Abstract
Objectives:
Thyroid transcription factor 1 (TTF-1) is routinely tested in the diagnostic evaluation of suspected lung cancers, is commonly expressed by lung adenocarcinomas, and may modulate lung cancer biology. We examined the role of TTF-1 as a predictive and prognostic marker in patients with advanced lung adenocarcinomas.
Materials and Methods:
We analyzed clinical, pathologic, and molecular features, treatments received, and overall survival obtained from the medical records of 479 consecutive patients at a single site with stage IV lung adenocarcinomas and evaluable TTF-1 expression. TTF-1 expression was determined by immunohistochemistry using antibody 8G7G3/1.
Results and Conclusion:
TTF-1 expression was evaluable in 479 (75%) of all patients reviewed, and was positive in 383 (80%, 95% CI 76 to 83%). Clinicopathologic features were similar between TTF-1 positive and TTF-1 negative tumors, except EGFR mutations were more common in TTF-1 positive cases (24% vs 6%, p<0.001). In univariate analysis, overall survival was significantly longer in patients with TTF-1 positive versus TTF-1 negative tumors (18 months vs 9 months, p<0.0001). In multivariate analysis, TTF-1 positivity remained associated with better overall survival (HR=0.38, p<0.0001), exceeding the prognostic impact of Karnofsky performance status >/= 80% (HR 0.62, p=0.0003) and receipt of first-line combination chemotherapy or targeted therapy (HR relative to single agent chemotherapy 0.59, p=0.05 and 0.51, p=0.05 respectively). Both patients with TTF-1 positive and TTF-1 negative cancers had longer durations of initial therapy when treated with pemetrexed-based chemotherapy. In patients with advanced lung adenocarcinomas, TTF-1 expression is associated with better survival but is not predictive of distinct benefit from pemetrexed-based chemotherapy.
Keywords: non-small-cell lung cancer, lung adenocarcinoma, thyroid transcription factor 1, biomarker, chemotherapy
1.0. Introduction
The identification of molecular and histologic subgroups of lung cancers that are selectively sensitive to particular therapeutic approaches have led to improvements in the outcomes of patients with tumors harboring specific genetic alterations, such as EGFR [1], ALK [2], HER2 [3], MET [4], BRAF [5], RET [6], and ROS1 [7]. Smoking-related lung cancers with greater somatic mutation burden have greater response to PD-1 blockade [8]. Lung adenocarcinomas have improved responsiveness to pemetrexed [9], as might those with RET [10] and ROS1 [11] re-arrangements. Overall, more precise understanding of the predictive and prognostic impact of distinct features of lung cancers has been critical to progress.
Thyroid transcription factor 1 (TTF-1) is one of the most commonly utilized immunohistochemical markers in the diagnosis of lung cancers. It is expressed in ~60-90% of lung adenocarcinomas, ~90% of small cell lung cancers and the majority of thyroid carcinomas, but is rarely expressed in adenocarcinomas of other sites or in squamous cell lung carcinomas [12-14]. TTF-1 is primarily used to distinguish adenocarcinomas of lung (and thyroid) origin from carcinomas of other sites and to distinguish lung adenocarcinomas and small cell lung cancers from squamous cell carcinomas [15]. Beyond its role in diagnosis, TTF-1 may also play a role in lung cancer biology, specifically as a relevant predictive and/or prognostic marker for patients with lung adenocarcinoma. TTF-1 is a lineage-specific transcription factor involved in morphogenesis, differentiation, and surfactant production of normal lung epithelial cells [16] and in cancer cells can act as both an oncogenic driver [17-19] and tumor suppressor [20, 21] in a context-dependant manner [22].
Given its role as a routine identifier of lung adenocarcinomas, which are generally more sensitive to pemetrexed-based chemotherapy than squamous cell carcinomas [9], we hypothesized that TTF-1 may further predict the subset of lung adenocarcinomas that are selectively sensitive to pemetrexed. Additionally, although the positive prognostic impact of TTF-1 expression in early stage lung adenocarcinomas has been reported [23-29], studies in advanced disease have been limited by small sample sizes and lack of control for genotype or treatment [30]. As a result, TTF-1 is not routinely examined or controlled for in clinical trials. To clarify the role of TTF-1 as a predictive and/or prognostic marker in patients with advanced lung adenocarcinomas, we examined the relationship between TTF-1 expression, molecular features, response to chemotherapy, and overall survival among a series of 633 consecutive patients with stage IV lung adenocarcinomas.
