Abstract
Nuts and vegetable oils are important sources of fat and of a wide variety of micronutrients and phytochemicals. Following their intake, several of their constituents, as well as their derived metabolites, are found in blood circulation and in urine. As a consequence, these could be used to assess the compliance to a dietary intervention or to determine habitual intake of nuts and vegetable oils. However, before these metabolites can be widely used as biomarkers of food intake (BFIs), several characteristics have to be considered, including specificity, dose response, time response, stability, and analytical performance. We have, therefore, conducted an extensive literature search to evaluate current knowledge about potential BFIs of nuts and vegetable oils. Once identified, the strengths and weaknesses of the most promising candidate BFIs have been summarized. Results from selected studies have provided a variety of compounds mainly derived from the fatty fraction of these foods, but also other components and derived metabolites related to their nutritional composition. In particular, α-linolenic acid, urolithins, and 5-hydroxyindole-3-acetic acid seem to be the most plausible candidate BFIs for walnuts, whereas for almonds they could be α-tocopherol and some catechin-derived metabolites. Similarly, several studies have reported a strong association between selenium levels and consumption of Brazil nuts. Intake of vegetable oils has been mainly assessed through the measurement of specific fatty acids in different blood fractions, such as oleic acid for olive oil, α-linolenic acid for flaxseed (linseed) and rapeseed (canola) oils, and linoleic acid for sunflower oil. Additionally, hydroxytyrosol and its metabolites were the most promising distinctive BFIs for (extra) virgin olive oil. However, most of these components lack sufficient specificity to serve as BFIs. Therefore, additional studies are necessary to discover new candidate BFIs, as well as to further evaluate the specificity, sensitivity, dose-response relationships, and reproducibility of these candidate biomarkers and to eventually validate them in other populations. For the discovery of new candidate BFIs, an untargeted metabolomics approach may be the most effective strategy, whereas for increasing the specificity of the evaluation of food consumption, this could be a combination of different metabolites.
Electronic supplementary material
The online version of this article (10.1186/s12263-019-0628-8) contains supplementary material, which is available to authorized users.
Keywords: Nuts, Oils, Biomarker, Intake, Metabolomics
Background
Western diets contain significant but varying amounts of nuts and vegetable oils. Both are natural plant foods rich in fat. Nuts have been a component of the human diet since pre-agricultural times [1]. In Western countries, nuts are consumed either raw or roasted as part of meals, as snacks, or as desserts. They are eaten whole (fresh or roasted), in salads, spreads (in both sweet and salty spreads), as oils or hidden in products, such as sauces, dairies, pastries, and baked goods [2]. Vegetable oils, which can be defined as “oils composed primarily of glycerides of fatty acids being obtained only from plant sources,” have been introduced more recently in Europe. Until the late nineteenth century, the olive was the only edible oil-bearing crop and its use was virtually restricted to the Mediterranean area, while the rest of the continent used animal fats as the principal source of cooking oil [3]. Due to technological developments, large-scale food production, and easier and cheaper transport, the consumption of olive oil and other vegetable oils increased [4].
Nuts are nutrient-dense foods and are rich sources of dietary fatty acids with a high ratio of unsaturated to saturated fatty acids [2]. Moreover, they contain many other nutrients and bioactive compounds, including high-quality proteins, fibers, minerals, tocopherols, phytosterols, and phenolic compounds [2]. The main fatty acids in nuts are oleic acid (C18:1), linoleic acid (C18:2), and α-linoleic acid (C18:3) [5, 6]. Vegetable oils are another important source of dietary fatty acid intake. Globally, the main oils in the human diet are derived from soya, palm, sunflower, and rape [7], although there is high variability depending on the local tradition of each region. These oils are mostly used for baking, frying, or as salad dressing [8]. Vegetable oils are rich sources of (n-9) monounsaturated fatty acids (MUFAs) and (n-6 and n-3) polyunsaturated fatty acids (PUFAs). Hydroxytyrosol [9] is a specific compound associated with olive oil consumption, which is believed to contribute to several of its beneficial health effects [10].
Many studies have investigated the potential health effects of nuts and vegetable oils. Previous epidemiologic studies on the health effects of nuts have shown that nut consumption is associated with a lower incidence of coronary heart disease in both men and women [11]. Additionally, intervention studies have shown an LDL-cholesterol-lowering effect of nut consumption, usually without any effect on HDL-cholesterol and triglycerides [12–14]. Likewise, it is known that isocaloric replacement of saturated fatty acids (SFAs) by MUFAs and PUFAs, which are most common in vegetable oils, is associated with a lower risk of developing cardiovascular diseases, which is partly mediated by lowering LDL-cholesterol [15].
Given the potential health benefits of both nuts and vegetable oils, it is important to find specific biomarkers of their intake. Currently, food frequency questionnaires (FFQs), food diaries, and 24-h dietary recalls are used as dietary assessment tools in studies on nutrition. However, these assessment tools are based on self-reporting by subjects and some of the drawbacks associated with self-reporting food consumption are, among others, that they rely on a correct estimation of portion size. Additionally, surveys based on retrospective methods (such as 24-h dietary recalls or FFQ) depend on the memory of the subject, which could lead to food omissions, while the prospective surveys (such as food diaries) could cause changes in eating behavior. They often focus on type, frequency, and serving size, but do not take into account information on food sources, food processing, or storage conditions. To illustrate, usually the presence of oil in processed foods or receipts is disregarded by consumers, whereas nuts are often hidden in processed foods (for example, in sauces, spreads, dairy products, etc.) and as such these products are easily missed with self-reported dietary assessment methods. Therefore, there is a growing interest in biomarkers of food intake (BFIs), which are a more objective reflection of dietary intake [16]. These biomarker-based measurements of dietary intake are independent of subjects’ memory, misreporting, or limitations of food composition databases and can improve intake measurements, contributing to better estimates of associations between diet and health outcomes. Therefore, the use of BFI as a complementary or alternative tool of the traditional instruments is one of the focus of current and future research topics in nutritional sciences.
This review has been developed as part of the Food Biomarkers Alliance (FoodBAll) consortium, supported by the Joint Programming Initiative “A Healthy Diet for a Healthy Life” [17]. The objective of this paper was to perform an extensive literature search of both observational and human intervention studies in order to describe which BFIs of both nuts and vegetable oils have been described until now.
Methodology
This review is focused on the most widely consumed types of nuts and vegetable oils. For nuts, walnuts, hazelnuts, pistachios, pecan nuts, macadamia nuts, cashews, and Brazil nuts were selected. Additionally, almonds and peanuts, although they are botanically classified as drupes and legumes, respectively, have also been included because of their nutritional profile. Among vegetable oils, olive, sunflower, flaxseed, and rapeseed oils were covered.
The review was conducted following the methodology harmonized within the FoodBAll consortium (http://foodmetabolome.org/) and recently described [18]. The search was conducted in three databases (PubMed, Scopus, and Web of Science) using the following combinations of grouped search terms: (biomarker* OR marker* OR metabolite* OR biokinetics OR biotransformation) AND (trial OR experiment OR study OR intervention) AND (human* OR men OR women OR patient* OR volunteer* OR participant*) AND (urine OR plasma OR serum OR blood OR excretion OR “adipose tissue” OR “fat tissue” OR “erythrocyte membrane*” OR phospholipid* OR “cholesterol ester*” OR “cholesteryl ester*” OR triglyceride* OR triacylglycerol*) AND (intake OR meal OR diet OR ingestion OR consumption OR eating OR drink* OR administration), together with specific keywords related to each food group, since searches were carried out separately for each food group. For nuts these were (nut OR nuts OR walnut* OR hazelnut* OR almond* OR pecan* OR macadamia* OR peanut* OR pistachio* OR cashew* OR “brazil nut”), whereas for vegetable oils they were (oil*) AND (olive* OR coconut* OR rapeseed* OR canola* OR sunflower* OR palm* OR flaxseed* OR linseed* OR sesame* OR corn* OR soybean* OR safflower* OR seed*). The mentioned keywords were used in the default fields of each database. They were [All fields], [Article Title/Abstract/Keywords], and [Topic] for PubMed, Scopus, and Web of Science, respectively.
Firstly, titles and abstracts were screened to determine whether they met the selection criteria. In case of doubt, the papers were also kept in the list of selected references, which were further evaluated using information included in the full text. Additional papers were identified from reference lists of selected papers and relevant reviews. Only papers in the English language were considered eligible, while no restriction was applied for publication dates (the last search was done in December 2017). Those papers identifying or using potential BFIs of nuts or vegetable oils measured in human biological samples were selected (i.e., animal studies were excluded). Those papers reporting duplicated data from the same study were excluded, with only one paper being retained for each study. The research papers identifying or using potential BFIs were selected by one or more skilled researchers. All candidate BFIs were merged in a unique list, which was further split according to their potentiality as promising candidate BFIs, either used alone (as a single BFI) or within a combination in a multi-metabolite biomarker panel. Those potentially good candidate BFIs were included in a first table together with the description of the corresponding studies where they were measured, while the others were grouped in a second table along with their associated references where the association with the food intake was described, as well as the main reason for exclusion.
Finally, a score system also developed within the FoodBAll consortium [19] was applied for those BFIs retained as potentially good candidates in order to systematically assess their current validity, as well as to pinpoint whether additional studies were still needed. It included eight items related to both analytical and biological aspects.
Results and discussion
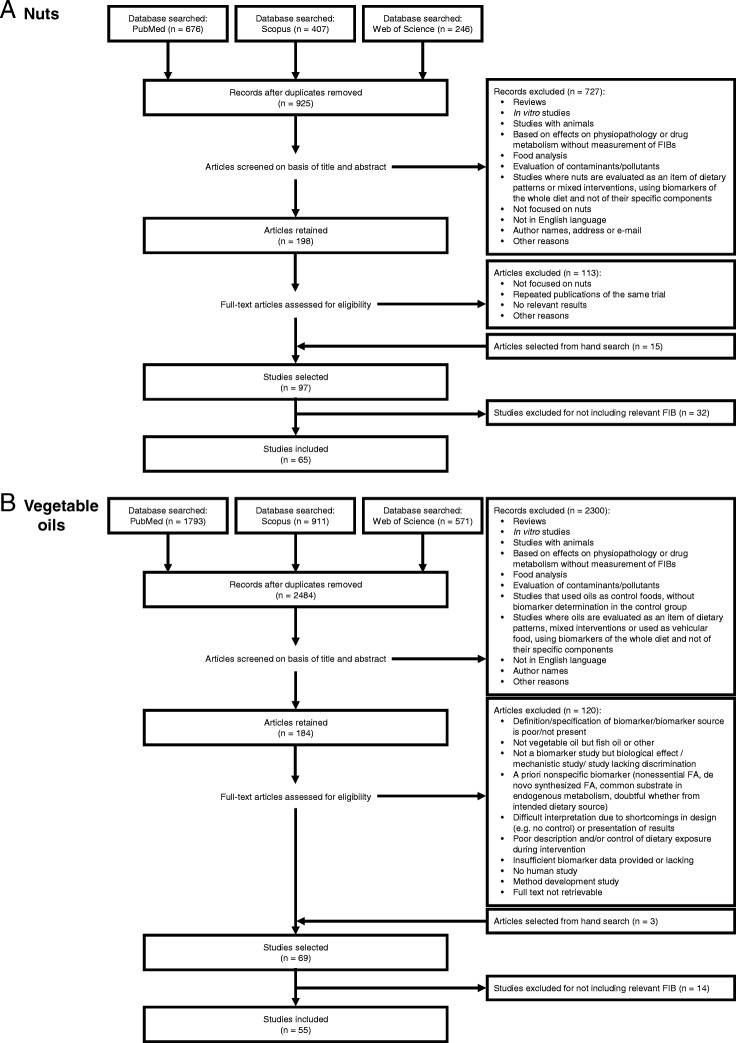
The literature has been extensively reviewed independently for nuts and vegetable oils intake biomarkers. Figure 1 presents an overview of the review and study selection process. Firstly, electronic searches were conducted using the Web of Science, PubMed, and Scopus databases. After excluding duplicated references, a total of 925 and 2484 articles were screened for nuts and vegetable oils, respectively. After title and abstract screening, a total of 97 and 69 articles were selected for providing information on potential candidate BFIs of consumption of nuts and vegetable oils, respectively. Further evaluation of the full-text papers reduced the results to 65 and 55 eligible papers to be included in the sections of nuts and vegetable oils, respectively. The results are successively presented below.
Fig. 1.
Flow diagram of study selection
Biomarkers of nut consumption
A description of selected studies reporting associations between nut intake and potentially relevant BFIs is given in Table 1. They are organized according to the types of nuts (walnuts, almonds, hazelnuts, pistachios, Brazil nuts, and mixed nuts), the study design [acute study (i.e., single-dose study), chronic intervention (i.e., follow-up after a continued supplementation for a specific frame of time) or observational study], the types of discriminating metabolites (fatty acids, polyphenol-derived metabolites, etc.) and the publication date. Most of the selected studies were focused on walnuts [12, 20–51], followed by Brazil nuts [52–64], while a lower number of studies were found for almonds [65–72], hazelnuts [73–75], pistachios [76–78], and mixed nuts [79–83]. The initial search also retrieved studies on pecan nuts [84, 85], macadamia nuts [86–88], cashews [89, 90], and peanuts [91–94], but none of them included any potentially relevant BFIs (see Additional file 1: Table S1 for the corresponding reasons). Therefore, they were not included in Table 1. Selected papers presented data from studies with different designs: most of them reported data from nutritional intervention studies, with acute [20–26, 52, 53] or chronic [12, 27–49, 54–62, 65–76, 79–83] intake of nuts, while four of them reported data from observational cohorts [50, 51, 63, 64]. The current available knowledge about different biological and analytical parameters that summarize the potential usefulness of each metabolite as a potential BFI is presented in Table 2, while the information on the food intake biomarkers of nuts considered nonrelevant is presented in Additional file 1: Table S1.
Table 1.
