Abstract
TALE has always had potential as a gene-editing and regulatory tool. However, with the advent of CRISPR/Cas9, an easier to use tool with the same function, TALE has recently been abandoned because of the time-consuming and low-efficiency process required for its construction. The off-target activity of CRISPR/Cas9 has been a challenge to its in vivo application. By contrast, TALE has been applied in vivo for gene editing and therapy because of its high targeting capability. To overcome the key limitation of the TALE technique, we developed a high-efficiency method for constructing custom TALEs. We created 62 new monomers and developed a new pipeline that enabled assembly of custom TALEs in just 1 day. We verified the new method by assembling nine TALEs targeting the promoters of two transcription factor genes: HNF4α and E47. The expression of the two endogenous genes in two cancer cells, HepG2 and PANC1, was activated by the constructed TALEs, which promoted differentiation of the two cancer cells. Using the new method, custom TALEs can be generated as easily and rapidly as CRISPR, thus promoting the wide application of TALE-based techniques.
Keywords: TALE, construction, rapid, one day
Introduction
Transcription activator-like effectors (TALEs) are type III effector proteins from plant-pathogenic bacteria of the genus Xanthomonas. TALE is a programmable DNA-binding domain that can theoretically target any sequence. All TALEs are composed of an N-terminal translocation domain, a C-terminal nuclear localization signal (NLS) with an acidic transcriptional activation domain, and a central tandem repeat DNA binding domain (DBD).1 The TALE DBD contains tandem repeats of 34 amino acid sequences (termed monomers) that are required for DNA recognition and binding.2, 3, 4 The naturally occurring TALEs have been found to have a variable number of monomers, ranging from 1.5 to 33.5.1 Although the sequence of each monomer is highly conserved, they differ primarily in two positions (the 12th and 13th) named as repeat variable diresidues (RVDs). Recent reports have found that the identity of these two residues determines the nucleotide binding specificity of each TALE repeat in a simple cipher that specifies the target base of each RVD (NI = A, HD = C, NG = T, NN = G).5, 6 Thus, each monomer targets one nucleotide, and the linear sequence of monomers in a TALE specifies the target DNA sequence in the 5′-to-3′ orientation.
According to these TALE-DNA molecular codes, biologists have assembled a TALE DBD and fused it to other functional protein domains to manipulate the genome, including gene regulation7, 8, 9, 10 and gene editing.10, 11, 12, 13, 14, 15, 16, 17 The TALE system, including TALE and TALE nuclease (TALEN), thus became powerful tools for genome operations. Especially, TALEN has been a leading technology for biomedical treatment. The TALE system has high targeting capability. For instance, in a study in which an iPS cell line was edited with TALEN, no mutagenic activity was detected at other genome sites homologous to the target site.18 Due to its high targeting effect, TALEN has been being employed to produce universal chimeric antigen receptor T (UCAR-T) cells by Cellectis19 and has been approved by the United States Food and Drug Administration (FDA) to be used in clinical cancer immunotherapy. The first patients to receive TALEN genetically engineered products have shown significant benefits.19 These patients, treated in 2015, are still completely free of cancer to this day.20
With the advent of CRISPR in 2013, CRISPR/Cas9 rapidly became the most widely used genetic manipulation technique because of its simplicity. Since then, the TALE system has been almost completely replaced by the CRISPR/Cas9 system. As compared with the CRISPR/Cas9 system, a key limitation of the TALE system is its cumbersome and tedious vector construction process, a time-consuming (about 1 week) procedure, even for skilled experimenters. To address the problem, many vector assembly methods have been developed, including those derived from Golden Gate cloning,11 regular cloning methods,21 and high-throughput methods.22 Because of the repetitive nature of TALEs, construction of the DNA-binding monomers is troublesome. Many groups used a hierarchical ligation strategy to overcome the difficulty of assembling monomers into ordered multimer arrays, taking advantage of degeneracy in codons surrounding the monomer junction and type II restriction enzymes.9, 10, 11, 12, 23, 24 However, these strategies are still limited by their relative low efficiency in constructing TALE expression vectors. Therefore, new TALE preparation methods as simple and rapid as CRISPR/Cas9 system are still in demand.
Currently, the most widely used TALE assembly kit is the Golden Gate TALEN and TAL Effector Kit 2.0 (Addgene). The monomers of this kit are plasmids contained in E. coli. In order to construct a custom TALE, the bacteria have to be amplified in culture, and various plasmids have to be purified. The purified plasmids are used to construct a custom TALE by using a Golden Gate digestion-ligation process that takes as long as 5 days (Figure S1). Moreover, the whole TALE assembly process has to undergo three bacterial transfections and colony selection and cultivation, which are all rate-limiting steps. Moreover, the efficiency of the digestion-ligation reaction with plasmids is low because a limited number of monomer molecules can be contained in a digestion-ligation reaction. In plasmid monomers, a large amount of plasmid backbone DNA is useless to TALE assembly, which prevents more effective monomer molecules from being added to the digestion-ligation reaction. In addition, the cutting efficiency of plasmid DNAs is lower than linear DNAs because of the compact supercoiled structure.25
Using the Addgene 2.0 assembly kit, we developed a new monomer library and developed a pipeline for rapidly assembly of custom TALEs that can constantly bind 18-bp targets. The new monomers can be used to assemble TALEs by the Golden Gate method.26, 27, 28 The monomer library consists of 60 base-determinant monomers and 2 novel linker monomers. All monomers are net linear dsDNA fragment ended with two constant sequences. All monomers can be easily produced and reproduced by high-fidelity PCR amplification in a 96-well PCR plate, using a pair of universal primers. To construct TALEs with these monomers, we designed a rapid assembly pipeline that can finish a custom TALE assembly in just 1 day. To verify new monomers and TALE assembly pipeline, we constructed nine TALEs targeting the promoters of two transcription factor genes: HNF4α and E47. The constructed TALEs could activate the expression of both exogenous reporter genes and endogenous genes. Moreover, the activation of endogenous HNF4α and E47 genes in HepG2 and PANC1 led to the differentiation of these cancer cells into normal liver or pancreas cells. Therefore, the monomer library and TALE assembly pipeline developed in this study can promote the wide application of TALE-based techniques.
Results
Preparation of TALE Monomer and Backbone
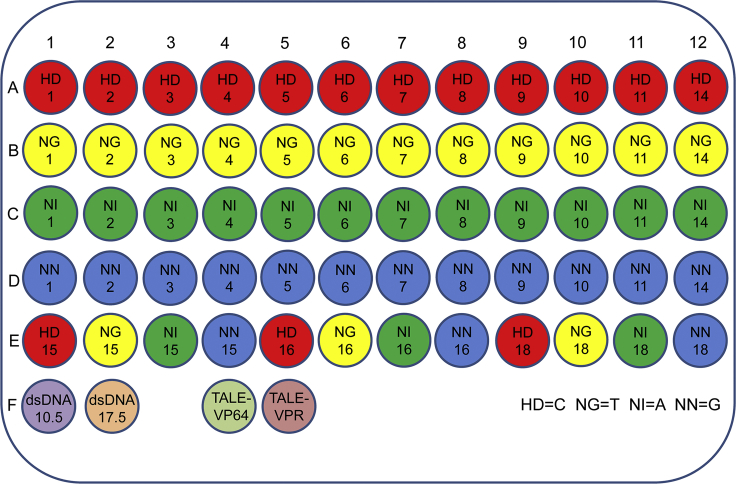
To overcome the limitations of the Addgene kit 2.0, we aimed to develop a new TALE construction strategy with plasmid-free monomers and a bacteria-free assembly pipeline. To this end, a set of PCR primers (Table S1) was designed, and 60 linear base-determinant monomers (Figure 1) were amplified from Addgene plasmid monomers (Figure S1A). Importantly, all PCR-produced linear monomers could then be reproduced by a pair of universal primers (Table S1) in a 96-well PCR plate (Figure 2). To eliminate intermediate bacteria transformations and receptor plasmids (pFUSA, pFUSB, and pLR) used in TALE assembly with the Addgene kit 2.0 (Figure S1), this study created two linking monomers, dsDNA10.5 and dsDNA17.5 (Figure 1), which could also be produced by PCR amplification, using universal primers in a 96-well PCR plate (Table S2). The linker monomer dsDNA10.5 was used to link monomers 10 and 11, and the linker monomer dsDNA17.5 was used to link monomers 17 and 18. In addition, the two linker monomers provided partial coding sequences that were originally harbored by pFUSA and pFUSB. Once the monomer 96-well PCR plate (Figure 1) was constructed, it could be kept for constructing any TALEs that could bind various 18-bp targets. The monomer plate (Figure 1) can be easily replicated by 96-well high-fidelity PCR amplification. The quality of replicates was easily checked by direct sequencing.
