Abstract
Background
Rural residents are less likely to receive screening for colorectal cancer (CRC) than urban residents. However, the mechanisms underlying this disparity, especially among people aged 50–64 years old with private health insurance, are not well understood. We examined the impact of travel time on stage at CRC diagnosis.
Methods
This retrospective cohort study used data from the Blue Cross and Blue Shield of Nebraska. Members of this private insurance company aged 50–64 years, diagnosed with CRC during the period 2012–2016, and continuously enrolled in the insurance plan for at least 6 months prior to CRC diagnosis, were selected for this study. Using Google Maps, we estimated patients’ travel time from their home ZIP code to the ZIP code of their colonoscopy provider. Using logistic regression, we analyzed the association between stage at CRC diagnosis, travel time, use of preventive services (i.e., check-ups or counseling to prevent or detect illness at an early stage) and patient characteristics.
Results
A total of 307 subjects met the inclusion criteria. People who had not used preventive services 6 months prior to CRC diagnosis had 2.80 (95% CI, 1.00–7.90) times the odds of metastatic CRC compared to those who had used these services. No statistically significant association was found between travel time and metastatic CRC diagnosis (P = 0.99; 95% CI, 0.98–1.01).
Conclusions
The fact that 13% of the study population presented with metastatic CRC suggests some noncompliance with preventive services such as screening guidelines. To increase screening uptake and reduce metastatic cases, employers should offer incentives for their employees to make use of preventive services such as CRC screening.
Keywords: Access to care, Colorectal cancer, Health services research, Geography, Private insurance
Background
Colorectal cancer (CRC) is the third most common cancer in the USA, preceded by lung and breast cancers in women, and lung and prostate cancers in men. It is the third leading cause of cancer death in the USA [1, 2]. Unlike breast cancer screening, screening for CRC not only leads to early detection of cancer, but also can prevent cancer from occurring [3, 4]. The large decline in both incidence and mortality from CRC in the USA over the past two decades has been attributed to the increased use of screening, especially colonoscopy [5]. Since the publication of the first guidelines for CRC screening in 1995, the screening rate has steadily increased from 35 to 62.4% in 2015 [6]. In 1998, Congress enacted a Medicare CRC screening benefit (i.e., Medicare is the federal health insurance program for individuals 65 years or older, certain younger individuals with disabilities and individuals with end-stage renal disease), but it was limited to fecal occult blood tests (FOBT) and sigmoidoscopy for symptomatic or high-risk individuals, and average-risk individuals with a positive FOBT who needed follow-up screening [7]. In 2001, the Medicare coverage extended to average-risk individuals nationwide for screening purposes [7, 8]. In 2010, the Affordable Care Act (ACA), or the comprehensive health care reform law enacted in March 2010, required private health insurance plans to cover preventive services recommended by the United States Preventive Services Task Force and graded ‘A’ or ‘B’, including CRC screening, with no out-of-pocket costs for members [9]. By 2011, 54 million privately insured Americans received additional preventive services coverage as required by the ACA, which included colonoscopy screening, mammograms, and Pap smears [10].
Despite its known benefits, CRC screening uptake has remained less than optimal. In 2015, only 65% of Americans eligible for screening were up to date on CRC screening [11], which is well below the 2014 Centers for Disease Control and Prevention’s Colorectal Cancer Program target of 80% [11], and the 70% goal set by Healthy People 2020 [12]. Further, individuals living in rural areas and other medically underserved populations have lower rates of CRC screening uptake. Studies have demonstrated that rural residents are 30% less likely to receive CRC screening than their urban counterparts [13, 14]. Individuals who are underinsured or those with lower socioeconomic status are also less likely to be up to date with CRC screening [15, 16]. While previous research generally suggests lower CRC screening rates among rural residents compared to urban residents, the mechanism to explain this disparity has not been well established. Nevertheless, the following factors are thought to be involved: lower income levels [16, 17], a higher percentage of people who are underinsured or uninsured [15, 18, 19], less awareness and understanding of CRC risks and benefits of CRC screening [20, 21], lack of physician recommendations on CRC screening [18, 22, 23], and distance to a CRC screening facility [24, 25]. For example, a population-based survey study conducted in Nebraska found that rural residents are more likely to perceive screening cost as a barrier, and that CRC cannot be prevented [20]. Unlike urban residents in Nebraska, rural residents are 60% less likely to receive any CRC screening, and 57% less likely to receive a colonoscopy [20].
Furthermore, the rural-urban disparities in access to cancer services is a global phenomenon. Prior international literature assessed the association between rural-urban status and cancer outcome [26]. For instance, Carriere et. Al., 2018 conducted an international systematic review to assess whether cancer survival among rural dwellers is less than their urban counterpart. Despite heterogeneities between studies (e.g., different definitions for rural-urban status and diverse studied geographic regions), rural residents were 5% less likely to survive cancer. Additionally, an Australian systematic review study found less mammography use among rural population which also have contributed to the lower breast cancer survival among women live in rural areas [27].
Many previous CRC and breast cancer screening studies were based on Medicare data, partly because it provides claims data for nearly every US resident over the age of 65 years. It can also provide more objective and reliable evidence for cancer screening use compared to self-reported survey data. However, substantial limitations of Medicare data are the restriction of the population to adults older than 65 years old and the representation of only fee-for-service Medicare beneficiaries. Given the consistently lower use of CRC screening among adults between 50 and 64 years of age [28], it is essential to elucidate factors associated with lower CRC screening in this younger age group. This is especially important given the increased incidence of CRC among individuals younger than 65 years of age [29].
Colonoscopy screening is associated with logistical hurdles such as taking time off work, and having another adult to accompany and transport the patient after the procedure. This is necessary since most patients will undergo conscious sedation and will miss a day of work [30]. Additionally, the unpleasant experience of bowel preparation before the procedure is a common obstacle [20, 23, 31, 32]. These barriers are more likely to be a perceived problem among rural residents since they are more likely to have to travel longer distances to receive a colonoscopy. According to the National Household Travel Survey, 41% of trips taken by rural residents in the US to receive medical or dental services were longer than 30 min in duration, while only 25% of trips taken by urban residents were longer than 30 min [33]. Distance to a screening facility has been shown to be a significant barrier for rural patients [25, 34].
Well-established evidence has suggested a relationship between screening and the prevention and early detection of CRC; therefore, this study was undertaken to assess the impact of travel time on stage at CRC diagnosis among privately insured, rural-dwelling people aged 50–64. We hypothesize that shorter travel time to colonoscopy facilities is associated with earlier stage of diagnosis (i.e., non-metastatic diagnoses) of CRC after accounting for other patient characteristics.
