Abstract
Environmental changes related to agricultural practices and activities can impact malaria transmission. In the objective to evaluate this impact on the human-vector contact, the level of human exposure to Anopheles vector bites was assess by an immuno-epidemiological indicator based on the assessment of the human IgG antibody response to the Anopheles gambiae gSG6-P1 salivary peptide, previously validated as a pertinent biomarker.
Two cross-sectional surveys were carried out in the dry and rainy season in three villages with intensive agricultural plantations (N'Zikro with rubber cultivation, Ehania-V5 and Ehania-V1 with palm oil exploitation) and in a control village without plantations (Ayébo). Overall, 775 blood samples were collected in filter papers from children aged 1 to 14 years-old for immunological analysis by ELISA. The IgG levels to the gSG6-P1 salivary peptide significantly differed between studied villages both in the dry and the rainy seasons (P < 0.0001) and were higher in agricultural villages compared to the control area. In particular, the level of specific IgG in Ehania-V5, located in the heart of palm oil plantations, was higher compared to other agricultural villages. Interestingly, the level of specific IgG levels classically increased between the dry and the rainy season in the control village (P < 0.0001) whereas it remained high in the dry season as observed in the rainy season in agricultural villages.
The present study indicated that rubber and oil palm plantations could maintain a high level of human exposure to Anopheles bites during both the dry and rainy seasons. These agricultural activities could therefore represent a permanent factor of malaria transmission risk.
Keywords: Anopheles, Agricultural activities, Biomarker, Antibody, Saliva, Malaria risk
1. Background
Malaria remains the most widespread and dangerous infection in terms of burden in the world and especially in Africa. Malaria was responsible for 500,000 deaths and 214 million clinical cases in 2016; children under 5 years of age and pregnant women being the most vulnerable population (WHO, 2017). Anopheles gambiae s.l is the primary malaria vector in Africa which transmits Plasmodium falciparum, the most Plasmodium species responsible for malaria morbidity/mortality (Houngbedji et al., 2015; Koudou et al., 2005; Nzeyimana et al., 2002).
In Côte d'Ivoire, malaria is the main cause of consultation in school health centers and represents 43% of the general consultation. The major Anopheles species are An. gambiae s.l, which as the most widespread malaria vector, and An. funestus. The role of An. gambiae s.l, and An. funestus on malaria transmission has been investigated several times in the north (Doannio et al., 2002), in the center zone (Diakité et al., 2010; Koudou et al., 2007) and in the southern forest zone (Adja et al., 2011).
Environmental changes related to agricultural practices and activities can impact on malaria transmission by influencing vector species composition, and density that are key factors for enhancing malaria transmission (Afrane et al., 2004; Henry et al., 2003). In the southeast Côte d'Ivoire, a large expansion of agricultural activities such as oil palm and rubber plantations were developed. Populations move in these areas for multiple reasons: living around, laboring, collecting forest products like palm oil fruits, collection of latex, etc., (Martens and Hall, 2000). Despite of socio-economic benefits, expansion of agriculture development schemes can aggravate the problem of mosquito-borne diseases by increasing also the number of breeding sites of mosquitoes. It could have therefore a direct influence on malaria transmission (Keiser et al., 2005). In this context, previous studies have demonstrated that agriculture (oil palm plantation, rubber plantation) can influence the diversity and density of mosquito fauna and by consequence, malaria transmission. For example, a study carried out in rubber cultivated area in Cameroon indicated that environmental modifications due to agro-industrial activities might have influenced vector distribution and the dynamics of malaria transmission (Bigoga et al., 2012). To improve the evaluation of malaria transmission/exposure according to the World Health Organization (WHO) recommendations, much effort was devoted to develop new indicators and methods at the individual level. Since last decade, several studies showed that the quantification of antibody (Ab) response to vector saliva in human populations could be a pertinent biomarker tool to assess the human exposure level to vector bites and thus to the risk of transmission of vector-borne diseases (Sagna et al., 2017). Specifically, the gSG6-P1 (An. gambiae Salivary Gland Protein-6 peptide 1) salivary peptide of An. gambiae has been identified as a pertinent candidate to evaluate the exposure of Anopheles bites (Poinsignon et al., 2008). Indeed, this salivary peptide is specific to Anopheles genus, antigenic, easy to synthesize and highly conserved between Anopheles mosquitoes (Poinsignon et al., 2010). In addition, human IgG responses to gSG6-P1 peptide was especially relevant as biomarker in a context of low exposure to Anopheles bites, such as urban malaria and dry season (Drame et al., 2012; Poinsignon et al., 2009; Sagna et al., 2013a, Sagna et al., 2013b). Such biomarker could be thus used i) to assess the heterogeneity of exposure level to Anopheles bites and malaria risk (Drame et al., 2012; Ya-Umphan et al., 2017) and ii) to evaluate the effectiveness of vector control strategies (Drame et al., 2010a, Drame et al., 2010b, Drame et al., 2013). For this reason, the study of Anopheles-human immunological relationships can be another approach for monitoring the real human-Anopheles bite contact and consequently identify individual at risk of malaria transmission.
The present study was conducted in four rural villages in Aboisso region, Southeastern Côte d'Ivoire. The aim was to evaluate the level of human exposure to Anopheles bites, and by consequence the risk of malaria transmission, and its evolution between the dry and rainy seasons. To evaluate Anopheles exposure in studied child population, the specific human IgG response to the Anopheles gSG6-P1 salivary peptide was used as an immuno-epidemiological biomarker tool.