Figure 1.
Schematic of Monomers in a 96-Well PCR Plate
Each monomer was prepared by PCR amplification by using monomer plasmids included in an Addgene kit as a template and different primers designed to amplify variant monomers (Table S1). In total, there were 60 base-determinant monomers and 2 linker monomers (dsDNA10.5 and dsDNA17.5). The position of each monomer is indicated in the well. The monomer plate can be easily regenerated by 96-well PCR amplification. This plate also contains two TALE backbone vectors, TALE-VP64 and TALE-VPR, which can be produced by E. coli DH5α transformation and extraction and added to the wells.
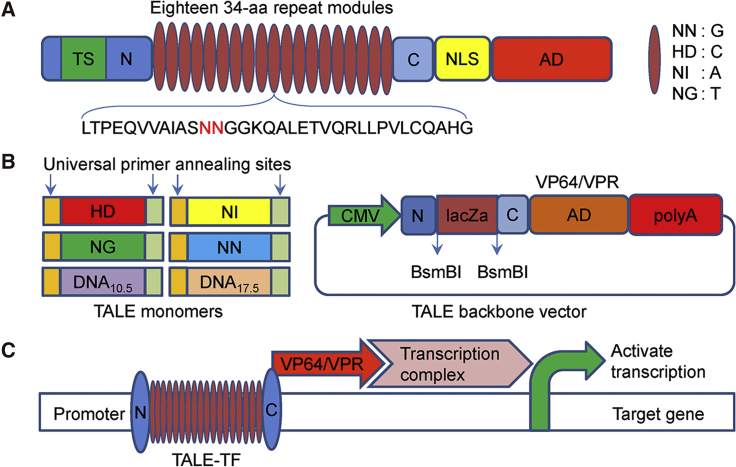
Figure 2.
Schematics of TALE Structure, Monomers, and TALE Backbone Vector for Assembling Custom TALEs
(A) Natural structure of TALEs derived from Xanthomonas sp. Each DNA binding module consists of 34 amino acids, where the repeat variable diresidues (RVDs) in the 12th and 13th amino acid positions of each repeat specify the DNA bases being targeted according to the cipher NG = T, HD = C, NI = A, and NN = G. (B) Monomers and TALE backbones for constructing custom TALEs. Each monomer ends with two constant sequences that provide the annealing sites of a pair of universal primers that can be used to regenerate the monomer library. CMV, cytomegalovirus promoter; N, non-repetitive N-terminus of TALE; C, non-repetitive C-terminus of TALE; BsmBI, type II restriction sites used for the insertion of a custom TALE DNA binding domain; LacZa, LacZa expression cassette for blue-white spot screening; NLS, nuclear localization signal; VP64, synthetic transcriptional activator derived from VP16 protein of herpes simplex virus; VPR, fusion of VP64, p65/RelA, and Rta. (C) Schematic of activating gene expression with TALEs. TALE-TF, TALE transcription factor.
To produce the final functional TALEs, we also constructed two TALE backbones, pTALE-VP64 and pTALE-VPR (fusion of VP64, p65/RelA, and Rta), which still harbored a LacZ expression cassette for easily screening the final positive TALE colonies with blue-white spot screening (Figure 2). In the construction of TALE backbones, the NLS and acidic transcription activation domain of wild-type hax3 in the wild-type TALE protein were replaced by a mammalian NLS derived from the simian virus 40 large T antigen and the synthetic transcription activation domain VP64 or VPR (VP64-p65-RTA) (Figure 2B), respectively. To enhance the expression of the final TALE in mammalian cells, the TALE backbones contained a strong cytomegalovirus (CMV) promoter (Figure 2).
Pipeline of TALE Construction
With the newly designed and produced monomers (Figures 1 and 2) and TALE backbones (Figure 2), a new pipeline was designed to construct a custom TALE in 1 day (Figure 3). This pipeline enables construction of a custom 18-bp TALE in two steps. First, four circular pentamers are prepared by Golden-Gate digestion-ligation reactions using new linear monomers, BsaI, and T4 DNA ligase (Figure 3). Second, the final TALE expression plasmid is prepared by a Golden-Gate digestion-ligation reaction using four pentamers, a TALE-backbone vector (TALE-VP64/VPR), BsmBI, and T4 DNA ligase. The final TALE plasmid can be obtained by one bacteria transformation, colony PCR identification, and plasmid extraction. The positive final TALE plasmid is further rapidly confirmed by EcoRI digestion identification. In comparison with the pipeline of the Addgene kit 2.0 (Figure S1B), two time-consuming bacteria transformations, overnight cultivation, and colony selections were removed. Moreover, we found that it was difficult to get white-positive colonies in these two steps when preparing TALEs with the Addgene kit. It was thus concluded that these two steps of the kit were error prone, which may be related to adding too many monomers in a Golden-Gate digestion-ligation reaction. For example, in the first two Golden-Gate digestion-ligation reactions, 10 and 7 monomers were added, respectively (Figure S1B). In addition to two receptor plasmids (pFUSA and pFUSB7), as many as 11 and 8 plasmids have to be included in two Golden-Gate digestion-ligation reactions. In contrast, a new pipeline was designed to contain only five short (about 100 bp) linear monomers in each of the first Golden-Gate digestion-ligation reactions, which is beneficial for enhancing digestion-ligation efficiency and fidelity.
Figure 3.
Pipeline for Constructing a TALE with New Monomers
Steps for the construction of a TALE are outlined. Time spent on each step is given. It can be seen that the whole TALE assembly procedure can be finished in 1 day. After a colony PCR screening, plasmid extraction, and EcoRI digestion confirmation, the usable final TALE can be obtained on the second day. The time taken by the process is identical to that taken by the current CRISPR.