Methods
Data sources
This is a retrospective cohort study using data from Blue Cross and Blue Shield of Nebraska (BCBSNE). BCBSNE is the largest private health insurer in Nebraska, serving over 700,000 people [33]. Data comprises claims from inpatient and outpatient facilities, and professional/office services, and include diagnosis and procedural codes, dates of service and the five-digit ZIP code for the provider. The data also contain members’ demographic information including age, gender, and their ZIP code of residence. BCBSNE captures members’ enrollment information including the beginning and end date of coverage. Rural-Urban Community Area Codes (RUCA) data and Google Maps were used to determine rurality and travel time [35, 36].
RUCA uses the U.S. Census Bureau’s Urbanized Area (UA) and Urbanized Cluster (UC) definitions supplemented with information on work commute and population density to characterize all of the census tracts regarding their rural and urban status [36]. The classification assigns metropolitan (i.e., primary commuting flow within an UA), micropolitan (i.e., large rural or primary flow within an UC of 10,000 to 49,999 individuals), small town (i.e., primary flow within an UC of 2500–9999 individuals) and rural commuting areas (i.e., primary flow to a tract outside a UA or UC) with numbers between 1 and 10. These numbers are subdivided into 21 secondary codes based on commuting flows. Although the original RUCA classification was based on census tract, it uses the ZIP code as its geographic unit. The latest RUCA codes are based on the 2010 decennial census and the 2006–2010 American Community Survey.
Furthermore, Google maps offer accurate driving directions between places at no cost. A program was developed (open-source programming language that is available on SAS) to make repeated calls to Google to obtain travel time information for any number of locations [37]. Subsequently, the program was tested using a nationally representative sample that cover 66,000 locations in the fifty states, the District of Columbia and Puerto Rico [35]. The program developer found a high correlation of Google maps with straight-line distance (r2 = 0.96) but with superior travel time estimate. While other GIS mapping systems offer greater sophistications and precision, it is at the expense of acquiring and managing specialized GIS software. The additional precision offered by other GIS, doesn’t provide more value to the research question raised on this study (i.e., nonemergency medical care) and to the state of Nebraska [35, 38].
Study population
Individuals included in the study were members of BCBSNE between January 1st, 2012, and June 30th, 2016; aged 50–64 years old with no prior CRC-related claims in the last 3 months; no prior cancers of any kind during the 6 months preceding the index diagnosis [39, 40]: The International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM) diagnosis codes 140–1529,1550–1958, or 1991–200; ICD-10-CM diagnosis codes C000-C179, C220-C768, or C80-C83; and were enrolled in BCBSNE for at least 6 months prior to the month in which their first diagnosis of CRC was identified. Figure 1 illustrates the eligibility criteria and the number of patients excluded for each criterion. Individuals were considered to be diagnosed with CRC if they had at least one inpatient or two outpatient claims with a primary diagnosis of CRC at two different visits. See the Appendix 1 for the codes used to identify the diagnosis of CRC [41]. We excluded members who were older than 65 years of age because the BCBSNE data likely did not contain claims for all of their Medicare-covered health services. We also excluded members with prior cancers (i.e., cancers at sites other than the colon/rectum) to ensure that the secondary malignant neoplasm originated from CRC [39, 40]. Any members residing outside the state of Nebraska were excluded because the study is confined to the residents of Nebraska.
Fig. 1.
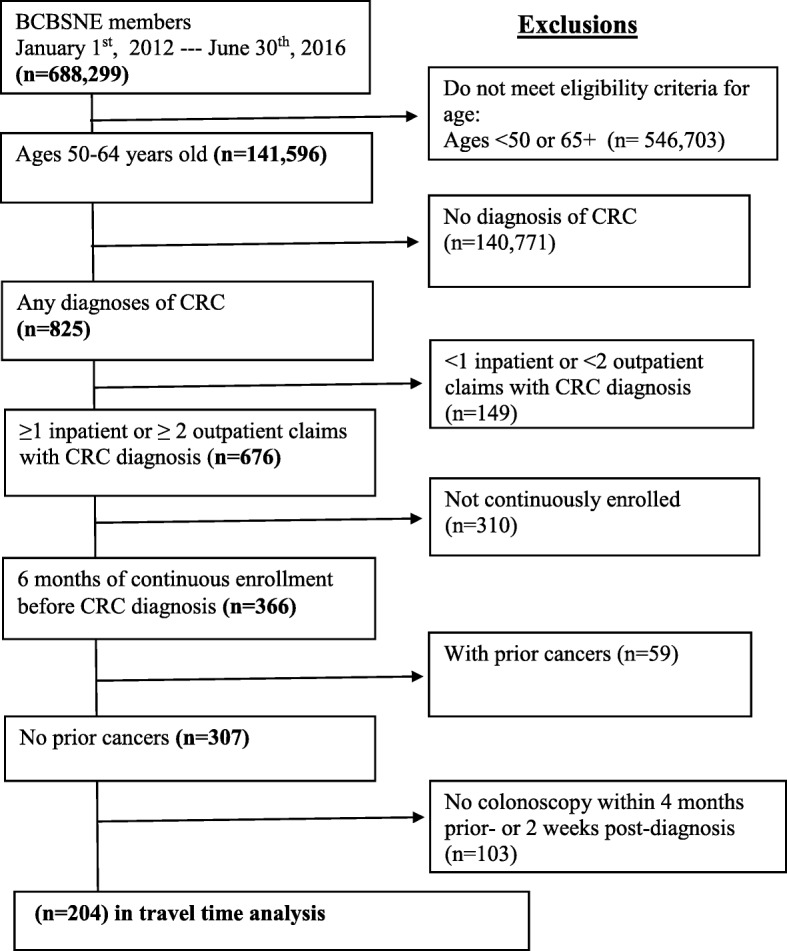
Eligibility criteria for the study population
Study variables
Patient characteristics
The beginning and end dates of coverage and services were extracted from patients’ enrollment files. Patient demographics including age, gender, and five-digit ZIP code of residence were extracted from their membership file. Utilization and clinical variables such as stage at diagnoses and preventive services use were derived from international classification of disease fields, and current procedural terminology fields from the claims file.
A dichotomous yes/no variable was also created to reflect members’ use of preventive services in the 6 months preceding the CRC diagnosis. Preventive services were defined as any health services such as check-ups or counseling to prevent or detect illness at an early stage when treatment is more viable [42]. Codes used to identify preventive services are detailed in the Appendix 2 [43]. In this study, individuals with at least one claim indicating the use of preventive services within 6 months prior to CRC diagnosis were considered to have used preventive services. Initial prevention medicine for a new patient, or periodic prevention medicine for an established patient aged 40–64 years old were examples of the codes used [44].
Travel time measurement
A patient’s ‘index’ colonoscopy was designated as the first time a patient had undergone a colonoscopy within 4 months prior to their CRC diagnosis. This colonoscopy was the basis on which both members’ and providers’ ZIP codes were defined to calculate travel time. The provider’s ZIP code was defined as that on the date of the index colonoscopy, and the member’s ZIP code as that for their place of residence when receiving the index colonoscopy.