2.0. Methods
2.1. Patients, Treatment, and Outcomes
After approval from the Institutional Review Board (WA-0007-13), we examined the medical records of 633 consecutive patients with newly diagnosed stage IV lung adenocarcinomas who were treated with systemic therapy at Memorial Sloan Kettering Cancer Center between January 2009 and December 2011. Retrospectively collected demographic and clinicopathologic details included age at diagnosis, sex, histology, TTF-1 expression, date of diagnosis of stage IV disease, performance status, and cigarette smoking history. Specific chemotherapy regimens were annotated and duration of initial treatment was quantified as time from first to last dose of treatment, with maintenance therapy duration considered part of initial treatment. Overall survival was calculated from the date of diagnosis of advanced stage disease to the date of death, or censored at last follow-up. Analyses of the predictive impact of TTF-1 on the benefit from pemetrexed-based initial chemotherapy were limited to those who received pemetrexed-based doublet or triplet chemotherapy. Those who received single therapies or concurrent targeted therapies were excluded. Patients in whom TTF-1 was unknown due to not having been tested as part of the clinical pathology evaluation were considered separately.
2.2. TTF-1 expression testing
TTF-1 expression was assessed as part of routine diagnostic evaluation. Expression was determined by immunohistochemistry (antibody clone 8G7G3/1, DAKO, Dilution 1:100) and recorded as a binary variable (positive = any nuclear reactivity in any tumor sample tested; negative = no reactivity; Supplemental Figure 1).
2.3. Statistical Analysis
Associations between TTF-1 expression and clinicopathologic characteristics were assessed using chi-squared and t-tests. The impact of TTF-1 on response to specific chemotherapies was assessed in patients who were treated with initial doublet or triplet cytotoxic chemotherapy. The effect of chemotherapy was estimated by evaluating the duration of treatment. The univariate impact of clinicopathologic characteristics and of TTF-1 expression on overall survival was assessed using Kaplan-Meier methods and compared using log-rank tests. Multivariate modeling was conducted using Cox proportional hazard regression to evaluate the impact of TTF-1 expression on overall survival with adjustment for potential confounders. Across all analyses, a threshold of <0.05 for two-sided p-value was considered significant.
3.0. Results
3.1. Clinical characteristics and association with TTF-1 expression
