Abstract
Background
Hyperuricemia (HUA) is very common in chronic kidney disease (CKD). HUA is associated with an increased risk of cardiovascular events and accelerates the progression of CKD. Our study aimed to explore the relationship between baseline serum uric acid levels and renal histopathological features.
Methods
One thousand seventy patients receiving renal biopsy in our center were involved in our study. The baseline characteristics at the time of the kidney biopsy were collected from Renal Treatment System (RTS) database, including age, gender, serum uric acid (UA), glomerular filtration rate (eGFR), serum creatinine (Cr), urea, albumin (Alb), 24 h urine protein quantitation (24 h-u-pro) and blood pressure (BP). Pathological morphological changes were evaluated by two pathologists independently. Statistical analysis was done using SPSS 21.0.
Results
Among 1070 patients, 429 had IgA nephropathy (IgAN), 641 had non-IgAN. The incidence of HUA was 38.8% (n = 415), 43.8% (n = 188), and 43.2% (n = 277) in all patients, patients with IgAN and non-IgAN patients, respectively. Serum uric acid was correlated with eGFR (r = − 0.418, p < 0.001), Cr (r = 0.391, p < 0.001), urea (r = 0.410, p < 0.001), 24-u-pro (r = 0.077, p = 0.022), systolic blood pressure (SBP) (r = 0.175, p < 0.001) and diastolic blood pressure (DBP) (r = 0.109, p = 0.001). Multivariate logistic regression analysis showed that after adjustment for Cr, age and blood pressure, HUA was a risk factor for segmental glomerulosclerosis (OR = 1.800, 95% CI:1.309–2.477) and tubular atrophy/interstitial fibrosis (OR = 1.802, 95% CI:1.005–3.232). HUA increased the area under curve (AUC) in diagnosis of segmental glomerulosclerosis.
Conclusions
Hyperuricemia is prevalent in CKD. The serum uric acid level correlates not only with clinical renal injury indexes, but also with renal pathology. Hyperuricemia is an independent risk factor for segmental glomerulosclerosis and tubular atrophy/interstitial fibrosis.
Keywords: Hyperuricemia, Histopathological features, Chronic kidney disease
Background
Uric acid is the product of purine metabolism in human body. 70% of uric acid in human body is excreted through kidneys. Uric acid is an intracellular oxidant when it is beyond the physiological range [1]. Hyperuricemia (HUA) is associated with endothelial dysfunction, vascular smooth muscle proliferation and interstitial inflammatory infiltration through a variety of mechanisms, such as inducing intracellular oxidative stress, mitochondrial dysfunction, inflammatory response and activation of the renin-angiotensin system (RAS) [2–7].
Hyperuricemia is a common phenomenon in patients with chronic kidney disease (CKD). Previous studies have shown that hyperuricemia was a risk factor for CKD [8, 9]. It can accelerate the progression of CKD [10–13], and increase the incidence of cardiovascular, cerebrovascular diseases and metabolic diseases [14–17]. However, the correlation between hyperuricemia and renal pathological changes is not entirely clear.
Previous studies suggested that HUA was associated with tubular interstitial lesions, and high uric acid levels indicated tubular interstitial lesions [18, 19]. However, the correlation between uric acid levels and glomerular sclerosis has not been studied. Our study aimed to investigate the correlation between uric acid and renal pathological changes, including both glomerular sclerosis and tubular interstitial lesions.
Methods
Study participants and data collection
Participants receiving renal biopsy in Sichuan Provincial People’s Hospital from January 2010 to December 2016 were screened. Those with adequate information on baseline characteristics in our Renal Treatment System (RTS) database were included in the current study. Exclusion criteria included inability to provide consent, enrollment in competing studies, pregnancy, familial hyperuricemia, transient hyperuricemia, primary gout, transient tubular injury, malignant hypertension, renal cancer, cirrhosis, recent chemotherapy or immunosuppressive therapy, organ transplantation, or dialysis treatment. A total of 1070 individuals (516 males and 554 females) were included in this study. The baseline demographic and clinical characteristics were collected at the time of renal biopsy from RTS database, including age, gender, serum uric acid, glomerular filtration rate (eGFR), serum creatinine, urea, albumin and 24 h urine protein quantitation (24 h-u-pro) and blood pressure. eGFR was estimated with CKD-EPI (CKD Epidemiology Collaboration) creatinine equation [20].
The study was approved by the Ethics Committee of the Sichuan Provincial People’s Hospital (Chengdu, China, No.2017–124). The de-identified data was obtained from RTS database. All patients gave fully informed written consent.
Diagnosis criteria
Hyperuricemia was defined as a fasting serum uric acid level greater than 420 μmol/L (7 mg/dl) for male and greater than 357 μmol/L (6 mg/dl) for female participants [21].
Renal pathological diagnosis was reviewed independently by two renal pathologists who were blinded to previous pathology reports and patients’ clinical outcomes. Segmental sclerosis of glomerulus was classified as segmental glomerulosclerosis group (S0) and non- segmental glomerulosclerosis group (S1). On the basis of extent of tubular atrophy/interstitial fibrosis, patients were divided into mild injury (T1), moderate injury (T2), and severe injury (T3) according to current literatures (0–25%, 26–50, > 50%) [22, 23].
Statistical analysis
Continuous data were presented as mean with standard deviation (SD) or median with interquartile ranges (IQR). Categorical variables were presented as proportions. Continuous data were compared by t-test or one-way ANOVA. Chi-square test was used to compare categorical variables between two groups. Pearson or Spearson correlation analysis was performed to calculate the correlation between uric acid and other clinical indicators. Logistic regression analysis was used to examine whether HUA was an independent predictor of segmental glomerulosclerosis or tubular atrophy/interstitial fibrosis. We also did sensitivity analyses to assess relationship between HUA and segmental glomerulosclerosis or tubular atrophy/interstitial fibrosis in several models. Receiver Operating characteristic Curves (ROC) was used and the area under curve (AUC) was analyzed to test whether HUA can increase the ability to diagnose glomerular segmental sclerosis and tubular atrophy/interstitial fibrosis. All analyses were performed using SPSS, version21.0. p value of less than 0.05 was considered statistically significant.