Travel time was calculated by measuring the time in minutes between the geographic centroid of each member’s ZIP code of residence and the provider’s ZIP code at the time of service. Travel time calculations were made using the Google Maps web page, using the SAS FILENAME URL method in SAS [35]. The method has a high correlation with straight-line distance (r2 = 0.96), but with a superior travel time estimate [35]. We measured travel time as a continuous variable, as well as four categories based on quartile distribution. For the rural-urban status definition, we used RUCA codes to assign each member’s residential status based on their residential ZIP code. Subsequently, we used these codes to classify members by rural–urban status using “Categorization C” as suggested by the publisher [45]. This categorization aggregates RUCA codes into urban and rural codes. The urban codes consist of a metropolitan area core, micropolitan or small-town high-commuting areas, or rural areas with a secondary commuting flow of 30 to 49% within an urban area. The rural codes consist of a micropolitan area core with secondary flow of 10–29% to an urban area, small-town, low-commuting areas, or rural areas with commuting to urban cluster areas.
Outcome variables
The primary outcome was the stage at CRC diagnosis, classified as metastatic versus non-metastatic CRC. This was based on the initial CRC diagnosis identified in the claims data. The following diagnosis codes were used to identify patients diagnosed with metastatic CRC at the time of initial cancer diagnosis: ICD-9-CM diagnosis codes 196.0, 196.1, 196.3, 196.5, 197.0–197.4, 197.6197.7, 198.0–198.8 or 199.0; and ICD-10-CM diagnosis codes C770, C771, C773, C774, C780.0-C784, C786, C787, C788, C790–C798 or C80. The first date of metastatic diagnosis was assigned as the ‘index’ metastatic diagnosis [39]. Those patients who did not have a metastatic CRC diagnosis at the time of initial cancer diagnosis were considered non-metastatic.
Data analysis
Age, gender, rural-urban status, colonoscopy use within 4 months prior to CRC diagnosis, use of preventive services, and travel time were compared between metastatic and non-metastatic groups using Wilcoxon’s rank-sum test for continuous variables, and the Chi-square (X2) test for categorical variables. Wald tests were used to assess the significance of predictors. We used the fractional polynomial method to examine any non-linear relationships between the log odds of metastatic diagnosis and continuous variables. We inspected the curves of the predictors against the dichotomous response and used the likelihood ratio test for improvement in fit against the assumed linear relationship. Lastly, we conducted a multivariate analysis to assess the relationship between travel time and metastatic CRC diagnosis, adjusting for gender, rural-urban status, and use of preventive services. All tests were two-tailed and with α = 0.05. We used SAS statistical software version 9.4 (SAS Institute Inc. Cary, NC) to conduct all analyses.
Results
The application of the inclusion and exclusion criteria resulted in a cohort of 307 patients (Fig. 1). Of the 307 members who met our eligibility criteria, we were able to identify that 66% (n = 204) had undergone a colonoscopy within the 4 months prior to CRC diagnosis; 13% (n = 27) of these presented with metastatic CRC. There were no differences in the measured characteristics between patients who used colonoscopy within the 4 months prior to CRC diagnosis and those who did not. Table 1 shows the characteristics of BCBSNE members diagnosed with CRC who made a claim for colonoscopy within the 4 months prior to CRC diagnosis. There were no significant differences between metastatic and non-metastatic cases. Most cases were diagnosed during the years 2013 and 2014 in both metastatic and non-metastatic groups. While the average number of months of enrollment before CRC diagnosis were similar between metastatic and non-metastatic cases (13.0 versus 13.53), the median number of months of enrollment was slightly higher among the metastatic group (26.0 versus 22.0).
Table 1.
Characteristics of BCBSNE members diagnosed with CRC who made a colonoscopy claim within 4 months prior to their CRC diagnosis (n = 204)
| Characteristics | Total (204) | Metastatic (27) | Non-metastatic (177) | ||||
|---|---|---|---|---|---|---|---|
| N | % | N | % | N | % | P | |
| Age | |||||||
| Mean (SD) | 57.50 | 4.0 | 57.56 | 4.56 | 57.48 | 4.15 | 0.91 |
| Median (SD) | 58.0 | 7.0 | 57.0 | 8.0 | 58.0 | 7.0 | |
| 50–54 | 56 | 27.0 | 7 | 26.0 | 49 | 28.0 | |
| 55–60 | 92 | 45.0 | 12 | 44.0 | 80 | 45.0 | |
| ≥ 61 | 56 | 28.0 | 8 | 30.0 | 48 | 27.0 | |
| Gender | |||||||
| Male | 112 | 54.90 | 17 | 63.0 | 95 | 53.67 | 0.37 |
| Female | 92 | 45.10 | 10 | 37.0 | 82 | 46.33 | |
| Member location | |||||||
| Rural | 110 | 53.92 | 11 | 41.0 | 99 | 56.0 | 0.14 |
| Urban | 94 | 46.08 | 16 | 59.0 | 78 | 44.0 | |
| Travel time (min) | |||||||
| Mean (SD) | 33.58 | 40.0 | 34.85 | 51.53 | 33.38 | 38.12 | 0.74 |
| Median (SD) | 19.0 | 27.0 | 18.0 | 17.0 | 19.0 | 28.0 | |
| Q1 | 11 | – | 13 | – | 11 | – | |
| Q2 | 19 | – | 18 | – | 19 | – | |
| Q3 | 38 | – | 30 | – | 39 | – | |
| Q4 | 227 | – | 223 | – | 227 | – | |
| Travel distance, (miles) | |||||||
| Mean (SD) | 29.0 | 43.0 | 30.0 | 58.0 | 29.0 | 40.50 | 0.74 |
| Median (SD) | 13.0 | 26.0 | 13.0 | 19.0 | 13.0 | 28.50 | |
| Q1 | 5.0 | – | 5.0 | – | 4.50 | – | |
| Q2 | 13.0 | – | 13.0 | – | 13.0 | – | |
| Q3 | 31.0 | – | 24.0 | – | 33.0 | – | |
| Q4 | 251.0 | – | 251.0 | – | 234.0 | – | |
| PCP access | |||||||
| PCP/10,000 mean | 11.03 | 8.10 | 12.33 | 12.08 | 10.82 | 7.31 | 0.95 |
| PCP/10,000 median | 10.08 | 8.51 | 7.82 | 13.58 | 10.24 | 8.19 | |
| Year of CRC diagnosis | |||||||
| 2012 | 33 | 16.0 | 7 | 26.0 | 26 | 15.0 | |
| 2013 | 50 | 25.0 | 4 | 15.0 | 46 | 26.0 | |
| 2014 | 62 | 30.0 | 11 | 41.0 | 51 | 29.0 | |
| 2015 | 39 | 19.0 | 5 | 18.0 | 34 | 19.0 | |
| 2016 | 20 | 10.0 | 0 | 0 | 20 | 11.0 | |
| Months of enrollment before CRC diagnosis | |||||||
| Mean (SD) | 26 | 13.50 | 25 | 13.0 | 26 | 13.53 | |
| Median (IQR) | 26 | 22.0 | 28 | 26.0 | 25 | 22.0 | |
Table 2 shows our analyses of the association between travel time and metastatic CRC among members who had undergone colonoscopy within 4 months prior to their diagnosis (n = 204). Mean and median travel times were very similar between the metastatic (mean = 34.85 min) and non-metastatic (mean = 33.38 min) groups. Among these members, 25% traveled a distance of a maximum of 6 miles, 50% traveled a distance of a maximum of 15 miles, and 75% traveled a distance of a maximum of 31 miles. While the median distance traveled by rural patients was 26 miles, urban patients traveled a median of 8 miles to get to a colonoscopy facility. For those who did not have claims for preventive services, the odds of being diagnosed with metastatic CRC was 2.80 (95% CI: 1.00–7.90) times greater than those who had claims for preventive services prior to CRC diagnosis.