Studies reporting associations between consumption and potential candidate food intake biomarkers for nuts
| Dietary factor [reference] | Study design | Number of subjects | Analytical method | Sample type | Discriminating metabolites/candidate biomarkers |
|---|---|---|---|---|---|
| Walnutsa [20] | Acute study | 8 | GC | Large TAG-rich lipoproteins | α-Linolenic acid |
| Walnutsa [21] | Acute study | 20 | GC | LDL cholesteryl esters | α-Linolenic acid |
| Walnuts [22] | Acute study | 40 | LC-MS | Urine | Urolithin B glucuronide |
| Walnutsa [23] | Acute study | 16 | HPLC | Urine | Urolithin A |
| Walnuts [24] | Acute study | 8 | Spectro-photometry | Urine | 5-Hydroxyindoleacetic acid |
| Walnuts [25] | Acute study | 3 | HPLC | Urine | 5-Hydroxyindoleacetic acid |
| Walnuts [26] | Acute study | 31 | LC-MS | Serum | 5-Hydroxyindoleacetic acid |
| Walnutsa [12] | Sustained intervention | 18 | GC | Serum cholesteryl esters | α-Linolenic acid |
| Walnutsa [27] | Sustained intervention | 16 | GC | Plasma | α-Linolenic acid |
| Walnutsa [28] | Sustained intervention | 21 | GC | Plasma TAG | α-Linolenic acid |
| Walnutsa [29] | Sustained intervention | 55 | GC | LDL cholesteryl esters | α-Linolenic acid |
| Walnutsa [30] | Sustained intervention | 18 | GC | Plasma | α-Linolenic acid |
| Walnuts [31] | Sustained intervention | 10 | TLC | LDL proteins | α-Linolenic acid |
| Walnutsa [32] | Sustained intervention | 40 | GC | Serum cholesteryl esters | α-Linolenic acid |
| Walnuts [33] | Sustained intervention | 90 | NR | Erythrocytes | α-Linolenic acid |
| Walnutsa [34] | Sustained intervention | 10 | GC | Blood drops | α-Linolenic acid |
| Walnuts [35] | Sustained intervention | 39 | NR | Erythrocytes | α-Linolenic acid |
| Walnutsa [36] | Sustained intervention | 25 | GC | Erythrocytes | Linolenic acid |
| Walnuts [37] | Sustained intervention | 50 | GC | Erythrocytes | α-Linolenic acid |
| Walnuts [38] | Sustained intervention | 25 | NR | Plasma phospholipids | α-Linolenic acid |
| Walnutsa [39] | Sustained intervention | 21 | GC | Erythrocytes | Linolenic acid |
| Walnuts [40] | Sustained intervention | 283 | GC | Erythrocytes | α-Linolenic acid |
| Walnutsa [41] | Sustained intervention | 18 | GC | Plasma | α-Linolenic acid |
| Walnuts [42] | Sustained intervention | 25 | GC-FID | Plasma phospholipids | α-Linolenic acid |
| Walnuts [43] | Sustained intervention | 109 | GC-FID | Serum | α-Linolenic acid |
| Walnutsa [44] | Sustained intervention | 45 | GC-FID | Erythrocytes | α-Linolenic acid |
| Walnutsa [45] | Sustained intervention | 20 | NR | Erythrocytes | α-Linolenic acid |
| Walnutsa [46] | Sustained intervention | 40 | GC | Plasma | α-Linolenic acid |
| Walnutsa [47] | Sustained intervention | 63 | LC-MS | Plasma / Urine / Prostate gland | Urolithin A glucuronide, urolithin B glucuronide (only in prostate gland) |
| Walnuts [48] | Sustained intervention | 10 | LC-MS | Urine / Feces | Urolithin A, urolithin A 3-glucuronide (only urine), isourolithin A, isourolithin A 3-glucuronide (only urine), urolithin B, urolithin B glurcuronide (only urine), urolithin C (only feces) |
| Walnutsa [49] | Sustained intervention | 20 | LC-MS | Urine / Feces | Urolithin A, urolithin A glucuronide (only urine), isourolithin A, isourolithin A glucuronide (only urine), urolithin B, urolithin B glucuronide (only urine), urolithin C (only feces) |
| Walnuts [50] | Observational study | 107 | LC-MS | Urine | 5-Hydroxyindole-3-acetic acid |
| Walnutsa [51] | Observational study | 381 | LC-MS | Urine | Urolithin A glucuronide / sulfate / sulfoglucuronide, urolithin B glucuronide, urolithin C sulfate, urolithin C glucuronide, hydroxyindoleacetic acid sulfate |
| Brazil nuts [52] | Acute study | 3 | LC-MS | Urine | Selenium |
| Brazil nuts [53] | Acute study | 2 | LC-ICP-MS | Urine | Selenium |
| Brazil nuts [54] | Sustained intervention | 15 | AAS | Plasma | Selenium |
| Brazil nuts [55] | Sustained intervention | 59 | AAS | Plasma | Selenium |
| Brazil nuts [56] | Sustained intervention | 81 | AAS | Plasma / Erythrocytes | Selenium |
| Brazil nuts [57] | Sustained intervention | 37 | AAS | Plasma / Erythrocytes | Selenium |
| Brazil nuts [58] | Sustained intervention | 91 | AAS | Plasma | Selenium |
| Brazil nuts [59] | Sustained intervention | 91 | ICP-MS | Plasma | Selenium |
| Brazil nuts [60] | Sustained intervention | 82 | AAS | Plasma / Erythrocytes / Urine (24 h) / Hair / Nails | Selenium |
| Brazil nuts [61] | Sustained intervention | 31 | AAS | Plasma | Selenium |
| Brazil nuts [62] | Sustained intervention | 32 | NS | Plasma | Selenium |
| Brazil nuts [63] | Observational study | 155 | ICP-MS | Blood | Selenium |
| Brazil nuts [64] | Observational study | 219 | ICP-MS | Whole-blood | Selenium |
| Almonds [65] | Sustained intervention | 16 | HPLC | Plasma / Erythrocytes | α-Tocopherol |
| Almonds [66] | Sustained intervention | 20 | HPLC | Plasma | α-Tocopherol |
| Almonds [67] | Sustained intervention | 60 | HPLC | Serum | α-Tocopherol |
| Almonds [68] | Sustained intervention | 24 | NR | Plasma | α-Tocopherol |
| Almonds [69] | Sustained intervention | 65 | HPLC-FLD | Plasma | α-Tocopherol |
| Almonds [70] | Sustained intervention | 22 | HPLC | Plasma | α-Tocopherol |
| Almonds [71] | Sustained intervention | 60 | HPLC | Plasma | α-Tocopherol |
| Almonds [72] | Sustained intervention | 45 | HPLC | Plasma | α-Tocopherol |
| Hazelnuts [73] | Sustained intervention | 48 | HPLC | Plasma | α-Tocopherol |
| Hazelnutsa [74] | Sustained intervention | 21 | HPLC | Serum / Isolated LDL | α-Tocopherol |
| Hazelnuts [75] | Sustained intervention | 72 | HPLC | Plasma | α-Tocopherol |
| Pistachios [77] | Sustained intervention | 28 | GC | Serum | β-Sitosterol |
| Pistachiosa [78] | Sustained intervention | 28 | HPLC | Serum | Lutein |
| Pistachiosa [76] | Sustained intervention | 54 | LC-MS | Plasma | Lutein-zeaxanthin |
| Mixed nuts (walnuts, almonds and hazelnuts) [79] | Sustained intervention | 27 | GC | Plasma | α-Linolenic acid |
| Mixed nuts (walnuts, almonds and hazelnuts) [80] | Sustained intervention | 375 | GC | Plasma | α-Linolenic acid |
| Mixed nuts (walnuts, almonds and hazelnuts) [81] | Sustained intervention | 42 | LC-MS | Urine | Urolithin A glucuronide / sulfate / sulfoglucuronide, hydroxyindoleacetic acid |
| Mixed nuts (walnuts, almonds and hazelnuts) [82] | Sustained intervention | 41 | LC-MS | Urine | Urolithin A, urolithin B |
| Mixed nuts (walnuts, almonds and hazelnuts) a [83] | Sustained intervention | 47 | LC-MS | Plasma | Urolithin A glucuronide |
AAS atomic absorption spectrometry, FID flame ionization detector, FLD fluorometric detection, GC gas chromatography, HPLC high-performance liquid chromatography, ICP inductively coupled plasma, MS mass spectrometry, NR not reported, TAG triacylglycerides, TLC thin-layer chromatography
aThe study includes other nonrelevant metabolites (included in Additional file 1: Table S1)
Table 2.
Validation scheme of potential food intake biomarkers for nuts
| Dietary factor [references] | Compound/metabolite | HMDB ID | Sample type | Criteria | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3a | 3b | 4 | 5 | 6 | 7 | 8 | ||||
| Walnuts [12, 20, 21, 28–45, 27, 46, 79, 80] | α-Linolenic acid | HMDB0001388 | Plasma / serum / erythrocytes | N | U | U | U | U | U | U | Y | U |
| Walnuts [22, 23, 48, 47, 49, 51, 82, 81, 83] | Urolithins: urolithin A (and phase II metabolites), isourolithin A (and phase II metabolites), urolithin B (and phase II metabolites), urolithin C (and phase II metabolites) | HMDB0013695 / HMDB0029222 / HMDB0060022 / HMDB0013696 / HMDB0041787 / HMDB0029218 |
Urine / plasma | N | U | Y | U | Y | U | U | Y | U |
| Walnuts [24, 50, 51, 81] | 5-Hydroxyindole-3-acetic acid | HMDB0000763 | Urine / serum | N | Y | Y | U | Y | U | U | Y | U |
| Almonds/hazelnuts [66–72] | α-Tocopherol | HMDB0001893 | Plasma / serum / erythrocytes | N | U | U | U | U | U | U | Y | U |
| Pistachios [77] | β-Sitosterol | HMDB0000852 | Serum | N | U | U | U | U | U | U | Y | U |
| Pistachios [76, 78] | Lutein-zeaxanthin | HMDB0003233/HMDB0002789 | Plasma / serum | N | U | U | U | U | U | U | Y | U |
| Brazil nuts [53–61] | Selenium | HMDB0001349 | Urine / plasma | N | Y | Y | Y | Y | U | U | Y | U |
HMDB human metabolome database, N no, U unknown, Y yes. Criteria: C1—Plausibility, Is the marker compound plausible as a specific BFI for the food or food group?; C2—Dose response, Is there a dose-response relationship at relevant intake levels of the targeted food?; C3—Time response, Is the biomarker kinetics described adequately to make a wise choice of sample type, frequency and time window?; C3a, single dose; C3b, multiple doses; C4, Robustness, Has the marker been shown to be robust after intake of complex meals reflecting dietary habits of the targeted population?; C5, Reliability, Has the marker been shown to compare well with other markers or questionnaire data for the same food/food group?; C6, Stability, Is the marker chemically and biologically stable during biospecimen collection and storage, making measurements reliable and feasible?; C7, Analytical performance, Are analytical variability (CV%), accuracy, sensitivity and specificity known as adequate for at least one reported analytical method?; C8, Reproducibility, Has the analysis been successfully reproduced in another laboratory?
Although most of the studies applied targeted approaches, the search strategy also retrieved some untargeted studies. Their inclusion or not in the present review was done based on the potentiality of the reported BFI, regardless of the analytical approach used. Therefore, some of the selected papers that used an untargeted strategy were retained as being particularly interesting because they discovered potentially relevant BFIs of nuts, whereas others were not further considered because they did not report any specific BFI. They were focused on walnuts [50, 51], almonds [95], pistachios [96], peanuts [97], and mixed nuts [81, 83, 98, 99]. Some of these studies reported results similar to the targeted approaches, confirming the relationships between walnut intake and urolithins, fatty acids and serotonin-derived metabolites [50, 51, 81, 83], and almond intake and catechin-derived metabolites [95] (see the corresponding subsections for more detailed information). Neither the latter study on catechin-derived metabolites nor targeted studies reporting results in the same direction [100–102] were retained among the studies reporting relevant candidate BFIs. This was because catechin-derived metabolites have broadly been reported to increase after the intake of other flavan-3-ol-rich food sources, including tea, cocoa, and red wine [103]. Guertin et al. (2014) [97] analyzed the correlations between serum metabolic profiles and peanut consumption according to data from FFQs in participants from the Prostate, Lung, Colorectal, and Ovarian Cancer Screening Trial (PLCO). With this approach, tryptophan betaine and 4-vinylphenol sulfate were proposed as candidate biomarkers of peanut intake. Both metabolites were also associated with nut intake in a case-control study [99]. Tryptophan betaine is an indole alkaloid previously also associated with peanut consumption after being detected in the breast milk of breastfeeding mothers [92]. However, it is also detected in legumes [104–106]. 4-vinylphenol is a catabolite generated by the direct decarboxylation of p-coumaric acid [107]. It would be interesting to go into greater depth with these metabolites in order to work out whether they could be considered as potential BFIs of peanuts. However, with the current knowledge, they could not be included in the list of the most promising candidate BFIs due to potential low specificity.
Walnuts
Nuts in general are a rich source of dietary fatty acids with a high unsaturated-to-saturated ratio. The main fatty acids in nuts are oleic acid (C18:1), linoleic acid (C18:2), and α-linolenic acid (C18:3, ALA). Walnuts are characterized by considerably higher amounts of ALA than other types of nuts (11.6% of total fatty acid composition for walnuts compared to < 0.7% for the others) [5, 6]. Such a composition explains the fact that among the different types of nuts, only walnut intake has been associated with ALA in blood both in studies only focused on consumption of walnuts [12, 20, 21, 27–46], and in studies with mixed nut intake that included walnuts [79, 80]. Linoleic acid (C18:2, LA) is the major PUFA present in most types of nuts (40–60% of total fatty acid composition for walnuts, pecans, peanuts, and Brazil nuts) [5, 6]. Therefore, it was consistently found in blood after walnut intake [12, 27–30, 32, 36, 39, 41, 45, 46, 108], and in studies with mixed nuts that included walnuts in their composition [109, 110]. Additionally, its presence in biological fluids was also associated with consumption of cashews [89], for which it is the second most abundant type of fatty acid (20.8%) [6]. Looking at the above-mentioned studies, ALA seems a better candidate biomarker of walnut intake than LA. Nevertheless, there are other food sources of ALA and LA, such as vegetable oils (flaxseed, linseed and rapeseed oils for ALA, and safflower, sunflower, soybean, and corn oil for LA), seeds, and animal products (see the section below dedicated to vegetable oils). This clearly means that the presence of neither ALA nor LA in biological fluids can solely indicate intake of nuts or walnuts. Additionally, both ALA and LA undergo biotransformations in the human body to longer-chain fatty acids [111], giving rise to eicosapentaenoic acid (C20:5, EPA) and docosahexaenoic acid (C22:6, DHA), respectively. Indeed, both of them have been reported after the intake of walnuts [27, 34, 109]. Also, in this case, a confounding factor may occur, as EPA and DHA are also related to fish consumption [112].
Oleic acid (C18:1) is the major MUFA present in most types of nuts (walnuts, almonds, peanuts, hazelnuts, macadamia nuts, and pecan nuts [5, 6]). As a consequence, higher amounts of this fatty acid have been observed in blood and urine after the intake of walnuts [28], almonds [113], hazelnuts [74, 114], pecan nuts [85], macadamia nuts [88], cashews [89, 90], and mixed nuts [98, 115]. This common presence in many types of nuts excludes oleic acid as a direct link to specific nut intake. Moreover, oleic acid has also been associated with olive oil intake (see the corresponding section below). In some targeted investigations, myristic acid (14:0) [12] and stearic acid (18:0) [44, 46], which are the major saturated fatty acids (SFAs) in walnuts, were reported in biological fluids after walnut intake [5]. However, myristic acid is also abundant in dairy products and has been proposed as a potential biomarker of dairy fat intake [116]. In summary, among the different types of fatty acids in walnuts, ALA is the most suitable candidate BFI for walnuts, although it is not specific for this food. For this reason, it seems necessary to perform a complementary search for other potential BFIs of walnuts that are not detected after the consumption of the other ALA food sources [117]. Importantly, McKay et al. [39] analyzed the percentage change in ALA levels compared to baseline levels following an ingestion of 21 g/day or 42 g/day of walnuts for 6 weeks. Although the magnitude of changes in ALA levels after 6 weeks seemed to be higher with the 42 g/day dose (which was the only dose that reached statistical significance compared to baseline), the authors did not make any reference to the potential differences (or not) between the two doses. Therefore, the dose-response association between walnut consumption and ALA levels needs to be further explored. Also, the time-response relationship needs to be further investigated, since neither of the available acute studies reporting levels of ALA after walnut consumption provided a kinetics description [20, 21], but rather they only provided data on one specific time point after consumption. Although the results of the present review did not find any observational study reporting positive associations between levels of ALA and walnut intake, the participants in the study of McKay et al. [39] were not instructed to limit the consumption of other n-3 fatty-rich foods (including fatty fish), thereby reflecting the robustness of this potential BFI in the general population, regardless of the background diet. As regards the analytical performance, various quantification methods using gas chromatography platforms have been developed [118, 119]. However, we could not find any report regarding the reliability (comparison with other BFIs or reference methods), stability during sample collection, storage and processing, or interlaboratory variation.
The appearance in biofluids of urolithins has been the subject of investigations by several authors. In terms of nuts, they have only been reported after intake of walnuts [22, 23, 47–49, 51] or mixed nuts including walnuts [81–83]. In most of these studies, the aglycone or phase II metabolites of urolithin A and B were the most frequently reported metabolites. Urolithins are the product of polymeric ellagitannins (ETs) metabolized by gut microbiota. Among different types of nuts, they are specific for walnuts, but they have also been reported after the intake of pomegranate, strawberries, raspberries, and blackberries. However, these additional foods do not provide important amounts of fatty acids. Therefore, through the employment of a multi-metabolite model, the presence of urolithins and fatty acids at the same time could reveal walnut intake with higher specificity [117]. With regard to the dose-response associations, although there are no studies with different doses of walnuts, one of the selected studies provided participants with different doses of ETs [22]. In that investigation, the subjects consumed different ET amounts through the intake of raspberries (422 mg of ellagic acid, EA), walnuts (191 mg of EA), strawberries (190 mg of EA), or red wine (5.4 mg of EA). The mean highest excretion of urolithins was observed in the walnut group and the lowest in the red wine group. Therefore, the excretion was not directly proportional to the amount of ETs consumed. Instead, it seems that the food matrix has an impact on the bioavailability and metabolism of ETs, which is expected since they exhibit a considerable structural diversity according to food source (i.e., pedunculagin is the major ET found in walnuts; while punicalagins and punicalins predominate in pomegranates; sanguiin H6, sanguiin H10, and lambertiancin C are the main ETs found in berries) [120]. Also, in this study, researchers detected these metabolites in samples collected 16 h after intake, whereas only trace amounts were detected in samples collected before this time point. The complete clearance of ET metabolism could not be estimated since these metabolites were still detected during the following 40 h, when the last sample was collected [22]. Urolithins have also been shown to be a discriminant of walnut consumption in observational studies [51], also highlighting their robustness as BFIs of walnuts in free-living conditions without dietary restrictions, and demonstrating that their levels from potential confounding foods are low. Also in this case, analytical methods have been reported for the quantification of these metabolites in biological samples [121], but we could not find any information related to their stability or interlaboratory reproducibility.