Results
Baseline clinical characteristics and pathological features
In the whole cohort, 429 (171 males and 258 females) of 1070 (516 males and 554 females) patients had biopsy proven IgAN. Patients with IgAN were younger, female predominant, had worse renal function, higher serum albumin level and lower 24 h-u-pro level, as compared to those with non-IgAN. The prevalence of hyperuricemia was 38.8% (n = 415), 43.8% (n = 188), and 43.2% (n = 277) in all patients, patients with IgAN and non-IgAN patients, respectively (p = 0.84, Table 1). Among the all participants (n = 1070), the majority of patients (812(75.9%)) did not have segmental glomerulosclerosis (Table 1, Fig. 1). The prevalence of tubular atrophy/interstitial fibrosis was 989 (92.4%), 68 (6.4%) and 13 (1.2%) for mild tubular atrophy/interstitial fibrosis, moderate tubular atrophy/interstitial fibrosis and severe tubular atrophy/interstitial fibrosis, respectively (Table 1, Fig. 2). The patients with IgAN had a higher ratio of segmental glomerulosclerosis and more serious situation of tubular atrophy/interstitial fibrosis than non-IgAN group (P < 0.001, Table 1).
Table 1.
Baseline clinical characteristics and pathological features
| Total | IgAN | non-IgAN | p-value | |
|---|---|---|---|---|
| n = 1070 | n = 429 | n = 641 | ||
| Age (years) | 38 ± 15 | 34 ± 12 | 40 ± 16 | <0.001 |
| Male (n, %) | 516 (48.2%) | 171 (39.9%) | 345 (53.8%) | <0.001 |
| Cr (μmol/L) | 84.2 ± 50.1 | 90.3 ± 50.8 | 80.1 ± 50.4 | 0.004 |
| eGFR (ml/min/1.73m2) | 98.1 ± 31.3 | 93.0 ± 32.9 | 101.6 ± 29.8 | <0.001 |
| Urea (mmol/L) | 6.5 ± 3.7 | 6.8 ± 3.7 | 6.3 ± 3.7 | 0.03 |
| Alb (g/L) | 33.2 ± 9.5 | 38.0 ± 6.7 | 30.0 ± 9.7 | <0.001 |
| UA (μmol/L) | 372.7 ± 104.1 | 382.4 ± 105.2 | 366.2 ± 102.8 | 0.01 |
| HUA (n,%) | 415 (38.8%) | 188 (43.8%) | 277 (35.4%) | 0.84 |
| 24 h-u-pro (g/d) | 1.6 (0.5,4.0) | 1.2 (0.5,2.3) | 2.2 (0.5,5.1) | <0.001 |
| SBP | 126.74 ± 17.87 | 126.13 ± 17.52 | 127.13 ± 18.09 | 0.40 |
| DBP | 78.01 ± 12.32 | 77.72 ± 17.16 | 78.19 ± 12.43 | 0.57 |
| hypertension(n,%) | 230 (21.5%) | 85 (19.8%) | 145 (22.6%) | 0.60 |
| Histopathological changes | ||||
| S0 (n,%) | 812 (75.9%) | 237 (55.2%) | 575 (89.7%) | <0.001 |
| S1 (n,%) | 258 (24.1%) | 192 (44.8%) | 66 (10.3%) | |
| T1 (n,%) | 989 (92.4%) | 369 (86.0%) | 620 (96.7%) | <0.001 |
| T2 (n,%) | 68 (6.4%) | 51 (11.9%) | 17 (2.7%) | |
| T3 (n,%) | 13 (1.2%) | 9 (2.1%) | 4 (0.6%) | |
Notes: Cr creatinine, eGFR estimated glomerular filtration rate, Alb albumin, UA uric acid, HUA hyperuricemia, 24 h-u-pro 24 h protein quantitation, SBP systolic blood pressure, DBP diastolic blood pressure, S0: non-segmental glomerular sclerosis, S1: segmental glomerular sclerosis, T1: mild tubular atrophy/interstitial fibrosis, T2: moderate tubular atrophy/interstitial fibrosis, T3: severe tubular atrophy/interstitial fibrosis
Fig. 1.
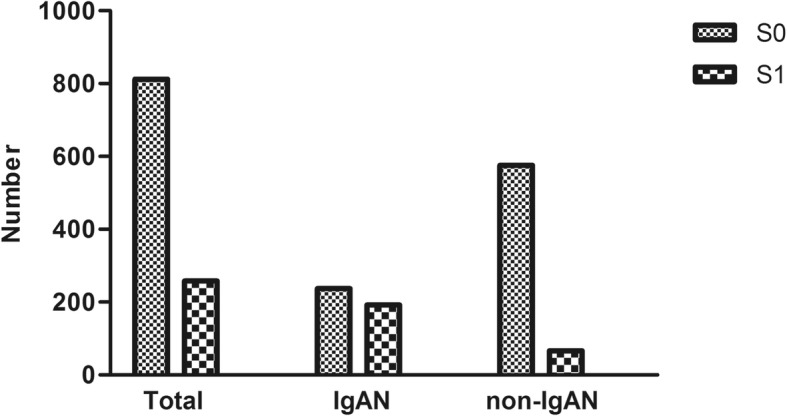
Distribution of segmental glomerular sclerosis. Notes: Number: number of patients. S0: non-segmental glomerular sclerosis. S1: segmental glomerular sclerosis
Fig. 2.