Table 2.
Logistic regressions of CRC patients who made a colonoscopy claim within 4 months prior to their CRC diagnosis (n = 204)
| Characteristics | Univariate Model | Multivariate Model | |
|---|---|---|---|
| Odds ratio (95% CI) | P | Odds ratio (95% CI) | |
| Age | 1.004 (0.91–1.01) | 0.91 | |
| Gender | |||
| Male | 1.0 | 1.0 | |
| Female | 0.68 (0.29–1.57) | 0.37 | 0.76 (0.32–1.80) |
| Member location | |||
| Rural | 1.0 | 1.0 | |
| Urban | 1.84 (0.82–4.20) | 0.14 | 2.14 (0.87–5.30) |
| Travel time (min) | |||
| Mean | 1.001 (0.99–1.01) | 0.74 | 0.99 (0.98–1.01) |
| Preventive services | |||
| Yes | 1.0 | 1.0 | |
| No | 2.81 (1.02–7.77) | 0.04 | 2.80 (1.00–7.90) |
The association between travel time and metastatic CRC diagnosis is adjusted for gender, rural–urban status, and use of preventive services
Discussion
The motivation to conduct this study was based on the notion that, unlike the Medicare population, people aged 50–64 years old more often have barriers related to work schedules, and are therefore less inclined to travel to a colonoscopy facility to be screened, leading to more frequent presentation with metastatic CRC [46–48]. Although our focus was primarily on the impact of rurality and travel times, we also examined the roles of demographic factors and use of preventive services; the characteristics of people in rural populations are different from those of the urban population, and access to services is of concern [20, 25, 49, 50].
We found no significant association between rural residency and late-stage at CRC diagnosis, which is comparable to results from recent studies that used cancer registry data from Iowa, Nebraska, and Georgia [50–52], with exceptions from a study that used 1998–2002 Illinois cancer registry data [53]. Also, the current study did not find a significant association between travel time and late-stage diagnosis. Again, the findings from this study generally confirm the results from the studies that used cancer registry data [51, 54]. Also, survey data generally indicate a lower adherence rate of CRC screening among rural residents compared to urban residents [13, 55].
Another explanation for the discrepancy between the survey studies and our results could be attributed to the differences between registry and claims data versus survey data. Survey data include insured, uninsured or underinsured individuals, while our sample consists of only insured individuals. Survey data can also suffer from potential biases (e.g., recall bias, social desirability bias), while registry data, and claims-based data, are more valid because of the accuracy required when reporting for a registry, and because claim’s reimbursement is dependent on patients’ health encounters. Additionally, registry data covers the entire state of Nebraska. While our sample is from BCBSNE, which is the largest private health insurance in Nebraska [56], the sample does not represent the entire privately insured population in Nebraska, nor the uninsured population. Another likely explanation is that there are widely varying degrees of rurality in different states. Rural Nebraska is very different from rural Alaska, or rural Kentucky, for example, where the differences between urban and rural populations in terms of use of health services may be much greater.
Our results indicate the key role of preventive services in the prevention or early detection of CRC. We found that patients who did not use preventive services within the 6 months prior to their CRC diagnosis were two times more likely to be diagnosed as having metastatic CRC compared to those who had availed of such services. This result likely reflects the notion that cancer screening communication between the patient and the provider may occur during an annual checkup or other routine care settings [57–59]. Not surprisingly, it also likely demonstrates that preventive visits are the main referral source for screening colonoscopies, whereas acute visits for CRC-related symptoms are the main referral source for diagnostic colonoscopies. Accordingly, non-distance barriers that can prevent or delay the receipt of preventive services should be alleviated. For instance, to encourage visits to a primary care physician, insurers/employers should consider removing cost-sharing during the initial CRC screening visits. An example of a potential cost that might incur during the initial visit is polyp removal [60, 61]. Co-insurance should also be removed from those undergoing follow-up colonoscopies after positive stool-based screening tests.
Compared to the older population (aged ≥65 years), CRC in the younger population appears to be a more aggressive disease [62–67]. It is more prevalent in the distal colon or rectum, tends to be poorly differentiated, more likely to be of mucinous and signet ring feature, and is typically diagnosed at later stages. These characteristics have been associated with a fast-growing CRC [68–70]. In addition to differences in molecular biology, the more aggressive nature among younger patients could be a result of lack of screening, non-compliance with screening guidelines, or failure to recognize and assess colonic symptoms among younger subjects [29, 69, 71, 72]. Non-compliance with colonoscopy screening has been linked to logistical barriers such as scheduling the colonoscopy procedure, or taking time off work to undergo colonoscopy, which is more common among the working-age population [73, 74].
Some limitations should be noted when interpreting the findings. First, we excluded 33.6% of the study sample for the travel time analysis because we were not able to identify whether these individuals had made colonoscopy claims within the 4 months prior to their CRC diagnosis. It is possible that some of these patients were diagnosed at the time of surgery because of an obstructed colon. Another possible explanation was that some of these cases could have been initially diagnosed with CRC prior to the study period, and then later presented with metastatic CRC that was diagnosed via radiologic imaging. Nonetheless, excluded cases were not significantly different from those included in all measured characteristics in this study. Second, our population is limited to individuals with private insurance from BCBSNE who live in the state of Nebraska, and our results may therefore not generalize to populations located in different states or with different types of insurance. Nonetheless, Nebraskans represent the US population relatively well in terms of sociodemographic characteristics except Nebraska has a higher proportion of whites than the U.S. average (79.0% vs. 60.7%) [75]. Third, we were unable to determine whether members bypassed the closest colonoscopy facility and voluntarily traveled longer distances to undergo screening at other facilities because BCBSNE data does not include all colonoscopy facilities in the state of Nebraska. Fourth, previous studies show that SES is proportionally associated with access to health services use [76]. In the current study, we were unable to control for the effect of SES. Nonetheless, the studied population is comprised of privately-insured population who we assumed is a homogenous population. We considered insurance status a proxy to SES because it has been found to be important in determining health outcomes in terms of access to health care [76]. According to US census data, 93.4% of Nebraskans have obtained a high school diploma and 59% of the households with median earnings of ≥ $45,000 [77]. Fifth, findings should be interpreted with cautions since the 6 months period for identifying preventive services use might not entirely capture the health behavior of an individual. For instance, one might use more health services only during or after an acute illness and thus, will be less likely to be captured within the window of 6 months. Ideally, we should examine the preventive services use within longer period of CRC diagnosis [44, 78]. Finally, it is possible that the current study is underpowered to find association between travel-time and stage at diagnosis.