Finally, walnut consumption has also been associated with an increase in the levels of 5-hydroxyindole-3-acetic acid (5-HIAA) [24–26, 50, 51, 81], which is a metabolite of the serotonin pathway. Walnuts have a higher serotonin content than other foods [24], and 5-HIAA has been described as a discriminant metabolite of walnut consumption in two independent observational studies [50, 51], which reinforces its plausibility as a robust BFI for walnuts. Feldman and Lee [24] reported a dose-dependent relationship between the ingested amount of walnuts and the urinary 5-HIAA excretion: 16 units of walnut consumption caused an excretion of 26.0 mg of 5-HIAA in 24 h in urine, while double the amount of walnuts caused excretion of 59 mg/24 h of 5-HIAA. A parallel observation was made when serotonin was provided by other food sources [25]. Additionally, in a more recent study, the authors also used different serotonin food sources [26]. However, the serum levels of 5-HIAA were higher in samples from subjects who consumed the richest source of serotonin (i.e., walnuts) in an amount proportional to the amount provided by each food source. It has been demonstrated that the levels of this metabolite increase within 2 h after the consumption of serotonin-containing foods, and from that moment the concentrations start to decrease, reaching the baseline values within 24 h [25, 26]. Again, analytical methods for the quantification of this metabolite in biological samples have been published [26], but we could not find any data about its stability during sample collection, storage and processing, or interlaboratory reproducibility. However, it has also been reported after intake of other foods such as bananas [24]. Although the contents of serotonin are much higher in walnuts (> 50 μg/g) than in these other potential sources (for instance, bananas contain about 15 μg/g) [24], it is important to consider also the size of a typical serving, since it will influence in the final absolute consumption. For example, the ingestion of serotonin through a typical dose of 30 g walnuts is approximately the same than the one obtained by the consumption of an average-sized banana of 120 g. Furthermore, 5-HIAA has also been reported after the consumption of a Jerte Valley cherry product [122]. The concentration of serotonin in other common nuts like almonds is low (≤ 0.6 μ/g) [24].
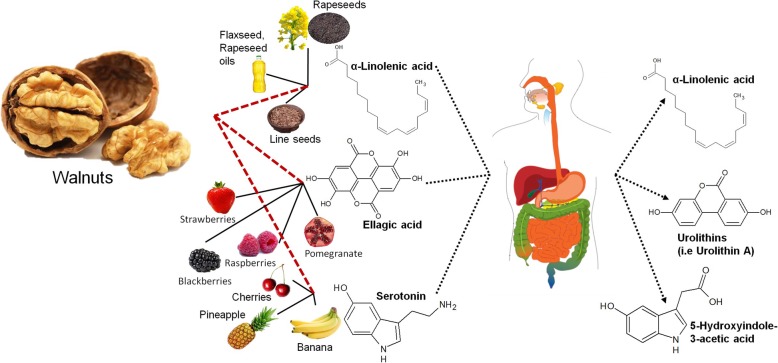
Therefore, as already highlighted in the previous paragraph, this is a clear example where multi-metabolite biomarker models may help to overcome the challenge of having a specific measurement [117]. This concept is outlined in Fig. 2, where it is schematized that although none of the candidates as BFIs for walnuts are highly specific when used as a single BFI (the most frequently used approach until now), the panel of them might be characteristic of no common food source other than walnuts.
Fig. 2.
Schematic diagram of combining medium-specific single biomarkers to create a more specific multi-metabolite biomarker panel
Almonds and hazelnuts
Another important group of nuts revised in this paper are almonds and hazelnuts, which are associated with increased levels of α-tocopherol [65–75]. Almonds and hazelnuts, respectively, have the highest concentrations of α-tocopherol among nuts [5, 6]. However, seeds and vegetable oils, green leafy vegetables, fortified cereals, and tomatoes are also important dietary sources of α-tocopherol [65].
Additionally, flavan-3-ol-derived metabolites have also been associated with almond consumption, although they are also characteristic for tea, wine, and cocoa intake [103]. Therefore, joint measurements of α-tocopherol and flavan-3-ol-derived metabolites could be used to obtain more specific information about almond intake. Nevertheless, additional untargeted metabolomics studies would be useful for proposing complementary metabolites to build multi-metabolite biomarker panels [117].
Pistachios
Among nuts, pistachios contain the highest levels of potassium, γ-tocopherol, vitamin K, phytosterols (mainly β-sitosterol), and xanthophyll carotenoids (lutein and zeaxanthin). The number of studies considering pistachio consumption and further metabolite measurements in biological fluids is very limited [76–78]. Lutein and zeaxanthin are particularly interesting compounds as they are very characteristic of pistachios, among other nuts, although they are also frequently present in a wide range of fruits, vegetables [in particular maize (corn) and green leafy vegetables such as spinach], and egg yolk [123, 124]. Two studies included a targeted quantitative analysis of these compounds in plasma after a dietary intervention with pistachios [76, 78]. An investigation by Hernandez-Alonso et al. [76] focused on the relationship between pistachio consumption and the improvement of cardiometabolic risk markers. In this crossover clinical trial, lutein and zeaxanthin, together with α-tocopherol, were proposed as indicators of pistachio intake to monitor the compliance with the dietary intervention. Volunteers were assigned to control diet or a pistachio-supplemented diet (57 g/day) for 4 months. These compounds were measured in fasting plasma at baseline, after a 2-week run-in period and then monthly until the end of each intervention period, and were shown to be statistically significant in the pistachio-supplemented group. However, different results were reported in a crossover, dose-response study performed by Kay et al. [78]. In this case, the researchers only found significant increases of lutein in serum after adding one or two daily servings of pistachios to their diets, whereas no changes from baseline levels in the concentrations of either zeaxanthin or α-tocopherol were observed.
In a study by Holligan et al. (2014), β-sitosterol in plasma was used to verify compliance with the diet (control diet vs diet with one serving of pistachios vs diet with two servings of pistachios for 4 weeks) [77]. The levels of β-sitosterol increased dose dependently and were found to be consistent with dietary approximations from daily questionnaires.
In summary, the above-mentioned reported investigations used the measurement of lutein, zeaxanthin, β-sitosterol, and α-tocopherols (pistachio components) to verify compliance with diets rich in pistachios. All of these compounds are common for many fruits and vegetables, as well as for other types of nuts, and thus cannot be considered specific metabolites of pistachio intake. Only one study was found that used an untargeted metabolomics approach to study the metabolic response in biological fluids after pistachio consumption [125]. However, it could not be included in the present review because it only reported on changes in endogenous metabolites. Therefore, additional complementary human trials with the use of untargeted metabolomics might reveal additional compounds or metabolites that could be suggested as potential biomarkers of intake.
Brazil nuts
Brazil nuts are one of the food sources with the highest contents of selenium. Accordingly, high levels of selenium have been reported in several studies after intake of Brazil nuts [52–64]. Although this essential mineral is found in many foods, the most relevant dietary source of selenium is Brazil nuts. However, it is important to keep in mind that it is also used in dietary supplements or in enriched foods, as well as that different geographical factors, such as selenium concentration in the soil (which varies from region to region), impact on the selenium content [126]. Selenium has also been observed to be a discriminant of Brazil nut consumption, independently of the background diet [63, 64]. The highest urinary selenium concentrations have been measured 4 h after consumption of Brazil nuts and even higher concentrations have been observed after repeated intakes [52]. Therefore, it remains to be clarified whether the use of only this compound is enough to measure the consumption of Brazil nuts or whether other complementary metabolites should be used jointly for reliable intake assessment.
Biomarkers of intake of vegetable oils
Biomarkers of vegetable oil intake have been studied most often by linking the intake of fatty acids from these oils to blood plasma and cell responses using controlled intervention studies [127–134]. The main oils studied were olive oil [127–158], flaxseed oil [159–173], rapeseed (canola) oil [157, 158, 174–179], and sunflower oil [157, 173, 178–180]. The study designs include acute studies [133–138, 140, 181], and parallel and crossover dietary intervention studies that vary in the level of control [127–132, 141–180]. These studies were often driven by examining the effects of fatty acids on cardiovascular risk factors such as changes in lipoproteins and hemodynamic factors in low- and high-risk subjects, thereby measuring compliance with dietary exposure. The biological specimens analyzed included plasma and plasma lipid fractions, such as cholesteryl esters and phospholipids, blood platelets, erythrocytes, and adipose tissue. In the case of (virgin) olive oil, the excretion of ingested polyphenols and their metabolites in urine and plasma was also studied. The information in regard to selected studies reporting associations between the consumption of vegetable oils and potential relevant BFIs is summarized in Table 3, while the information regarding the putative BFIs for vegetable oils is given in Table 4 and the information concerning the potential BFIs of vegetable oils that were considered nonrelevant is given in Additional file 1: Table S2.
Table 3.
Studies reporting associations between consumption and potential candidate food intake biomarkers for vegetable oils
| Dietary factor [reference] | Study design | Number of subjects | Analytical method | Sample type | Discriminating metabolites/candidate biomarkers |
|---|---|---|---|---|---|
| Olive oil [127] | Sustained intervention | 11 | GC-FID | Erythrocytes | Oleic acid |
| Olive oil [128] | Sustained intervention | 12 | GC-FID | Plasma | Oleic acid |
| Olive oila [129] | Sustained intervention | 30 | GC-FID | Plasma | Oleic acid |
| Olive oil [130] | Sustained intervention | 21 | GC-FID | Plasma/platelets | Oleic acid |
| Olive oil [131] | Sustained intervention | 16 | GC-FID | Erythrocytes | Oleic acid |
| Olive oil | Sustained intervention | 30 | GC-FID | Plasma/PBMC | Oleic acid |
| Olive oil, extra virgin [133] | Acute study | 10 | GC | Plasma—TAG | Oleic acid |
| Olive oil, pomace and refined [134] | Acute study | 10 | GC | Serum—TRL | Oleic acid |
| Olive oil, virgina [135] | Acute study | 11 | UPLC-MS; GC-MS | Urine | Hydroxytyrosol |
| Olive oil, different phenolic contenta [136] | Acute study | 12 | GC-MS | Urine | Hydroxytyrosol, 3-O-methy-hydroxytyrosol |
| Olive oil, enriched or virgina [137] | Acute study | 13 | UPLC-MS/MS | Plasma | Hydroxytyrosol sulfate |
| Olive oil, virgin [138] | Acute study | 13 | UPLC-MS/MS | Plasma | Hydroxytyrosol sulfate |
| Olive oil, high phenolic content [139] | Acute study | 12 | UPLC-MS/MS | Plasma | Hydroxytyrosol sulfate, hydroxytyrosol acetate sulfate |
| Olive oil, virgin, moderate and high phenolic content [140] | Acute study | 13 | UPLC-MS/MS | Plasma | Hydroxytyrosol sulfate, hydroxytyrosol acetate sulfate |
| Olive oil, extra virgin [141] | Sustained intervention | 10 | GC-FID | Plasma and cells | Oleic acid |
| Olive oil, extra virgin [142] | Sustained intervention | 424 | GC | Plasma | Oleic acid |
| Olive oil, different phenolica compound content [143] | Sustained intervention | 30 | GC-MS | Urine | Hydroxytyrosol |
| Olive oil, different phenol contenta [144] | Sustained intervention | 200 | GC-MS | Urine | Hydroxytyrosol |
| Olive oil, high-phenol vs low-phenol extra virgina [145] | Sustained intervention | 10 | GC-MS | Urine | Hydroxytyrosol |
| Olive oils, different phenolic contenta [146] | Sustained intervention | 30 | HPLC | Urine | Hydroxytyrosol |
| Olive oil, different phenolic contenta [147] | Sustained intervention | 38 | GC-MS | Urine | Hydroxytyrosol |
| Olive oil, refined, common and virgina [148] | Sustained intervention | 33 | HPLC | Urine | Hydroxytyrosol |
| Olive oil, extra virgina [149] | Sustained intervention | 20 | HPLC-MS | Plasma | Hydroxytyrosol |
| Olive oil, extra virgin [80] | Sustained intervention | 750 | GC-MS | Urine | Hydroxytyrosol |
| Olive oil, different phenolic compound contenta [150] | Sustained intervention | 28 | GC-MS | Urine | Hydroxytyrosol, O-methylhydroxytyrosol |
| Olive oil, with different phenolic contentsa [151] | Sustained intervention | 182 | GC-MS/MS | Urine | Hydroxytyrosol, 3-O-methylhydroxytyrosol |
| Olive oil, virgin and refineda [152] | Sustained intervention | 36 | HPLC-MS/MS | Plasma-LDL | Hydroxytyrosol sulfate |
| Olive oil, virgin, enriched [153] | Sustained intervention | 33 | UHPLC-MS/MS | Urine | Hydroxytyrosol sulfate |
| Olive oil, high polyphenol contenta [154] | Sustained intervention | 51 | UHPLC-MS/MS | Plasma-HDL | Hydroxytyrosol sulfate |
| Olive oil, virgin, high phenolica [155] | Sustained intervention | 5 | UPLC-MS/MS | Urine | Hydroxytyrosol sulfate, hydroxytyrosol acetate sulfate |
| Olive oil, virgin, enriched [156] | Sustained intervention | 33 | UPLC-MS/MS | Plasma/urine | Hydroxytyrosol sulfate, hydroxytyrosol acetate sulfate |
| Olive oil, virgin (OO); rapeseed oil (RO); sunflower oil (SO) [157] | Sustained intervention | 18 | GC-FID | Plasma | Oleic acid (OO, RO), linoleic acid (SO, RO) |
| Olive oil (OO), canola oil (CO) a [158] | Sustained intervention | 14 | GC-FID | Plasma | Oleic acid (OO, α-Linolenic acid (CO) |
| Flaxseed oil [159] | Sustained intervention | 16 | GC-FID | Plasma/PBMC | α-Linolenic acid |
| Flaxseed oil [160] | Sustained intervention | 30 | GC-FID | Plasma | α-Linolenic acid |
| Flaxseed oil [161] | Sustained intervention | 46 | GC-FID | Platelets | α-Linolenic acid |
| Flaxseed oil [162] | Sustained intervention | 28 | GC-FID | Plasma | α-Linolenic acid |
| Flaxseed oil [163] | Sustained intervention | 17 | GC-FID | Plasma/platelets | α-Linolenic acid |
| Flaxseed oil [164] | Sustained intervention | 20 | GC | Plasma/erythrocytes | α-Linolenic acid |
| Flaxseed oil [165] | Sustained intervention | 51 | GC | Erythrocytes | α-Linolenic acid |
| Flaxseed oil [166] | Sustained intervention | 62 | GLC | Erythrocytes | α-Linolenic acid |
| Flaxseed oil [167] | Sustained intervention | 86 | GC | Plasma | α-Linolenic acid |
| Flaxseed oil [168] | Sustained intervention | 34 | GC | Plasma | α-Linolenic acid |
| Flaxseed oil [169] | Sustained intervention | 98 | GC | Erythrocytes | α-Linolenic acid |
| Flaxseed oil [170] | Sustained intervention | 26 | GC-FID | Plasma | α-Linolenic acid |
| Flaxseed oil [171] | Sustained intervention | 37 | GC | Erythrocytes | α-Linolenic acid |
| Flaxseed oil [172] | Sustained intervention | 15 | GC-FID | Serum | α-Linolenic acid |
| Flaxseed oil (FO); sunflower oil (SO) [173] | Sustained intervention | 10 | GC-FID | Platelets | α-Linolenic acid (FO); linoleic acid (SO) |
| Rapeseed oil [177] | Sustained intervention | 40 | GC-FID | Plasma | α-Linolenic acid |
| Canola oila [174] | Sustained intervention | 14 | GC-FID | Breastmilk | α-Linolenic acid |
| Canola oil [175] | Sustained intervention | 130 | GC-FID | Plasma | α-Linolenic acid |
| Canola oil [176] | Sustained intervention | 130 | GC-FID | Plasma | α-Linolenic acid |
| Canola oil (CO); sunflower oil (SO) a [178] | Sustained intervention | 8 | GC-FID | Plasma | α-Linolenic acid (CO); linoleic acid (SO) |
| Canola oil (CO); sunflower oil (SO) [179] | Sustained intervention | 8 | GC-FID | Platelets | α-Linolenic acid (CO); linoleic acid (SO) |
| Sunflower oil [180] | Sustained intervention | 37 | GC | Plasma-CE/ASAT | Linoleic acid |
ASAT abdominal subcutaneous adipose tissue, CE cholesterol esters, FID flame ionization detector, GC gas chromatography, GLC gas-liquid chromatography, HPLC high-performance liquid chromatography, MS mass spectrometry, MS/MS tandem mass spectrometry, PBMC peripheral blood mononuclear cell, TAG triacylglycerides, UHPLC ultra-high performance liquid chromatography
aThe study includes other nonrelevant metabolites (included in Additional file 1: Table S2)
Table 4.