2. Methods
2.1. Study site and population
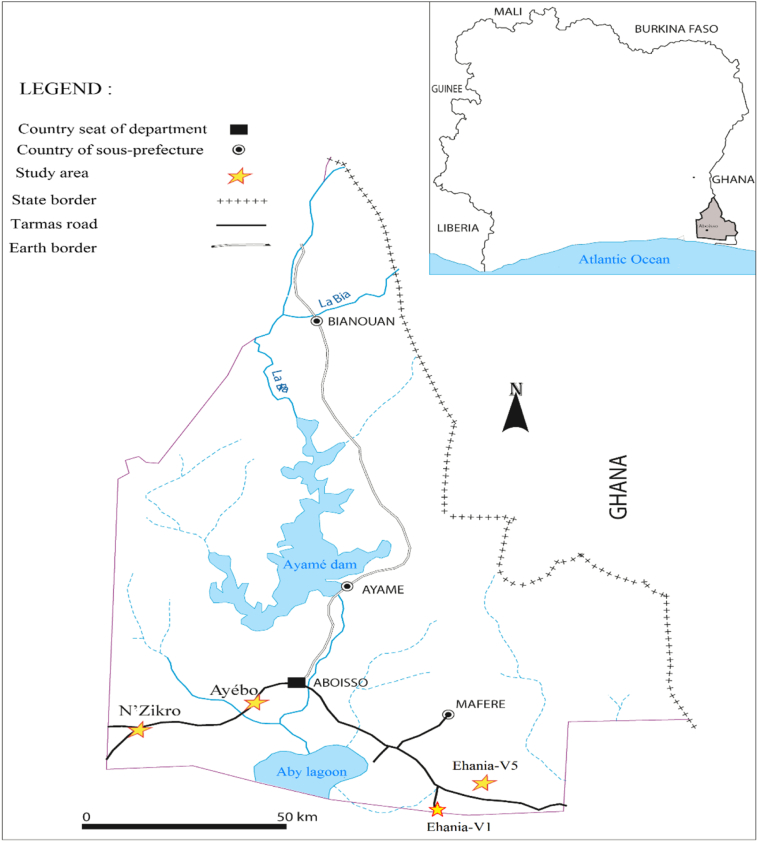
The study was conducted in four villages of the Aboisso health district (05°28′ north latitude and 03°12′ west longitude) in the Southeast of Côte d'Ivoire (Fig. 1) in December 2013 during the dry season and in July 2014 during the rainy season. The climate is punctuated by two major seasons: the long rainy season from April to July, with the peak of rainfalls in June, while the long dry season extends from October to March. The annual rainfall average is 1848 mm with an annual temperature average of 27 °C. These villages are mostly inhabited by indigenous Agni cohabiting with non-native Dioula, Ghanaian, Burkinabe, Nigerien and Nigerian families. In this area, economic activities are dominated by food crops and large agricultural establishments such as cash crops (rubber, oil palm, pineapple). In this area, the previous entomological survey (2012−2013) indicated that An. gambiae s.l was the only Anopheles species and by the consequences the only species of malaria vector (personal communication).
Fig. 1.
Map of Aboisso department and the four studied villages are represented by stars.
Four villages were selected on the basis on agriculture practices:
-
(i)
Ehania-V1 is far from 140 km to Abidjan (Geographical coordinates: longitude: 5°14′ N, latitude: 2°46′ W), and 60 km to Ghana (Fig. 1). It is surrounded by the largest industrial oil palm plantation (30,000 ha) belonging to the PALMCI company which yield constitutes 32% of the annual oil palm yield of the country. The company plays a role of guidance and supervision of 8097 out-growers. The presence of primary rainforest which provides strong vegetation can be mentioned.
-
(ii)
Ehania-V5 (5°13′60″ N; 2°46′0″ W) is located in the middle of industrial palm oil plantations (Elaeis guineensis) where some inhabited houses are located within 15 m of oil palm trees. The residencies are mainly composed of traditional house and also few ordinarily modern houses.
-
(iii)
N'zikro (7°32′30.0″ N; 5°4′12″ W) is far from 88 km east of Abidjan. It is a large area including traditional rubber (Hevea brasiliensis) fields (200 ha) which are closed to the village in the southeast and north parts. On the south-western area of N'zikro, an industrial palm oil plantation of PALMCI extends to 3.600 ha. Mostly people of this village live within the immediate rubber plantations environment because the last inhabited house was 50 m away from rubber plantation. The residencies are composed of traditional and ordinarily modern houses.
-
(iv)
Ayébo (5°26′27″ N; 3°15′52″ W) is located at 106 km from Abidjan and at 18.5 km from N'zikro. This village is considered as control site in our study as no industrial or extensive agricultural activities are practiced in the immediate surrounds. The limited cultivation is yams, coffee and plantain very far from 800 m to the village.
2.2. Studied population
Two cross-sectional surveys were conducted in December 2013 (dry season) and in July 2014 (rainy season) in the four villages studied. Overall, 775 children aged from 1 to 14 years living in theses villages during the last two months before each survey were included and distributed as follows: i) 200 children in N'zikro, (99 in dry season and 101 in rainy season); ii) 198 in Ayébo (100 in dry and 98 in rainy season); iii) 198 in Ehania-V1, (100 in dry and 98 in rainy season) and iv) 179 in Ehania-V5 (81 in dry and 98 in rainy season). Children surveyed in the dry season were not the same as those surveyed in the rainy season.
Inclusion criteria were the absence of clinical sign of severe malaria according to WHO definition and of any other disease that can cause an obvious febrile illness. Moreover, standardized questionnaires were individually administrated for assessing epidemiological information such as the use of individual protection against mosquito bites (Insecticide-Treated Nets - ITN - use in particular), the sex and the age. During each visit, a dried blood spot (DBS) was collected from each included individual on Whatman 3MM filter paper for immunological analysis. All filter papers were kept at 4 °C before use.