Conclusions
This study did not find an association between the travel time taken for patients to reach colonoscopy facilities and the metastatic stage of their cancer at diagnosis. The fact that 13% of this privately insured working-age population presented with metastatic CRC suggests some non-compliance with screening guidelines. It is also possible that in this young cohort, some cases presented with an aggressive and fast-growing disease, with the potential development of metastatic CRC between screening colonoscopies. Nevertheless, it is possible that this working-age population faces logistic barriers that prevent them from taking time off work to undergo screening. To increase screening use and reduce cases of metastatic CRC, employers should offer incentives (e.g., removing cost-sharing during initial visits, or for follow-up colonoscopy among individuals with FOBT positive test) for their employees to undergo preventive services such as CRC screening.
Acknowledgements
The authors acknowledge the Blue Cross and Blue Shield of Nebraska for providing the data. The authors also thank Dr. Nicole de Rosa (University of Nebraska Medical Center) for her clinical recommendations.
Funding
Not applicable.
Availability of data and materials
The data that support the findings of this study are available from BCBSNE but restrictions apply to the availability of these data, which were used under license for the current study, and so are not publicly available. Data are however available from the authors upon reasonable request and with permission of BCBSNE.
Abbreviations
- ACA
Affordable Care Act
- BCBSNE
Blue Cross Blue Shield of Nebraska
- CRC
Colorectal Cancer
- FOBT
Fecal Occult Blood Test
- ICD
International Classification of Diseases
- RUCA
Rural Urban Commuting Area
Appendix 1
Table 3.
ICD codes used in identifying the diagnosis of CRC
| ICD code-9 and 10 | Description |
|---|---|
| 153.0/C183 | Malignant neoplasm of hepatic flexure |
| 153.1/C184 | Malignant neoplasm of transverse colon |
| 153.2/C186 | Malignant neoplasm of descending colon |
| 153.3/C187 | Malignant neoplasm of sigmoid colon |
| 153.4/C180 | Malignant neoplasm of cecum |
| 153.6/C182 | Malignant neoplasm of ascending colon |
| 153.7/C185 | Malignant neoplasm of splenic flexure |
| 153.8/C188 | Malignant neoplasm of other specified sites of large intestine |
| 153.9/C189 | Malignant neoplasm of colon, unspecified site |
| 154.0/C19 | Malignant neoplasm of rectosigmoid junction |
| 154.1/C20 | Malignant neoplasm of rectum |
| 154.8/C218 | Malignant neoplasm of other sites of rectum, rectosigmoid junction, and anus |
Appendix 2
Table 4.
ICD and CPT codes used in identifying preventive services use
| ICD code-9 and 10 | Description | CPT code | Description |
|---|---|---|---|
| V700 | General medical examination, routine medical examination at health care facility | 99,386 | initial preventive medicine new patient 40-64 yrs |
| V708 | General med. Exam., other specified general medical examinations | 99,396 | periodic preventive med established patient 40-64 yrs |
| V709 | General med. Exam., unspecified general medical examination | ||
| Z0000 | Encounter for general adult medical examination | ||
| Z008 | Encounter for other general exam | ||
| V7231 | Routine gynecological examination | ||
| V7232 | Pap smear confirmation | ||
| Z0141 | Encounter for routine gynecological examination | ||
| Z01411 | Encounter for routine gynecological examination with abnormal findings | ||
| Z01419 | Encounter for routine gynecological examination, no abnormal findings | ||
| Z0142 | Confirms pap smear test |
Authors’ contributions
MA designed the study, performed the analyses and wrote the manuscript. MA, AS, KMMI, SW and MC helped with the study design and assisted with the manuscript preparation. JM helped in reviewing data analysis. All authors helped with manuscript revisions. SW and AS helped with data acquisition. All authors have read and approved the submitted manuscript.
Ethics approval and consent to participate
The University of Nebraska Medical Center Institutional Review Board (IRB# 366–1) approved this study.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Mesnad Alyabsi, Phone: +966(11)429-4444, Email: alyabsime@ngha.med.sa.
Mary Charlton, Email: mary-charlton@uiowa.edu.
Jane Meza, Email: jmeza@unmc.edu.
K. M. Monirul Islam, Email: kmislam@unmc.edu.
Amr Soliman, Email: asoliman@med.cuny.edu.
Shinobu Watanabe-Galloway, Email: swatanabe@unmc.edu.
References
- 1.Kohler BA, Sherman RL, Howlader N, Jemal A, Ryerson AB, Henry KA, Boscoe FP, Cronin KA, Lake A, Noone AM, et al. Annual Report to the Nation on the Status of Cancer, 1975–2011, Featuring Incidence of Breast Cancer Subtypes by Race/Ethnicity, Poverty, and State. J Natl Cancer Inst. 2015;107(6):djv048. doi: 10.1093/jnci/djv048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Siegel R, Desantis C, Jemal A. Colorectal cancer statistics, 2014. CA Cancer J Clin. 2014;64(2):104–117. doi: 10.3322/caac.21220. [DOI] [PubMed] [Google Scholar]
- 3.Allison JE. The effect of fecal occult-blood screening on the incidence of colorectal cancer. N Engl J Med. 2001;344(13):1022. [PubMed] [Google Scholar]
- 4.Winawer SJ, Zauber AG, Ho MN, O'Brien MJ, Gottlieb LS, Sternberg SS, Waye JD, Schapiro M, Bond JH, Panish JF. Prevention of colorectal cancer by colonoscopic polypectomy. The National Polyp Study Workgroup. N Engl J Med. 1993;329(27):1977–1981. doi: 10.1056/NEJM199312303292701. [DOI] [PubMed] [Google Scholar]
- 5.Welch HG, Robertson DJ. Colorectal Cancer on the decline--why screening Can't explain it all. N Engl J Med. 2016;374(17):1605–1607. doi: 10.1056/NEJMp1600448. [DOI] [PubMed] [Google Scholar]
- 6.White A, Thompson TD, White MC, Sabatino SA, de Moor J, Doria-Rose PV, Geiger AM, Richardson LC. Cancer screening test use - United States, 2015. MMWR Morb Mortal Wkly Rep. 2017;66(8):201–206. doi: 10.15585/mmwr.mm6608a1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Medicare Claims Processing [https://www.cms.gov/Regulations-and-Guidance/Guidance/Transmittals/downloads/r52cp.pdf]. Accessed 12 Dec 2017.