Validation scheme of potential food intake biomarkers for vegetable oils
| Dietary factor [references] | Compound/metabolite | HMDB ID | Sample type | Criteria | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3a | 3b | 4 | 5 | 6 | 7 | 8 | ||||
| Olive oil [128, 133, 134, 141, 142, 157, 158] | Oleic acid | HMDB0000207 | Plasma/blood cells | N | U | Y | Y | Y | U | U | Y | U |
| Olive oil, (extra) virgin [80, 135, 136, 144–150, 143, 151] | Hydroxytyrosol | HMDB0005784 | Urine/plasma | Y | Y | Y | U | Y | U | U | Y | U |
| Olive oil, (extra) virgin [137, 138, 153–155, 152, 156] | Hydroxytyrosol sulfate | – | Urine/plasma | Y | Y | Y | U | Y | Y | U | Y | U |
| Olive oil, (extra) virgin [139, 140, 155, 156] | Hydroxytyrosol acetate sulfate | – | Urine/plasma | Y | U | Y | U | Y | U | U | Y | U |
| Olive oil, (extra) virgin [136, 150, 151] | 3-O-Methylhydroxytyrosol | – | Urine | Y | Y | U | U | Y | U | U | Y | U |
| Flaxseed/linseed oil [160–165] | α-Linolenic acid | HMDB0001388 | Plasma/serum/erythrocytes/platelets | N | Y | U | Y | Y | U | U | Y | U |
| Rapeseed/canola oil [158, 175–178, 174, 179] | α-Linolenic acid | HMDB0001388 | Plasma/platelets/breast milk | N | Y | Y | U | Y | U | U | Y | U |
| Sunflower oil [157, 173, 179, 178, 180] | Linoleic acid | HMDB0000673 | Plasma/platelets/subcutaneous adipose tissue | N | U | U | U | Y | U | U | Y | U |
HMDB human metabolome database, N no, U unknown, Y yes. Criteria: C1—Plausibility, Is the marker compound plausible as a specific BFI for the food or food group?; C2—Dose response, Is there a dose-response relationship at relevant intake levels of the targeted food?; C3—Time response, Is the biomarker kinetics described adequately to make a wise choice of sample type, frequency and time window?; C3a, single dose; C3b, multiple doses; C4, Robustness, Has the marker been shown to be robust after intake of complex meals reflecting the dietary habits of the targeted population?; C5, Reliability, Has the marker been shown to compare well with other markers or questionnaire data for the same food/food group?; C6, Stability, Is the marker chemically and biologically stable during biospecimen collection and storage, making measurements reliable and feasible?; C7, Analytical performance, Are analytical variability (CV%), accuracy, sensitivity and specificity known as adequate for at least one reported analytical method?; C8, Reproducibility, Has the analysis been successfully reproduced in another laboratory?
Olive oil
Olive oil is obtained from the fruits of the olive tree (Olea europaea) and its fatty acid constituent is predominantly oleic acid [C18:1(n-9)], and depending on type (refined, virgin, extra virgin oil), variable amounts of unsaponifiable fatty acids are present [182].
Several markers of (virgin) olive oil consumption have been identified in urine and blood, including tyrosol, hydroxytyrosol, and their metabolites. Dose-response relationships for the excretion of tyrosol and hydroxytyrosol in urine were observed in several studies using either a 1-day [136] or a 3-week crossover design [143, 144, 146–148]. Excretion of tyrosol and/or hydroxytyrosol was maintained when olive oil was included as an ingredient in the daily diet [80, 143, 145–147, 149–151]. For acute intakes of extra virgin olive oil, time-response relationships were described in plasma [183] and urine [135]. Most of the tyrosol, hydroxytyrosol, and metabolites were excreted within 6 h after administration of the dose. In a 4-week single-arm study, plasma hydroxytyrosol increased about fivefold after daily administration of 50 mL of extra virgin olive oil [149]. Also, (hydroxy)-tyrosol metabolites (3-O-methylhydroxytyrosol, homovanillic acid, homovanillic alcohol, and hydroxytyrosol sulfate) were identified in urine in a dose-dependent manner [136, 138, 140, 184]. After 3 weeks or more of daily ingestion of olive oils with varying phenolic content, these and other metabolites (hydroxytyrosol acetate sulfate, homovanillic alcohol sulfate, homovanillic acid sulfate, hydroxytyrosol sulfate, hydroxytyrosol acetate sulfate, and homovanillic acid glucuronide) increased in plasma [152, 154, 156] and urine [145, 149–151]. Ingestion of a single dose of olive oil with moderate to high phenolic content also resulted in an increase in the amount of metabolites in both urine [135] and plasma [137–139]. The increase in plasma metabolites occurred within 6 h after dosing. Hydroxytyrosol and its metabolites 3-O-methylhydroxytyrosol, hydroxytyrosol sulfate, and hydroxytyrosol acetate sulfate are probably specific for (extra) virgin olive oil [185]. Tyrosol is not only present in olives but also in wine. Homovanillic acid, homovanillyl alcohol, and their conjugated metabolites are also less specific: e.g., homovanillic acid is a dopamine metabolite occurring in human body fluids, while homovanillyl alcohol can be detected in honey as it is a constituent of the mandibular secretion of honeybees [185].
The effect of olive oil intake on change in the fatty acid profile in blood cells and plasma lipid fractions has also been studied both for acute intakes and during prolonged feeding. Acute changes in the amount of plasma C18:1(n-9) were observed within 3–4 h after a meal [133, 134]. Prolonged consumption of diets moderate to high in olive oil resulted in increases in the amount of oleic acid in plasma, plasma lipid fractions, and erythrocytes, as was shown in single-arm studies, crossover studies, and parallel studies that lasted 2–8 weeks [127–131, 141, 157]. A time response for repeated intakes of olive oil was also described [132, 141].
Flaxseed oil
Flaxseed oil or linseed oil is the oil obtained from the seed of the flax plant (Linum usitatissimum L.) and is known for its considerable amounts (> 50% of total fat) of ALA. Parallel or crossover feeding trials, lasting 2–12 weeks, with flaxseed oil in the daily diet showed increased incorporation of ALA in platelets and erythrocyte membranes and elevated levels in plasma lipid fractions [157, 159–171, 173]. A limited number of studies described a time-related increase [164, 166, 167] and a dose-dependent change [163, 169] in the biomedia. In several of these studies, changes in the level of elongation and desaturation products (stearidonic acid -C18:4(n-3)-, eicosatetraenoic -C20:4(n-3)-, EPA, and DHA) were also observed depending on the duration of the feeding.
Rapeseed (canola) oil
Oils produced from Brassica oilseeds are very low in erucic acid nowadays (C22:1 n-9), thanks to improvements in plant-breeding programs to grow low erucic acid cultivars [186]. The majority of fatty acids in rapeseed/canola oil are MUFAs, mainly oleic acid. The PUFA fraction consists of variable amounts of LA and ALA. The amount of ALA is much lower in rapeseed oil than in flaxseed oil, but the human consumption of rapeseed, either direct or as part of edible fats and other manufactured food, is higher. Biomarkers of the intake of rapeseed oil have focused on ALA. In several crossover studies ranging from 2.5 to 6 weeks in duration, levels of ALA in plasma lipid fractions and blood platelets increased after consumption of diets with increased levels of ALA from rapeseed or canola oil [157, 158, 177–179]. A dose-dependent increase was observed in one study [163]. Sampling the breastmilk of lactating women from 6 to 24 h up to 7 days after a dose of 40 g of canola oil revealed significantly increased amounts of ALA in the breast milk within 10 h [174].
Sunflower oil
Oil of the seeds of the sunflower (Helianthus annuus L.) is nutritionally valued by its high amounts of LA. Global consumption of sunflower oil ranks fourth after palm oil, rapeseed oil, and soybean oil [7]. Only high-oleic sunflower oil (HOSO) was known until a few decades ago. Newer sunflower hybrids yielding oils with high oleic acid content became available on the market more recently [187]. Crossover or parallel feeding studies ranging from 2.5 to 8 weeks with sunflower oil as a discerned source of fat in the diet showed increased levels of linoleic acid in plasma lipid fractions, platelets, and subcutaneous adipose tissue at the end of the intervention [157, 173, 178–180, 188]. For sunflower oil with a high oleic acid content, increased amounts of oleic acid in plasma lipid fractions and erythrocytes were observed after 3–5 weeks of feeding [131, 188–192]. To the best of our knowledge, acute- or repeated-intake time-response relationships have not been described for sunflower oil.
Other oils
A limited number of studies were found regarding other common oils such as safflower oil, corn oil, coconut oil, and soybean oil. These studies show that after prolonged feeding (of several weeks or longer), plasma/serum lipid fractions emerge as a potential putative biomarker [112, 158, 174, 192–199]. Data from these studies showed that in general, increasing the amounts of dietary fatty acids increase the level of fatty acids in blood lipid fractions, cell membranes, and adipose tissue. This is in line with the work of Hodson et al. [200], who reviewed the fatty acid composition of biological specimens as a biomarker of dietary intake. Fatty acids in biological specimens not synthesized endogenously [essential (n-6) and (n-3) fatty acids] correlate well with the intake of vegetable oils high in these fatty acids. The response, therefore, is specific for the fatty acid but not for the vegetable oil consumed. An inconvenient factor in studying fatty acids as biomarkers is that an increase in the level of one fatty acid inevitably leads to a decrease in the level of one or more other fatty acids. Furthermore, oils high in essential fatty acids, such as C18:3 (n-3) in flaxseed oil, generally increase incorporation and elevate the level of their fatty acid elongation products such as EPA and DHA. These observations were not taken into account in this review since the level of distinctiveness of such putative biomarkers progressively diminishes when other foods and food groups have similar components and are part of the same (endogenous) biochemical pathways.
Conclusions
The most plausible candidate biomarkers for walnut intake are ALA, urolithins, and HIAA. Since these metabolites can also be detected after the intake of other foods, a combined model with all three metabolites could be a feasible solution for accurately monitoring walnut intake. In the case of almonds, α-tocopherol could potentially be a good candidate; however, here again a combination with other metabolites, such as catechin-derived metabolites, may improve the prediction of almond intake. For Brazil nuts, selenium may be a good candidate biomarker of intake, but it is a mineral widely distributed among other food sources. Thus, further untargeted metabolomics studies could be useful for finding additional candidate biomarkers with which to construct a multi-metabolite biomarker model. Similar needs exist for hazelnuts, macadamia nuts, peanuts, pecan nuts, and pistachios.
In regard to vegetable oils, several biomarkers of their intake have been described but none of them has been validated against other markers for the same food or food group. In the case of (virgin) olive oil, the most promising distinctive biomarker is hydroxytyrosol and its metabolites. In vegetable oils other than olive oil, fatty acids have been studied frequently, but these components lack sufficient distinctive sensitivity and specificity as biomarkers of the intake of vegetable oils. They represent a marker of the fatty acid itself rather than of the vegetable oil ingested. The analytical methods used in the reviewed literature can in general be considered sensitive and specific. Further discovery and validation studies are needed, which could focus on components in the unsaponifiable part of the oils.
Therefore, additional studies are necessary to discover new candidate BFIs, as well as to further evaluate the specificity, sensitivity, dose-response relationships, and reproducibility of these candidate biomarkers and to eventually validate them in other populations. For the discovery of new candidate BFIs, an untargeted metabolomics approach may be the most effective strategy, whereas for increasing the specificity of the evaluation of food consumption, this could be a combination of different metabolites.
Additional file
Table S1. Nonrelevant food intake biomarkers for nuts. Table S2. Nonrelevant food intake biomarkers for vegetable oils. (PDF 111 kb)
Acknowledgements
Not applicable.
Funding
FoodBAll is a project funded by the BioNH call (grant number 529051002) under the Joint Programming Initiative “A Healthy Diet for a Healthy Life.” The project is funded nationally by the respective research councils; the work was funded in part by a grant from the Spanish National Grants from the Ministry of Economy and Competitiveness (MINECO) (PCIN-2014-133; 2015-238 MINECO, Spain), an award of 2014SGR1566 from the Generalitat de Catalunya’s Agency AGAUR and funds from CIBERFES (co-funded by the FEDER program from the EU) to CAL.
Availability of data and materials
Not applicable.
Abbreviations
- ALA
α-Linolenic acid
- BFIs
Biomarkers of food intake
- DHA
Docosahexaenoic acid
- EA
Ellagic acid
- EPA
Eicosapentaenoic acid
- FFQs
Food frequency questionnaires
- HIAA
Hydroxyindole-acetic acid
- HOSO
High-linoleic sunflower oil
- LA
Linoleic acid
- MUFAs
Monounsaturated fatty acids
- PUFAs
Polyunsaturated fatty acids
- SFAs
Saturated fatty acids
Authors’ contributions
MGA and PH coordinated and wrote the sections about nuts and vegetable oils, respectively; MGA, SEA, JdG, MU, and CAL carried out the literature search on nuts; PH, ML, JdG, and US carried out the literature search on oils; MCJO and SJLB wrote the introduction. All the authors commented critically on the manuscript, and read and approved the final manuscript.
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
JMG received unrestricted research grants from Unilever for studies of fatty acids in the Alpha Omega Cohort. The other authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Mar Garcia-Aloy, Email: margarcia@ub.edu.
Paul J. M. Hulshof, Email: paul.hulshof@wur.nl
Sheila Estruel-Amades, Email: sheilaestruel@ub.edu.
Maryse C. J. Osté, Email: m.c.j.oste@umcg.nl
Maria Lankinen, Email: maria.lankinen@uef.fi.
Johanna M. Geleijnse, Email: marianne.geleijnse@wur.nl
Janette de Goede, Email: janettedegoede78@gmail.com.
Marynka Ulaszewska, Email: maria.ulaszewska@fmach.it.
Fulvio Mattivi, Email: fulvio.mattivi@unitn.it.
Stephan J. L. Bakker, Email: s.j.l.bakker@umcg.nl
Ursula Schwab, Email: ursula.schwab@uef.fi.
Cristina Andres-Lacueva, Email: candres@ub.edu.