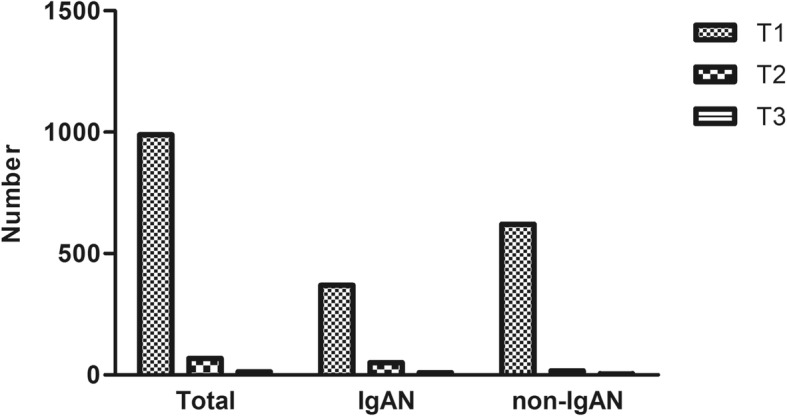
Distribution tubular atrophy / interstitial fibrosis. Notes: Number: number of patients. T1: mild tubular atrophy / interstitial fibrosis. T2: moderate tubular atrophy / interstitial fibrosis. T3: severe tubular atrophy / interstitial fibrosis
Uric acid and renal pathological features
Participants with segmental glomerulosclerosis had higher level of uric acid and worse renal function than those without segmental glomerulosclerosis in the whole cohort, IgAN and non-IgAN cohort (Table 2). In the whole cohort, the number of mild tubular atrophy/interstitial fibrosis, moderate tubular atrophy/interstitial fibrosis and severe tubular atrophy/interstitial fibrosis was 989, 68 and 13, respectively. Patients whose tubular interstitial lesions were more serious, had higher uric acid and lower renal function (Table 2). Among the 1070 patients, uric acid was correlated with eGFR (r = − 0.418, p < 0.001), Cr (r = 0.391, p < 0.001), urea (r = 0.410, p < 0.001), 24-u-pro (r = 0.077, p = 0.022), systolic blood pressure (r = 0.175, p < 0.001) and diastolic blood pressure (r = 0.109, p = 0.001). Uric acid was also correlated with segmental glomerulosclerosis (r = 0.117, P < 0.001) and tubular atrophy/interstitial fibrosis (r = 0.190, P < 0.001) in the whole cohort.
Table 2.
Uric acid and renal pathological features
| Total | S0 | S1 | p-value | T1 | T2 | T3 | p-value |
|---|---|---|---|---|---|---|---|
| (n = 1070) | (n = 812) | (n = 258) | (n = 989) | (n = 68) | (n = 13) | ||
| age | 38.67 ± 15.51 | 34.87 ± 11.53 | <0.001 | 37.64 ± 14.99 | 38.91 ± 11.54 | 41.15 ± 9.54 | 0.55 |
| SBP | 126.42 ± 17.55 | 127.74 ± 18.84 | 0.33 | 126.24 ± 17.79 | 131.18 ± 15.83△ | 142.17 ± 24.07# | 0.001 |
| DBP | 77.62 ± 11.98 | 79.23 ± 13.29 | 0.09 | 77.67 ± 12.29 | 81.05 ± 10.00△ | 88.08 ± 18.46 | 0.002 |
| UA | 365.8 ± 102.2 | 394.3 ± 107.1 | <0.001 | 367.0 ± 101.3 | 436.6 ± 114.1△ | 469.1 ± 103.0# | <0.001 |
| Cr | 81.5 ± 52.7 | 92.7 ± 43.2 | 0.002 | 78.1 ± 40.7 | 146.2 ± 78.0△ | 221.8 ± 117.4# | <0.001 |
| eGFR | 101.0 ± 30.4 | 89.2 ± 32.7 | <0.001 | 102.0 ± 28.7 | 54.5 ± 23.5△ | 35.3 ± 18.1#ο | <0.001 |
| Urea | 6.3 ± 3.7 | 7.2 ± 3.6 | <0.001 | 6.2 ± 3.3 | 9.9 ± 5.6△ | 14.0 ± 5.5# | <0.001 |
| Alb | 32.0 ± 9.8 | 37.1 ± 7.3 | <0.001 | 33.0 ± 9.7 | 35.2 ± 7.4 | 35.6 ± 6.1 | 0.12 |
| 24 h-u-pro | 1.7 (0.4, 4.3) | 1.5 (0.7, 2.8) | <0.001 | 1.6 (0.5, 4.0) | 1.9 (1.0, 3.6) | 3.7 (1.9, 4.6) | 0.89 |
| IgAN(n = 429) | (n = 237) | (n = 192) | (n = 369) | (n = 51) | (n = 9) | ||
| age | 35.23 ± 12.30 | 33.22 ± 10.25 | 0.07 | 34.09 ± 11.77 | 35.73 ± 9.73 | 36.78 ± 6.03 | 0.51 |
| SBP | 126.73 ± 16.64 | 125.43 ± 18.53 | 0.48 | 125.21 ± 17.48 | 130.74 ± 15.12 | 136.88 ± 24.86 | 0.03 |
| DBP | 77.35 ± 11.30 | 78.16 ± 13.13 | 0.53 | 76.95 ± 12.06 | 81.53 ± 10.09△ | 87.00 ± 19.03 | 0.006 |
| UA | 374.6 ± 101.2 | 392.0 ± 109.5 | 0.09 | 373.1 ± 101.1 | 435.3 ± 115.3△ | 464.9 ± 97.2# | <0.001 |
| Cr | 89.7 ± 56.6 | 91.0 ± 42.8 | 0.79 | 79.6 ± 33.0 | 152.4 ± 86.2△ | 177.6 ± 57.4# | <0.001 |
| eGFR | 94.9 ± 33.0 | 90.7 ± 32.6 | 0.19 | 99.6 ± 29.2 | 53.9 ± 24.4△ | 42.2 ± 16.4# | <0.001 |
| Urea | 6.6 ± 3.7 | 7.1 ± 3.7 | 0.11 | 6.2 ± 2.8 | 10.1 ± 6.0△ | 13.6 ± 4.5# | <0.001 |
| Alb | 37.7 ± 7.5 | 38.6 ± 5.6 | 0.17 | 38.4 ± 6.7 | 36.1 ± 6.9△ | 34.9 ± 4.5 | 0.02 |
| 24 h-u-pro | 1.0 (0.4, 2.0) | 1.4 (0.7, 2.5) | 0.52 | 1.0 (0.5, 2.1) | 2.0 (0.9, 4.0)△ | 3.7 (2.0, 4.8) | <0.001 |