Of 633 patients examined, 479 (75%) patients had lung adenocarcinomas that were assessed for TTF-1 expression, of which 383 (80%, 95% CI 76 to 83%) were TTF-1 positive. Clinical features of patients with TTF-1 positive tumors were similar to those with TTF-1 negative tumors (Table 1), and both groups were similar to the group of 154 patients who did not have TTF-1 assessed (Supplemental Table 1). EGFR mutations were more common in tumors that were TTF-1 positive compared to those that were TTF-1 negative (24% vs 6%, p<0.001).
Table 1:
Clinical characteristics of patients with Stage IV lung adenocarcinomas
| TTF-1 Positive | TTF-1 Negative | P | |
|---|---|---|---|
| Number of Patients | 383 (80%) | 96 (20%) | |
| Age (years) | |||
| Median | 63 | 63 | |
| Range | 18-87 | 24-84 | 0.80 |
| Sex | |||
| Male | 172 (45%) | 53 (55%) | 0.086 |
| Smoking | |||
| Never | 99 (26%) | 19 (20%) | |
| Former | 193 (50%) | 45 (47%) | |
| Current | 91 (24%) | 32 (33%) | 0.139 |
| Karnofsky Performance Status | |||
| ≤70 | 98 (26%) | 35 (36%) | |
| 80+ | 285 (74%) | 61 (64%) | 0.041 |
| Genotype | |||
| Unknown | 54 (14%) | 19 (20%) | 0.203 |
| KRAS mutant | 81 (21%) | 26 (27%) | 0.219 |
| EGFR mutant | 92 (24%) | 6 (6%) | <0.001 |
| ALK-rearranged | 9 (2%) | 1 (1%) | 0.695 |
| EGFR/KRAS WT | 153 (40%) | 45 (47%) | 0.247 |
3.2. Univariate association of TTF-1 with overall survival in all patients and in molecular or therapy-defined subgroups
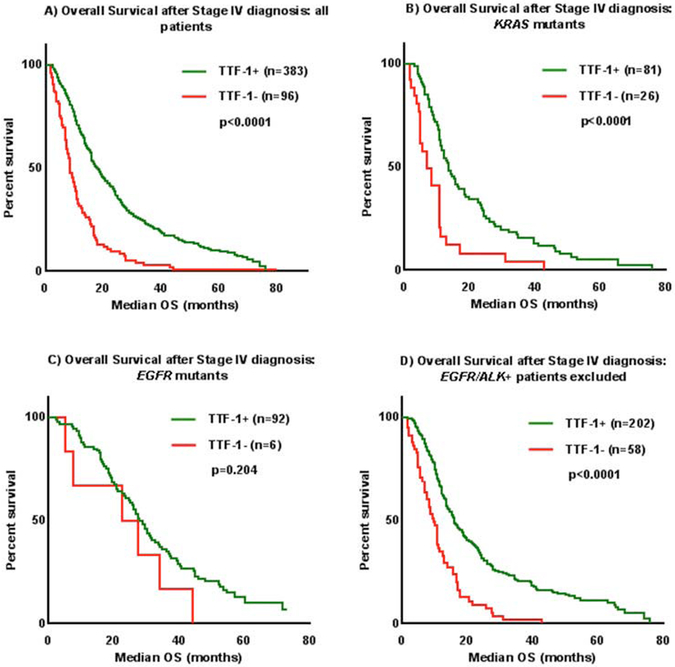
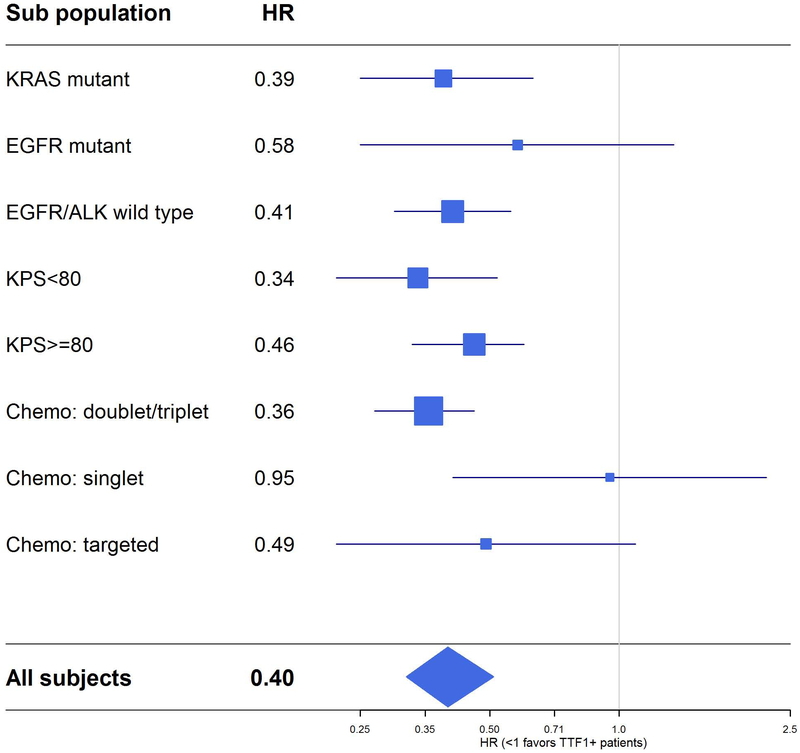
Overall, median survival was twice as long in patients with TTF-1 positive tumors compared to those with TTF-1 negative tumors (18 months vs 9 months, p<0.0001) (Figure 1A). Given the potential confounding effects of molecular subgroups, treatment received, and clinical features such as functional status on overall survival, the impact of TTF-1 was further examined within these specific subgroups. Among those with KRAS mutations (n=107), survival was longer in TTF-1 positive patients (13 months vs 7 months p<0.0001, Figure 1B). In 98 individuals with tumors with EGFR sensitizing mutations, survival with and without TTF-1 expression was comparable (28 months vs 25 months, p=0.20, Figure 1C). When excluding those with known targetable somatic alterations in EGFR or ALK (or those with unknown EGFR/ALK status), survival was significantly longer in those who were TTF-1 positive (n=260, 16 months vs 10 months, p<0.0001, Figure 1D). Among those who received initial doublet or triplet cytotoxic chemotherapy, TTF-1 expression was associated with increased survival (n=368, 16 months vs 9 months, p<0.0001, Figure 2), but not in those who received only single agent chemotherapy or who received molecularly targeted therapy (p=0.90 and p=0.081, respectively, Supplemental Figure 2). In those with either Karnofsky performance status >/= 80% or Karnofsky Performance Status < 80% functional status, TTF-1 was associated with improved survival (Figure 3).