References
- 1.Eaton SB, Konner M. Paleolithic nutrition. A consideration of its nature and current implications. N Engl J Med. 1985;312:283–289. doi: 10.1056/NEJM198501313120505. [DOI] [PubMed] [Google Scholar]
- 2.Ros E. Health benefits of nut consumption. Nutrients. 2010;2:652–682. doi: 10.3390/nu2070652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Grigg D. The European diet: regional variations in food consumption in the 1980s. Geoforum. 1993;24:277–289. doi: 10.1016/0016-7185(93)90021-9. [DOI] [Google Scholar]
- 4.Cordain L, Eaton SB, Sebastian A, Mann N, Lindeberg S, Watkins BA, et al. Origins and evolution of the Western diet: health implications for the 21st century. Am J Clin Nutr. 2005;81:341–354. doi: 10.1093/ajcn.81.2.341. [DOI] [PubMed] [Google Scholar]
- 5.Maguire LS, O’Sullivan SM, Galvin K, O’Connor TP, O’Brien NM. Fatty acid profile, tocopherol, squalene and phytosterol content of walnuts, almonds, peanuts, hazelnuts and the macadamia nut. Int J Food Sci Nutr. 2004;55:171–178. doi: 10.1080/09637480410001725175. [DOI] [PubMed] [Google Scholar]
- 6.Ryan E, Galvin K, O’Connor TP, Maguire AR, O’Brien NM. Fatty acid profile, tocopherol, squalene and phytosterol content of Brazil, pecan, pine, pistachio and cashew nuts. Int J Food Sci Nutr. 2006;57:219–228. doi: 10.1080/09637480600768077. [DOI] [PubMed] [Google Scholar]
- 7.United States Department of Agriculture F.A.S . Consumption of vegetable oils worldwide from 2013/14 to 2017/2018, by oil type (in million metric tons) 2017. [Google Scholar]
- 8.Sayon-Orea C, Carlos S, Martínez-Gonzalez MA. Does cooking with vegetable oils increase the risk of chronic diseases?: a systematic review. Br J Nutr. 2015;113:S36–S48. doi: 10.1017/S0007114514002931. [DOI] [PubMed] [Google Scholar]
- 9.Miró-Casas E, Covas M-I, Fitó M, Farré-Albadalejo M, Marrugat J, de la Torre R. Tyrosol and hydroxytyrosol are absorbed from moderate and sustained doses of virgin olive oil in humans. Eur J Clin Nutr. 2003;57:186–190. doi: 10.1038/sj.ejcn.1601532. [DOI] [PubMed] [Google Scholar]
- 10.Linseisen J, Bergström E, Gafá L, González C, Thiébaut A, Trichopoulou A, et al. Consumption of added fats and oils in the European prospective investigation into cancer and nutrition (EPIC) centres across 10 European countries as assessed by 24-hour dietary recalls. Public Health Nutr. 2002;5:1227. doi: 10.1079/PHN2002401. [DOI] [PubMed] [Google Scholar]
- 11.Aune D, Keum N, Giovannucci E, Fadnes LT, Boffetta P, Greenwood DC, et al. Nut consumption and risk of cardiovascular disease, total cancer, all-cause and cause-specific mortality: a systematic review and dose-response meta-analysis of prospective studies. BMC Med. 2016;14:207. doi: 10.1186/s12916-016-0730-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sabate J, Fraser GE, Burke K, Knutsen SF, Bennett H, Lindsted KD. Effects of walnuts on serum lipid levels and blood pressure in normal men. N Engl J Med. 1993;328:603–607. doi: 10.1056/NEJM199303043280902. [DOI] [PubMed] [Google Scholar]
- 13.Sabaté J, Oda K, Ros E. Nut consumption and blood lipid levels: a pooled analysis of 25 intervention trials. Arch Intern Med. 2010;170:821–827. doi: 10.1001/archinternmed.2010.79. [DOI] [PubMed] [Google Scholar]
- 14.Banel DK, Hu FB. Effects of walnut consumption on blood lipids and other cardiovascular risk factors: a meta-analysis and systematic review. Am J Clin Nutr. 2009;90:56–63. doi: 10.3945/ajcn.2009.27457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sacks FM, Lichtenstein AH, Wu JHY, Appel LJ, Creager MA, Kris-Etherton PM, et al. Dietary fats and cardiovascular disease: a presidential advisory from the American Heart Association. Circulation. 2017;136:e1–23. doi: 10.1161/CIR.0000000000000510. [DOI] [PubMed] [Google Scholar]
- 16.Garcia-Aloy M, Andres-Lacueva C. Food intake biomarkers for increasing the efficiency of dietary pattern assessment through the use of metabolomics: unforeseen research requirements for addressing current gaps. J Agric Food Chem. 2018;66:5–7. doi: 10.1021/acs.jafc.7b05586. [DOI] [PubMed] [Google Scholar]
- 17.Brouwer-Brolsma E, Brennan L, Drevon CA, van Kranen H, Manach C, Dragsted LO, et al. Combining traditional dietary assessment methods with novel metabolomics techniques: current efforts by the food biomarker alliance. Proc Nutr Soc. 2017;76:619–627. doi: 10.1017/S0029665117003949. [DOI] [PubMed] [Google Scholar]
- 18.Praticò G, Gao Q, Scalbert A, Vergères G, Kolehmainen M, Manach C, et al. Guidelines for biomarker of food intake reviews (BFIRev): how to conduct an extensive literature search for biomarker of food intake discovery. Genes Nutr. 2018;13:3. doi: 10.1186/s12263-018-0592-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dragsted LOLO, Gao Q, Scalbert A, Vergères G, Kolehmainen M, Manach C, et al. Validation of biomarkers of food intake: critical assessment of candidate biomarkers. Genes Nutr. 2018;13:14. doi: 10.1186/s12263-018-0603-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bellido C, López-Miranda J, Blanco-Colio LM, Pérez-Martínez P, Muriana FJ, Martín-Ventura JL, et al. Butter and walnuts, but not olive oil, elicit postprandial activation of nuclear transcription factor kappaB in peripheral blood mononuclear cells from healthy men. Am J Clin Nutr. 2004;80:1487–1491. doi: 10.1093/ajcn/80.6.1487. [DOI] [PubMed] [Google Scholar]
- 21.Fuentes F, López-Miranda J, Pérez-Martínez P, Jiménez Y, Marín C, Gómez P, et al. Chronic effects of a high-fat diet enriched with virgin olive oil and a low-fat diet enriched with α-linolenic acid on postprandial endothelial function in healthy men. Br J Nutr. 2008;100:159–165. doi: 10.1017/S0007114508888708. [DOI] [PubMed] [Google Scholar]
- 22.Cerdá B, Tomás-Barberán FA, Espín JC. Metabolism of antioxidant and chemopreventive ellagitannins from strawberries, raspberries, walnuts, and oak-aged wine in humans: identification of biomarkers and individual variability. J Agric Food Chem. 2005;53:227–235. doi: 10.1021/jf049144d. [DOI] [PubMed] [Google Scholar]
- 23.Haddad EH, Gaban-Chong N, Oda K, Sabaté J. Effect of a walnut meal on postprandial oxidative stress and antioxidants in healthy individuals. Nutr J. 2014;13:4. doi: 10.1186/1475-2891-13-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Feldman JM, Lee EM. Serotonin content of foods: effect on urinary excretion of 5-hydroxyindoleacetic acid. Am J Clin Nutr. 1985;42:639–643. doi: 10.1093/ajcn/42.4.639. [DOI] [PubMed] [Google Scholar]
- 25.Mashige F, Matsushima Y, Kanazawa H, Sakuma I, Takai N, Bessho F, et al. Acidic catecholamine metabolites and 5-hydroxyindoleacetic acid in urine: the influence of diet. Ann Clin Biochem. 1996;33:43–49. doi: 10.1177/000456329603300106. [DOI] [PubMed] [Google Scholar]
- 26.Tohmola N, Johansson A, Sane T, Renkonen R, Hämäläinen E, Itkonen O. Transient elevation of serum 5-HIAA by dietary serotonin and distribution of 5-HIAA in serum protein fractions. Ann Clin Biochem. 2015;52(Pt 4):428–433. doi: 10.1177/0004563214554842. [DOI] [PubMed] [Google Scholar]
- 27.Abbey M, Noakes M, Belling GB, Nestel PJ. Partial replacement of saturated fatty acids with almonds or walnuts lowers total plasma cholesterol and low-density-lipoprotein cholesterol. Am J Clin Nutr. 1994;59:995–999. doi: 10.1093/ajcn/59.5.995. [DOI] [PubMed] [Google Scholar]
- 28.Chisholm A, Mann J, Skeaff M, Frampton C, Sutherland W, Duncan A, et al. A diet rich in walnuts favourably influences plasma fatty acid profile in moderately hyperlipidaemic subjects. Eur J Clin Nutr. 1998;52:12–16. doi: 10.1038/sj.ejcn.1600507. [DOI] [PubMed] [Google Scholar]
- 29.Zambón D, Sabaté J, Muñoz S, Campero B, Casals E, Merlos M, et al. Substituting walnuts for monounsaturated fat improves the serum lipid profile of hypercholesterolemic men and women. A randomized crossover trial. Ann Intern Med. 2000;132:538–546. doi: 10.7326/0003-4819-132-7-200004040-00005. [DOI] [PubMed] [Google Scholar]
- 30.Almario RU, Vonghavaravat V, Wong R, Kasim-Karakas SE. Effects of walnut consumption on plasma fatty acids and lipoproteins in combined hyperlipidemia. Am J Clin Nutr. 2001;74:72–79. doi: 10.1093/ajcn/74.1.72. [DOI] [PubMed] [Google Scholar]
- 31.Muñoz S, Merlos M, Zambón D, Rodríguez C, Sabaté J, Ros E, et al. Walnut-enriched diet increases the association of LDL from hypercholesterolemic men with human HepG2 cells. J Lipid Res. 2001;42:2069–2076. [PubMed] [Google Scholar]
- 32.Iwamoto M, Imaizumi K, Sato M, Hirooka Y, Sakai K, Takeshita A, et al. Serum lipid profiles in Japanese women and men during consumption of walnuts. Eur J Clin Nutr. 2002;56:629–637. doi: 10.1038/sj.ejcn.1601400. [DOI] [PubMed] [Google Scholar]
- 33.Sabaté J, Cordero-Macintyre Z, Siapco G, Torabian S, Haddad E. Does regular walnut consumption lead to weight gain? Br J Nutr. 2005;94:859–864. doi: 10.1079/BJN20051567. [DOI] [PubMed] [Google Scholar]
- 34.Marangoni F, Colombo C, Martiello A, Poli A, Paoletti R, Galli C. Levels of the n-3 fatty acid eicosapentaenoic acid in addition to those of alpha linolenic acid are significantly raised in blood lipids by the intake of four walnuts a day in humans. Nutr Metab Cardiovasc Dis. 2007;17:457–461. doi: 10.1016/j.numecd.2006.02.004. [DOI] [PubMed] [Google Scholar]
- 35.Simon JA, Tanzman JS, Sabaté J. Lack of effect of walnuts on serum levels of prostate specific antigen: a brief report. J Am Coll Nutr. 2007;26:317–320. doi: 10.1080/07315724.2007.10719617. [DOI] [PubMed] [Google Scholar]
- 36.Rajaram S, Haddad EH, Mejia A, Sabate J. Walnuts and fatty fish influence different serum lipid fractions in normal to mildly hyperlipidemic individuals: a randomized controlled study. Am J Clin Nutr. 2009;89:1657S–1663S. doi: 10.3945/ajcn.2009.26736S. [DOI] [PubMed] [Google Scholar]
- 37.Tapsell LC, Batterham MJ, Teuss G, Tan S-Y, Dalton S, Quick CJ, et al. Long-term effects of increased dietary polyunsaturated fat from walnuts on metabolic parameters in type II diabetes. Eur J Clin Nutr. 2009;63:1008–1015. doi: 10.1038/ejcn.2009.19. [DOI] [PubMed] [Google Scholar]
- 38.Nguyen L, Rajaram S, Mohan S, Sabaté J. Effect of plant and marine sources of n-3 fatty acids on markers of bone turnover in healthy adults. FASEB J. 2010;24:946.7. doi: 10.1096/fj.10-154484. [DOI] [Google Scholar]
- 39.McKay DL, Chen C-YO, Yeum K-J, Matthan NR, Lichtenstein AH, Blumberg JB. Chronic and acute effects of walnuts on antioxidant capacity and nutritional status in humans: a randomized, cross-over pilot study. Nutr J. 2010;9:21. doi: 10.1186/1475-2891-9-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wu H, Pan A, Yu Z, Qi Q, Lu L, Zhang G, et al. Lifestyle counseling and supplementation with flaxseed or walnuts influence the Management of Metabolic Syndrome. J Nutr. 2010;140:1937–1942. doi: 10.3945/jn.110.126300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Damasceno NRT, Perez-Heras A, Serra M, Cofan M, Sala-Vila A, Salas-Salvado J, et al. Crossover study of diets enriched with virgin olive oil, walnuts or almonds. Effects on lipids and other cardiovascular risk markers. Nutr Metab Cardiovasc Dis. 2011;21 Suppl 1:S14–S20. doi: 10.1016/j.numecd.2010.12.006. [DOI] [PubMed] [Google Scholar]
- 42.Chiang Y-L, Haddad E, Rajaram S, Shavlik D, Sabate J. The effect of dietary walnuts compared to fatty fish on eicosanoids, cytokines, soluble endothelial adhesion molecules and lymphocyte subsets: a randomized, controlled crossover trial. Prostaglandins Leukot Essent Fatty Acids. 2012;87:111–117. doi: 10.1016/j.plefa.2012.07.007. [DOI] [PubMed] [Google Scholar]
- 43.Robbins WA, Xun L, FitzGerald LZ, Esguerra S, Henning SM, Carpenter CL. Walnuts improve semen quality in men consuming a Western-style diet: randomized control dietary intervention trial. Biol Reprod. 2012;87:101. doi: 10.1095/biolreprod.112.101634. [DOI] [PubMed] [Google Scholar]
- 44.Campos Mondragón MG, Oliart Ros RM, Angulo Guerrero JO. Inflammatory markers in patients with metabolic syndrome after the intake of fatty acids n-3 and conjugated linoleic acid (CLA) Nutr Clínica y Dietética Hosp. 2013;33:7–17. [Google Scholar]
- 45.Burns-Whitmore B, Haddad E, Sabaté J, Rajaram S. Effects of supplementing n-3 fatty acid enriched eggs and walnuts on cardiovascular disease risk markers in healthy free-living lacto-ovo-vegetarians: a randomized, crossover, free-living intervention study. Nutr J. 2014;13:29. doi: 10.1186/1475-2891-13-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wu L, Piotrowski K, Rau T, Waldmann E, Broedl UC, Demmelmair H, et al. Walnut-enriched diet reduces fasting non-HDL-cholesterol and apolipoprotein B in healthy Caucasian subjects: a randomized controlled cross-over clinical trial. Metabolism. 2014;63:382–391. doi: 10.1016/j.metabol.2013.11.005. [DOI] [PubMed] [Google Scholar]
- 47.González-Sarrías A, Giménez-Bastida JA, García-Conesa MT, Gómez-Sánchez MB, García-Talavera NV, Gil-Izquierdo A, et al. Occurrence of urolithins, gut microbiota ellagic acid metabolites and proliferation markers expression response in the human prostate gland upon consumption of walnuts and pomegranate juice. Mol Nutr Food Res. 2010;54:311–322. doi: 10.1002/mnfr.200900152. [DOI] [PubMed] [Google Scholar]
- 48.Garcia-Villalba R, Espin JC, Tomas-Barberan FA, García-Villalba R, Espín JC, Tomás-Barberán FA. Chromatographic and spectroscopic characterization of urolithins for their determination in biological samples after the intake of foods containing ellagitannins and ellagic acid. J Chromatogr A. 2015;1428:162–175. doi: 10.1016/j.chroma.2015.08.044. [DOI] [PubMed] [Google Scholar]
- 49.Romo-Vaquero M, García-Villalba R, González-Sarrías A, Beltrán D, Tomás-Barberán FA, Espín JC, et al. Interindividual variability in the human metabolism of ellagic acid: contribution of Gordonibacter to urolithin production. J Funct Foods. 2015;17:785–791. doi: 10.1016/j.jff.2015.06.040. [DOI] [Google Scholar]
- 50.Andersen M-BS, Kristensen M, Manach C, Pujos-Guillot E, Poulsen SK, Larsen TM, et al. Discovery and validation of urinary exposure markers for different plant foods by untargeted metabolomics. Anal Bioanal Chem. 2014;406:1829–1844. doi: 10.1007/s00216-013-7498-5. [DOI] [PubMed] [Google Scholar]