This study followed ethical principles recommended by the Edinburgh revision of the Helsinki Declaration. The Director of Health District of Aboisso administrative Department and authorities of each studied village were informed about objectives, procedures and benefits of the study. Approval, including the use of oral consent, was obtained from the different village authorities and the Departmental Director of Health of Aboisso district before starting data collection. This study was approved by the National Malaria Control Program (NMCP) of Côte d'Ivoire. In addition, the participation of children in the study including blood sample collection was done after the oral informed consent of each parent or guardian of children. Sick children were treated according to the National Policy.
2.3. Salivary peptide gSG6-P1
The gSG6-P1 peptide was selected as previously described (Poinsignon et al., 2008). It was synthesized and purified (>95%) by Genepep SA (Saint-Jean de Védas, France). The peptide was shipped in lyophilized form and then suspended in 0.22 μm ultra-filtered water and frozen at −20 °C until use.
2.4. Evaluation of anti-human IgG level to gSG6-P1 antigen by ELISA
ELISA (Enzyme-linked immunosorbent assays) were carried out on DBS to measure the IgG Ab level to the gSG6-P1 salivary peptide as previously described (Drame et al., 2010b).
All ELISA conditions were determined after several preliminary experiments to obtain optimal dilutions of all reagents. Briefly, Maxisorp plates (Nunc, Roskilde, Danemark) were coated with gSG6-P1 (20 μg/ml) in Phosphate Buffered Saline (PBS). Plates were blocked with 300 μl/well of Protein-Free Blocking-Buffer (Pierce, Thermo Scientific, France). After washing (distilled water + Tween 0.1%), each DBS eluate was incubated in duplicate at +4 °C overnight at a 1/20 dilution in PBS-Tween 1% + BSA 1% (Sigma-Aldrich). Mouse biotinylated Ab to human IgG (BD Pharmingen, San Diego CA, USA) was incubated at 1/1000 dilution in PBS-Tween 1% + BSA 1% (1 h 30 at 37 °C) and peroxidase-conjugated streptavidin (Amersham, les Ulis, France) was then added (1/2000; 1 h at 37 °C). Colorimetric development was carried out using ABTS (2.2-azino-bis (3 ethylbenzthiazoline 6-sulfonic acid) diammonium; (Sigma Aldrich), pH = 4, containing 0.003% H2O2) and absorbance (OD) was measured at 405 nm. In parallel, each tested sample was assessed in a blank well containing no salivary peptide antigen (ODn) to measure non-specific reactions. A known positive control was included on each ELISA plate to control the plate-to-plate variation as well as reproducibility of the test. Individual results were expressed as the ΔOD value calculated according to the formula: ΔOD = ODx − ODn, where ODx represented the mean of individual OD values in the two wells containing antigen and ODn the OD value in well without antigen.
2.5. Statistical analysis
All data were analyzed with GraphPad Prism5 software (San Diego, CA). After ensuring that ΔOD values were not normally distributed, the non-parametric tests were used to compare ΔOD between groups. Mann-Whitney test was used for the comparison of Ab levels between two independent groups and the Kruskal-Wallis test was used for the comparisons between more than two independent groups. The Dunn's posttest was used for multiple paired comparisons between villages. All differences were considered significant at P < 0.05.
3. Results
3.1. IgG levels to gSG6-P1 salivary peptide according to the village in dry and rainy seasons
Specific IgG levels to gSG6-P1 were compared in children between the four villages studied in the dry and rainy seasons (Fig. 2). The results showed a high heterogeneity of IgG Ab levels within and between villages both in both the dry and rainy seasons.
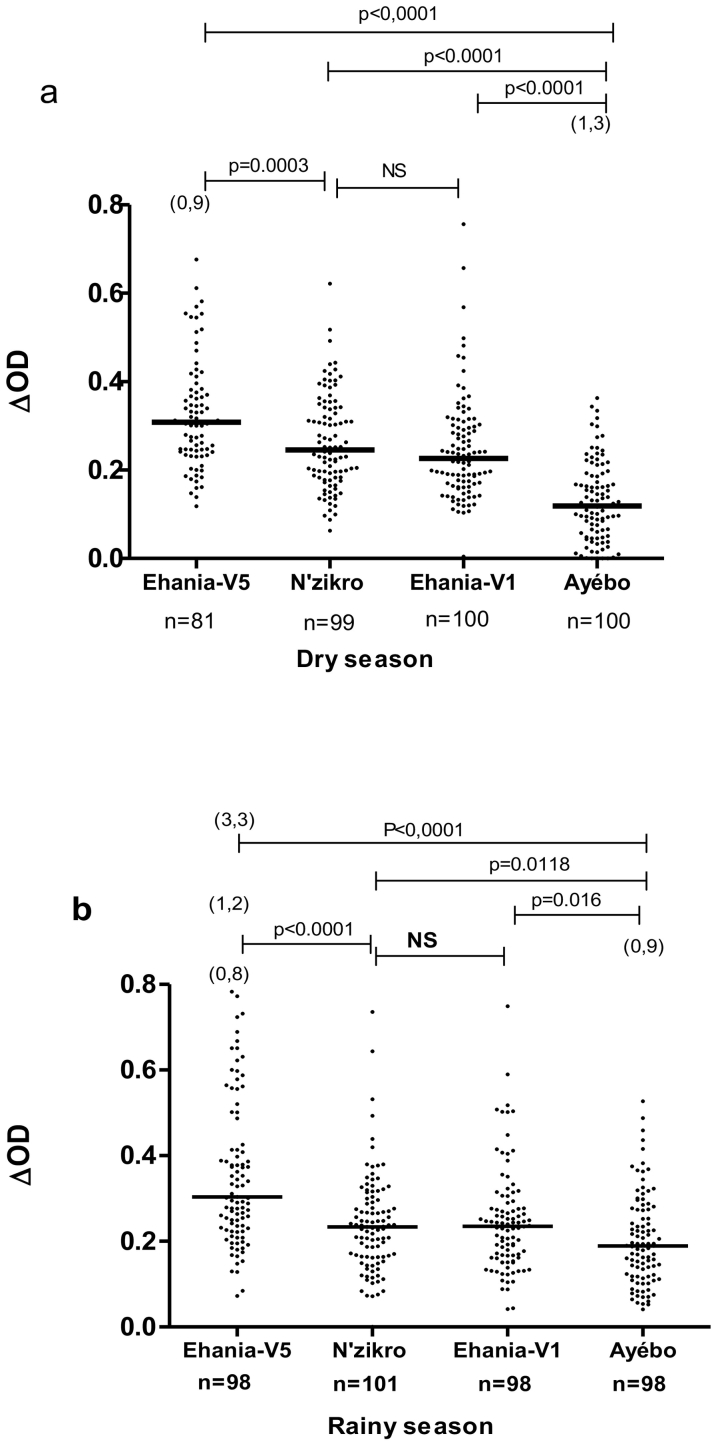
Fig. 2.
