. 2014;36(3):277–283. doi: 10.1016/j.genhosppsych.2014.01.010. [DOI] [PubMed] [Google Scholar]
- 34.Hodge A, Almeida OP, English DR, Giles GG, Flicker L. Patterns of dietary intake and psychological distress in older Australians: benefits not just from a Mediterranean diet. Int Psychogeriatr. 2013;25(3):456–466. doi: 10.1017/S1041610212001986. [DOI] [PubMed] [Google Scholar]
- 35.Stedile N, Canuto R, Col CD, Sene JS, Stolfo A, Wisintainer GN, et al. Dietary total antioxidant capacity is associated with plasmatic antioxidant capacity, nutrient intake and lipid and DNA damage in healthy women. Int J Food Sci Nutr. 2016;67(4):479–488. doi: 10.3109/09637486.2016.1164670. [DOI] [PubMed] [Google Scholar]
- 36.Nayak B, Liu RH, Tang J. Effect of processing on phenolic antioxidants of fruits, vegetables, and grains—a review. Crit Rev Food Sci Nutr. 2015;55(7):887–918. doi: 10.1080/10408398.2011.654142. [DOI] [PubMed] [Google Scholar]
- 37.Richard A, et al. Associations between fruit and vegetable consumption and psychological distress: results from a population-based study. BMC Psychiatry. 2015;15(1):213. doi: 10.1186/s12888-015-0597-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wang Y, Yang M, Lee S-G, Davis CG, Kenny A, Koo SI, et al. Plasma total antioxidant capacity is associated with dietary intake and plasma level of antioxidants in postmenopausal women. J Nutr Biochem. 2012;23(12):1725–1731. doi: 10.1016/j.jnutbio.2011.12.004. [DOI] [PubMed] [Google Scholar]
- 39.Valtuena S, Pellegrini N, Franzini L, Bianchi MA, Ardigo D, Del Rio D, et al. Food selection based on total antioxidant capacity can modify antioxidant intake, systemic inflammation, and liver function without altering markers of oxidative stress. Am J Clin Nutr. 2008;87(5):1290–1297. doi: 10.1093/ajcn/87.5.1290. [DOI] [PubMed] [Google Scholar]
- 40.Wang Y, Yang M, Lee S-G, Davis CG, Koo SI, Fernandez ML, et al. Diets high in total antioxidant capacity improve risk biomarkers of cardiovascular disease: a 9-month observational study among overweight/obese postmenopausal women. Eur J Nutr. 2014;53(6):1363–1369. doi: 10.1007/s00394-013-0637-0. [DOI] [PubMed] [Google Scholar]
- 41.Prior RL, Gu L, Wu X, Jacob RA, Sotoudeh G, Kader AA, et al. Plasma antioxidant capacity changes following a meal as a measure of the ability of a food to alter in vivo antioxidant status. J Am Coll Nutr. 2007;26(2):170–181. doi: 10.1080/07315724.2007.10719599. [DOI] [PubMed] [Google Scholar]
- 42.Wang Y, Chung S-J, Song WO, Chun OK. Estimation of daily proanthocyanidin intake and major food sources in the US diet–3. Int J Nutr. 2011;141(3):447–452. doi: 10.3945/jn.110.133900. [DOI] [PubMed] [Google Scholar]
- 43.Khalil A, Gaudreau P, Cherki M, Wagner R, Tessier DM, Fulop T, et al. Antioxidant-rich food intakes and their association with blood total antioxidant status and vitamin C and E levels in community-dwelling seniors from the Quebec longitudinal study NuAge. Exp Gerontol. 2011;46(6):475–481. doi: 10.1016/j.exger.2011.02.002. [DOI] [PubMed] [Google Scholar]
- 44.Serafini M, Del Rio D. Understanding the association between dietary antioxidants, redox status and disease: is the total antioxidant capacity the right tool? Redox Rep. 2004;9(3):145–152. doi: 10.1179/135100004225004814. [DOI] [PubMed] [Google Scholar]
- 45.Bub A, Watzl B, Abrahamse L, Delincee H, Adam S, Wever J, et al. Moderate intervention with carotenoid-rich vegetable products reduces lipid peroxidation in men. J Nutr. 2000;130(9):2200–2206. doi: 10.1093/jn/130.9.2200. [DOI] [PubMed] [Google Scholar]
- 46.Pellegrini N, Riso P, Porrini M. Tomato consumption does not affect the total antioxidant capacity of plasma. Nutrition. 2000;16(4):268–271. doi: 10.1016/s0899-9007(99)00305-6. [DOI] [PubMed] [Google Scholar]
- 47.Cumurcu BE, Ozyurt H, Etikan I, Demir S, Karlidag R. Total antioxidant capacity and total oxidant status in patients with major depression: impact of antidepressant treatment. Psychiatry Clin Neurosci. 2009;63(5):639–645. doi: 10.1111/j.1440-1819.2009.02004.x. [DOI] [PubMed] [Google Scholar]
- 48.Milaneschi Y, et al. The relationship between plasma carotenoids and depressive symptoms in older persons. World J Biol Psychiatry. 2012;13(8):588–598. doi: 10.3109/15622975.2011.597876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Craft N, et al. Carotenoid, tocopherol, and retinol concentrations in elderly human brain. Exp Anim. 2004;21:22. [PubMed] [Google Scholar]
- 50.Chang C-C, et al. Reduction of dorsolateral prefrontal cortex gray matter in late-life depression. Psychiatry Res. 2011;193(1):1–6. doi: 10.1016/j.pscychresns.2011.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Prior RL, Wu X. Diet antioxidant capacity: relationships to oxidative stress and health. Am J Biomed Sci. 2013;5(2):126–139. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.