Construction of Custom TALEs
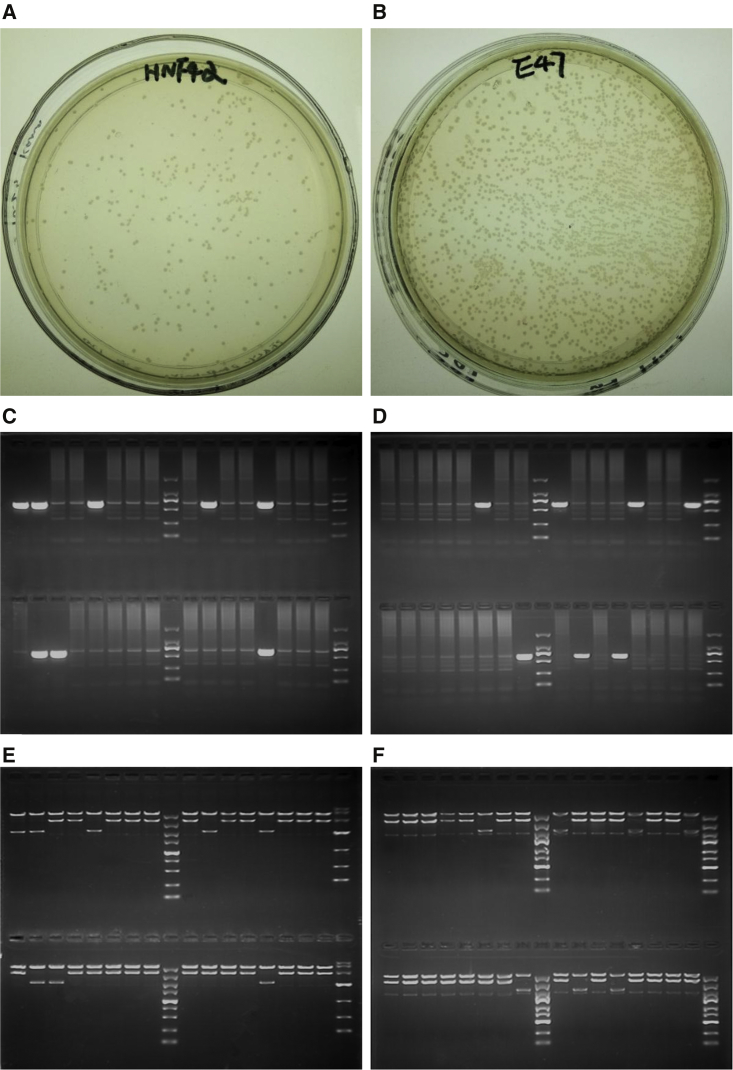
To verify the newly produced monomers and TALE assembly pipeline, we assembled nine TALEs targeting promoters of human HNF4α and E47 by following the assembly steps described in Materials and Methods. The assembled TALEs were named HNF4α-TALE1/2/3-VP64, HNF4α-TALE1/2/3-VPR, and E47-TALE1/2/3-VPR. E. coli was transformed by the final TALEs and cultivated on solid agar. The results showed that many white spots were produced (Figures 4A and 4B). No blue spots were seen on the agar plate. The colony PCR detection of randomly picked colonies confirmed that the insert size was correct, and typically over 80% of colonies were bona fide positive colonies (Figures 4C and 4D; Figure S2). These data indicate the high efficiency of constructing custom TALEs with new monomers (Figure 1), backbone vectors (Figure 2), and pipeline (Figure 3). The subsequent EcoRI digestion of extracted plasmids further confirmed the results of colony PCR, which indicated that typically 80% of white spots were correctly and successfully assembled in the final TALEs (Figures 4E and 4F). We also assembled another TALE, HNF4α-TALE-VP64, targeted to the same site as HNF4α-TALE-VPR, in order to compare the activity of two transactivation domains: VP64 and VPR.
Figure 4.
Constructing Custom TALEs with New Monomers and Pipeline
(A and B) E. coli colonies on solid agar that were transformed with HNF4α-TALE1-VPR (A) and E47-TALE1-VPR (B). (C and D) Colony PCR detection of four groups of randomly selected white spots. HNF4α-TALE1-VPR (C) and E47-TALE1-VPR (D) groups contained eight colonies. Full-length PCR products should be 2,051 bp long. However, PCR products were often less prominent, whereas the “ladder effect” represented a robust indicator of successful assembly. (E and F) Colony identification by EcoRI digestion. All colonies picked from HNF4α-TALE1-VPR (E) and E47-TALE1-VPR (F) were cultivated for extracting plasmid. The extracted plasmids were digested with EcoRI, which cut out the full-length TALE of 3,537 bp. The incorrectly assembled TALEs produced a 2,143-bp band in EcoRI digestion. It can be seen that the identification results of colony PCR were in agreement with those of EcoRI digestion.
Activation of Exogenous Gene with TALEs
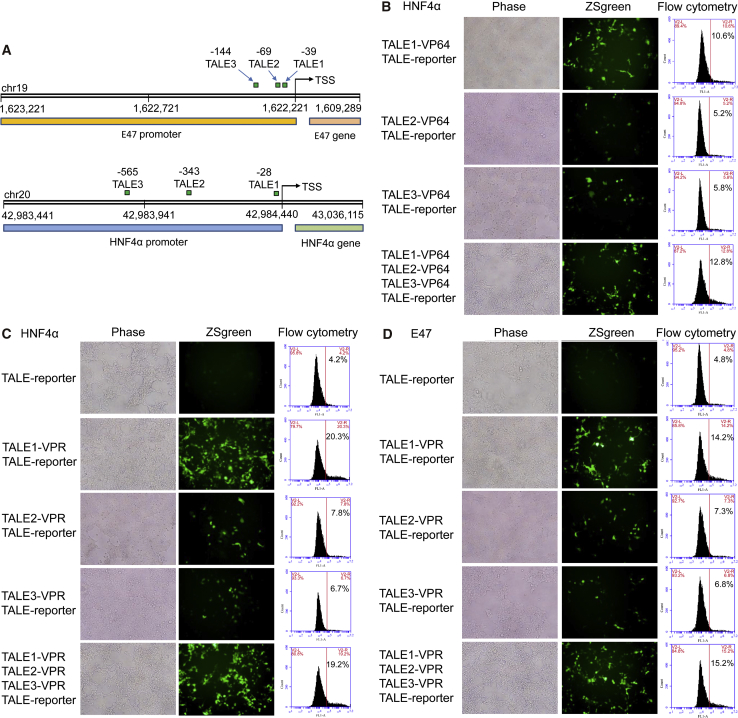
To check if the constructed TALEs would function in mammalian cells, a fluorescence reporter construct was prepared by cloning the HNF4α/E47 promoter that contained the TALE-binding sites and a minimal CMV promoter upstream of a reporter gene, ZsGreen (HNF4α/E47-TALE reporter). Three TALEs targeting three different sites in the HNF4α and E47 promoters were assembled (Figure 5A). The 293T cells were co-transfected by an assembled TALE and corresponding TALE reporters. The results revealed that the expression of corresponding TALE reporters was activated by all TALEs (Figures 5B–5D); however, the activation level was not the same (Figures 5B–5D). It was revealed that the TALEs targeting the sites near the transcription start sites (TSSs) showed the highest transcriptional activation activity (HNF4α-TALE1-VP64/VPR and E47-TALE1-VPR; Figures 5B–5D). In comparison, the TALE-VPR activated much higher expression of ZsGreen than did TALE-VP64 (Figures 5B and 5C). In contrast, the control transfection did not activate reporter gene expression (Figure S3). These data indicate that the TALEs constructed with new monomers, backbone vectors, and pipeline were functional.
Figure 5.
Activating Exogenous Reporter Gene with TALEs
293T cells were co-transfected with a TALE-VPR/VP64 plasmid and its corresponding reporter plasmid expressing ZsGreen. (A) Positions of TALE targets in the promoters of HNF4α and E47 genes. (B) Cells cotransfected HNF4α-TALE1/2/3-VP64 and HNF4α-TALE-reporter. (C) Cells cotransfected with HNF4α-TALE1/2/3-VPR and HNF4α-TALE-reporter. (D) Cells cotransfected with E47-TALE1/2/3-VPR and E47-TALE-reporter.