- 51.Garcia-Aloy M, Llorach R, Urpi-Sarda M, Tulipani S, Estruch R, Martínez-González MA, et al. Novel multimetabolite prediction of walnut consumption by a urinary biomarker model in a free-living population: the PREDIMED study. J Proteome Res. 2014;13:3476–3483. doi: 10.1021/pr500425r. [DOI] [PubMed] [Google Scholar]
- 52.Dumont E, Ogra Y, Suzuki KT, Vanhaecke F, Cornelis R. Liquid chromatography–electrospray ionization tandem mass spectrometry for on-line characterization, monitoring and isotopic profiling of the main selenium-metabolite in human urine after consumption of Se-rich and Se-enriched food. Anal Chim Acta. 2006;555:25–33. doi: 10.1016/j.aca.2005.08.063. [DOI] [Google Scholar]
- 53.Terol A, Ardini F, Basso A, Grotti M. Determination of selenium urinary metabolites by high temperature liquid chromatography-inductively coupled plasma mass spectrometry. J Chromatogr A. 2015;1380:112–119. doi: 10.1016/j.chroma.2014.12.071. [DOI] [PubMed] [Google Scholar]
- 54.Strunz CC, Oliveira TV, Vinagre JCM, Lima A, Cozzolino S, Maranhão RC. Brazil nut ingestion increased plasma selenium but had minimal effects on lipids, apolipoproteins, and high-density lipoprotein function in human subjects. Nutr Res. 2008;28:151–155. doi: 10.1016/j.nutres.2008.01.004. [DOI] [PubMed] [Google Scholar]
- 55.Thomson CD, Chisholm A, McLachlan SK, Campbell JM. Brazil nuts: an effective way to improve selenium status. Am J Clin Nutr. 2008;87:379–384. doi: 10.1093/ajcn/87.2.379. [DOI] [PubMed] [Google Scholar]
- 56.Stockler-Pinto MB, Mafra D, Farage NE, Boaventura GT, Cozzolino SMF. Effect of Brazil nut supplementation on the blood levels of selenium and glutathione peroxidase in hemodialysis patients. Nutrition. 2010;26:1065–1069. doi: 10.1016/j.nut.2009.08.006. [DOI] [PubMed] [Google Scholar]
- 57.Cominetti C, de Bortoli MC, Garrido ABJ, Cozzolino SMF. Brazilian nut consumption improves selenium status and glutathione peroxidase activity and reduces atherogenic risk in obese women. Nutr Res. 2012;32:403–407. doi: 10.1016/j.nutres.2012.05.005. [DOI] [PubMed] [Google Scholar]
- 58.Huguenin GVB, Moreira ASB, Siant’Pierre TD, Gonçalves RA, Rosa G, Oliveira GMM, et al. Effects of dietary supplementation with Brazil nuts on microvascular endothelial function in hypertensive and dyslipidemic patients: a randomized crossover placebo-controlled trial. Microcirculation. 2015;22:687–699. doi: 10.1111/micc.12225. [DOI] [PubMed] [Google Scholar]
- 59.Huguenin GVB, Oliveira GMM, Moreira ASB, Saint’Pierre TD, Goncalves RA, Pinheiro-Mulder AR, et al. Improvement of antioxidant status after Brazil nut intake in hypertensive and dyslipidemic subjects. Nutr J. 2015;14:54. doi: 10.1186/s12937-015-0043-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Martens IBG, Cardoso BR, Hare DJ, Niedzwiecki MM, Lajolo FM, Martens A, et al. Selenium status in preschool children receiving a Brazil nut-enriched diet. Nutrition. 2015;31:1339–1343. doi: 10.1016/j.nut.2015.05.005. [DOI] [PubMed] [Google Scholar]
- 61.Rita Cardoso B, Apolinário D, da Silva BV, Busse AL, Magaldi RM, Jacob-Filho W, et al. Effects of Brazil nut consumption on selenium status and cognitive performance in older adults with mild cognitive impairment: a randomized controlled pilot trial. Eur J Nutr. 2016;55:107–116. doi: 10.1007/s00394-014-0829-2. [DOI] [PubMed] [Google Scholar]
- 62.Hu Y, McIntosh GH, Le Leu RK, Somashekar R, Meng XQ, Gopalsamy G, et al. Supplementation with Brazil nuts and green tea extract regulates targeted biomarkers related to colorectal cancer risk in humans. Br J Nutr. 2016;116:1901–1911. doi: 10.1017/S0007114516003937. [DOI] [PubMed] [Google Scholar]
- 63.Lemire M, Fillion M, Barbosa F, Guimarães JRD, Mergler D. Elevated levels of selenium in the typical diet of Amazonian riverside populations. Sci Total Environ. 2010;408:4076–4084. doi: 10.1016/j.scitotenv.2010.05.022. [DOI] [PubMed] [Google Scholar]
- 64.Rayman MP, Bath SC, Westaway J, Williams P, Mao J, Vanderlelie JJ, et al. Selenium status in U.K. pregnant women and its relationship with hypertensive conditions of pregnancy. Br J Nutr. 2015;113:249–258. doi: 10.1017/S000711451400364X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Jambazian PR, Haddad E, Rajaram S, Tanzman J, Sabaté J. Almonds in the diet simultaneously improve plasma α-tocopherol concentrations and reduce plasma lipids. J Am Diet Assoc. 2005;105:449–454. doi: 10.1016/j.jada.2004.12.002. [DOI] [PubMed] [Google Scholar]
- 66.Hollis J, Mattes R. Effect of chronic consumption of almonds on body weight in healthy humans. Br J Nutr. 2007;98:651–656. doi: 10.1017/S0007114507734608. [DOI] [PubMed] [Google Scholar]
- 67.Li N, Jia X, Chen C-YO, Blumberg JB, Song Y, Zhang W, et al. Almond consumption reduces oxidative DNA damage and lipid peroxidation in male smokers. J Nutr. 2007;137:2717–2722. doi: 10.1093/jn/137.12.2717. [DOI] [PubMed] [Google Scholar]
- 68.Chen C-YO, Liu J-F, Liu Y-H, Chen C-M. Almonds ameliorate risk factors of cardiovascular disease in type 2 diabetes. FASEB J. 2009;23:563.32. doi: 10.1096/fj.09-133587. [DOI] [Google Scholar]
- 69.Wien M, Bleich D, Raghuwanshi M, Gould-Forgerite S, Gomes J, Monahan-Couch L, et al. Almond consumption and cardiovascular risk factors in adults with prediabetes. J Am Coll Nutr. 2010;29:189–197. doi: 10.1080/07315724.2010.10719833. [DOI] [PubMed] [Google Scholar]
- 70.Richmond K, Williams S, Mann J, Brown R, Chisholm A. Markers of cardiovascular risk in postmenopausal women with type 2 diabetes are improved by the daily consumption of almonds or sunflower kernels: a feeding study. ISRN Nutr. 2013;2013:626414. doi: 10.5402/2013/626414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Choudhury K, Clark J, Griffiths HR. An almond-enriched diet increases plasma alpha-tocopherol and improves vascular function but does not affect oxidative stress markers or lipid levels. Free Radic Res. 2014;48:599–606. doi: 10.3109/10715762.2014.896458. [DOI] [PubMed] [Google Scholar]
- 72.Chen C-YO, Holbrook M, Duess M-A, Dohadwala MM, Hamburg NM, Asztalos BF, et al. Effect of almond consumption on vascular function in patients with coronary artery disease: a randomized, controlled, cross-over trial. Nutr J. 2015;14:61. doi: 10.1186/s12937-015-0049-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Tey SL, Brown RC, Chisholm AW, Delahunty CM, Gray AR, Williams SM. Effects of different forms of hazelnuts on blood lipids and alpha-tocopherol concentrations in mildly hypercholesterolemic individuals. Eur J Clin Nutr. 2011;65:117–124. doi: 10.1038/ejcn.2010.200. [DOI] [PubMed] [Google Scholar]
- 74.Orem A, Yucesan FB, Orem C, Akcan B, Kural BV, Alasalvar C, et al. Hazelnut-enriched diet improves cardiovascular risk biomarkers beyond a lipid-lowering effect in hypercholesterolemic subjects. J Clin Lipidol. 2013;7:123–131. doi: 10.1016/j.jacl.2012.10.005. [DOI] [PubMed] [Google Scholar]
- 75.Tey SL, Robinson T, Gray AR, Chisholm AW, Brown RC. Do dry roasting, lightly salting nuts affect their cardioprotective properties and acceptability? Eur J Nutr. 2017;56:1025–1036. doi: 10.1007/s00394-015-1150-4. [DOI] [PubMed] [Google Scholar]
- 76.Hernandez-Alonso P, Salas-Salvado J, Baldrich-Mora M, Juanola-Falgarona M, Bullo M. Beneficial effect of pistachio consumption on glucose metabolism, insulin resistance, inflammation, and related metabolic risk markers: a randomized clinical trial. Diabetes Care. 2014;37:3098–3105. doi: 10.2337/dc14-1431. [DOI] [PubMed] [Google Scholar]
- 77.Holligan SD, West SG, Gebauer SK, Kay CD, Kris-Etherton PM. A moderate-fat diet containing pistachios improves emerging markers of cardiometabolic syndrome in healthy adults with elevated LDL levels. Br J Nutr. 2014;112:744–752. doi: 10.1017/S0007114514001561. [DOI] [PubMed] [Google Scholar]
- 78.Kay CD, Gebauer SK, West SG, Kris-Etherton PM. Pistachios increase serum antioxidants and lower serum oxidized-LDL in hypercholesterolemic adults. J Nutr. 2010;140:1093–1098. doi: 10.3945/jn.109.117366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Casas-Agustench P, López-Uriarte P, Bulló M, Ros E, Cabré-Vila JJ, Salas-Salvadó J. Effects of one serving of mixed nuts on serum lipids, insulin resistance and inflammatory markers in patients with the metabolic syndrome. Nutr Metab Cardiovasc Dis. 2011;21:126–135. doi: 10.1016/j.numecd.2009.08.005. [DOI] [PubMed] [Google Scholar]
- 80.Estruch R, Ros E, Salas-Salvadó J, Covas M-I, Corella D, Arós F, et al. Primary prevention of cardiovascular disease with a Mediterranean diet. N Engl J Med. 2013;368:1279–1290. doi: 10.1056/NEJMoa1200303. [DOI] [PubMed] [Google Scholar]
- 81.Tulipani S, Llorach R, Jáuregui O, López-Uriarte P, Garcia-Aloy M, Bullo M, et al. Metabolomics unveils urinary changes in subjects with metabolic syndrome following 12-week nut consumption. J Proteome Res. 2011;10:5047–5058. doi: 10.1021/pr200514h. [DOI] [PubMed] [Google Scholar]
- 82.Tulipani S, Urpi-Sarda M, García-Villalba R, Rabassa M, López-Uriarte P, Bulló M, et al. Urolithins are the main urinary microbial-derived phenolic metabolites discriminating a moderate consumption of nuts in free-living subjects with diagnosed metabolic syndrome. J Agric Food Chem. 2012;60:8930–8940. doi: 10.1021/jf301509w. [DOI] [PubMed] [Google Scholar]
- 83.Mora-Cubillos X, Tulipani S, Garcia-Aloy M, Bulló M, Tinahones FJ, Andres-Lacueva C. Plasma metabolomic biomarkers of mixed nuts exposure inversely correlate with severity of metabolic syndrome. Mol Nutr Food Res. 2015;59:2480–90 [DOI] [PubMed]
- 84.Hudthagosol C, Haddad EH, McCarthy K, Wang P, Oda K, Sabate J. Pecans acutely increase plasma postprandial antioxidant capacity and catechins and decrease LDL oxidation in humans. J Nutr. 2011;141:56–62. doi: 10.3945/jn.110.121269. [DOI] [PubMed] [Google Scholar]
- 85.Rajaram S, Burke K, Connell B, Myint T, Sabate J. A monounsaturated fatty acid-rich pecan-enriched diet favorably alters the serum lipid profile of healthy men and women. J Nutr. 2001;131:2275–2279. doi: 10.1093/jn/131.9.2275. [DOI] [PubMed] [Google Scholar]
- 86.Garg ML, Blake RJ, Wills RBH. Macadamia nut consumption lowers plasma total and LDL cholesterol levels in hypercholesterolemic men. J Nutr. 2003;133:1060–1063. doi: 10.1093/jn/133.4.1060. [DOI] [PubMed] [Google Scholar]
- 87.Hiraoka-Yamamoto J, Ikeda K, Negishi H, Mori M, Hirose A, Sawada S, et al. Serum lipid effects of a monounsaturated (palmitoleic) fatty acid-rich diet based on macadamia nuts in healthy, young japanese women. Clin Exp Pharmacol Physiol. 2004;31:S37–S38. doi: 10.1111/j.1440-1681.2004.04121.x. [DOI] [PubMed] [Google Scholar]
- 88.Griel AE, Cao Y, Bagshaw DD, Cifelli AM, Holub B, Kris-Etherton PM. A macadamia nut-rich diet reduces total and LDL-cholesterol in mildly hypercholesterolemic men and women. J Nutr. 2008;138:761–767. doi: 10.1093/jn/138.4.761. [DOI] [PubMed] [Google Scholar]
- 89.Abdullah MMH, Cyr A, Lepine M-C, Labonte M-E, Couture P, Jones PJH, et al. Recommended dairy product intake modulates circulating fatty acid profile in healthy adults: a multi-Centre cross-over study. Br J Nutr. 2015;113:435–444. doi: 10.1017/S0007114514003894. [DOI] [PubMed] [Google Scholar]
- 90.Mah E, Schulz JA, Kaden VN, Lawless AL, Rotor J, Mantilla LB, et al. Cashew consumption reduces total and LDL cholesterol: a randomized, crossover, controlled-feeding trial. Am J Clin Nutr. 2017;105:1070–1078. doi: 10.3945/ajcn.116.150037. [DOI] [PubMed] [Google Scholar]
- 91.Ghadimi Nouran M, Kimiagar M, Abadi A, Mirzazadeh M, Harrison G. Peanut consumption and cardiovascular risk. Public Health Nutr. 2010;13:1581–1586. doi: 10.1017/S1368980009992837. [DOI] [PubMed] [Google Scholar]
- 92.Keller BO, Wu BTF, Li SSJ, Monga V, Innis SM. Hypaphorine is present in human milk in association with consumption of legumes. J Agric Food Chem. 2013;61:7654–7660. doi: 10.1021/jf401758f. [DOI] [PubMed] [Google Scholar]
- 93.Seal AJ, Creeke PI, Dibari F, Cheung E, Kyroussis E, Semedo P, et al. Low and deficient niacin status and pellagra are endemic in postwar Angola. Am J Clin Nutr. 2007;85:218–224. doi: 10.1093/ajcn/85.1.218. [DOI] [PubMed] [Google Scholar]
- 94.Lemaitre RN, Fretts AM, Sitlani CM, Biggs ML, Mukamal K, King IB, et al. Plasma phospholipid very-long-chain saturated fatty acids and incident diabetes in older adults: the cardiovascular health study. Am J Clin Nutr. 2015;101:1047–1054. doi: 10.3945/ajcn.114.101857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Llorach R, Garrido I, Monagas M, Urpi-Sarda M, Tulipani S, Bartolome B, et al. Metabolomics study of human urinary metabolome modifications after intake of almond (Prunus dulcis (Mill.) D.A. Webb) skin polyphenols. J Proteome Res. 2010;9:5859–5867. doi: 10.1021/pr100639v. [DOI] [PubMed] [Google Scholar]
- 96.Nieman DC, Scherr J, Luo B, Meaney MP, Dreau D, Sha W, et al. Influence of pistachios on performance and exercise-induced inflammation, oxidative stress, immune dysfunction, and metabolite shifts in cyclists: a randomized, crossover trial. PLoS One. 2014;9:e113725. doi: 10.1371/journal.pone.0113725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Guertin KA, Moore SC, Sampson JN, Huang W-Y, Xiao Q, Stolzenberg-Solomon RZ, et al. Metabolomics in nutritional epidemiology: identifying metabolites associated with diet and quantifying their potential to uncover diet-disease relations in populations. Am J Clin Nutr. 2014;100:208–217. doi: 10.3945/ajcn.113.078758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Vázquez-Fresno R, Llorach R, Urpi-Sarda M, Lupianez-Barbero A, Estruch R, Corella D, et al. Metabolomic pattern analysis after mediterranean diet intervention in a nondiabetic population: a 1- and 3-year follow-up in the PREDIMED study. J Proteome Res. 2015;14:531–540. doi: 10.1021/pr5007894. [DOI] [PubMed] [Google Scholar]