- 8.Phillips KA, Liang SY, Ladabaum U, Haas J, Kerlikowske K, Lieberman D, Hiatt R, Nagamine M, Van Bebber SL. Trends in colonoscopy for colorectal cancer screening. Med Care. 2007;45(2):160–167. doi: 10.1097/01.mlr.0000246612.35245.21. [DOI] [PubMed] [Google Scholar]
- 9.Coverage of Colonoscopies Under the Affordable Care Act's Prevention Benefit. [https://www.kff.org/health-costs/report/coverage-of-colonoscopies-under-the-affordable-care/]. Accessed 12 Dec 2017.
- 10.Sommers B, Wilson L. Fifty-four million additional Americans are receiving preventive services without cost-sharing under the affordable care act. In: Issue Brief, Office of the Assistant Secretary for Planning and Evaluation: In; 2012.
- 11.Klabunde C, Joseph D, King J, White A, Plescia M. Morbidity and Mortality Weekly Report (MMWR). vol. 62. 2013. Vital Signs: Colorectal Cancer Screening Test Use — United States, 2012; pp. 881–888. [PMC free article] [PubMed] [Google Scholar]
- 12.Centers for Disease Control and Prevention Vital signs: colorectal cancer screening, incidence, and mortality--United States, 2002-2010. MMWR Morb Mortal Wkly Rep. 2011;60(26):884–889. [PubMed] [Google Scholar]
- 13.Anderson AE, Henry KA, Samadder NJ, Merrill RM, Kinney AY. Rural vs urban residence affects risk-appropriate colorectal cancer screening. Clin Gastroenterol Hepatol. 2013;11(5):526–533. doi: 10.1016/j.cgh.2012.11.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ojinnaka CO, Choi Y, Kum HC, Bolin JN. Predictors of colorectal Cancer screening: does rurality play a role? J Rural Health. 2015;31(3):254–268. doi: 10.1111/jrh.12104. [DOI] [PubMed] [Google Scholar]
- 15.Meissner HI, Breen N, Klabunde CN, Vernon SW. Patterns of colorectal cancer screening uptake among men and women in the United States. Cancer Epidemiol Biomark Prev. 2006;15(2):389–394. doi: 10.1158/1055-9965.EPI-05-0678. [DOI] [PubMed] [Google Scholar]
- 16.Pollack LA, Blackman DK, Wilson KM, Seeff LC, Nadel MR. Colorectal cancer test use among Hispanic and non-Hispanic U.S. populations. Prev Chronic Dis. 2006;3(2):A50. [PMC free article] [PubMed] [Google Scholar]
- 17.Carlos RC, Underwood W, Fendrick AM, Bernstein SJ. Behavioral associations between prostate and colon cancer screening. J Am Coll Surg. 2005;200(2):216–223. doi: 10.1016/j.jamcollsurg.2004.10.015. [DOI] [PubMed] [Google Scholar]
- 18.Honda K. Factors associated with colorectal cancer screening among the US urban Japanese population. Am J Public Health. 2004;94(5):815–822. doi: 10.2105/ajph.94.5.815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tang TS, Solomon LJ, McCracken LM. Barriers to fecal occult blood testing and sigmoidoscopy among older Chinese-American women. Cancer Pract. 2001;9(6):277–282. doi: 10.1046/j.1523-5394.2001.96008.x. [DOI] [PubMed] [Google Scholar]
- 20.Hughes AG, Watanabe-Galloway S, Schnell P, Soliman AS. Rural-urban differences in colorectal Cancer screening barriers in Nebraska. J Community Health. 2015;40(6):1065–1074. doi: 10.1007/s10900-015-0032-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Klabunde CN, Schenck AP, Davis WW. Barriers to colorectal cancer screening among Medicare consumers. Am J Prev Med. 2006;30(4):313–319. doi: 10.1016/j.amepre.2005.11.006. [DOI] [PubMed] [Google Scholar]
- 22.Gilbert A, Kanarek N. Colorectal cancer screening: physician recommendation is influential advice to Marylanders. Prev Med. 2005;41(2):367–379. doi: 10.1016/j.ypmed.2005.01.008. [DOI] [PubMed] [Google Scholar]
- 23.Janz NK, Wren PA, Schottenfeld D, Guire KE. Colorectal cancer screening attitudes and behavior: a population-based study. Prev Med. 2003;37(6 Pt 1):627–634. doi: 10.1016/j.ypmed.2003.09.016. [DOI] [PubMed] [Google Scholar]
- 24.Bennett KJ, Probst JC, Bellinger JD. Receipt of cancer screening services: surprising results for some rural minorities. J Rural Health. 2012;28(1):63–72. doi: 10.1111/j.1748-0361.2011.00365.x. [DOI] [PubMed] [Google Scholar]
- 25.Coughlin SS, Thompson TD. Colorectal cancer screening practices among men and women in rural and nonrural areas of the United States, 1999. J Rural Health. 2004;20(2):118–124. doi: 10.1111/j.1748-0361.2004.tb00017.x. [DOI] [PubMed] [Google Scholar]
- 26.Carriere R, Adam R, Fielding S, Barlas R, Ong Y, Murchie P. Rural dwellers are less likely to survive cancer - an international review and meta-analysis. Health Place. 2018;53:219–227. doi: 10.1016/j.healthplace.2018.08.010. [DOI] [PubMed] [Google Scholar]
- 27.Leung J, McKenzie S, Martin J, McLaughlin D. Effect of rurality on screening for breast cancer: a systematic review and meta-analysis comparing mammography. Rural Remote Health. 2014;14(2):2730. [PubMed] [Google Scholar]
- 28.Siegel, R.L., Miller, K.D., Fedewa, S.A., Ahnen, D.J., Meester, R.G., Barzi, A. and Jemal, A., 2017. Colorectal cancer statistics, 2017. CA: a cancer journal for clinicians, 67(3), pp.177-93. [DOI] [PubMed]
- 29.Ahnen DJ, Wade SW, Jones WF, Sifri R, Mendoza Silveiras J, Greenamyer J, Guiffre S, Axilbund J, Spiegel A, You YN. The increasing incidence of young-onset colorectal cancer: a call to action. Mayo Clin Proc. 2014;89(2):216–224. doi: 10.1016/j.mayocp.2013.09.006. [DOI] [PubMed] [Google Scholar]
- 30.Levin B, Lieberman DA, McFarland B, Smith RA, Brooks D, Andrews KS, Dash C, Giardiello FM, Glick S, Levin TR, et al. Screening and surveillance for the early detection of colorectal cancer and adenomatous polyps, 2008: a joint guideline from the American Cancer Society, the US multi-Society task force on colorectal Cancer, and the American College of Radiology. CA Cancer J Clin. 2008;58(3):130–160. doi: 10.3322/CA.2007.0018. [DOI] [PubMed] [Google Scholar]
- 31.Harewood GC, Wiersema MJ, Melton LJ. A prospective, controlled assessment of factors influencing acceptance of screening colonoscopy. Am J Gastroenterol. 2002;97(12):3186–3194. doi: 10.1111/j.1572-0241.2002.07129.x. [DOI] [PubMed] [Google Scholar]
- 32.Walsh JM, Kaplan CP, Nguyen B, Gildengorin G, McPhee SJ, Pérez-Stable EJ. Barriers to colorectal cancer screening in Latino and Vietnamese Americans. Compared with non-Latino white Americans. J Gen Intern Med. 2004;19(2):156–166. doi: 10.1111/j.1525-1497.2004.30263.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Probst JC, Laditka SB, Wang JY, Johnson AO. Effects of residence and race on burden of travel for care: cross sectional analysis of the 2001 US National Household Travel Survey. BMC Health Serv Res. 2007;7:40. doi: 10.1186/1472-6963-7-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.James TM, Greiner KA, Ellerbeck EF, Feng C, Ahluwalia JS. Disparities in colorectal cancer screening: a guideline-based analysis of adherence. Ethn Dis. 2006;16(1):228–233. [PubMed] [Google Scholar]
- 35.Boscoe FP, Henry KA, Zdeb MS. A Nationwide Comparison of Driving Distance Versus Straight-Line Distance to Hospitals. Prof Geogr. 2012;64(2):188–96. [DOI] [PMC free article] [PubMed]
- 36.Using RUCA Data [http://depts.washington.edu/uwruca/ruca-uses.php]. Accessed 7 Mar 2017.