IgG response to gSG6-P1 according to villages in dry season and rainy season: Individual IgG Ab response are presented for dry season (a) and rainy season (b) Points indicate individual IgG level (ΔOD) and bars indicate the median value for each village. Numbers in parentheses above the dot plots indicates values above ΔOD = 0.8. Statistical significant differences between two villages are indicated (the Kruskal-Wallis U test non-parametric test).
In the dry season (Fig. 2a), the level of specific IgG response significantly differed between the four villages (P < 0.0001; Kruskal-Wallis test). The statistical analysis reported that the levels of IgG response to gSG6-P1 in children from agricultural villages were significantly higher than those from Ayébo, the “control village” without agricultural practices in its immediate environment (p < 0.0001). The median of specific IgG Ab level in children from Ehania-V5 agricultural village was higher than those from children from others agricultural villages (p < 0.001, Kruskal-Wallis test). No significant difference in specific IgG levels was observed between children from N'zikro and those from Ehania-V1 village (p = 0.123; Mann-Whitney test).
In the rainy season (Fig. 2b), similar pattern was also observed but in a lesser extent. The median of specific IgG levels in children significantly varied according to villages (p < 0.0001; Kruskal-Wallis test). The comparison of the level of IgG Ab response between the agricultural villages showed that it was significantly higher in Ehania-V5 compared to N'zikro and Ehania-V1 (p < 0.0001; Kruskal-Wallis test). In contrast, the level of IgG response was similar in N'zikro and Ehania-V1 villages (p = 0.9627; Mann-Whitney test). The IgG level in Ayébo was significantly lower than those in Ehania-V5 (p < 0.0001; Mann-Whitney test), in N'zikro (p = 0.012, Mann-Whitney test) and in Ehania-V1 (p = 0.016: Mann-Whitney test).
3.2. IgG levels to gSG6-P1 salivary peptide in age groups according to villages in dry and rainy season
IgG levels to gSG6-P1 salivary peptide were evaluated according to three age groups (1–4; 5–9 and 10–14 years old; Fig. 3) in the dry (Fig. 3a) and rainy (Fig. 3b) seasons. The same trend as observed for the whole population studied was observed according to villages in all age groups. First, the specific IgG responses differed significantly between villages in all age groups (p < 0.0001, Kruskal-Wallis test) both in the dry and rainy seasons. Secondly, the multiple pairwise comparisons between villages showed that specific IgG responses were significantly higher in agricultural villages than in Ayébo village in all age groups in the dry and rainy seasons (p < 0.0001, Mann-Whitney test, data not shown).
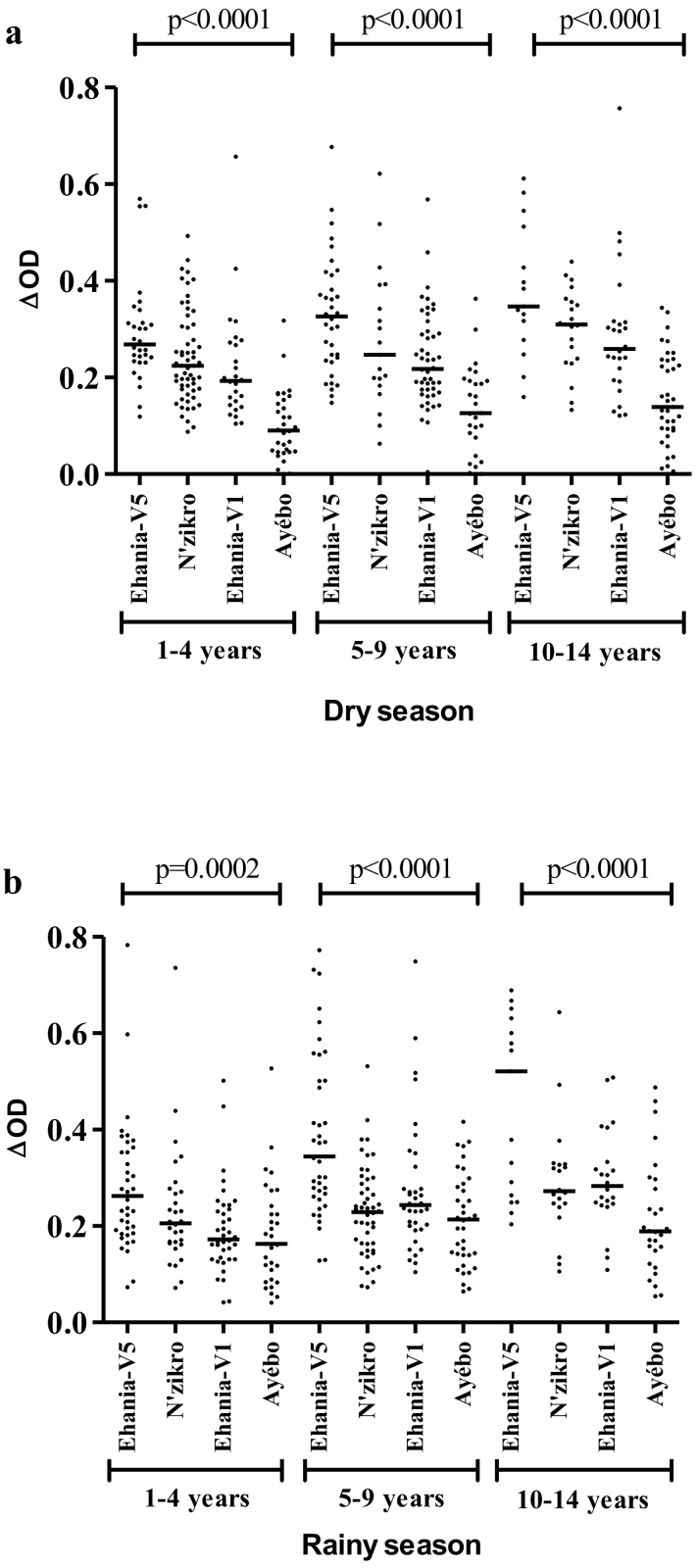
Fig. 3.