Activation of Endogenous Gene with TALEs
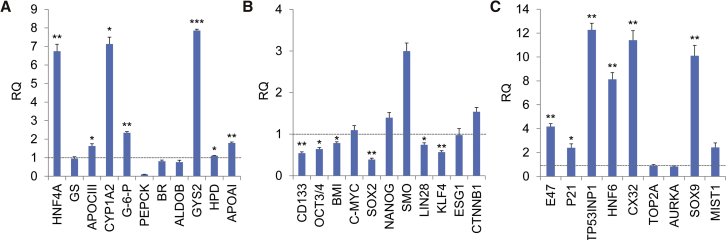
To find whether the constructed TALEs could activate the endogenous gene expression in mammalian cells, we first transfected HepG2 cells with HNF4α-TALE-VPR. The qPCR detection of HNF4α expression revealed that its expression was highly activated by HNF4α-TALE-VPR (Figure 6A). In addition, the expression of several characteristic hepatocyte markers was induced, including APOCIII, CYP1Α2, G-6-P, GYS2, APOAI, and HPD (Figure 6A). At the same time, the expression of multiple stemness genes was significantly downregulated, including CD133, OCT3/4, BMI, SOX2, KLF4, and LIN28 (Figure 6B). Therefore, activation of endogenous HNF4α gene by the constructed TALE-VPR induced differentiation of hepatoma cells.
Figure 6.
Activating Endogenous Genes with TALEs
HepG2 and PANC1 cells were transfected with HNF4α/E47-TALE1-VPR. (A) Expression of HNF4α and characteristic hepatocyte markers in HepG2 cells. (B) Expression of “stemness” genes in HepG2 cells. (C) Expression of E47, cell cycle arrest-related genes (p21 and TP53INP1), and E47 target genes (HNF6, SOX9, CX32, and MIST1) in PANC1 cells. Data analysis was performed with the Applied Biosystems StepOne software v2.3, and Ct values of detected genes were normalized with that of β-actin. The relative expression level of target mRNAs was calculated as relative quantity (RQ) according to the equation: RQ = 2−ΔΔCt. Student’s t test was used to check statistical significance. *p < 0.05, **p < 0.01, ***p < 0.001. The dash lines refer to RQ values of negative controls.
Similarly, the PANC1 cells were transfected with E47-TALE-VPR. The qPCR detection of E47 expression revealed that its expression was highly activated by E47-TALE-VPR (Figure 6C). Moreover, the expression of the cyclin-dependent kinase inhibitor p21 and the stress response protein TP53INP1, which play critical roles in cell-cycle arrest, was significantly upregulated. E47 expression also induced upregulation of the gap junction protein connexin 32 (CX32) and the ductal genes SOX9 and HNF6 in PANC1 cells. Meanwhile, E47 induced MIST1, which can regulate CX32 expression in PANC1 cells. E47 also downregulated the expression of the pancreatic ductal adenocarcinoma-associated cell cycle activators topoisomerase2A (Top2A) and aurora kinase A (AURKA).
Differentiation of Cancer Cells with TALEs
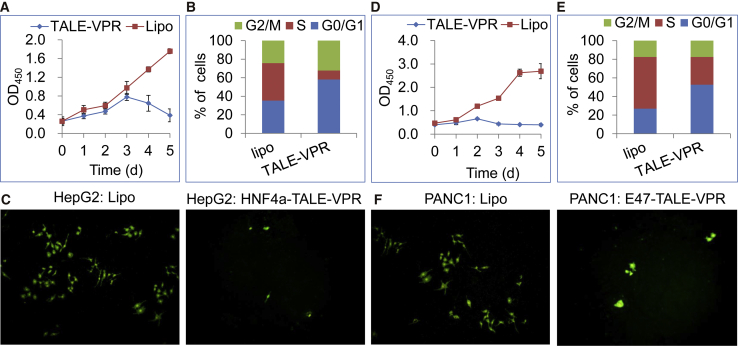
To explore whether the TALE-activated gene expression would result in phenotypic changes of the cells, cell viability, cycle, and migration were determined. The dynamic detection of cell viability revealed that HNF4α-TALE-VPR transfection reduced the cell viability of HepG2 cells on the third day after transfection (Figure 7A). Remarkably, on the fifth day after transfection, cell viability reduced more than 50%. The measurement of the cell cycle indicated that HNF4α-TALE-VPR transfection resulted in significant cell cycle arrest in the HepG2 cells (Figure 7B). On the third day after infection, only 9.66% of HNF4α-TALE-VPR-transfected HepG2 cells were in the S phase, whereas as many as 40.36% of the control cells were in the S phase (Figure 7B; Figure S4). The cell migration assay revealed that HNF4α-TALE-VPR transfection led to significant inhibition of HepG2 cell migration (Figure 7C. These phenotypic changes suggest that HNF4α-TALE-VPR-activated HNF4α expression induced differentiation of HepG2 cells into normal hepatocytes, which is in agreement with the significant upregulation of characteristic hepatocyte markers (Figure 6A) and the significant downregulation of stemness genes (Figure 6B).
Figure 7.
Assays of Cell Viability, Cycle, and Migration
HepG2 and PANC1 cells were transfected with HNF4α/E47-TALE1-VPR. (A) HepG2 cell viability. (B) HepG2 cell cycle. (C) HepG2 cell migration. (D) PANC1 cell viability. (E) PANC1 cell cycle. (F) PANC1 cell migration. CCK-8 was used to determine the viability of cells at different time points. Flow cytometry was used to detect the cell cycle. The percentage of cells in individual cell cycle phases was determined. A Transwell assay was used to detect cell migration.
The cell viability, cycle, and migration of PANC1 cells were also examined. Similarly, the results indicated that E47-TALE-VPR transfection resulted in significant reduction of cell viability (Figure 7C), cycle proliferation (Figure 7D; Figure S3), and migration (Figure 7E) in the PANC1 cells. These phenotypic changes were in agreement with the significant upregulation of P21, TP53INP1, and MIST1 genes, indicating that E47 reprogrammed aggressive PANC1 cells to a quiescent acinar state by restoring the expression of P21, TP53INP1, and MIST1. Mist1 is a key bHLH transcription factor that controls the acinar maturation program.
Activation of Edited Endogenous Genes with TALEs
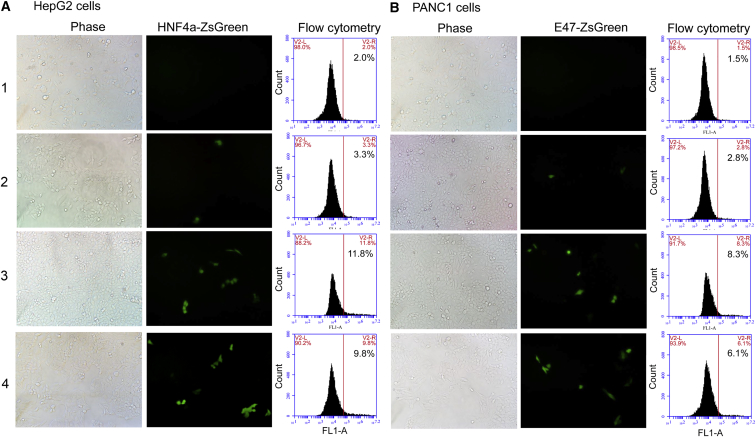
To further characterize the activation of endogenous genes at the protein level by TALE-TF, four TALEN vectors (Table S5) were constructed with a new method for fusing the ZsGreen gene to endogenous genes by homology-directed repair (HDR). The HNF4a and E47 genes in the HepG2 and PANC1 cells were edited by co-transfecting cells with a pair of TALEN vectors and a homologous donor, respectively (Figure 8). The edited HNF4a and E47 genes were respectively activated by transfecting the edited HepG2 and PANC1 cells with HNF4α-TALE1-VPR and E47-TALE1-VPR. The results indicate that the expression of HNF4α-ZsGreen protein was significantly activated by HNF4α-TALE1-VPR in the edited HepG2 cells (Figure 8A). Similarly, the expression of E47-ZsGreen protein was significantly activated by E47-TALE1-VPR in the edited PANC1 cells (Figure 8B). To edit and activate endogenous genes simultaneously, HepG2 and PANC1 cells were also respectively co-transfected with a pair of TALEN vectors: a homologous donor and TALE1-VPR. Similar gene activation was observed in two cells (Figure 8).