Figure 1:
TTF-1 expression was positively associated with Overall Survival (OS) after Stage IV diagnosis in (A) all patients, (B) KRAS mutants, (C) EGFR mutants, and (D) a subset of subjects with EGFR/ALK+ patients excluded.
Figure 2:
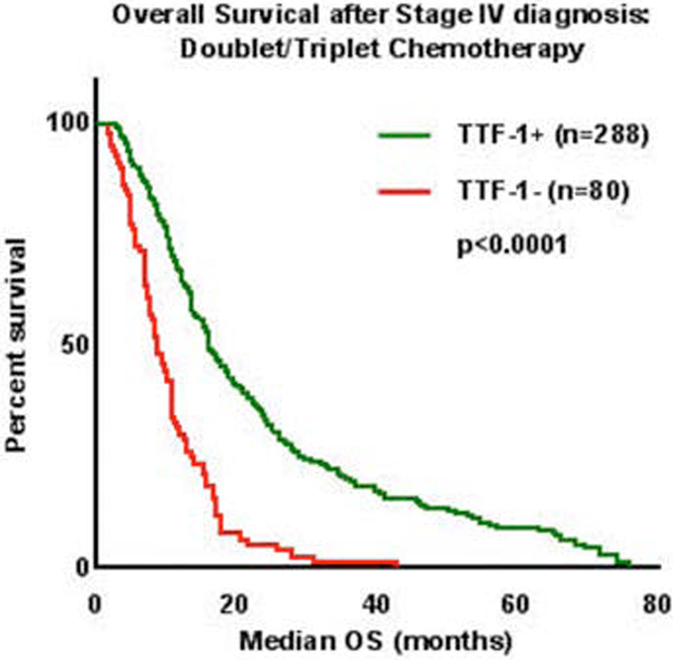
TTF-1 expression was significantly associated with Overall Survival (OS) after Stage IV diagnosis in patients who received doublet or triplet chemotherapy.
Figure 3:
TTF-1 expression was positively associated with overall survival in all patients and in each molecular, performance status, and therapy-defined subgroup. The size of the blue squares is proportional to the size of the corresponding subgroups.
3.3. TTF-1 Prognostic Impact: Multivariate association with overall survival
We constructed a multivariate regression model using variables known to affect survival, including age, sex, smoking status, performance status, molecular subgroups, and treatment received. Adjusting for these variables, TTF-1 positivity improved survival (HR 0.38, 95% CI 0.29 to 0.51, p<0.0001), to an extent exceeding that of Karnofsky Performance Status>/= 80% (HR 0.62, 95% CI 0.48 to 0.80, p=0.0003) and receipt of combination chemotherapy (HR relative to single agent chemotherapy 0.59, 95% CI 0.35 to 1.01, p=0.054) or targeted therapy (HR relative to single agent chemotherapy 0.51, 95% CI 0.26 to 1.00, p=0.051) (Table 2).
Table 2:
Multivariate Cox proportional hazards regression of overall survival (excluding patients with unknown mutation, n=368)
| Variable | HR | 95% CI | P | |
|---|---|---|---|---|
| Lower | upper | |||
| Age at diagnosis (<80 vs. >=80) | 0.82 | 0.51 | 1.31 | 0.396 |
| Sex (female vs. male) | 0.92 | 0.74 | 1.16 | 0.493 |
| Smoking status (Never vs. current/former) | 0.72 | 0.54 | 0.96 | 0.026 |
| TTF-1 (+ vs. −) | 0.38 | 0.29 | 0.51 | <.0001 |
| Karnofsky Performance Status (>=80 vs. <80) | 0.62 | 0.48 | 0.80 | 0.0003 |
| Mutation status | ||||
| ALK rearranged vs wild type | 0.76 | 0.36 | 1.58 | 0.756 |
| EGFR mutant vs wild type | 0.94 | 0.61 | 1.44 | 0.938 |
| KRAS mutant vs wild type | 1.41 | 1.08 | 1.84 | 0.011 |
| Initial chemotherapy | ||||
| Doublet/triplet vs singlet | 0.59 | 0.35 | 1.01 | 0.054 |
| Targeted vs singlet | 0.51 | 0.26 | 1.00 | 0.051 |
3.4. TTF-1 Predictive Impact: Benefit of initial pemetrexed-based chemotherapy
We examined the duration of treatment on initial pemetrexed-based chemotherapy as a surrogate of progression-free survival. This analysis was performed in TTF-1 positive and TTF-1 negative patients separately in order to circumvent the (now demonstrated) confounding impact of TTF-1 expression on survival. We hypothesized that an improvement in duration of treatment only among those with TTF-1 expression may be indicative of a predictive interaction between TTF-1 and pemetrexed-based therapy. However, there was improvement in duration of treatment among both TTF-1 positive and TTF-1 negative patients treated with pemetrexed-based chemotherapy compared to those treated with non-pemetrexed-based (Supplemental Table 2) doublet or triplet chemotherapy (among TTF-1 positive patients: HR=0.42, 95% CI 0.29 to 0.59, p<0.0001, Table 3A; among TTF-1 negative patients: HR=0.55, 95% CI 0.28-1.06, p=0.074, Table 3B). In a Cox proportional hazard model, the interaction between TTF-1 expression and pemetrexed chemotherapy was not significant (p=0.430).