| non-IgAN (n = 641) | (n = 575) | (n = 66) | (n = 620) | (n = 17) | (n = 4) | ||
| age | 40.09 ± 16.45 | 39.73 ± 13.60 | 0.84 | 39.75 ± 16.25 | 48.47 ± 11.49△ | 51.00 ± 8.98 | 0.03 |
| SBP | 126.31 ± 17.90 | 133.89 ± 18.40 | 0.002 | 126.81 ± 17.94 | 132.50 ± 18.40 | 152.75 ± 21.42#ο | 0.009 |
| DBP | 77.72 ± 12.24 | 82.08 ± 13.38 | 0.009 | 78.07 ± 12.41 | 79.57 ± 9.95 | 90.25 ± 19.87 | 0.13 |
| UA | 362.2 ± 102.4 | 401. ± 100.3 | 0.003 | 363.4 ± 101.4 | 440.4 ± 113.6△ | 478.6 ± 130.7# | <0.001 |
| Cr | 78.1 ± 50.6 | 97.7 ± 44.5 | 0.003 | 77.3 ± 44.7 | 127.3 ± 41.5△ | 321.3 ± 165.3 | <0.001 |
| eGFR | 103.5 ± 28.9 | 84.7 ± 32.6 | <0.001 | 103.4 ± 28.3 | 56.4 ± 20.9△ | 19.8 ± 11.3#ο | <0.001 |
| Urea | 6.2 ± 3.7 | 7.3 ± 3.4 | 0.02 | 6.2 ± 3.6 | 9.5 ± 3.9△ | 14.7 ± 8.0 | <0.001 |
| Alb | 29.6 ± 9.7 | 32.6 ± 9.5 | 0.02 | 29.8 ± 9.7 | 32.6 ± 8.4 | 37.1 ± 9.6 | 0.17 |
| 24 h-u-pro | 2.3 (0.4, 5.1) | 1.8 (1.0, 4.5) | 0.44 | 2.3 (0.4, 5.1) | 1.6 (1.1, 4.8) | 3.3 (1.8, 3.3) | 0.86 |
Notes: △:T1 vs.T2, p<0.05, #: T1 vs. T3, p<0.05, Ο: T2 vs. T3, p<0.05, UA uric acid, Cr creatinine, eGFR estimated glomerular filtration rate, Alb albumin, 24 h-u-pro 24 h protein quantitation, SBP systolic blood pressure, DBP diastolic blood pressure
Hyperuricemia and renal pathological changes
Univariate logistic regression analysis showed that hyperuricemia was associated with segmental glomerulosclerosis (OR = 1.918, 95% CI:1.444–2.546) and tubular atrophy/interstitial fibrosis (OR = 3.279, 95% CI:2.037–5.276). Multivariate logistic regression analysis confirmed this finding (segmental glomerulosclerosis (OR = 1.800, 95% CI:1.309–2.477) (Table 3) and tubular atrophy/interstitial fibrosis (OR = 1.802, 95% CI:1.005–3.232)) after adjustment for serum creatinine, age and blood pressure. Furthermore, hyperuricemia remained a risk factor for segmental glomerulosclerosis after adjustment for other models, such as Cr + 24 h-u-pro + age + BP, Cr + Alb + age + BP, eGFR +Alb + age + BP (Table 3).
Table 3.
Logistic analysis for predictors of segmental glomerulosclerosis
| OR1 (95% CI) | OR2 (95% CI) | OR3 (95% CI) | OR4 (95% CI) | OR 5 (95% CI) | |
|---|---|---|---|---|---|
| HUA | 1.800△ (1.309–2.477) | 1.771△ (1.250–2.509) | 1.812△ (1.297–2.533) | 1.400 (0.975–2.011) | 1.422△ (1.003–2.016) |
Notes: △: p<0.05, OR1: adjusted for Cr + age + BP, OR2: adjusted for Cr + 24 h-u-pro + age + BP, OR3: adjusted for Cr + Alb + age + BP, OR4: adjusted for eGFR + 24 h-u-pro + age + BP, OR5: adjusted for eGFR +Alb + age + BP, HUA hyperuricemia, Cr creatinine, eGFR estimated glomerular filtration rate, Alb albumin, 24 h-u-pro 24 h protein quantitation, BP blood pressure
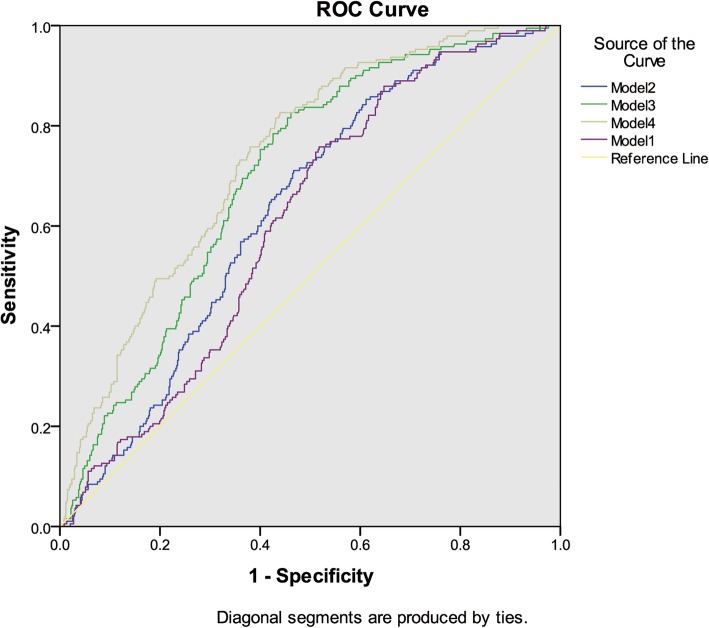
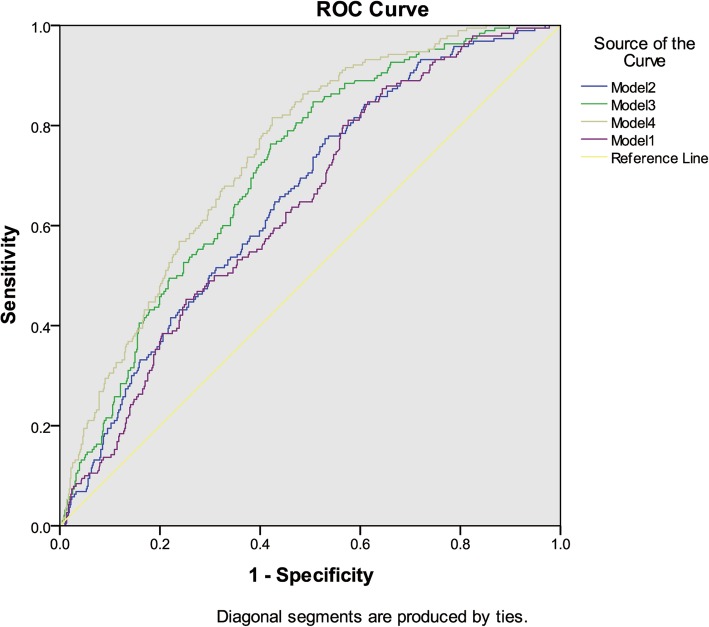
The predictors, which were statistical significant from logistic regression analysis, were used to study their ability to diagnose segmental glomerulosclerosis with Receiver Operating Characteristic curves in four models. We analyzed the area under the curve and compared the difference between models with HUA and models without HUA. When considering the variable of hyperuricemia, the area under the curve was larger than that without hyperuricemia (Figs. 3 & 4, Table 4). Compared with the other three models, model 4 (HUA + eGFR + Alb + age + BP) had the largest area under the curve. In model 4, AUC changed from 0.738 to 0.741 after adding hyperuricemia to the model (Table 4).