- 99.Playdon MC, Sampson JN, Cross AJ, Sinha R, Guertin KA, Moy KA, et al. Comparing metabolite profiles of habitual diet in serum and urine. Am J Clin Nutr. 2016;104:776–789. doi: 10.3945/ajcn.116.135301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Milbury PE, Chen C-Y, Blumberg JB. Temporal effects of almond skin polyphenols on plasma biomarkers of redox status. Milbury et al. 21 (5): A159—the FASEB journal. FASEB J. 2007;21:352.6. [Google Scholar]
- 101.Urpi-Sarda M, Monagas M, Khan N, Lamuela-Raventos RM, Santos-Buelga C, Sacanella E, et al. Epicatechin, procyanidins, and phenolic microbial metabolites after cocoa intake in humans and rats. Anal Bioanal Chem. 2009;394:1545–1556. doi: 10.1007/s00216-009-2676-1. [DOI] [PubMed] [Google Scholar]
- 102.Garrido I, Urpi-Sarda M, Monagas M, Gómez-Cordovés C, Martín-Alvarez PJ, Llorach R, et al. Targeted analysis of conjugated and microbial-derived phenolic metabolites in human urine after consumption of an almond skin phenolic extract. J Nutr. 2010;140:1799–1807. doi: 10.3945/jn.110.124065. [DOI] [PubMed] [Google Scholar]
- 103.Monagas M, Urpi-Sarda M, Sánchez-Patán F, Llorach R, Garrido I, Gómez-Cordovés C, et al. Insights into the metabolism and microbial biotransformation of dietary flavan-3-ols and the bioactivity of their metabolites. Food Funct. 2010;1:233–253. doi: 10.1039/c0fo00132e. [DOI] [PubMed] [Google Scholar]
- 104.Lu C-T, Tang H-F, Sun X-L, Wen A-D, Zhang W, Ma N. Indole alkaloids from chickpea seeds (Cicer arietinum L.) 2010. [Google Scholar]
- 105.Tsopmo A, Muir AD. Chemical profiling of lentil (Lens culinaris Medik.) cultivars and isolation of compounds. J Agric Food Chem. 2010;58:8715–8721. doi: 10.1021/jf101412y. [DOI] [PubMed] [Google Scholar]
- 106.Hofinger M, Monseur X, Pais M, Jarreau FX. Further confirmation of the presence of indolylacrylic acid in lentil seedlings and identification of hypaphorine as its precursor. Phytochemistry. 1975;14:475–477. doi: 10.1016/0031-9422(75)85112-0. [DOI] [Google Scholar]
- 107.Grando MS, Versini G, Nicolini G, Mattivi F. Selective use of wine yeast strains having different volatile phenols production. Vitis. 1993;32:43–50. [Google Scholar]
- 108.Fitschen PJ, Rolfhus KR, Winfrey MR, Allen BK, Manzy M, Maher MA. Cardiovascular effects of consumption of black versus English walnuts. J Med Food. 2011;14:890–898. doi: 10.1089/jmf.2010.0169. [DOI] [PubMed] [Google Scholar]
- 109.Ortega FJ, Cardona-Alvarado MI, Mercader JM, Moreno-Navarrete JM, Moreno M, Sabater M, et al. Circulating profiling reveals the effect of a polyunsaturated fatty acid-enriched diet on common microRNAs. J Nutr Biochem. 2015;26:1095–1101. doi: 10.1016/j.jnutbio.2015.05.001. [DOI] [PubMed] [Google Scholar]
- 110.Chisholm A, Mc Auley K, Mann J, Williams S, Skeaff M. Cholesterol lowering effects of nuts compared with a canola oil enriched cereal of similar fat composition. Nutr Metab Cardiovasc Dis. 2005;15:284–292. doi: 10.1016/j.numecd.2005.01.006. [DOI] [PubMed] [Google Scholar]
- 111.Wennberg M, Vessby B, Johansson I, Andersen LF, Solvoll K, Johansson LRK, et al. Evaluation of relative intake of fatty acids according to the northern Sweden FFQ with fatty acid levels in erythrocyte membranes as biomarkers. Public Health Nutr. 2009;12:1477. doi: 10.1017/S1368980008004503. [DOI] [PubMed] [Google Scholar]
- 112.Mozaffarian D, Wu JHY. (n-3) fatty acids and cardiovascular health: are effects of EPA and DHA shared or complementary? J Nutr. 2012;142:614S–625S. doi: 10.3945/jn.111.149633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Sabaté J, Haddad E, Tanzman JS, Jambazian P, Rajaram S. Serum lipid response to the graduated enrichment of a step I diet with almonds: a randomized feeding trial. Am J Clin Nutr. 2003;77:1379–1384. doi: 10.1093/ajcn/77.6.1379. [DOI] [PubMed] [Google Scholar]
- 114.Deon V, Del Bo’ C, Guaraldi F, Abello F, Belviso S, Porrini M, et al. Effect of hazelnut on serum lipid profile and fatty acid composition of erythrocyte phospholipids in children and adolescents with primary hyperlipidemia: a randomized controlled trial. Clin Nutr. 2017;37:1193–201 [DOI] [PubMed]
- 115.Nishi SK, Kendall CWC, Bazinet RP, Bashyam B, Ireland CA, Augustin LSA, et al. Nut consumption, serum fatty acid profile and estimated coronary heart disease risk in type 2 diabetes. Nutr Metab Cardiovasc Dis. 2014;24:845–852. doi: 10.1016/j.numecd.2014.04.001. [DOI] [PubMed] [Google Scholar]
- 116.Wolk A, Furuheim M, Vessby B. Fatty acid composition of adipose tissue and serum lipids are valid biological markers of dairy fat intake in men. J Nutr. 2001;131:828–833. doi: 10.1093/jn/131.3.828. [DOI] [PubMed] [Google Scholar]
- 117.Garcia-Aloy M, Rabassa M, Casas-Agustench P, Hidalgo-Liberona N, Llorach R, Andres-Lacueva C. Novel strategies for improving dietary exposure assessment: multiple-data fusion is a more accurate measure than the traditional single-biomarker approach. Trends Food Sci Technol. 2017;69:220–229. doi: 10.1016/j.tifs.2017.04.013. [DOI] [Google Scholar]
- 118.Bondia-Pons I, Castellote AI, López-Sabater MC. Comparison of conventional and fast gas chromatography in human plasma fatty acid determination. J Chromatogr B. 2004;809:339–344. doi: 10.1016/j.jchromb.2004.07.002. [DOI] [PubMed] [Google Scholar]
- 119.Glaser C, Demmelmair H, Koletzko B. High-throughput analysis of fatty acid composition of plasma glycerophospholipids. J Lipid Res. 2010;51:216–221. doi: 10.1194/jlr.D000547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Garcia-Muñoz C, Vaillant F. Metabolic fate of Ellagitannins: implications for health, and research perspectives for innovative functional foods. Crit Rev Food Sci Nutr. 2014;54:1584–1598. doi: 10.1080/10408398.2011.644643. [DOI] [PubMed] [Google Scholar]
- 121.Nuñez-Sánchez MA, García-Villalba R, Monedero-Saiz T, García-Talavera NV, Gómez-Sánchez MB, Sánchez-Álvarez C, et al. Targeted metabolic profiling of pomegranate polyphenols and urolithins in plasma, urine and colon tissues from colorectal cancer patients. Mol Nutr Food Res. 2014;58:1199–1211. doi: 10.1002/mnfr.201300931. [DOI] [PubMed] [Google Scholar]
- 122.Garrido M, Espino J, González-Gómez D, Lozano M, Barriga C, Paredes SD, et al. The consumption of a Jerte Valley cherry product in humans enhances mood, and increases 5-hydroxyindoleacetic acid but reduces cortisol levels in urine. Exp Gerontol. 2012;47:573–580. doi: 10.1016/j.exger.2012.05.003. [DOI] [PubMed] [Google Scholar]
- 123.Sommerburg O, Keunen JE, Bird AC, van Kuijk FJ. Fruits and vegetables that are sources for lutein and zeaxanthin: the macular pigment in human eyes. Br J Ophthalmol. 1998;82:907–910. doi: 10.1136/bjo.82.8.907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Eisenhauer B, Natoli S, Liew G, Flood V, Eisenhauer B, Natoli S, et al. Lutein and zeaxanthin—food sources, bioavailability and dietary variety in age-related macular degeneration protection. Nutrients. 2017;9:120. doi: 10.3390/nu9020120. [DOI] [PubMed] [Google Scholar]
- 125.Hernández-Alonso P, Cañueto D, Giardina S, Salas-Salvadó J, Cañellas N, Correig X, et al. Effect of pistachio consumption on the modulation of urinary gut microbiota-related metabolites in prediabetic subjects. J Nutr Biochem. 2017;45:48–53. doi: 10.1016/j.jnutbio.2017.04.002. [DOI] [PubMed] [Google Scholar]
- 126.Wan J, Zhang M, Adhikari B. Advances in selenium-enriched foods: from the farm to the fork. Trends Food Sci Technol. 2018;76:1–5. doi: 10.1016/j.tifs.2018.03.021. [DOI] [Google Scholar]
- 127.Pagnan A, Corrocher R, Ambrosio GB, Ferrari S, Guarini P, Piccolo D, et al. Effects of an olive-oil-rich diet on erythrocyte membrane lipid composition and cation transport systems. Clin Sci (Lond) 1989;76:87–93. doi: 10.1042/cs0760087. [DOI] [PubMed] [Google Scholar]
- 128.Sola R, Baudet MF, Motta C, Maillé M, Boisnier C, Jacotot B. Effects of dietary fats on the fluidity of human high-density lipoprotein: influence of the overall composition and phospholipid fatty acids. Biochim Biophys Acta. 1990;1043:43–51. doi: 10.1016/0005-2760(90)90108-A. [DOI] [PubMed] [Google Scholar]
- 129.Sanders K, Johnson L, O’Dea K, Sinclair AJ. The effect of dietary fat level and quality on plasma lipoprotein lipids and plasma fatty acids in normocholesterolemic subjects. Lipids. 1994;29:129–138. doi: 10.1007/BF02537152. [DOI] [PubMed] [Google Scholar]
- 130.Choudhury N, Tan L, Truswell AS. Comparison of palmolein and olive oil: effects on plasma lipids and vitamin E in young adults. Am J Clin Nutr. 1995;61:1043–1051. doi: 10.1093/ajcn/61.5.1043. [DOI] [PubMed] [Google Scholar]
- 131.Ruíz-Gutiérrez V, Muriana FJ, Guerrero A, Cert AM, Villar J. Plasma lipids, erythrocyte membrane lipids and blood pressure of hypertensive women after ingestion of dietary oleic acid from two different sources. J Hypertens. 1996;14:1483–1490. doi: 10.1097/00004872-199612000-00016. [DOI] [PubMed] [Google Scholar]
- 132.Yaqoob P, Knapper JA, Webb DH, Williams CM, Newsholme EA, Calder PC. Effect of olive oil on immune function in middle-aged men. Am J Clin Nutr. 1998;67:129–135. doi: 10.1093/ajcn/67.1.129. [DOI] [PubMed] [Google Scholar]
- 133.Svensson J, Rosenquist A, Ohlsson L. Postprandial lipid responses to an alpha-linolenic acid-rich oil, olive oil and butter in women: a randomized crossover trial. Lipids Health Dis. 2011;10:106. doi: 10.1186/1476-511X-10-106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Cabello-Moruno R, Martinez-Force E, Montero E, Perona JS. Minor components of olive oil facilitate the triglyceride clearance from postprandial lipoproteins in a polarity-dependent manner in healthy men. Nutr Res. 2014;34:40–47. doi: 10.1016/j.nutres.2013.10.003. [DOI] [PubMed] [Google Scholar]
- 135.Khymenets O, Farré M, Pujadas M, Ortiz E, Joglar J, Covas MI, et al. Direct analysis of glucuronidated metabolites of main olive oil phenols in human urine after dietary consumption of virgin olive oil. Food Chem. 2011;126:306–314. doi: 10.1016/j.foodchem.2010.10.044. [DOI] [Google Scholar]
- 136.Weinbrenner T, Fitó M, Farré Albaladejo M, Saez GT, Rijken P, Tormos C, et al. Bioavailability of phenolic compounds from olive oil and oxidative/antioxidant status at postprandial state in healthy humans. Drugs Exp Clin Res. 2004;30:207–212. [PubMed] [Google Scholar]
- 137.Suárez M, Valls RM, Romero M-P, Macià A, Fernández S, Giralt M, et al. Bioavailability of phenols from a phenol-enriched olive oil. Br J Nutr. 2011;106:1691–1701. doi: 10.1017/S0007114511002200. [DOI] [PubMed] [Google Scholar]
- 138.Valls R-M, Farràs M, Suárez M, Fernández-Castillejo S, Fitó M, Konstantinidou V, et al. Effects of functional olive oil enriched with its own phenolic compounds on endothelial function in hypertensive patients. A randomised controlled trial. Food Chem. 2015;167:30–35. doi: 10.1016/j.foodchem.2014.06.107. [DOI] [PubMed] [Google Scholar]
- 139.Rubió L, Macià A, Valls RM, Pedret A, Romero M-P, Solà R, et al. A new hydroxytyrosol metabolite identified in human plasma: hydroxytyrosol acetate sulphate. Food Chem. 2012;134:1132–1136. doi: 10.1016/j.foodchem.2012.02.192. [DOI] [PubMed] [Google Scholar]
- 140.Farràs M, Valls RM, Fernández-Castillejo S, Giralt M, Solà R, Subirana I, et al. Olive oil polyphenols enhance the expression of cholesterol efflux related genes in vivo in humans. A randomized controlled trial. J Nutr Biochem. 2013;24:1334–1339. doi: 10.1016/j.jnutbio.2012.10.008. [DOI] [PubMed] [Google Scholar]
- 141.Vicario IM, Malkova D, Lund EK, Johnson IT. Olive oil supplementation in healthy adults: effects in cell membrane fatty acid composition and platelet function. Ann Nutr Metab. 1998;42:160–169. doi: 10.1159/000012729. [DOI] [PubMed] [Google Scholar]
- 142.Mayneris-Perxachs J, Sala-Vila A, Chisaguano M, Castellote AI, Estruch R, Covas MI, et al. Effects of 1-year intervention with a Mediterranean diet on plasma fatty acid composition and metabolic syndrome in a population at high cardiovascular risk. PLoS One. 2014;9:e85202. doi: 10.1371/journal.pone.0085202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Marrugat J, Covas M-I, Fitó M, Schröder H, Miró-Casas E, Gimeno E, et al. Effects of differing phenolic content in dietary olive oils on lipids and LDL oxidation. Eur J Nutr. 2004;43:140–147. doi: 10.1007/s00394-004-0452-8. [DOI] [PubMed] [Google Scholar]
- 144.Covas M-I, de la Torre K, Farré-Albaladejo M, Kaikkonen J, Fitó M, López-Sabater C, et al. Postprandial LDL phenolic content and LDL oxidation are modulated by olive oil phenolic compounds in humans. Free Radic Biol Med. 2006;40:608–616. doi: 10.1016/j.freeradbiomed.2005.09.027. [DOI] [PubMed] [Google Scholar]
- 145.Salvini S, Sera F, Caruso D, Giovannelli L, Visioli F, Saieva C, et al. Daily consumption of a high-phenol extra-virgin olive oil reduces oxidative DNA damage in postmenopausal women. Br J Nutr. 2006;95:742–751. doi: 10.1079/BJN20051674. [DOI] [PubMed] [Google Scholar]
- 146.Gimeno E, de la Torre-Carbot K, Lamuela-Raventós RM, Castellote AI, Fitó M, de la Torre R, et al. Changes in the phenolic content of low density lipoprotein after olive oil consumption in men. A randomized crossover controlled trial. Br J Nutr. 2007;98:1243–1250. doi: 10.1017/S0007114507778698. [DOI] [PubMed] [Google Scholar]
- 147.Machowetz A, Gruendel S, Garcia A, Harsch I, Covas M-I, Zunft H-J, et al. Effect of olive oil consumption on serum resistin concentrations in healthy men. Horm Metab Res. 2008;40:697–701. doi: 10.1055/s-2008-1078728. [DOI] [PubMed] [Google Scholar]
- 148.Perona JS, Fitó M, Covas M-I, Garcia M, Ruiz-Gutierrez V. Olive oil phenols modulate the triacylglycerol molecular species of human very low-density lipoprotein. A randomized, crossover, controlled trial. Metabolism. 2011;60:893–899. doi: 10.1016/j.metabol.2010.08.010. [DOI] [PubMed] [Google Scholar]
- 149.Oliveras-López M-J, Innocenti M, Martín Bermudo F, López-García de la Serrana H, Mulinacci N. Effect of extra virgin olive oil on glycaemia in healthy young subjects. Eur J Lipid Sci Technol. 2012;114:999–1006. doi: 10.1002/ejlt.201100368. [DOI] [Google Scholar]
- 150.Fitó M, Cladellas M, de la Torre R, Martí J, Muñoz D, Schröder H, et al. Anti-inflammatory effect of virgin olive oil in stable coronary disease patients: a randomized, crossover, controlled trial. Eur J Clin Nutr. 2008;62:570–574. doi: 10.1038/sj.ejcn.1602724. [DOI] [PubMed] [Google Scholar]