IgG response to gSG6-P1 according to villages in age group in dry and rainy season: Specific IgG responses are presented for dry season (a) and rainy season (b). Points indicate individual IgG level (ΔOD) and bars indicate the median value for each village. Statistical significant differences between two villages are indicated (the Kruskal-Wallis U test non-parametric test).
According to age groups, the levels of specific IgG in children of 10–14 years old of Ehania-V5 village were significantly higher than those recorded in 1–4 years and 5–9 both in the dry and rainy season (p < 0.0001).
3.3. Effect of seasons on IgG levels to gSG6-P1 salivary peptide
In the objective to highlight the direct influence of seasons (dry versus rainy), combined or not with agricultural activities, the evolution of specific IgG response was separately compared in each village studied (Fig. 4).
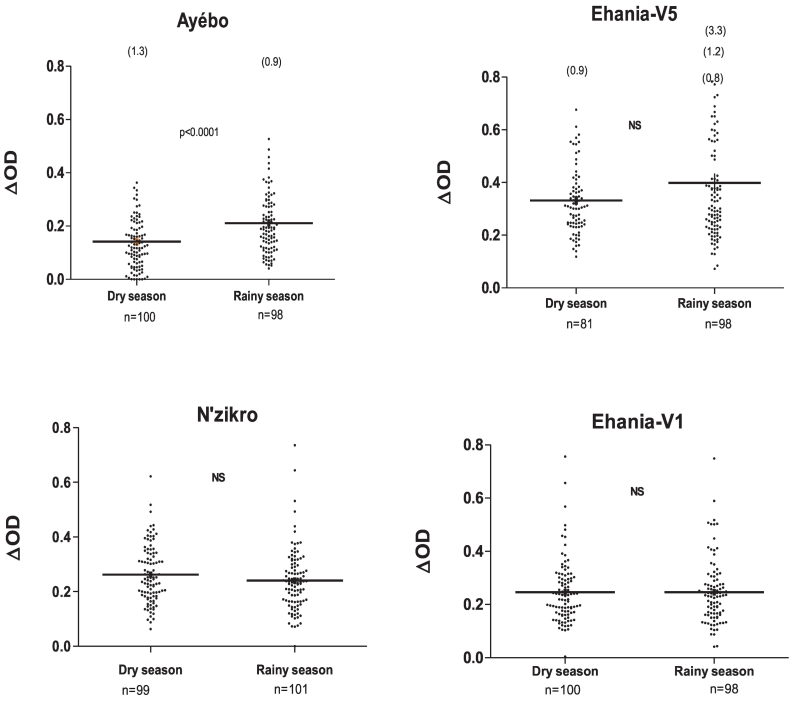
Fig. 4.
Impact of seasons on IgG response to gSG6-P1 according to villages: The results are presented in dry season and in rainy season in Ayébo (a), N'zikro (b), Ehania-V1 (c) and Ehania-V5 (d). Points indicate individual specific IgG level and bars indicate the median value for each village. Numbers in parentheses above the dot plots indicates values above ΔOD = 0.8. Statistical significant differences between dry and rainy season are indicated for each village (P value of the Mann-Whitney non-parametric test).
In Ayébo (control village), the results showed significant variations of IgG responses to gSG6-P1 between the seasons. Indeed, specific IgG levels significantly increased in the rainy season compared to the dry season, (p < 0.0001, Mann-Whitney test). In contrast, in agricultural villages (N'zikro, Ehania-V1 and Enania-V5), no significant variation of specific IgG levels was observed between seasons whatever the type of plantations (palm oil, rubber plantation) considered.
3.4. Socio-epidemiological factors on IgG levels to gSG6-P1 salivary peptide
The level of IgG to gSG6-P1 peptide was analyzed according to major socio-epidemiological characteristics (age, sex, and ITNs use) which could modulate the level of human exposure to Anopheles bites, both during the dry and rainy seasons (Table 1).
Table 1.