Figure 8.
Activating Exogenous Reporter Gene with TALEs
(A) Editing and activating the endogenous HNF4α gene in HepG2 cells. (B) Editing and activating the endogenous E47 gene in PANC1 cells. (1) Cells transfected only by Lipofectamine as the control. (2) HepG2/PANC1 cells co-transfected by HNF4α/E47-TALEN and HOM-HNF4α/E47-ZsGreen for editing HNF4α/E47 gene to form fused HNF4α/E47-ZsGreen gene in genome. (3) Edited HepG2/PANC1 cells transfected by HNF4α/E47-TALE1-VPR for activating the fused HNF4α/E47-ZsGreen gene in genome. (4) HepG2/PANC1 cells co-transfected by HNF4a/E47-TALEN, HOM-HNF4α/E47-ZsGreen and HNF4α/E47-TALE1-VPR.
Discussion
The TALE system has been a promising gene operation tool that has high DNA-binding specificity. However, the low-efficiency and time-consuming vector construction process has always prevented its wide application. For overcoming this limitation, we greatly improved both monomers and the assembly pipeline of the Addgene kit. First, we made a new monomer library that consisted of 60 base-determinant monomers and 2 linker monomers. All base-determinant monomers were prepared by PCR amplification by using Addgene kit plasmid monomers as templates. Two linker monomers were chemically synthesized. Importantly, all new monomers ended in two constant sequences and could be amplified by high-fidelity PCR using a pair of universal primers. One advantage of this plasmid-free monomer library is that it can be easily reproduced by high-fidelity PCR amplification in a 96-well PCR plate in a high-throughput format, eliminating the need to produce a plasmid monomer library by time-consuming bacteria cultivation and plasmid DNA extraction with the Addgene kit. Another advantage is that the linear monomers have no redundant plasmid sequences and thus allow more monomer molecules to be included in the first digestion-ligation reaction, which is beneficial for forming more circular pentamers in high efficiency. Second, this study made a new pipeline for assembling custom TALEs using new monomers. Following this pipeline, custom TALEs can be rapidly and easily assembled in just one day. In fact, with the off-the-shelf monomer plate, starting from a given target sequence, TALE preparation can be finished in as few as 2 days. These improvements should promote new wide application of TALE.
A limitation of the TALE assembly method developed in this study is that it can only be used to assemble TALEs with 18-bp binding sites. This study focused on TALEs of this length because TALEs were typically built with 18 repeats of 34 amino acids. With the constant T base, TALEs constructed by the new method can bind 19-bp target sequences in genomes. This is a frequently-used length of TALEs that carries high specificity and low cost. However, with the same strategy as was used to make linear monomers in this study, other monomers can be similarly manufactured for preparing longer TALEs if needed.
The gene transcriptional activation level is related to transactivation domains and TALE-targeting position. We used two different transcriptional activation domains: VP64 and VPR.9, 29 The results indicate that VPR always had higher transcriptional activation activity than VP64, in agreement with results obtained with CRISPR.29 We therefore activated endogenous genes with TALE-VPR. In the future, more potent activation systems such as Suntag can be tried for use in TALEs.29 To knock down gene expression, the transcriptional repression domain (RD) such as the Krüppel-associated box transcriptional repression domain (KRAB) can be fused to the TALE.30 In addition, other epigenetic functional domains, such as DNMT3a (DNA methyltransferase), EZH2 (histone 3 lysine 27 methyltransferase), and LSD1 (histone demethylase) can be fused to the TALE for making variant gene regulators (GRs).31 These GRs have wide application in basic research and biomedicine. The relative position of TALE targets to the TSS has a significant effect on TALE activation effect. The TALE-VPR regulation to endogenous genes is also dependent on the chromatin state.32, 33 For these reasons, this study typically built several TALE-VPRs for each target gene. It was found that well-functioning TALEs were always positioned in the proximal promoter region.
In this study, we selected HNF4α and E47 as target genes to activate using the constructed TALEs because it has been reported that activation of these two transcription factors leads to differentiation of the cancer cells HepG2 and PANC1.34, 35 To see the functional results of the constructed TALEs, we transfected HepG2 and PANC1 cells with HNF4α-TALE-VPR and E47-TALE-VPR, respectively. We found that the two genes were significantly activated by the transfected TALE-VPRs. Correspondingly, the expression of some target genes regulated by these two transcription factors was also regulated, indicating the regulatory functions of these two activated transcription factors. Measurements of cell viability, cycle, and migration revealed that the TALEs-transfected cells underwent significant phenotypic changes, including cycle arrest and reduced viability and migration. HNF4α is a central regulator of the differentiated hepatocyte phenotype, and forced re-expression of HNF4α was sufficient to overcome repression of the hepatic phenotype in dedifferentiated hepatoma cells.35 TALE-VPR targeting the HNF4α promoter significantly upregulated HNF4α expression in HepG2 cells, which led to the significant upregulation of hepatocyte marker genes and downregulation of stemness genes. For example, CD133 is currently thought to serve as a target for identifying cancer stem or progenitor cells in a portion of hepatocellular carcinoma (HCC).36, 37, 38 Upregulation of HNF4α expression induces downregulation of CD133 and some genes that are highly expressed in human embryonic stem cells and are involved in the establishment or maintenance of pluripotency,38, 39, 40 including OCT3/4, BMI, SOX2, KLF4, LIN28, and ESG1. The current study revealed that E47 regulated its target genes, such as p21 and TP53INP1 in PANC1 cells,41, 42 in agreement with a previous report that E47 upregulated expression of p21 and TP53INP1 genes in all tested PANC1 cell lines associated with G0/G1 arrest.34 These data suggest that the TALE-VPRs targeting HNF4α and E47 promoters have a potential clinical application as gene therapy reagents.
Conclusions
To overcome key technical limitations of TALE application, we developed a fast and efficient strategy for preparing TALE expression plasmids in just 1 day. We manufactured a new set of linear monomers that could be easily reproduced by high-fidelity PCR amplification in a 96-well PCR plate in a high-throughput format. With new monomers, any 18-bp TALE expression plasmids could be rapidly assembled using a newly designed pipeline. This method can promote wide application of TALE-based tools.
Materials and Methods
Materials and Reagents
The chemical and biochemical reagents used in this study and their manufacturers are listed in detail in the Supplemental Information.
Preparation of Plasmids
The VP64 and VPR fragments were amplified by PCR from pcDNA-dCas9-VP64 and pcDNA-dCas9-VPR, using PrimeSTAR HS DNA Polymerase (Takara Bio), according to the manufacturer’s instructions, in which the EcoRI and NotI sites were introduced at the two ends. The PCR primers were VP64-F/R and VPR-F/R (Table S3). The fragments were ligated into pPIRES2-EGFP to produce pPIE-VP64 and pPIE-VPR. The TAL(LacZa) fragment was recovered from pTAL2 (Addgene kit) with EcoRI, which was ligated into pPIE-VP64 and pPIE-VPR to produce the TALE backbone vector pTALE-VP64 and pTALE-VPR. The 1,000-bp promoter fragments of HNF4α and E47 genes were amplified by PCR from HepG2 and PANC1 cell genomic DNA (gDNA) using PrimeSTAR HS DNA Polymerase, in which the XhoI and HindIII sites were introduced at the two ends. The PCR primers were HNF4α-F/R and E47-F/R (Table S3). The fragments were ligated into pEZX-miniCMV-ZsGreen to produce the reporter vectors pHNF4α-TALE-reporter and pE47-TALE-reporter. The maps of the plasmids are shown in the Supplemental Information. All PCR programs were run on a MasterCycler Pro (Eppendorf). For preparing high-quality plasmids, all plasmids were amplified in E. coli DH5α and extracted with the EndoFree Plasmid kits (QIAGEN), according to the manufacturer’s instructions. All plasmids were further verified by DNA sequencing.