Fig. 3.
Diagnosis of segmental glomerular sclerosis without HUA. Notes: Model 1: Cr + age + BP. Model 2: Cr + 24 h-u-pro + age + BP. Model 3: Cr + Alb + age + BP. Model 4: eGFR +Alb + age + BP
Fig. 4.
Diagnosis of segmental glomerular sclerosis with HUA. Notes: Model 1: HUA + Cr + age + BP. Model 2: HUA + Cr + 24 h-u-pro + age + BP. Model 3: HUA + Cr + Alb + age + BP. Model 4: HUA + eGFR +Alb + age + BP
Table 4.
Specificity and sensitivity for predicting segmental glomerulosclerosis
| Model 1 | Model 2 | Model 3 | Model 4 | |||||
|---|---|---|---|---|---|---|---|---|
| HUA | HUA | HUA | HUA | HUA | HUA | HUA | HUA | |
| absent | present | absent | present | absent | present | absent | present | |
| Sensitivity | 0.758 | 0.815 | 0.711 | 0.773 | 0.837 | 0.774 | 0.828 | 0.819 |
| Specificity | 0.493 | 0.429 | 0.531 | 0.466 | 0.535 | 0.571 | 0.559 | 0.573 |
| AUC (SE) | 0.617 (0.020) | 0.6433 (0.020) | 0.629 (0.021) | 0.652 (0.021) | 0.700 (0.019) | 0.716 (0.019) | 0.738 (0.019) | 0.741 (0.019) |
Notes: HUA hyperuricemia, Cr creatinine, eGFR estimated glomerular filtration rate, Alb albumin, 24 h-u-pro 24 h protein quantitation, BP blood pressure. Model 1: Cr + age + BP, Model 2: Cr + 24 h-u-pro + age + BP, Model 3: Cr + Alb + age + BP, Model 4: eGFR +Alb + age + BP, AUC area under the curve, SE standard error
Discussion
Our study included 1070 patients with chronic kidney disease who received renal biopsy. The overall prevalence of HUA was 38.8%, suggesting that uric acid lowering treatment may be beneficial for more than one third of the patients. We attempted to divide the 1070 patients into different subgroups according to renal pathology, such as IgAN, membranous nephropathy (MN) group, focal segmental glomerulosclerosis (FSGS), etc. However, preliminary data analysis revealed that other groups except IgAN had similar clinical features in the current cohort. Moreover, the small number of cases of individual group, is not conducive to the statistical analysis. Finally, we divided all patients into IgAN and non-IgAN and found that the prevalence of HUA was higher in IgAN than in non-IgAN.
In the studied cohort, we found that the more serious the histological injury was, the worse renal function were, which were in accordance with previous studies [22–24]. We also found that uric acid was associated with renal pathological changes. High uric acid levels are associated with poorer kidney function. In order to further investigate the correlation between uric acid and histological damage of kidney, we performed logistic regression analysis for all patients. The results showed that after adjustment for Cr, age and blood pressure, HUA was still a risk factor for segmental glomerulosclerosis (OR = 1.800, 95% CI:1.309–2.477) and tubular atrophy/interstitial fibrosis (OR = 1.802,95% CI:1.005–3.232). Furthermore, we built four different models as sensitivity analysis, and found that HUA was still a risk factor for segmental glomerulosclerosis in all four models (Table 3). However, we did not find a significant association between HUA and tubular atrophy/interstitial fibrosis. In model 4, if the index of HUA was added, the area under curve increased from 0.738 to 0.741 (Table 4). Although this increase was not significant, it could improve the value of diagnosis to some extents.