- 151.Machowetz A, Poulsen HE, Gruendel S, Weimann A, Fitó M, Marrugat J, et al. Effect of olive oils on biomarkers of oxidative DNA stress in northern and southern Europeans. FASEB J. 2007;21:45–52. doi: 10.1096/fj.06-6328com. [DOI] [PubMed] [Google Scholar]
- 152.de la Torre-Carbot K, Chávez-Servín JL, Jaúregui O, Castellote AI, Lamuela-Raventós RM, Nurmi T, et al. Elevated circulating LDL phenol levels in men who consumed virgin rather than refined olive oil are associated with less oxidation of plasma LDL. J Nutr. 2010;140:501–508. doi: 10.3945/jn.109.112912. [DOI] [PubMed] [Google Scholar]
- 153.Farràs M, Castañer O, Martín-Peláez S, Hernáez Á, Schröder H, Subirana I, et al. Complementary phenol-enriched olive oil improves HDL characteristics in hypercholesterolemic subjects. A randomized, double-blind, crossover, controlled trial. The VOHF study. Mol Nutr Food Res. 2015;59:1758–1770. doi: 10.1002/mnfr.201500030. [DOI] [PubMed] [Google Scholar]
- 154.Fernández-Ávila C, Montes R, Castellote AI, Chisaguano AM, Fitó M, Covas MI, et al. Fast determination of virgin olive oil phenolic metabolites in human high-density lipoproteins. Biomed Chromatogr. 2015;29:1035–1041. doi: 10.1002/bmc.3389. [DOI] [PubMed] [Google Scholar]
- 155.Serra A, Rubió L, Macià A, Valls R-M, Catalán Ú, de la Torre R, et al. Application of dried spot cards as a rapid sample treatment method for determining hydroxytyrosol metabolites in human urine samples. Comparison with microelution solid-phase extraction. Anal Bioanal Chem. 2013;405:9179–9192. doi: 10.1007/s00216-013-7322-2. [DOI] [PubMed] [Google Scholar]
- 156.Rubió L, Farràs M, de La Torre R, Macià A, Romero M-P, Valls RM, et al. Metabolite profiling of olive oil and thyme phenols after a sustained intake of two phenol-enriched olive oils by humans: identification of compliance markers. Food Res Int. 2014;65:59–68. doi: 10.1016/j.foodres.2014.05.009. [DOI] [Google Scholar]
- 157.Larsen LF, Jespersen J, Marckmann P. Are olive oil diets antithrombotic? Diets enriched with olive, rapeseed, or sunflower oil affect postprandial factor VII differently. Am J Clin Nutr. 1999;70:976–982. doi: 10.1093/ajcn/70.6.976. [DOI] [PubMed] [Google Scholar]
- 158.Schwab US, Vogel S, Lammi-Keefe CJ, Ordovas JM, Schaefer EJ, Li Z, et al. Varying dietary fat type of reduced-fat diets has little effect on the susceptibility of LDL to oxidative modification in moderately hypercholesterolemic subjects. J Nutr. 1998;128:1703–1709. doi: 10.1093/jn/128.10.1703. [DOI] [PubMed] [Google Scholar]
- 159.Kelley DS, Nelson GJ, Love JE, Branch LB, Taylor PC, Schmidt PC, et al. Dietary alpha-linolenic acid alters tissue fatty acid composition, but not blood lipids, lipoproteins or coagulation status in humans. Lipids. 1993;28:533–537. doi: 10.1007/BF02536085. [DOI] [PubMed] [Google Scholar]
- 160.Mantzioris E, James MJ, Gibson RA, Cleland LG. Dietary substitution with an α-linolenic acid-rich vegetable oil increases eicosapentaenoic acid concentrations in tissues. Am J Clin Nutr. 1994;59:1304–1309. doi: 10.1093/ajcn/59.6.1304. [DOI] [PubMed] [Google Scholar]
- 161.Freese R, Mutanen M. Alpha-linolenic acid and marine long-chain n-3 fatty acids differ only slightly in their effects on hemostatic factors in healthy subjects. Am J Clin Nutr. 1997;66:591–598. doi: 10.1093/ajcn/66.3.591. [DOI] [PubMed] [Google Scholar]
- 162.Goh YK, Jumpsen JA, Ryan EA, Clandinin MT. Effect of omega 3 fatty acid on plasma lipids, cholesterol and lipoprotein fatty acid content in NIDDM patients. Diabetologia. 1997;40:45–52. doi: 10.1007/s001250050641. [DOI] [PubMed] [Google Scholar]
- 163.Li D, Sinclair A, Wilson A, Nakkote S, Kelly F, Abedin L, et al. Effect of dietary α-linolenic acid on thrombotic risk factors in vegetarian men. Am J Clin Nutr. 1999;69:872–882. doi: 10.1093/ajcn/69.5.872. [DOI] [PubMed] [Google Scholar]
- 164.Cao J, Schwichtenberg KA, Hanson NQ, Tsai MY. Incorporation and clearance of omega-3 fatty acids in erythrocyte membranes and plasma phospholipids. Clin Chem. 2006;52:2265–2272. doi: 10.1373/clinchem.2006.072322. [DOI] [PubMed] [Google Scholar]
- 165.Nelson TL, Stevens JR, Hickey MS. Inflammatory markers are not altered by an eight week dietary α-linolenic acid intervention in healthy abdominally obese adult males and females. Cytokine. 2007;38:101–106. doi: 10.1016/j.cyto.2007.05.010. [DOI] [PubMed] [Google Scholar]
- 166.Barceló-Coblijn G, Murphy EJ, Othman R, Moghadasian MH, Kashour T, Friel JK. Flaxseed oil and fish-oil capsule consumption alters human red blood cell n–3 fatty acid composition: a multiple-dosing trial comparing 2 sources of n–3 fatty acid. Am J Clin Nutr. 2008;88:801–809. doi: 10.1093/ajcn/88.3.801. [DOI] [PubMed] [Google Scholar]
- 167.Kaul N, Kreml R, Austria JA, Richard MN, Edel AL, Dibrov E, et al. A comparison of fish oil, flaxseed oil and hempseed oil supplementation on selected parameters of cardiovascular health in healthy volunteers. J Am Coll Nutr. 2008;27:51–58. doi: 10.1080/07315724.2008.10719674. [DOI] [PubMed] [Google Scholar]
- 168.Taylor CG, Noto AD, Stringer DM, Froese S, Malcolmson L. Dietary milled flaxseed and flaxseed oil improve N-3 fatty acid status and do not affect glycemic control in individuals with well-controlled type 2 diabetes. J Am Coll Nutr. 2010;29:72–80. doi: 10.1080/07315724.2010.10719819. [DOI] [PubMed] [Google Scholar]
- 169.Dewell A, Marvasti FF, Harris WS, Tsao P, Gardner CD. Low- and high-dose plant and marine (n-3) fatty acids do not affect plasma inflammatory markers in adults with metabolic syndrome. J Nutr. 2011;141:2166–2171. doi: 10.3945/jn.111.142240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Neukam K, De Spirt S, Stahl W, Bejot M, Maurette J-M, Tronnier H, et al. Supplementation of flaxseed oil diminishes skin sensitivity and improves skin barrier function and condition. Skin Pharmacol Physiol. 2011;24:67–74. doi: 10.1159/000321442. [DOI] [PubMed] [Google Scholar]
- 171.Kontogianni MD, Vlassopoulos A, Gatzieva A, Farmaki A-E, Katsiougiannis S, Panagiotakos DB, et al. Flaxseed oil does not affect inflammatory markers and lipid profile compared to olive oil, in young, healthy, normal weight adults. Metabolism. 2013;62:686–693. doi: 10.1016/j.metabol.2012.11.007. [DOI] [PubMed] [Google Scholar]
- 172.Kawakami Y, Yamanaka-Okumura H, Naniwa-Kuroki Y, Sakuma M, Taketani Y, Takeda E. Flaxseed oil intake reduces serum small dense low-density lipoprotein concentrations in Japanese men: a randomized, double blind, crossover study. Nutr J. 2015;14:39. doi: 10.1186/s12937-015-0023-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Allman MA, Pena MM, Pang D. Supplementation with flaxseed oil versus sunflowerseed oil in healthy young men consuming a low fat diet: effects on platelet composition and function. Eur J Clin Nutr. 1995;49:169–178. [PubMed] [Google Scholar]
- 174.Francois CA, Connor SL, Wander RC, Connor WE. Acute effects of dietary fatty acids on the fatty acids of human milk. Am J Clin Nutr. 1998;67:301–308. doi: 10.1093/ajcn/67.2.301. [DOI] [PubMed] [Google Scholar]
- 175.Senanayake VK, Pu S, Jenkins DA, Lamarche B, Kris-Etherton PM, West SG, et al. Plasma fatty acid changes following consumption of dietary oils containing n-3, n-6, and n-9 fatty acids at different proportions: preliminary findings of the canola oil multicenter intervention trial (COMIT) Trials. 2014;15:136. doi: 10.1186/1745-6215-15-136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Pu S, Eck P, Jenkins DJA, Connelly PW, Lamarche B, Kris-Etherton PM, et al. Interactions between dietary oil treatments and genetic variants modulate fatty acid ethanolamides in plasma and body weight composition. Br J Nutr. 2016;115:1012–1023. doi: 10.1017/S0007114515005425. [DOI] [PubMed] [Google Scholar]
- 177.Valsta LM, Salminen I, Aro A, Mutanen M. Alpha-linolenic acid in rapeseed oil partly compensates for the effect of fish restriction on plasma long chain n-3 fatty acids. Eur J Clin Nutr. 1996;50:229–235. [PubMed] [Google Scholar]
- 178.Corner EJ, Bruce VM, McDonald BE. Accumulation of eicosapentaenoic acid in plasma phospholipids of subjects fed canola oil. Lipids. 1990;25:598–601. doi: 10.1007/BF02536008. [DOI] [PubMed] [Google Scholar]
- 179.Weaver BJ, Corner EJ, Bruce VM, McDonald BE, Holub BJ. Dietary canola oil: effect on the accumulation of eicosapentaenoic acid in the alkenylacyl fraction of human platelet ethanolamine phosphoglyceride. Am J Clin Nutr. 1990;51:594–598. doi: 10.1093/ajcn/51.4.594. [DOI] [PubMed] [Google Scholar]
- 180.Rosqvist F, Iggman D, Kullberg J, Cedernaes J, Johansson H-E, Larsson A, et al. Overfeeding polyunsaturated and saturated fat causes distinct effects on liver and visceral fat accumulation in humans. Diabetes. 2014;63:2356–2368. doi: 10.2337/db13-1622. [DOI] [PubMed] [Google Scholar]
- 181.Rubió L, Valls R-M, Macià A, Pedret A, Giralt M, Romero M-P, et al. Impact of olive oil phenolic concentration on human plasmatic phenolic metabolites. Food Chem. 2012;135:2922–2929. doi: 10.1016/j.foodchem.2012.07.085. [DOI] [PubMed] [Google Scholar]
- 182.Boskou D. Olive oil. In: Gunstone FD, editor. Vegetable oils in food technology. Oxford: Wiley-Blackwell; 2011. pp. 243–271. [Google Scholar]
- 183.Bonanome A, Pagnan A, Caruso D, Toia A, Xamin A, Fedeli E, et al. Evidence of postprandial absorption of olive oil phenols in humans. Nutr Metab Cardiovasc Dis. 2000;10:111–120. [PubMed] [Google Scholar]
- 184.Caruso D, Visioli F, Patelli R, Galli C, Galli G. Urinary excretion of olive oil phenols and their metabolites in humans. Metabolism. 2001;50:1426–1428. doi: 10.1053/meta.2001.28073. [DOI] [PubMed] [Google Scholar]
- 185.Wishart DS, Knox C, Guo AC, Eisner R, Young N, Gautam B, et al. HMDB: a knowledgebase for the human metabolome. Nucleic Acids Res. 2009;37(Database issue):D603–D610. doi: 10.1093/nar/gkn810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Przybylski R, Eskin NAM. Oil Composition and Properties. In: Daun JK, Eskin NAM, Hickling D, editors. Canola: Chemistry, Production, Processing, and Utilization. AOCS Press; 2011;245–80.
- 187.Grompone MA. Vegetable oils in food technology. Oxford: Wiley-Blackwell; 2011. Sunflower oil; pp. 137–167. [Google Scholar]
- 188.Solà R, La Ville AE, Richard JL, Motta C, Bargalló MT, Girona J, et al. Oleic acid rich diet protects against the oxidative modification of high density lipoprotein. Free Radic Biol Med. 1997;22:1037–1045. doi: 10.1016/S0891-5849(96)00490-X. [DOI] [PubMed] [Google Scholar]
- 189.Choudhury N, Truswell S, McNeil Y. Comparison of plasma lipids and vitamin E in young and middle-aged subjects on potato crisps fried in palmolein and highly oleic sunflower oil. Ann Nutr Metab. 1997;41:137–148. doi: 10.1159/000177989. [DOI] [PubMed] [Google Scholar]
- 190.Cater NB, Heller HJ, Denke MA. Comparison of the effects of medium-chain triacylglycerols, palm oil, and high oleic acid sunflower oil on plasma triacylglycerol fatty acids and lipid and lipoprotein concentrations in humans. Am J Clin Nutr. 1997;65:41–45. doi: 10.1093/ajcn/65.1.41. [DOI] [PubMed] [Google Scholar]
- 191.Denke MA, Grundy SM. Comparison of effects of lauric acid and palmitic acid on plasma lipids and lipoproteins. Am J Clin Nutr. 1992;56:895–898. doi: 10.1093/ajcn/56.5.895. [DOI] [PubMed] [Google Scholar]
- 192.Wardlaw GM, Snook JT. Effect of diets high in butter, corn oil, or high-oleic acid sunflower oil on serum lipids and apolipoproteins in men. Am J Clin Nutr. 1990;51:815–821. doi: 10.1093/ajcn/51.5.815. [DOI] [PubMed] [Google Scholar]
- 193.Tomasch R, Wagner K-H, Elmadfa I. Antioxidative power of plant oils in humans: the influence of α- and γ-tocopherol. Ann Nutr Metab. 2001;45:110–115. doi: 10.1159/000046715. [DOI] [PubMed] [Google Scholar]
- 194.Lemcke-Norojärvi M, Kamal-Eldin A, Appelqvist L-A, Dimberg LH, Öhrvall M, Vessby B. Corn and sesame oils increase serum γ-tocopherol concentrations in healthy Swedish women. J Nutr. 2001;131:1195–1201. doi: 10.1093/jn/131.4.1195. [DOI] [PubMed] [Google Scholar]
- 195.Lee TC, Ivester P, Hester AG, Sergeant S, Case L, Morgan T, et al. The impact of polyunsaturated fatty acid-based dietary supplements on disease biomarkers in a metabolic syndrome/diabetes population. Lipids Health Dis. 2014;13:196. doi: 10.1186/1476-511X-13-196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Cox C, Mann J, Sutherland W, Chisholm A, Skeaff M. Effects of coconut oil, butter, and safflower oil on lipids and lipoproteins in persons with moderately elevated cholesterol levels. J Lipid Res. 1995;36:1787–1795. [PubMed] [Google Scholar]
- 197.Uusitalo U, Feskens EJ, Tuomilehto J, Dowse G, Haw U, Fareed D, et al. Fall in total cholesterol concentration over five years in association with changes in fatty acid composition of cooking oil in Mauritius: cross sectional survey. BMJ. 1996;313:1044–1046. doi: 10.1136/bmj.313.7064.1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Abraham RD, Riemersma RA, Elton RA, Macintyre C, Oliver MF. Effects of safflower oil and evening primrose oil in men with a low dihomo-gamma-linolenic level. Atherosclerosis. 1990;81:199–208. doi: 10.1016/0021-9150(90)90067-S. [DOI] [PubMed] [Google Scholar]
- 199.Kuhnt K, Fuhrmann C, Köhler M, Kiehntopf M, Jahreis G. Dietary echium oil increases long-chain n-3 PUFAs, including docosapentaenoic acid, in blood fractions and alters biochemical markers for cardiovascular disease independently of age, sex, and metabolic syndrome. J Nutr. 2014;144:447–460. doi: 10.3945/jn.113.180802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Hodson L, Skeaff CM, Fielding BA. Fatty acid composition of adipose tissue and blood in humans and its use as a biomarker of dietary intake. Prog Lipid Res. 2008;47:348–380. doi: 10.1016/j.plipres.2008.03.003. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Nonrelevant food intake biomarkers for nuts. Table S2. Nonrelevant food intake biomarkers for vegetable oils. (PDF 111 kb)
Data Availability Statement
Not applicable.