Table 3:
Response to Pemetrexed-based and Non-Pemetrexed based chemotherapies in (A) TTF-1 positive patients and (B) TTF-1 negative patients.
| A) TTF-1+ patients: | ||||
|---|---|---|---|---|
| Pemetrexed- based doublet/triplet chemotherapy |
Non-pemetrexed- based doublet/triplet chemotherapy |
Hazard Ratio (95% CI) |
P | |
| n | 249 (86%) | 39 (14%) | ||
| Median duration of initial chemotherapy | 5.7 mo | 3.4 mo | 0.41 (0.29-0.59) | <0.0001 |
| Median overall survival | 16.0 mo | 14.2 mo | 0.82 (0.58-1.16) | 0.27 |
| B) TTF-1- patients: | ||||
| Pemetrexed- based doublet/triplet chemotherapy |
Non-pemetrexed- based doublet/triplet chemotherapy |
Hazard Ratio (95% CI) |
P | |
| n | 69 (86%) | 11 (14%) | ||
| Median duration of initial chemotherapy | 3.1 mo | 2.4 mo | 0.55 (0.29-1.06) | 0.072 |
| Median overall survival | 8.5 mo | 9.3 mo | 1.0 (0.53-1.9) | 0.97 |
4.0. Discussion
We examined a large set of consecutive patients diagnosed with stage IV lung adenocarcinomas to determine the prognostic and predictive impact of TTF-1. We found that the median survival of patients with TTF-1 positive tumors was twice as long as TTF-1 negative patients. The prognostic impact of TTF-1 was seen in nearly every subgroup examined, including those with KRAS mutations, those without targetable EGFR or ALK alterations, those treated with standard doublet/triplet initial chemotherapy, and those with either good or poor performance status. The lack of association between TTF-1 positivity and survival seen in patients with EGFR mutations or those who received targeted therapy may be a result of either the very small numbers of these patients in the TTF-1 negative group (of the 98 known EGFR mutant patients, only 6 were TTF-1 negative), which limits the power of this comparison, or may result from the impact of molecularly targeted therapies, which supersedes the prognostic impact of TTF-1 in these patients. The increased proportion of EGFR mutations in TTF-1 positive tumors compared to TTF-1 negative tumors seen in our series was expected given previous findings which detail the rarity of EGFR positive tumors which are also TTF-1 negative [23, 31-33]. The lack of association between TTF-1 positivity and survival seen in patients who received singlet initial chemotherapy is likely a result of small sample size (n=31) and poor overall health at the time this subgroup of patients began initial chemotherapy, which likely warranted this less aggressive treatment. The substantial improvement in overall survival which was consistent across nearly all subgroups was further emphasized in multivariate analysis of clinical and treatment features associated with survival. The prognostic impact of TTF-1 was similar to, or greater than, that of every variable examined including, remarkably, the receipt of doublet/triplet chemotherapy, targeted therapy, or good performance status. In sum, our data demonstrate that TTF-1 is a profound, independent prognostic variable in patients with advanced lung adenocarcinomas.