Influence of sociodemographic characteristics on IgG levels (median) to gSG6-P1 salivary peptide.
| Dry season |
Rainy season |
||||||
|---|---|---|---|---|---|---|---|
| N | Median | P values | N | Median | P values | ||
| Ayébo | |||||||
| Age (years) | 1–4 | 32 | 0.090 | 0.070 | 31 | 0.163 | 0.271 |
| 5–9 | 29 | 0.126 | 38 | 0.213 | |||
| 10–14 | 39 | 0.139 | 29 | 0.189 | |||
| Gender | M | 50 | 0.153 | 0.059 | 40 | 0.166 | 0.128 |
| F | 50 | 0.097 | 58 | 0.200 | |||
| ITNs | Yes | 19 | 0.136 | 0.871 | 19 | 0.157 | 0.321 |
| No | 81 | 0.1175 | 79 | 0.194 | |||
| N'zikro | |||||||
| Age | 1–4 | 58 | 0.223 | 0.041 | 30 | 0.206 | 0.039 |
| 5–9 | 19 | 0.246 | 51 | 0.228 | |||
| 10–14 | 22 | 0.31 | 20 | 0.240 | |||
| Gender | M | 47 | 0.245 | 0.756 | 43 | 0.23 | 0.915 |
| F | 52 | 0.238 | 58 | 0.237 | |||
| ITNs | Yes | 8 | 0.211 | 0.166 | 85 | 0.224 | 087 |
| No | 91 | 0.251 | 98 | 0.232 | |||
| Ehania V1 | |||||||
| Age | 1–4 | 26 | 0.193 | 0.065 | 39 | 0.171 | <0.0001 |
| 5–9 | 48 | 0.218 | 37 | 0.244 | |||
| 10–14 | 26 | 0.259 | 22 | 0.28 | |||
| Gender | M | 47 | 0.232 | 0.944 | 55 | 0.231 | 0.960 |
| F | 53 | 0.22 | 43 | 0.242 | |||
| ITNs | Yes | 35 | 0.191 | 0.033 | 38 | 0.233 | 0.521 |
| No | 65 | 0.247 | 60 | 0.238 | |||
| Ehania V5 | |||||||
| Age | 1–4 | 30 | 0.268 | 0.079 | 41 | 0.258 | 0.003 |
| 5–9 | 36 | 0.326 | 41 | 0.344 | |||
| 10–14 | 15 | 0.347 | 17 | 0.520 | |||
| Gender | M | 34 | 0.290 | 0.488 | 52 | 0.296 | 0.856 |
| F | 47 | 0.312 | 47 | 0.311 | |||
| ITNs | Yes | 31 | 0.277 | 0.189 | 25 | 0.236 | 0.003 |
| No | 50 | 0.325 | 74 | 0.357 | |||
N: represents the number of children according to sociodemographic characteristics.
In the dry season, no significant difference was observed in specific IgG level according to age group in Ayébo, Ehania-V1 and Ehania-V5 (p > 0.05). Only in N'zikro village, the specific IgG levels significantly differed between age groups (p = 0.041). The median of specific IgG levels progressively increased from 0.223 to 0.310 in young to older children, respectively.
Related to gender, the statistical analysis showed no significant difference of specific IgG levels according to all villages in the dry season (p > 0.05).
No significant difference was also observed in specific IgG levels according to the ITNs use (YES/NO) in Ayébo, N'zikro and Ehania-V5 (p > 0.05). In Ehania-V1, a significant difference was observed according to ITNs use with lower IgG level for children declaring to use an ITN (p = 0.033).
In the rainy season, significant differences were observed according to age groups in N'zikro, Ehania-V1 and Ehania-V5 villages and specific IgG level increased with age (p < 0.001). No significant difference of specific IgG Ab levels according to gender was observed in all villages in this season (p > 0.05). Only in Ehania-V5 village, the level of specific IgG was significantly lower in ITNs users (p = 0.003) as observed in the dry season.
Furthermore, the impact of the use of ITNs on specific IgG levels was compared between Ayébo (control village) and cumulated agricultural villages (data not shown). Our analyses indicated that specific IgG levels remained significantly higher in agricultural villages compared to control village in the dry season (p < 0.0001) and in the rainy season (p = 0.026) in children who declared to sleep under ITNs. These results suggested no impact of ITNs use on the difference of specific IgG level according to agricultural practices.
4. Discussion
This study described for the first time the use of Anopheles gSG6-P1 salivary biomarker to evaluate the impact of agricultural practices on the level of human exposure to malaria vector bites by comparing immunological results in agricultural villages and a control village without agricultural activity in its immediate environment. One major advantage of such biomarker is to assess the level of human exposure at individual level compared to classical entomological methods which give indirect information related to the density of mosquito at a geographic level.
The results of the present study showed variations of specific IgG level to gSG6-P1 within and between the different agro-ecosystems. This suggests that the IgG response to gSG6-P1 salivary peptide could be an adequate indicator to identify the specific populations at higher risk of malaria transmission in the same geographical area. Similar trend was mentioned by Sagna et al. in villages in northern Senegal. These authors observed that IgG levels to gSG6-P1 highly varied according to villages, discriminating the heterogeneity of Anopheles exposure between villages in the same region (Sagna et al., 2013b).
Children living in agricultural villages significantly developed higher IgG responses to the gSG6-P1 than those from Ayébo, a control village without agricultural plantations in its immediate environment. These results were observed both in the dry and rainy seasons. Some studies have shown that agricultural practices could be associated with a high level of Anopheles bites which impact on malaria transmission (Somboon et al., 1998; Yasuoka and Levins, 2007). Palm oil and rubber plantations could constitute artificial environments with their own ecosystem, which could favor Anopheles populations and by consequences, malaria transmission (Pluess et al., 2009).