Preparation of Monomers
Monomers were prepared by high-fidelity PCR amplification. The plasmid monomers of the Addgene kit were used as templates. The primers for amplifying each linear monomer are shown in Table S1. High-fidelity PCR amplification was performed using PrimeSTAR HS DNA Polymerase, according to the manufacturer’s instructions. Each PCR reaction (240 μL) contained 48 ng plasmid monomer, 10 μM forward and reverse primers (Table S1), and 1× PrimeSTAR HS DNA Polymerase. The PCR products were purified with the MinElute Gel Extraction kit, according to the manufacturer’s instructions. The PCR templates for amplifying the two linker monomers dsDNA10.5 and dsDNA17.5 were chemically synthesized (oligo DNA10.5 and DNA17.5 in Table S2). The double-stranded DNA10.5 and DNA17.5 (dsDNA10.5 and dsDNA17.5) were amplified by high-fidelity PCR with universal primers (Table S1), using PrimeSTAR HS DNA Polymerase. Once the first set of linear monomers was prepared by high-fidelity PCR amplification, as described above, all linear monomers could be reproduced by high-throughput in 96-well plates by high-fidelity PCR amplification, using a pair of universal primers (Table S1).
Construction of Custom TALEs
Custom TALEs were prepared according to new pipelines schematically shown in Figure 3. First, according to a 19-bp target sequence of 5ʹ-T0N1N2N3N4N5N6N7N8N9N10N11N12N13N14N15N16N17N18-3ʹ (where N = A, G, T, or C) and the amino acid-coding rules of the monomers (NI = A, HD = C, NG = T, and NN = G), four digestion-ligation reactions for producing pentamers (N1-N2-N3-N4-N5, N6-N7-N8-N9-N10, dsDNA10.5-N11-N12-N13-N14, and N15-N16-N17-dsDNA17.5-N18) were set up. The 20 μL reactions contained 10 U BsaI, 400 U T4 DNA ligase, 1× T4 DNA ligase buffer, 2 μg BSA, and 200 ng of each of five monomers. The reactions were incubated in a thermocycler for 10 cycles of 37°C for 5 min and 16°C for 10 min. The enzymes were inactivated at 50°C for 5 min and 80°C for 5 min. The remained linear DNA was removed by adding 1 μL Plasmid-Safe nuclease (10 U/μL; Epibio) and 1 μL ATP (10 mM) to each pentamer reaction and incubating at 37°C for 30 min. The Plasmid-Safe nuclease was inactivated at 70°C for 30 min. The pentamers were purified on a PCR cleaning column and collected in water at a concentration of 100 ng/μL. Second, four pentamers were cloned into TALE backbones by another digestion-ligation reaction. A 20 μL reaction contained 75 ng TALE backbone (pTALE-VP64 or pTALE-VPR), 10 U BsmBI, 400 U T4 DNA ligase, 1× T4 DNA ligase buffer, and 200 ng of each of four pentamers. The reaction was incubated in a thermocycler for 10 cycles of 37°C for 5 min and 16°C for 10 min. The reactions were then incubated at 50°C for 5 min and 80°C for 5 min to inactivate enzymes. Third, E. coli DH5α was transformed with the TALE products and cultivated overnight on lysogeny broth (LB) agar containing kanamycin, X-gal, and IPTG at 37°C. The positive colonies were identified by colony PCR with primers TAL-F and TAL-R (Table S2) using 1× premix Taq. Finally, the selected colonies were cultivated overnight in liquid LB medium containing 100 μg/mL kanamycin at 37°C. A small number of plasmids was prepared with the QIAprep Spin Miniprep Kit (QIAGEN) and verified by EcoRI digestion. Large quantities of plasmids were extracted with the EndoFree Plasmid kit (QIAGEN), according to the manufacturer’s instructions.
Cell Culture and Transfection
Cells were cultured with DMEM containing 10% fetal bovine serum (FBS), 100 U/mL penicillin, and 100 μg/mL streptomycin in a humidified incubator with 5% CO2 at 37°C. Cells were seeded in a 96-well plate at a density of 3,000 cells/well, a 24-well plate at a density of 5 × 104 cells/well, or a 6-well plate at a density of 2 × 105 cells/well and cultivated for 24 h. Cells were then transfected with plasmids by using Lipofectamine 2000 (Thermo Fisher Scientific), according to the manufacturer’s instruction. All vectors used for transfection were isolated using EndoFree Plasmid kits. The transfected cells were cultured for an additional 48 or 72 h.
Activation of Reporter Construct with TALEs
293T cells cultivated in 24-well plates were co-transfected with 400 ng of TALE vector (HNF4α-TALE-VP64, HNF4α-TALE-VPR, or E47-TALE-VPR) and 400 ng of ZsGreen reporter vector (HNF4α-TALE-reporter or E47-TALE-reporter). Cells were photographed with a fluorescence microscope (IX51 with a DP71 camera; Olympus) and quantitatively analyzed with a flow cytometer (FACSCalibur; BD).
Detection of Gene Expression
Cells cultivated in 24-well plates were transfected by TALE-VPR. The elution buffer of the EndoFree Plasmid kit was used as blank control. After 48 h, the total RNA was extracted by Trizol. The cDNA was prepared using the FastKing RT Kit (with gDNase; Tiangen Biotech), according to the manufacturer’s instructions. The gene expression was detected by qPCR using 2× Fast SYBR Green Master Mix (Applied Biosystems), according to the manufacturer’s instructions. β-Actin was used as an internal reference to analyze the relative mRNA expression of different genes. PCR primers are shown in Table S4. The qPCR programs were run on a real-time PCR machine, StepOne Plus (Applied Biosystems). Each qPCR detection was performed in at least three technical replicates. Melting curve analysis was performed. Data analysis was performed using the Applied Biosystems StepOne software v2.3, and Ct values were normalized with that of β-actin. The relative expression level of target mRNAs was calculated as relative quantity (RQ) according to the equation: RQ = 2-ΔΔCt. Student’s t test was used to check the statistical significance.
Detection of Cell Viability and Cycle
Cells cultivated in 96-well plates were transfected with 200 ng TALE-VPR plasmid, using Lipofectamine 2000. The transfected cells were cultivated for variant time. Cell viability was detected with CCK8 (Biosharp), according to the manufacturer’s instruction. The optical density (OD) value was read by a BioTek plate reader at the wavelength of 450 nm. The cell cycle was analyzed by flow cytometry. Cells were collected by trypsinization, resuspended with 40 μL 4% paraformaldehyde, cells were added with 1 μL 100 μg/mL DAPI, and incubated at room temperature for 30 min. The cells were precipitated by centrifugation, washed once with PBS, then resuspended in PBS, and analyzed with a flow cytometer (FACSCalibur; BD). G0/G1, S, and G2/M phase were generated by modeling data with ModFitLT software (Verity Software House).
Detection of Cell Migration
Cells cultivated in 24-well plates were transfected with 800 ng of TALE-VPR plasmid using Lipofectamine 2000. The transfected cells were cultivated for 48 h, then collected by trypsinization and resuspended in serum-free DMEM at a final concentration of 1 × 105 cells/mL. The cells were added to the upper chamber at the volume of 200 μL per well. DMEM containing 10% FBS and 50 μg/mL fibronectin was added to the lower chamber at the volume of 600 μL per well. Cells were incubated at 37°C for 48 h. The Transwell was removed. The lower chamber was washed once with PBS and 100 μL of PBS was added, containing 10 mg/mL acridine orange. After staining for 30 min at room temperature, the cells were washed three times with PBS, photographed with fluorescence microscope IX51 or DP71 (Olympus).