In recent years, with the lifestyle modifications, the prevalence of hyperuricemia (HUA) is increasing, and the prevalence of HUA in Chinese adults ranged from 8.4 to 13.3% [25, 26]. Our study showed that patients with glomerulonephritis have an even higher prevalence of HUA, indicating a considerable number of population might benefit from uric acid lowering interventions. HUA is not only an independent risk factor for CKD [8, 9], but isalso associated with an increased risk of CKD progression [10, 11] and cardiovascular outcomes [14, 15]. Moreover, the renal pathological changes are also one of major prognostic predictors for CKD progression. The more serious the lesion is, the worse the renal prognosis is [22–24]. The pathological examination is deemed to be a gold standard for the evaluation of the extent of chronic kidney damages. However, it relies on renal biopsy, which is an invasive examination. In some clinical settings, this invasive method might be contraindicated in or refused by the patients. Looking for a clinical biochemical indicator to assist with evaluating the necessity of performing renal biopsy in guiding clinical management. Uric acid seems to be a potential indicator in this regard. After multiple logistic regression and sensitivity analyses, HUA was found independently associated with segmental glomerulosclerosis and tubular atrophy/interstitial fibrosis. This can be further validated in prospective studies in the future.
The relationship between HUA and renal pathological features could be explained by the mechanisms how HUA injures the kidneys. HUA can lead to injury in target organs, such as glomerular sclerosis, glomerular hypertension, glomerulosclerosis, interstitial lesions, and acute kidney injury [27]. HUA can also directly affect the renal interstitium and lead to fibrosis by inducing the transdifferentiation of glomerular epithelial cells [6]. Uric acid is closely related to the progression of kidney disease [28]. After the treatment of HUA, eGFR increased, proteinuria decreased and renal function improved [29–32]. Histological changes in kidney are associated with a variety of factors, not just uric acid [6]. The underlying implication of HUA and renal pathological changes could somehow be explained by uric acid metabolism in the kidney. The glomerulus is a mass of capillary network. Uric acid crystals are deposited in renal tubules and renal interstitium, causing kidney diseases. HUA can induce oxidative stress and endothelial dysfunction, causing renal vasoconstriction, glomerular hypertension, renal blood flow reduction [5, 7, 12]. It also activates the RAS system, leading to glomerulosclerosis and interstitial fibrosis [33, 34].
Due to the nature of retrospective cross-sectional study, there are some limitations in our study. Firstly, we were unable to draw a causal relationship between uric acid and renal pathological changes. Secondly, some confounders were not collected and included in our analyses, which may have an impact on the results. However, in our study, we found that HUA was associated with glomerulosclerosis and tubulointerstitial injury, which could be helpful in predicting glomerulosclerosis and tubulointerstitial injury in clinical practice especially for patients not going to or not willing to have renal biopsy. The results also raise that HUA as a potential treatment target as recommended by current guidelines might be helpful with renal sclerosis, which needs large scale prospective studies to prove.
Conclusions
Hyperuricemia is prevalent in CKD. Uric acid correlates not only with clinical renal injury indexes, but also with renal pathology. Hyperuricemia is independently associated with segmental glomerulosclerosis and tubular atrophy/interstitial fibrosis.
Acknowledgements
This study was supported by Health and Family Planning Commission of Sichuan Province Research Project (17PJ062), Youth Science and Technology Creative Research Groups of Sichuan Province (2015TD0013), Sichuan Science and Technology Department support project (2015SZ0245). Amanda Y Wang was supported by National Heart Foundation post-doctoral fellowship.
We are grateful to all the subjects who participated in this study.
Funding
This study was supported by Health and Family Planning Commission of Sichuan Province Research Project (17PJ062), Youth Science and Technology Creative Research Groups of Sichuan Province (2015TD0013), Sichuan Science and Technology Department support project (2015SZ0245). Amanda Y Wang was supported by National Heart Foundation post-doctoral fellowship.
Availability of data and materials
The datasets used and analysed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- 24 h-u-pro
24 h protein quantitation
- Alb
Albumin
- AUC
Area under curve
- CKD
Chronic kidney disease
- Cr
Creatinine
- DBP
Diastolic blood pressure
- eGFR
Glomerular filtration rate
- HUA
Hyperuricemia
- IgAN
IgA nephropathy
- ROC
Receiver Operating characteristic Curves
- RTS
Renal Treatment System
- SBP
Systolic blood pressure
- UA
Uric acid
Authors’ contributions
FSL collected and processed the data, helped with the study design and drafted the manuscript. ZP read the pathological section. WX participated in the study design and coordination. HDQ performed the statistical analysis. HDQ, WAY, LGS, and WL conceived the study, participated in its design and coordination, and helped to draft the manuscript. All authors read and approved the final manuscript.
Ethics approval and consent to participate
The study was approved by the Ethics Committee of the Sichuan Provincial People’s Hospital (Chengdu, China, No.2017–124). The de-identified data was obtained from RTS database. All patients gave fully informed written consent.
Consent for publication
Not applicable.
Competing interests
All authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Shulei Fan, Email: fsl0311@163.com.
Ping Zhang, Email: zhangping415@126.com.
Amanda Ying Wang, Email: awang@georgeinstitute.org.au.
Xia Wang, Email: xwang@georgeinstitute.org.au.
Li Wang, Email: scwangli62@163.com.
Guisen Li, Email: guisenli@163.com.
Daqing Hong, Email: hongdaqing11@126.com.