The high IgG response to gSG6-P1 in Ehania-V5 compared to others agricultural villages suggests that children living in this village were more exposed to Anopheles bites. The immediate environment, characterized by rural area and also by exclusive palm plantation zone (Ehania-V5 is located in the heart of industrial palm oil plantations) could explain this pattern. Besides of this, ripe palm fruits are collected by PALMCI trucks and in most cases it could create deep wheel tracks on the ground. With rainfall, such tracks could probably present potential breeding sites for An. gambiae (Tanga et al., 2011). Indeed, the Ehania-V5 results, especially in rainy season, indicated substantial variations of anti-gSG6-P1 IgG level between individuals within this village. Even if entomological data were not available in the present study, previous study clearly indicated a positive association between the exposure levels to An. gambiae bites, estimated by classical entomological methods and the specific IgG levels reflecting the real contact between human populations and Anopheles mosquitoes (Drame et al., 2012). One hypothesis of the high level of human exposition of mosquito bites observed in N'zikro, could involve the presence of many bowls (1.15 l) on each rubber tree. Indeed, bowls are used to collect the latex which coagulates on the bottom. Such bowls retain rainfall and could thus act also as Anopheles breeding sites (Assako-Assako et al., 2005). Nevertheless, future entomological studies on the potential breeding sites in the trees have to be performed to validate this hypothesis in our studied area.
In the objective to highlight the classical association between human exposure to mosquito bites and the season, the evolution of IgG levels to gSG6-P1 salivary peptide was compared in Ayébo “control village” according to seasons. Specific IgG responses significantly increased in the rainy season compared to the dry season. High temperature during the dry season can reduce the survival of mosquitoes and, in contrast, during the rainy season, the rain classically creates breeding sites on the soil. According to our immunological results, children living in Ayébo were more bitten by of Anopheles vector during the rainy season than the dry season. It was a classical observation because it is well known that Anopheles populations are higher during the rainy season in Côte d'Ivoire context (Dossou-yovo, 2000). This season-dependent effect was also observed in Northern Senegal where the anti-gSG6-P1 IgG levels were significantly higher in the rainy season than in the dry season (Poinsignon et al., 2010; Sagna et al., 2013b; Drame et al., 2013). The evolution of specific IgG response was then compared within each agricultural village between the dry and rainy seasons. IgG level responses in agricultural villages remained higher than Ayébo village in both the seasons. Interestingly, in contrast to Ayebo control village, no significant difference in specific IgG response was observed between both the seasons in the agricultural villages. This finding suggested an influence of agricultural activities on the evolution of IgG response to gSG6-P1, biomarker of the real human-Anopheles contact, according to the season. In others terms, agro-ecosystem villages seemed to maintain higher human exposure to vector bites in the dry season, at a similar level that observed in the rainy season in areas without agricultural activities. Children in agro-ecosystem sites could be thus permanently and highly exposed to Anopheles bites both in the dry and rainy seasons. Similar trends were recorded by Afrane et al., in Kumassi Ghana, using entomological methods. The authors found that EIR (entomological inoculation rate) were higher in agricultural areas than in non-agricultural areas, whatever the season (Afrane et al., 2004). In the same way, another study in Cameroon reported that malaria transmission occurred both in the dry and rainy seasons with the intensities peaking in the dry season, in rubber cultivated area (Bigoga et al., 2012). In the present study, environmental modifications due to agriculture activities might have influenced the Anopheles vector distribution and the dynamics of malaria transmission in the three agricultural villages. Similar results were also reported by Nzeyimana et al. in extensive cultivation of rice in south-western forest of Côte d'Ivoire (Nzeyimana et al., 2002) and by Mutero et al. in agro-ecosystem of rice in central Kenya (Mutero et al., 2004) which favored high malaria transmission. High human exposure to malaria transmission, whatever the season, can induce a high risk for all residents, especially for travelers and other non-immune workers. The comparison of IgG levels to gSG6-P1 peptide in children only using ITNs between Ayébo and agricultural areas showed that specific IgG responses remained significantly higher in agricultural villages compared to control village. Globally, this observation suggests no impact of ITNs use on the difference of specific IgG level according to agricultural practices. It indicated that ITNs use could not be considered as variation factor to explain agricultural-dependant results of the present study.
Nevertheless, this result could also involve i) the low used of ITNs observed in agricultural areas by children, which do not allow efficient protection against Anopheles bites (Koudou et al., 2010) and/or ii) the changes of Anopheles behavior which could bitten individuals outdoor during day period without ITN coverage (the evening before to go to sleep and early morning), as previously suggested (Moiroux et al., 2014). In addition, these results suggests that higher exposure to Anopheles bites in agricultural villages, and by consequences higher nighttime nuisance, did not seem to elicit a higher ITNs use by children whatever the season. According to the results of the present study, it could be thus recommended that ITN implementation and use by populations, or other vector control strategies, could be increased or favored in areas with agricultural activities and plantation companies, and this whatever the seasons.
5. Conclusion
This study showed that agricultural activities, such as rubber and palm cultivations, could maintain a high level of human exposure to Anopheles bites. These agricultural practices could therefore represent a permanent risk of malaria transmission whatever the season. People living in agricultural villages could be at high risk of malaria during the whole year, and combined vector control strategies could be implemented to reduce Anopheles vector densities in these particular cultivated areas.
Conflict of interest
The authors declare that they have no competing interests.