The prognostic impact of TTF-1 has been previously described in series of surgically resected lung cancers but appears to be underutilized, perhaps as a result of the relatively small sample size and lack of control for histology and stage in previous series [26, 32]. For example, in a series of 89 lung adenocarcinomas, Barletta et al. [26] found that TTF-1 expression was associated with improved survival, but histologic characteristics of these patients were not examined, and the impact of disease stage on the association between TTF-1 expression and overall survival was not considered. Similarly, Zhao et al. [31] reported a significant association between TTF-1 and survival, but only when combining stage III and stage IV cancers together, and there was no significant survival impact found in patients with stage I extent of disease. To circumvent these limitations, others have performed meta-analyses, which have generally favored a positive association between TTF-1 expression and survival, but inclusion of heterogeneous disease stages, histologies, and treatments have prevented routine application [25] [27]. More recently, Zhang et al. [23] examined the association between TTF-1 and survival in a large series of consecutive patients with stage I-III lung adenocarcinomas. Although only 43% of patients had survival follow-up for more than 12 months and receipt of adjuvant chemotherapy was not incorporated in multivariate survival analysis, an association between TTF-1 expression and survival was found (multivariate HR of TTF-1 negative vs positive 1.553, p=0.043) [30].
In addition to investigating the prognostic significance of TTF-1 expression, we also explored whether TTF-1 expression predicted improved benefit from pemetrexed-based chemotherapy in patients with lung adenocarcinomas. Sun et al. [33] reported longer progression-free survival and overall survival for pemetrexed-based chemotherapy were associated with TTF-1 positivity in nonsquamous NSCLCs. However, histology was not well characterized, and this study only included patients treated with pemetrexed. Grønberg et al. [34] did not observe an association between TTF-1 expression and response to pemetrexed-based chemotherapy, although the study included various subtypes of NSCLCs and did not investigate progression-free survival. In our series, there was a significantly longer duration of treatment with pemetrexed-based chemotherapy in both TTF-1 positive and TTF-1 negative patients compared to TTF-1 matched patients treated with other chemotherapies. As pemetrexed-based chemotherapy has an established benefit in patients with lung adenocarcinomas [9], our data favors the conclusion that TTF-1 expression does not predict a distinct benefit from pemetrexed chemotherapy.
Although our study describes TTF-1 expression in binary terms (present vs absent), greater granularity in the prognostic and predictive impact of TTF-1 in lung adenocarcinomas may be derived if the degree and distribution of expression had been quantified. In a small series of 89 patients, Barletta et al. [26] characterized TTF-1 expression that was high vs low vs absent, and found that patients with resected tumors expressing high or low TTF-1 had similar survival, which was improved compared to those that were TTF-1 absent. They also found a subset of patients to have copy number amplification of TTF-1 (NKX2-1) determined by fluorescence in situ hybridization. Patients with TTF-1 negative or TTF-1 amplified tumors had poorer survival than those who were TTF-1 immunohistochemistry high/low and TTF-1 not amplified.
The binary classification used here mirrors how TTF-1 is used as a routine diagnostic test when differentiating adenocarcinomas from squamous cell carcinomas of the lung, and thus permits generalizability and ease of use without the need for additional testing or reporting by pathologists. However, one of the limitations of this study is that TTF-1 expression was not assessed in all patients. We note that the clinical characteristics of patients with TTF-1 status unknown were largely similar to those of patients with known TTF-1 status, with the exception of age, which was slightly greater in TTF-1 unknown patients, and of the increased frequency of unknown genotype in TTF-1 unknown patients (Supplemental Table 1). Insufficient tissue availability may have limited the feasibility of TTF-1 and other molecular testing. Additionally, survival among patients with TTF-1 status unknown was intermediate to patients with TTF-1 positive and negative tumors (Supplemental Figure 3). Overall, these data suggest that patients with unknown TTF-1 status likely comprised a population similar to patients with known TTF-1 status, and that the conclusions of our series are not selectively skewed by those with missing TTF-1 data. The retrospective nature of this analysis and the large scale of this series did not permit performing formal RECIST reads to determine objective response to initial therapy and we have instead used duration of treatment as a surrogate for progression-free survival. We aimed to compensate, in part, for this retrospective analysis by examining a consecutive series of patients with advanced lung adenocarcinomas. Fortunately, the retrospective evaluation permitted long-term overall survival follow-up without the need for significant data censoring.
The identification of important clinicopathologic factors which impact survival have enabled clinicians to more accurately assess prognosis and stratify for risk in clinical trials. Controlling for these factors permits improved reliability, reproducibility, and interpretation of studies. Age, sex, histology, smoking history, and molecular status are often collected in clinical trial databases but only some of these measures have actual prognostic or predictive impacts. Our data emphasize the substantial prognostic impact of TTF-1 and propose that this data should be routinely captured and reported. Moving forward, these data will hopefully spur further research to examine the physiologic mechanism underlying the impact of TTF-1 in the biology of lung cancers and whether indeed there may be opportunity to precisely identify therapies for patients with TTF-1 expressing lung cancers.