References
- 1.Diamond HS, Paolino JS. Evidence for a postsecretory reabsorptive site for uric acid in man. J Clin Invest. 1973;52(6):1491–1499. doi: 10.1172/JCI107323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Aroor AR, Jia G, Habibi J, Sun Z, Ramirez-Perez FI, Brady B, Chen D, Martinez-Lemus LA, Manrique C, Nistala R, et al. Uric acid promotes vascular stiffness, maladaptive inflammatory responses and proteinuria in western diet fed mice. Metab Clin Exp. 2017;74:32–40. doi: 10.1016/j.metabol.2017.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cai W, Duan XM, Liu Y, Yu J, Tang YL, Liu ZL, Jiang S, Zhang CP, Liu JY, Xu JX. Uric acid induces endothelial dysfunction by activating the HMGB1/RAGE signaling pathway. Biomed Res Int. 2017;2017:4391920. doi: 10.1155/2017/4391920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Corry DB, Eslami P, Yamamoto K, Nyby MD, Makino H, Tuck ML. Uric acid stimulates vascular smooth muscle cell proliferation and oxidative stress via the vascular renin-angiotensin system. J Hypertens. 2008;26(2):269–275. doi: 10.1097/HJH.0b013e3282f240bf. [DOI] [PubMed] [Google Scholar]
- 5.Zoccali C, Maio R, Mallamaci F, Sesti G, Perticone F. Uric acid and endothelial dysfunction in essential hypertension. Journal of the American Society of Nephrology : JASN. 2006;17(5):1466–1471. doi: 10.1681/ASN.2005090949. [DOI] [PubMed] [Google Scholar]
- 6.Ryu ES, Kim MJ, Shin HS, Jang YH, Choi HS, Jo I, Johnson RJ, Kang DH. Uric acid-induced phenotypic transition of renal tubular cells as a novel mechanism of chronic kidney disease. American journal of physiology Renal physiology. 2013;304(5):F471–F480. doi: 10.1152/ajprenal.00560.2012. [DOI] [PubMed] [Google Scholar]
- 7.Sanchez-Lozada LG, Tapia E, Santamaria J, Avila-Casado C, Soto V, Nepomuceno T, Rodriguez-Iturbe B, Johnson RJ, Herrera-Acosta J. Mild hyperuricemia induces vasoconstriction and maintains glomerular hypertension in normal and remnant kidney rats. Kidney Int. 2005;67(1):237–247. doi: 10.1111/j.1523-1755.2005.00074.x. [DOI] [PubMed] [Google Scholar]
- 8.Iseki K, Ikemiya Y, Inoue T, Iseki C, Kinjo K, Takishita S. Significance of hyperuricemia as a risk factor for developing ESRD in a screened cohort. American journal of kidney diseases : the official journal of the National Kidney Foundation. 2004;44(4):642–650. doi: 10.1016/S0272-6386(04)00934-5. [DOI] [PubMed] [Google Scholar]
- 9.Li L, Yang C, Zhao Y, Zeng X, Liu F, Fu P. Is hyperuricemia an independent risk factor for new-onset chronic kidney disease?: a systematic review and meta-analysis based on observational cohort studies. BMC Nephrol. 2014;15:122. doi: 10.1186/1471-2369-15-122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mohandas R, Johnson RJ. Uric acid levels increase risk for new-onset kidney disease. Journal of the American Society of Nephrology : JASN. 2008;19(12):2251–2253. doi: 10.1681/ASN.2008091012. [DOI] [PubMed] [Google Scholar]
- 11.Obermayr RP, Temml C, Gutjahr G, Knechtelsdorfer M, Oberbauer R, Klauser-Braun R. Elevated uric acid increases the risk for kidney disease. Journal of the American Society of Nephrology : JASN. 2008;19(12):2407–2413. doi: 10.1681/ASN.2008010080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jalal DI, Decker E, Perrenoud L, Nowak KL, Bispham N, Mehta T, Smits G, You Z, Seals D, Chonchol M, et al. Vascular function and uric acid-lowering in stage 3 CKD. Journal of the American Society of Nephrology : JASN. 2017;28(3):943–952. doi: 10.1681/ASN.2016050521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Moe OW. Posing the question again: does chronic uric acid nephropathy exist? Journal of the American Society of Nephrology : JASN. 2010;21(3):395–397. doi: 10.1681/ASN.2008101115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bos MJ, Koudstaal PJ, Hofman A, Witteman JC, Breteler MM. Uric acid is a risk factor for myocardial infarction and stroke: the Rotterdam study. Stroke. 2006;37(6):1503–1507. doi: 10.1161/01.STR.0000221716.55088.d4. [DOI] [PubMed] [Google Scholar]
- 15.Keenan T, Zhao W, Rasheed A, Ho WK, Malik R, Felix JF, Young R, Shah N, Samuel M, Sheikh N, et al. Causal assessment of serum urate levels in Cardiometabolic diseases through a Mendelian randomization study. J Am Coll Cardiol. 2016;67(4):407–416. doi: 10.1016/j.jacc.2015.10.086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Perez-Ruiz F, Martinez-Indart L, Carmona L, Herrero-Beites AM, Pijoan JI, Krishnan E. Tophaceous gout and high level of hyperuricaemia are both associated with increased risk of mortality in patients with gout. Ann Rheum Dis. 2014;73(1):177–182. doi: 10.1136/annrheumdis-2012-202421. [DOI] [PubMed] [Google Scholar]
- 17.Puddu P, Puddu GM, Cravero E, Vizioli L, Muscari A. Relationships among hyperuricemia, endothelial dysfunction and cardiovascular disease: molecular mechanisms and clinical implications. J Cardiol. 2012;59(3):235–242. doi: 10.1016/j.jjcc.2012.01.013. [DOI] [PubMed] [Google Scholar]