- 37.Driving Distances and Drive Times using SAS and Google Maps [http://www.sascommunity.org/wiki/Driving_Distances_and_Drive_Times_using_SAS_and_Google_Maps]. Accessed 7 Mar 2017.
- 38.Lee RC. Current approaches to shortage area designation. J Rural Health. 1991;7(4 Suppl):437–450. [PubMed] [Google Scholar]
- 39.Paramore LC, Thomas SK, Knopf KB, Cragin LS, Fraeman KH. Estimating costs of care for patients with newly diagnosed metastatic colorectal cancer. Clin Colorectal Cancer. 2006;6(1):52–58. doi: 10.3816/CCC.2006.n.021. [DOI] [PubMed] [Google Scholar]
- 40.Song X, Zhao Z, Barber B, Gregory C, Schutt D, Gao S. Characterizing medical care by disease phase in metastatic colorectal cancer. Am J Manag Care. 2011;17 Suppl 5 Developing:SP20–25. [PubMed]
- 41.Setoguchi S, Solomon DH, Glynn RJ, Cook EF, Levin R, Schneeweiss S. Agreement of diagnosis and its date for hematologic malignancies and solid tumors between medicare claims and cancer registry data. Cancer Causes Control. 2007;18(5):561–569. doi: 10.1007/s10552-007-0131-1. [DOI] [PubMed] [Google Scholar]
- 42.What is Preventive Care? [https://www.cdc.gov/prevention/]. Accessed 10 Mar 2017.
- 43.Fenton JJ, Franks P, Reid RJ, Elmore JG, Baldwin LM. Continuity of care and cancer screening among health plan enrollees. Med Care. 2008;46(1):58–62. doi: 10.1097/MLR.0b013e318148493a. [DOI] [PubMed] [Google Scholar]
- 44.Fenton JJ, Cai Y, Weiss NS, Elmore JG, Pardee RE, Reid RJ, Baldwin LM. Delivery of cancer screening: how important is the preventive health examination? Arch Intern Med. 2007;167(6):580–585. doi: 10.1001/archinte.167.6.580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.RUCA Data [http://depts.washington.edu/uwruca/ruca-codes.php]. Accessed 7 Mar 2017.
- 46.Cokkinides VE, Chao A, Smith RA, Vernon SW, Thun MJ. Correlates of underutilization of colorectal cancer screening among U.S. adults, age 50 years and older. Prev Med. 2003;36(1):85–91. doi: 10.1006/pmed.2002.1127. [DOI] [PubMed] [Google Scholar]
- 47.Tessaro I, Mangone C, Parkar I, Pawar V. Knowledge, barriers, and predictors of colorectal cancer screening in an Appalachian church population. Prev Chronic Dis. 2006;3(4):A123. [PMC free article] [PubMed] [Google Scholar]
- 48.Vernon SW. Participation in colorectal cancer screening: a review. J Natl Cancer Inst. 1997;89(19):1406–1422. doi: 10.1093/jnci/89.19.1406. [DOI] [PubMed] [Google Scholar]
- 49.Sankaranarayanan J, Watanabe-Galloway S, Sun J, Qiu F, Boilesen EC, Thorson AG. Age and rural residence effects on accessing colorectal cancer treatments: a registry study. Am J Manag Care. 2010;16(4):265–273. [PubMed] [Google Scholar]
- 50.Sankaranarayanan J, Watanabe-Galloway S, Sun J, Qiu F, Boilesen E, Thorson AG. Rurality and other determinants of early colorectal cancer diagnosis in Nebraska: a 6-year cancer registry study, 1998-2003. J Rural Health. 2009;25(4):358–365. doi: 10.1111/j.1748-0361.2009.00244.x. [DOI] [PubMed] [Google Scholar]
- 51.Charlton ME, Matthews KA, Gaglioti A, Bay C, McDowell BD, Ward MM, Levy BT. Is travel time to colonoscopy associated with late-stage colorectal Cancer among Medicare beneficiaries in Iowa? J Rural Health. 2016;32(4):363–73. [DOI] [PMC free article] [PubMed]
- 52.Hines R, Markossian T, Johnson A, Dong F, Bayakly R. Geographic residency status and census tract socioeconomic status as determinants of colorectal cancer outcomes. Am J Public Health. 2014;104(3):e63–e71. doi: 10.2105/AJPH.2013.301572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.McLafferty S, Wang F. Rural reversal? Rural-urban disparities in late-stage cancer risk in Illinois. Cancer. 2009;115(12):2755–2764. doi: 10.1002/cncr.24306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Scoggins JF, Fedorenko CR, Donahue SM, Buchwald D, Blough DK, Ramsey SD. Is distance to provider a barrier to care for medicaid patients with breast, colorectal, or lung cancer? J Rural Health. 2012;28(1):54–62. doi: 10.1111/j.1748-0361.2011.00371.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Cole AM, Jackson JE, Doescher M. Urban-rural disparities in colorectal cancer screening: cross-sectional analysis of 1998-2005 data from the centers for disease Control's behavioral risk factor surveillance study. Cancer Med. 2012;1(3):350–356. doi: 10.1002/cam4.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Company profile [https://www.nebraskablue.com/about/company-profile/history]. Accessed 10 May 2017.