Gene Editing and Activation by TALEs
A pair of TALEN vectors was respectively designed for editing endogenous HNF4α and E47 genes. The target sequences of these TALENs were shown in Table S5. The TALEN vectors were constructed using the simplified TALE vector assembly method developed in this study. To fuse a ZsGreen coding sequence to the endogenous HNF4α and E47 genes by HDR, the ZsGreen coding sequence with two homologous arms (HOM-HNF4a-ZsGreen and HOM-E47-ZsGreen) were prepared by PCR amplification, using the primers listed in Table S6. The HNF4α gene in HepG2 cell was edited by co-transfecting HepG2 cell with a pair of TALEN vectors (Left-HNF4a-TALEN and Right-HNF4a-TALEN) and homologous donor (HOM-HNF4a-ZsGreen). The E47 gene in PANC1 cell was edited by co-transfecting PANC1 cells with a pair of TALEN vectors (Left-E47-TALEN and Right-E47-TALEN) and homologous donor (HOM-E47-ZsGreen). To activate expression of endogenous HNF4α-ZsGreen and E47-ZsGreen genes, HepG2 and PANC1 cells were respectively transfected with HNF4α-TALE1-VPR and E47-TALE1-VPR. To edit and activate endogenous genes simultaneously, HepG2 and PANC1 cells were also respectively co-transfected with a pair of TALEN vectors, a homologous donor, and TALE1-VPR. The cells were photographed with a fluorescence microscope and quantified with flow cytometry.
Author Contributions
S.Z. performed most of the experiments. H.C. performed PCR amplification. J.W. conceived of and directed the study. J.W. and S.Z. wrote the manuscript.
Conflicts of Interest
The authors declare no competing interests.
Acknowledgments
This work was supported by the National Natural Science Foundation of China (61571119).
Footnotes
Supplemental Information can be found with this article online at https://doi.org/10.1016/j.omtm.2019.02.004.
Supplemental Information
References
- 1.Boch J., Bonas U. Xanthomonas AvrBs3 family-type III effectors: discovery and function. Annu. Rev. Phytopathol. 2010;48:419–436. doi: 10.1146/annurev-phyto-080508-081936. [DOI] [PubMed] [Google Scholar]
- 2.Römer P., Hahn S., Jordan T., Strauss T., Bonas U., Lahaye T. Plant pathogen recognition mediated by promoter activation of the pepper Bs3 resistance gene. Science. 2007;318:645–648. doi: 10.1126/science.1144958. [DOI] [PubMed] [Google Scholar]
- 3.Kay S., Hahn S., Marois E., Wieduwild R., Bonas U. Detailed analysis of the DNA recognition motifs of the Xanthomonas type III effectors AvrBs3 and AvrBs3Deltarep16. Plant J. 2009;59:859–871. doi: 10.1111/j.1365-313X.2009.03922.x. [DOI] [PubMed] [Google Scholar]
- 4.Römer P., Strauss T., Hahn S., Scholze H., Morbitzer R., Grau J., Bonas U., Lahaye T. Recognition of AvrBs3-like proteins is mediated by specific binding to promoters of matching pepper Bs3 alleles. Plant Physiol. 2009;150:1697–1712. doi: 10.1104/pp.109.139931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Boch J., Scholze H., Schornack S., Landgraf A., Hahn S., Kay S., Lahaye T., Nickstadt A., Bonas U. Breaking the code of DNA binding specificity of TAL-type III effectors. Science. 2009;326:1509–1512. doi: 10.1126/science.1178811. [DOI] [PubMed] [Google Scholar]
- 6.Moscou M.J., Bogdanove A.J. A simple cipher governs DNA recognition by TAL effectors. Science. 2009;326:1501. doi: 10.1126/science.1178817. [DOI] [PubMed] [Google Scholar]
- 7.Miller J.C., Tan S., Qiao G., Barlow K.A., Wang J., Xia D.F., Meng X., Paschon D.E., Leung E., Hinkley S.J. A TALE nuclease architecture for efficient genome editing. Nat. Biotechnol. 2011;29:143–148. doi: 10.1038/nbt.1755. [DOI] [PubMed] [Google Scholar]
- 8.Morbitzer R., Römer P., Boch J., Lahaye T. Regulation of selected genome loci using de novo-engineered transcription activator-like effector (TALE)-type transcription factors. Proc. Natl. Acad. Sci. USA. 2010;107:21617–21622. doi: 10.1073/pnas.1013133107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhang F., Cong L., Lodato S., Kosuri S., Church G.M., Arlotta P. Efficient construction of sequence-specific TAL effectors for modulating mammalian transcription. Nat. Biotechnol. 2011;29:149–153. doi: 10.1038/nbt.1775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Geissler R., Scholze H., Hahn S., Streubel J., Bonas U., Behrens S.E., Boch J. Transcriptional activators of human genes with programmable DNA-specificity. PLoS ONE. 2011;6:e19509. doi: 10.1371/journal.pone.0019509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cermak T., Doyle E.L., Christian M., Wang L., Zhang Y., Schmidt C., Baller J.A., Somia N.V., Bogdanove A.J., Voytas D.F. Efficient design and assembly of custom TALEN and other TAL effector-based constructs for DNA targeting. Nucleic Acids Res. 2011;39:e82. doi: 10.1093/nar/gkr218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Li T., Huang S., Zhao X., Wright D.A., Carpenter S., Spalding M.H., Weeks D.P., Yang B. Modularly assembled designer TAL effector nucleases for targeted gene knockout and gene replacement in eukaryotes. Nucleic Acids Res. 2011;39:6315–6325. doi: 10.1093/nar/gkr188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wood A.J., Lo T.W., Zeitler B., Pickle C.S., Ralston E.J., Lee A.H., Amora R., Miller J.C., Leung E., Meng X. Targeted genome editing across species using ZFNs and TALENs. Science. 2011;333:307. doi: 10.1126/science.1207773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Christian M., Cermak T., Doyle E.L., Schmidt C., Zhang F., Hummel A., Bogdanove A.J., Voytas D.F. Targeting DNA double-strand breaks with TAL effector nucleases. Genetics. 2010;186:757–761. doi: 10.1534/genetics.110.120717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hockemeyer D., Wang H., Kiani S., Lai C.S., Gao Q., Cassady J.P., Cost G.J., Zhang L., Santiago Y., Miller J.C. Genetic engineering of human pluripotent cells using TALE nucleases. Nat. Biotechnol. 2011;29:731–734. doi: 10.1038/nbt.1927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tesson L., Usal C., Ménoret S., Leung E., Niles B.J., Remy S., Santiago Y., Vincent A.I., Meng X., Zhang L. Knockout rats generated by embryo microinjection of TALENs. Nat. Biotechnol. 2011;29:695–696. doi: 10.1038/nbt.1940. [DOI] [PubMed] [Google Scholar]
- 17.Sun N., Liang J., Abil Z., Zhao H. Optimized TAL effector nucleases (TALENs) for use in treatment of sickle cell disease. Mol. Biosyst. 2012;8:1255–1263. doi: 10.1039/c2mb05461b. [DOI] [PubMed] [Google Scholar]
- 18.Park C.Y., Kim J., Kweon J., Son J.S., Lee J.S., Yoo J.E., Cho S.R., Kim J.H., Kim J.S., Kim D.W. Targeted inversion and reversion of the blood coagulation factor 8 gene in human iPS cells using TALENs. Proc. Natl. Acad. Sci. USA. 2014;111:9253–9258. doi: 10.1073/pnas.1323941111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Qasim W., Amrolia P.J., Samarasinghe S., Ghorashian S., Zhan H., Stafford S., Butler K., Ahsan G., Gilmour K., Adams S. First Clinical Application of Talen Engineered Universal CAR19 T Cells in B-ALL. Blood. 2015;126:2046. [Google Scholar]
- 20.Qasim W., Zhan H., Samarasinghe S., Adams S., Amrolia P., Stafford S., Butler K., Rivat C., Wright G., Somana K. Molecular remission of infant B-ALL after infusion of universal TALEN gene-edited CAR T cells. Sci. Transl. Med. 2017;9:eaaj2013. doi: 10.1126/scitranslmed.aaj2013. [DOI] [PubMed] [Google Scholar]
- 21.Sander J.D., Cade L., Khayter C., Reyon D., Peterson R.T., Joung J.K., Yeh J.R.J. Targeted gene disruption in somatic zebrafish cells using engineered TALENs. Nat. Biotechnol. 2011;29:697–698. doi: 10.1038/nbt.1934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Reyon D., Tsai S.Q., Khayter C., Foden J.A., Sander J.D., Joung J.K. FLASH assembly of TALENs for high-throughput genome editing. Nat. Biotechnol. 2012;30:460–465. doi: 10.1038/nbt.2170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Weber E., Gruetzner R., Werner S., Engler C., Marillonnet S. Assembly of designer TAL effectors by Golden Gate cloning. PLoS ONE. 2011;6:e19722. doi: 10.1371/journal.pone.0019722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Morbitzer R., Elsaesser J., Hausner J., Lahaye T. Assembly of custom TALE-type DNA binding domains by modular cloning. Nucleic Acids Res. 2011;39:5790–5799. doi: 10.1093/nar/gkr151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.New England BioLabs. Cleavage of Supercoiled DNA. https://www.neb.com/tools-and-resources/selection-charts/cleavage-of-supercoiled-dna.