- 18.Mazzali M, Kanellis J, Han L, Feng L, Xia YY, Chen Q, Kang DH, Gordon KL, Watanabe S, Nakagawa T, et al. Hyperuricemia induces a primary renal arteriolopathy in rats by a blood pressure-independent mechanism. American journal of physiology Renal physiology. 2002;282(6):F991–F997. doi: 10.1152/ajprenal.00283.2001. [DOI] [PubMed] [Google Scholar]
- 19.Zhou J, Chen Y, Liu Y, Shi S, Li X, Wang S, Zhang H. Plasma uric acid level indicates tubular interstitial leisions at early stage of IgA nephropathy. BMC Nephrol. 2014;15:11. doi: 10.1186/1471-2369-15-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF, 3rd, Feldman HI, Kusek JW, Eggers P, Van Lente F, Greene T, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150(9):604–612. doi: 10.7326/0003-4819-150-9-200905050-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Weisman MH, Gano AD, Jr, Gabriel SE, Hochberg MC, Kavanaugh A, Ofman JJ, Prashker M, Suarez-Almazor ME, Yelin E, Nakelsky SD, et al. Reading and interpreting economic evaluations in rheumatoid arthritis: an assessment of selected instruments for critical appraisal. J Rheumatol. 2003;30(8):1739–1747. [PubMed] [Google Scholar]
- 22.Working Group of the International Ig ANN, the renal pathology S. Cattran DC, Coppo R, Cook HT, Feehally J, Roberts IS, Troyanov S, Alpers CE, Amore A, et al. The Oxford classification of IgA nephropathy: rationale, clinicopathological correlations, and classification. Kidney Int. 2009;76(5):534–545. doi: 10.1038/ki.2009.243. [DOI] [PubMed] [Google Scholar]
- 23.Working Group of the International Ig ANN, The renal pathology S. Roberts IS, Cook HT, Troyanov S, Alpers CE, Amore A, Barratt J, Berthoux F, Bonsib S, et al. The Oxford classification of IgA nephropathy: pathology definitions, correlations, and reproducibility. Kidney Int. 2009;76(5):546–556. doi: 10.1038/ki.2009.168. [DOI] [PubMed] [Google Scholar]
- 24.Jiang L, Liu G, Lv J, Huang C, Chen B, Wang S, Zou W, Zhang H, Wang H. Concise semiquantitative histological scoring system for immunoglobulin a nephropathy. Nephrology. 2009;14(6):597–605. doi: 10.1111/j.1440-1797.2008.01083.x. [DOI] [PubMed] [Google Scholar]
- 25.Zhang L, Wang F, Wang L, Wang W, Liu B, Liu J, Chen M, He Q, Liao Y, Yu X, et al. Prevalence of chronic kidney disease in China: a cross-sectional survey. Lancet. 2012;379(9818):815–822. doi: 10.1016/S0140-6736(12)60033-6. [DOI] [PubMed] [Google Scholar]
- 26.Liu R, Han C, Wu D, Xia X, Gu J, Guan H, Shan Z, Teng W. Prevalence of hyperuricemia and gout in mainland China from 2000 to 2014: a systematic review and meta-analysis. Biomed Res Int. 2015;2015:762820. doi: 10.1155/2015/762820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Johnson RJ, Nakagawa T, Jalal D, Sanchez-Lozada LG, Kang DH, Ritz E. Uric acid and chronic kidney disease: which is chasing which? Nephrology, dialysis, transplantation : official publication of the European Dialysis and Transplant Association - European Renal Association. 2013;28(9):2221–2228. doi: 10.1093/ndt/gft029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chonchol M, Shlipak MG, Katz R, Sarnak MJ, Newman AB, Siscovick DS, Kestenbaum B, Carney JK, Fried LF. Relationship of uric acid with progression of kidney disease. American journal of kidney diseases : the official journal of the National Kidney Foundation. 2007;50(2):239–247. doi: 10.1053/j.ajkd.2007.05.013. [DOI] [PubMed] [Google Scholar]
- 29.Hira D, Chisaki Y, Noda S, Araki H, Uzu T, Maegawa H, Yano Y, Morita SY, Terada T. Population pharmacokinetics and therapeutic efficacy of Febuxostat in patients with severe renal impairment. Pharmacology. 2015;96(1–2):90–98. doi: 10.1159/000434633. [DOI] [PubMed] [Google Scholar]
- 30.Levy G, Cheetham TC. Is it time to start treating asymptomatic hyperuricemia? American journal of kidney diseases : the official journal of the National Kidney Foundation. 2015;66(6):933–935. doi: 10.1053/j.ajkd.2015.09.002. [DOI] [PubMed] [Google Scholar]
- 31.McGowan B, Bennett K, Silke C, Whelan B. Adherence and persistence to urate-lowering therapies in the Irish setting. Clin Rheumatol. 2016;35(3):715–721. doi: 10.1007/s10067-014-2823-8. [DOI] [PubMed] [Google Scholar]
- 32.Sircar D, Chatterjee S, Waikhom R, Golay V, Raychaudhury A, Chatterjee S, Pandey R. Efficacy of Febuxostat for slowing the GFR decline in patients with CKD and asymptomatic hyperuricemia: a 6-month, double-blind, randomized, placebo-controlled trial. American journal of kidney diseases : the official journal of the National Kidney Foundation. 2015;66(6):945–950. doi: 10.1053/j.ajkd.2015.05.017. [DOI] [PubMed] [Google Scholar]
- 33.Nakagawa T, Mazzali M, Kang DH, Kanellis J, Watanabe S, Sanchez-Lozada LG, Rodriguez-Iturbe B, Herrera-Acosta J, Johnson RJ. Hyperuricemia causes glomerular hypertrophy in the rat. Am J Nephrol. 2003;23(1):2–7. doi: 10.1159/000066303. [DOI] [PubMed] [Google Scholar]
- 34.Dobrian AD, Schriver SD, Prewitt RL. Role of angiotensin II and free radicals in blood pressure regulation in a rat model of renal hypertension. Hypertension. 2001;38(3):361–366. doi: 10.1161/01.HYP.38.3.361. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and analysed during the current study are available from the corresponding author on reasonable request.