- 57.Dolan NC, Ramirez-Zohfeld V, Rademaker AW, Ferreira MR, Galanter WL, Radosta J, Eder MM, Cameron KA. The effectiveness of a physician-only and physician-patient intervention on colorectal Cancer screening discussions between providers and African American and Latino patients. J Gen Intern Med. 2015;30(12):1780–1787. doi: 10.1007/s11606-015-3381-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Laiyemo AO, Adebogun AO, Doubeni CA, Ricks-Santi L, McDonald-Pinkett S, Young PE, Cash BD, Klabunde CN. Influence of provider discussion and specific recommendation on colorectal cancer screening uptake among U.S. adults. Prev Med. 2014;67:1–5. doi: 10.1016/j.ypmed.2014.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Mosen DM, Feldstein AC, Perrin NA, Rosales AG, Smith DH, Liles EG, Schneider JL, Meyers RE, Elston-Lafata J. More comprehensive discussion of CRC screening associated with higher screening. Am J Manag Care. 2013;19(4):265–271. [PMC free article] [PubMed] [Google Scholar]
- 60.Green BB, Coronado GD, Devoe JE, Allison J. Navigating the murky waters of colorectal cancer screening and health reform. Am J Public Health. 2014;104(6):982–986. doi: 10.2105/AJPH.2014.301877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Peterse EFP, Meester RGS, Gini A, Doubeni CA, Anderson DS, Berger FG, Zauber AG, Lansdorp-Vogelaar I. Value of waiving coinsurance for colorectal Cancer screening in Medicare beneficiaries. Health Aff (Millwood) 2017;36(12):2151–2159. doi: 10.1377/hlthaff.2017.0228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Domergue J, Ismail M, Astre C, Saint-Aubert B, Joyeux H, Solassol C, Pujol H. Colorectal carcinoma in patients younger than 40 years of age. Montpellier Cancer institute experience with 78 patients. Cancer. 1988;61(4):835–840. doi: 10.1002/1097-0142(19880215)61:4<835::aid-cncr2820610432>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- 63.Dozois EJ, Boardman LA, Suwanthanma W, Limburg PJ, Cima RR, Bakken JL, Vierkant RA, Aakre JA, Larson DW. Young-onset colorectal cancer in patients with no known genetic predisposition: can we increase early recognition and improve outcome? Medicine (Baltimore) 2008;87(5):259–263. doi: 10.1097/MD.0b013e3181881354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Fairley TL, Cardinez CJ, Martin J, Alley L, Friedman C, Edwards B, Jamison P. Colorectal cancer in U.S. adults younger than 50 years of age, 1998-2001. Cancer. 2006;107(5 Suppl):1153–1161. doi: 10.1002/cncr.22012. [DOI] [PubMed] [Google Scholar]
- 65.Lee PY, Fletcher WS, Sullivan ES, Vetto JT. Colorectal cancer in young patients: characteristics and outcome. Am Surg. 1994;60(8):607–612. [PubMed] [Google Scholar]
- 66.Schellerer VS, Merkel S, Schumann SC, Schlabrakowski A, Förtsch T, Schildberg C, Hohenberger W, Croner RS. Despite aggressive histopathology survival is not impaired in young patients with colorectal cancer : CRC in patients under 50 years of age. Int J Color Dis. 2012;27(1):71–79. doi: 10.1007/s00384-011-1291-8. [DOI] [PubMed] [Google Scholar]
- 67.You YN, Xing Y, Feig BW, Chang GJ, Cormier JN. Young-onset colorectal cancer: is it time to pay attention? Arch Intern Med. 2012;172(3):287–289. doi: 10.1001/archinternmed.2011.602. [DOI] [PubMed] [Google Scholar]
- 68.Alici S, Aykan NF, Sakar B, Bulutlar G, Kaytan E, Topuz E. Colorectal cancer in young patients: characteristics and outcome. Tohoku J Exp Med. 2003;199(2):85–93. doi: 10.1620/tjem.199.85. [DOI] [PubMed] [Google Scholar]
- 69.Ballester V, Rashtak S, Boardman L. Clinical and molecular features of young-onset colorectal cancer. World J Gastroenterol. 2016;22(5):1736–1744. doi: 10.3748/wjg.v22.i5.1736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.O'Connell JB, Maggard MA, Liu JH, Etzioni DA, Livingston EH, Ko CY. Do young colon cancer patients have worse outcomes? World J Surg. 2004;28(6):558–562. doi: 10.1007/s00268-004-7306-7. [DOI] [PubMed] [Google Scholar]
- 71.Society AC. Colorectal Cancer Facts & Figures, 2017–2019. Atlanta: American Cancer Society;2017. 2017. [Google Scholar]
- 72.Trivers KF, Shaw KM, Sabatino SA, Shapiro JA, Coates RJ. Trends in colorectal cancer screening disparities in people aged 50-64 years, 2000-2005. Am J Prev Med. 2008;35(3):185–193. doi: 10.1016/j.amepre.2008.05.021. [DOI] [PubMed] [Google Scholar]
- 73.Denberg TD, Melhado TV, Coombes JM, Beaty BL, Berman K, Byers TE, Marcus AC, Steiner JF, Ahnen DJ. Predictors of nonadherence to screening colonoscopy. J Gen Intern Med. 2005;20(11):989–995. doi: 10.1111/j.1525-1497.2005.00164.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Subramanian S, Klosterman M, Amonkar MM, Hunt TL. Adherence with colorectal cancer screening guidelines: a review. Prev Med. 2004;38(5):536–550. doi: 10.1016/j.ypmed.2003.12.011. [DOI] [PubMed] [Google Scholar]
- 75.United States Census Bureau . QuickFacts Nebraska. 2017. [Google Scholar]
- 76.Singer BH, Ryff CD, Council NR . The influence of inequality on Health Outcomes. 2001. [Google Scholar]
- 77.U.S. Census Bureau: Selected economic characteristics, 2013–2017 American Community Survey 5-year estimates. 2017. Retrieved from: https://factfinder.census.gov/faces/nav/jsf/pages/community_facts.xhtml?src=bkmk. Accessed 16 Dec 2018.
- 78.Fenton JJ, Elmore JG, Buist DS, Reid RJ, Tancredi DJ, Baldwin LM. Longitudinal adherence with fecal occult blood test screening in community practice. Ann Fam Med. 2010;8(5):397–401. doi: 10.1370/afm.1133. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from BCBSNE but restrictions apply to the availability of these data, which were used under license for the current study, and so are not publicly available. Data are however available from the authors upon reasonable request and with permission of BCBSNE.