Supplementary Material
Highlights:
TTF-1 positivity is associated with improved survival in lung adenocarcinomas.
The prognostic impact of TTF-1 exceeds nearly all other clinicopathologic features.
TTF-1 is not predictive of distinct benefit from pemetrexed chemotherapy.
We advocate that TTF-1 should be routinely tested and reported in clinical trials.
Acknowledgements:
Funding: This work was supported by the Memorial Sloan Kettering Cancer Center Support Grant/Core Grant (P30 CA008748).
Footnotes
Conflict of interest disclosure: None of the authors report a relevant potential conflict of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References:
- 1.Riely GJ and Yu HA, EGFR: The Paradigm of an Oncogene-Driven Lung Cancer. Clin Cancer Res, 2015. 21(10): p. 2221–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Shaw AT, et al. , Crizotinib versus chemotherapy in advanced ALK-positive lung cancer. N Engl J Med, 2013. 368(25): p. 2385–94. [DOI] [PubMed] [Google Scholar]
- 3.Kris MG, et al. , Targeting HER2 aberrations as actionable drivers in lung cancers: phase II trial of the pan-HER tyrosine kinase inhibitor dacomitinib in patients with HER2-mutant or amplified tumors. Ann Oncol, 2015. 26(7): p. 1421–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Paik PK, et al. , Response to MET inhibitors in patients with stage IV lung adenocarcinomas harboring MET mutations causing exon 14 skipping. Cancer Discov, 2015. 5(8): p. 842–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Planchard D, et al. , Dabrafenib in patients with BRAF-positive advanced non-small-cell lung cancer: a single-arm, multicentre, open-label, phase 2 trial. Lancet Oncol, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Drilon A, et al. , Response to Cabozantinib in patients with RET fusion-positive lung adenocarcinomas. Cancer Discov, 2013. 3(6): p. 630–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shaw AT, et al. , Crizotinib in ROS1-rearranged non-small-cell lung cancer. N Engl J Med, 2014. 371(21): p. 1963–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rizvi NA, et al. , Mutational landscape determines sensitivity to PD-1 blockade in non-small cell lung cancer. Science, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Scagliotti GV, et al. , Phase III study comparing cisplatin plus gemcitabine with cisplatin plus pemetrexed in chemotherapy-naive patients with advanced-stage non-small-cell lung cancer. J Clin Oncol, 2008. 26(21): p. 3543–51. [DOI] [PubMed] [Google Scholar]
- 10.Drilon A, et al. , Clinical outcomes with pemetrexed-based systemic therapies in RET-rearranged lung cancers. Ann Oncol, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chen YF, et al. , Efficacy of Pemetrexed-Based Chemotherapy in Patients with ROS1 Fusion-Positive Lung Adenocarcinoma Compared with in Patients Harboring Other Driver Mutations in East Asian Populations. J Thorac Oncol, 2016. [DOI] [PubMed] [Google Scholar]
- 12.Brunnstrom H, et al. , Immunohistochemistry in the differential diagnostics of primary lung cancer: an investigation within the Southern Swedish Lung Cancer Study. Am J Clin Pathol, 2013. 140(1): p. 37–46. [DOI] [PubMed] [Google Scholar]
- 13.Mukhopadhyay S and Katzenstein AL, Comparison of monoclonal napsin A, polyclonal napsin A, and TTF-1 for determining lung origin in metastatic adenocarcinomas. Am J Clin Pathol, 2012. 138(5): p. 703–11. [DOI] [PubMed] [Google Scholar]
- 14.Folpe AL, et al. , Thyroid transcription factor-1: immunohistochemical evaluation in pulmonary neuroendocrine tumors. Mod Pathol, 1999. 12(1): p. 5–8. [PubMed] [Google Scholar]
- 15.Rekhtman N, et al. , Immunohistochemical algorithm for differentiation of lung adenocarcinoma and squamous cell carcinoma based on large series of whole-tissue sections with validation in small specimens. Mod Pathol, 2011. 24(10): p. 1348–59. [DOI] [PubMed] [Google Scholar]
- 16.Boggaram V, Thyroid transcription factor-1 (TTF-1/Nkx2.1/TITF1) gene regulation in the lung. Clinical Science, 2009. 116(1-2): p. 27–35. [DOI] [PubMed] [Google Scholar]