- 26.Engler C., Kandzia R., Marillonnet S. A one pot, one step, precision cloning method with high throughput capability. PLoS ONE. 2008;3:e3647. doi: 10.1371/journal.pone.0003647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Engler C., Gruetzner R., Kandzia R., Marillonnet S. Golden gate shuffling: a one-pot DNA shuffling method based on type IIs restriction enzymes. PLoS ONE. 2009;4:e5553. doi: 10.1371/journal.pone.0005553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Weber E., Engler C., Gruetzner R., Werner S., Marillonnet S. A modular cloning system for standardized assembly of multigene constructs. PLoS ONE. 2011;6:e16765. doi: 10.1371/journal.pone.0016765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chavez A., Tuttle M., Pruitt B.W., Ewen-Campen B., Chari R., Ter-Ovanesyan D., Haque S.J., Cecchi R.J., Kowal E.J.K., Buchthal J. Comparison of Cas9 activators in multiple species. Nat. Methods. 2016;13:563–567. doi: 10.1038/nmeth.3871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhang Z., Wu E., Qian Z., Wu W.S. A multicolor panel of TALE-KRAB based transcriptional repressor vectors enabling knockdown of multiple gene targets. Sci. Rep. 2014;4:7338. doi: 10.1038/srep07338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wang H., Guo R., Du Z., Bai L., Li L., Cui J., Li W., Hoffman A.R., Hu J.-F. Epigenetic Targeting of Granulin in Hepatoma Cells by Synthetic CRISPR dCas9 Epi-suppressors. Mol. Ther. Nucleic Acids. 2018;11:23–33. doi: 10.1016/j.omtn.2018.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Murakami M.T., Sforça M.L., Neves J.L., Paiva J.H., Domingues M.N., Pereira A.L., Zeri A.C., Benedetti C.E. The repeat domain of the type III effector protein PthA shows a TPR-like structure and undergoes conformational changes upon DNA interaction. Proteins. 2010;78:3386–3395. doi: 10.1002/prot.22846. [DOI] [PubMed] [Google Scholar]
- 33.Scholze H., Boch J. TAL effectors are remote controls for gene activation. Curr. Opin. Microbiol. 2011;14:47–53. doi: 10.1016/j.mib.2010.12.001. [DOI] [PubMed] [Google Scholar]
- 34.Kim S., Lahmy R., Riha C., Yang C., Jakubison B.L., van Niekerk J., Staub C., Wu Y., Gates K., Dong D.S. The basic helix-loop-helix transcription factor E47 reprograms human pancreatic cancer cells to a quiescent acinar state with reduced tumorigenic potential. Pancreas. 2015;44:718–727. doi: 10.1097/MPA.0000000000000328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yin C., Lin Y., Zhang X., Chen Y.X., Zeng X., Yue H.Y., Hou J.L., Deng X., Zhang J.P., Han Z.G., Xie W.F. Differentiation therapy of hepatocellular carcinoma in mice with recombinant adenovirus carrying hepatocyte nuclear factor-4alpha gene. Hepatology. 2008;48:1528–1539. doi: 10.1002/hep.22510. [DOI] [PubMed] [Google Scholar]
- 36.Yin S., Li J., Hu C., Chen X., Yao M., Yan M., Jiang G., Ge C., Xie H., Wan D. CD133 positive hepatocellular carcinoma cells possess high capacity for tumorigenicity. Int. J. Cancer. 2007;120:1444–1450. doi: 10.1002/ijc.22476. [DOI] [PubMed] [Google Scholar]
- 37.Suetsugu A., Nagaki M., Aoki H., Motohashi T., Kunisada T., Moriwaki H. Characterization of CD133+ hepatocellular carcinoma cells as cancer stem/progenitor cells. Biochem. Biophys. Res. Commun. 2006;351:820–824. doi: 10.1016/j.bbrc.2006.10.128. [DOI] [PubMed] [Google Scholar]
- 38.Ma S., Chan K.W., Hu L., Lee T.K., Wo J.Y., Ng I.O., Zheng B.J., Guan X.Y. Identification and characterization of tumorigenic liver cancer stem/progenitor cells. Gastroenterology. 2007;132:2542–2556. doi: 10.1053/j.gastro.2007.04.025. [DOI] [PubMed] [Google Scholar]
- 39.Yu J., Vodyanik M.A., Smuga-Otto K., Antosiewicz-Bourget J., Frane J.L., Tian S., Nie J., Jonsdottir G.A., Ruotti V., Stewart R. Induced pluripotent stem cell lines derived from human somatic cells. Science. 2007;318:1917–1920. doi: 10.1126/science.1151526. [DOI] [PubMed] [Google Scholar]
- 40.Takahashi K., Tanabe K., Ohnuki M., Narita M., Ichisaka T., Tomoda K., Yamanaka S. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell. 2007;131:861–872. doi: 10.1016/j.cell.2007.11.019. [DOI] [PubMed] [Google Scholar]
- 41.Joshi U.S., Dergham S.T., Chen Y.Q., Dugan M.C., Crissman J.D., Vaitkevicius V.K., Sarkar F.H. Inhibition of pancreatic tumor cell growth in culture by p21WAF1 recombinant adenovirus. Pancreas. 1998;16:107–113. doi: 10.1097/00006676-199803000-00001. [DOI] [PubMed] [Google Scholar]
- 42.Gironella M., Seux M., Xie M.J., Cano C., Tomasini R., Gommeaux J., Garcia S., Nowak J., Yeung M.L., Jeang K.T. Tumor protein 53-induced nuclear protein 1 expression is repressed by miR-155, and its restoration inhibits pancreatic tumor development. Proc. Natl. Acad. Sci. USA. 2007;104:16170–16175. doi: 10.1073/pnas.0703942104. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.