- 17.Weir BA, et al. , Characterizing the cancer genome in lung adenocarcinoma. Nature, 2007. 450(7171): p. 893–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Maeda Y, et al. , Kras(G12D) and Nkx2-1 haploinsufficiency induce mucinous adenocarcinoma of the lung. J Clin Invest, 2012. 122(12): p. 4388–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yamaguchi T, et al. , NKX2-1/TITF1/TTF-1-Induced ROR1 is required to sustain EGFR survival signaling in lung adenocarcinoma. Cancer Cell, 2012. 21(3): p. 348–61. [DOI] [PubMed] [Google Scholar]
- 20.Saito RA, et al. , Thyroid transcription factor-1 inhibits transforming growth factor-beta-mediated epithelial-to-mesenchymal transition in lung adenocarcinoma cells. Cancer Res, 2009. 69(7): p. 2783–91. [DOI] [PubMed] [Google Scholar]
- 21.Winslow MM, et al. , Suppression of lung adenocarcinoma progression by Nkx2-1. Nature, 2011. 473(7345): p. 101–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yamaguchi T, et al. , NKX2-1/TTF-1: an enigmatic oncogene that functions as a double-edged sword for cancer cell survival and progression. Cancer Cell, 2013. 23(6): p. 718–23. [DOI] [PubMed] [Google Scholar]
- 23.Zhang Y, et al. , Negative Thyroid Transcription Factor 1 Expression Defines an Unfavorable Subgroup of Lung Adenocarcinomas. J Thorac Oncol, 2015. 10(10): p. 1444–50. [DOI] [PubMed] [Google Scholar]
- 24.Winslow MM, et al. , Suppression of lung adenocarcinoma progression by Nkx2-1. Nature, 2011. 473(7345): p. 101–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Berghmans T, et al. , Thyroid transcription factor 1--a new prognostic factor in lung cancer: a meta-analysis. Ann Oncol, 2006. 17(11): p. 1673–6. [DOI] [PubMed] [Google Scholar]
- 26.Barletta JA, et al. , Clinical significance of TTF-1 protein expression and TTF-1 gene amplification in lung adenocarcinoma. Modern Pathology, 2008. 21: p. 335a–335a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Qian HH, et al. , Prognostic value of TTF-1 expression in patients with non-small cell lung cancer: A meta-analysis. Clin Chim Acta, 2015. 451(Pt B): p. 208–14. [DOI] [PubMed] [Google Scholar]
- 28.Barlesi F, et al. , Positive thyroid transcription factor 1 staining strongly correlates with survival of patients with adenocarcinoma of the lung. Br J Cancer, 2005. 93(4): p. 450–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Anagnostou VK, et al. , Thyroid transcription factor 1 is an independent prognostic factor for patients with stage I lung adenocarcinoma. J Clin Oncol, 2009. 27(2): p. 271–8. [DOI] [PubMed] [Google Scholar]
- 30.Kadota K, et al. , Thyroid transcription factor-1 expression is an independent predictor of recurrence and correlates with the IASLC/ATS/ERS histologic classification in patients with stage I lung adenocarcinoma. Cancer, 2013. 119(5): p. 931–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhao Q, et al. , Thyroid transcription factor-1 expression is significantly associated with mutations in exon 21 of the epidermal growth factor receptor gene in Chinese patients with lung adenocarcinoma. OncoTargets and therapy, 2015. 8: p. 2469–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Xu XY, et al. , Analysis of clinical characteristics and differential diagnosis of the lung biopsy specimens in 99 adenocarcinoma cases and 111 squamous cell carcinoma cases: utility of an immunohistochemical panel containing CK5/6, CK34betaE12, p63, CK7 and TTF-1. Pathol Res Pract, 2014. 210(10): p. 680–5. [DOI] [PubMed] [Google Scholar]
- 33.Sun JM, et al. , Significance of thymidylate synthase and thyroid transcription factor 1 expression in patients with nonsquamous non-small cell lung cancer treated with pemetrexed-based chemotherapy. J Thorac Oncol, 2011. 6(8): p. 1392–9. [DOI] [PubMed] [Google Scholar]
- 34.Gronberg BH, et al. , Associations between TS, TTF-1, FR-alpha, FPGS, and overall survival in patients with advanced non-small-cell lung cancer receiving pemetrexed plus carboplatin or gemcitabine plus carboplatin as first-line chemotherapy. J Thorac Oncol, 2013. 8(10): p. 1255–64. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.