Authors' contributions
Cécile Agnimou Malanfoua Sadia-Kacou, Maurice Akré Adja, Benjamin Guibehi Koudou, Yao Tano and Franck Remoue conceived and designed the study. Cécile Agnimou Malanfoua Sadia-Kacou, collected data, performed all ELISA and drafted the manuscript. Benjamin Guibehi Koudou and Maurice Akré Adja, coordinated the field activities and read the manuscript. Emmanuel Elanga Ndille has the responsibility to train CAMSK to ELISA technology and to follow the results. Cécile Agnimou Malanfoua Sadia-Kacou, Anne Poinsignon, André Barembaye Sagna and Emmanuel Elanga Ndille interpreted the data and revised the manuscript. Céline Mabot Yobo, contributed to data analysis and revised the manuscript. Tano Yao, supervised the study. All authors read and approved the final manuscript.
Acknowledgements
The authors gratefully acknowledge the population of N'Zikro, Ayébo and the Ehania villages of Integrated Agricultural Unit (IAU) of the PALM-CI industry and, especially their participants and their parents. The authors also thank gratefully the village authorities of the study sites and the administrative authorities of the Ehania IAU for accepting and supporting this study in their localities. The authors thank Dr. Adonis N'Guessan, head doctor of Ehania IAU hospital for his support, Coulibaly Bamoro and Adou Arsène for their help to realize the map of study area. This study was integrated to malaria surveys and had all the support of the Malaria National Control Program, which has provided us with anti-malarial and facilitated collaboration with study sites health agents. This collaboration was also facilitated by the Aboisso Departmental and Regional Health Director.
Contributor Information
Anne Poinsignon, Email: anne.poinsignon@ird.fr.
Franck Remoue, Email: franck.remoue@ird.fr.
References
- Adja A.M., N'goran E.K., Koudou B.G., Dia I., Kengne P., Fontenille D., Chandre F. Contribution of Anopheles funestus, An. gambiae and An. nili (Diptera: Culicidae) to the perennial malaria transmission in the southern and western forest areas of Cote d'Ivoire. Ann. Trop. Med. Parasitol. 2011;105:13–24. doi: 10.1179/136485910X12851868780388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Afrane Y.A., Klinkenberg E., Drechsel P., Owusu-Daaku K., Garms R., Kruppa T. Does irrigated urban agriculture influence the transmission of malaria in the city of Kumasi, Ghana? Acta Trop. 2004;89:125–134. doi: 10.1016/j.actatropica.2003.06.001. [DOI] [PubMed] [Google Scholar]
- Assako-Assako R.J., Bley D., Simard F. Apport des sciences sociales et de l'entomologie dans l'analyse de l'endemicite du paludisme a HEVECAM, une agroindustrie du Sud-Cameroun. Revue Internationale de Géologie, de Géographie et d'Ecologie Tropicales. 2005;30(1):101–114. [Google Scholar]
- Bigoga J.D., Nanfack F.M., Awono-Ambene P.H., Patchoke S., Atangana J., Otia V.S., Fondjo E., Moyou R.S., Leke R.G. Seasonal prevalence of malaria vectors and entomological inoculation rates in the rubber cultivated area of Niete, South Region of Cameroon. Parasit. Vectors. 2012;5:197. doi: 10.1186/1756-3305-5-197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diakité N.R., Adja A.M., Von S.T., Utzinger J., N'goran E.K. Situation épidémiologique avant la mise en eau du barrage hydroagricole de cinq villages de Bouaké, centre Côte d'Ivoire. Bull. Soc. Pathol. Exot. 2010;103:22–28. doi: 10.1007/s13149-009-0029-4. [DOI] [PubMed] [Google Scholar]
- Doannio J.M., Dossou-Yovo J., Diarrassouba S., Rakotondraibe M.E., Chauvancy G., Chandre F., Riviere F., Carnevale P. Dynamics of malaria transmission in Kafine, a rice growing village in a humid savannah area of Cote d'Ivoire. Bull. Soc. Pathol. Exot. 2002;95:11–16. [PubMed] [Google Scholar]
- Dossou-Yovo, J. 2000. Etude éthologique des moustiques vecteurs du paludisme en rapport avec les aspects parasitologiques de la transmission du Plasmodium dans la région de Bouaké. Thèse de Doctorat d'Etat en Entomologie Médicale.
- Drame, P. M., Poinsignon, A., Besnard, P., Cornelie, S., Le Mire, J., Toto, J. C., Foumane, V., Dos-Santos, M. A., Sembene, M., Fortes, F., Simondon, F., Carnevale, P. & Remoue, F. 2010a. Human antibody responses to the Anopheles salivary gSG6-P1 peptide: a novel tool for evaluating the efficacy of ITNs in malaria vector control. PLoS One, 5, e15596. [DOI] [PMC free article] [PubMed]
- Drame P.M., Poinsignon A., Besnard P., Le Mire J., Dos-Santos M.A., Sow C.S., Cornelie S., Foumane V., Toto J.C., Sembene M., Boulanger D., Simondon F., Fortes F., Carnevale P., Remoue F. Human antibody response to Anopheles gambiae saliva: an immuno-epidemiological biomarker to evaluate the efficacy of insecticide-treated nets in malaria vector control. Am. J. Trop. Med. Hyg. 2010;83:115–121. doi: 10.4269/ajtmh.2010.09-0684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drame P.M., Machault V., Diallo A., Cornelie S., Poinsignon A., Lalou R., Sembene M., Dos Santos S., Rogier C., Pages F., Le Hesran J.Y., Remoue F. IgG responses to the gSG6-P1 salivary peptide for evaluating human exposure to Anopheles bites in urban areas of Dakar region. Senegal. Malar J. 2012;11:72. doi: 10.1186/1475-2875-11-72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drame P.M., Diallo A., Poinsignon A., Boussari O., Dos Santos S., Machault V., Lalou R., Cornelie S., Lehesran J.Y., Remoue F. Evaluation of the effectiveness of malaria vector control measures in urban settings of Dakar by a specific anopheles salivary biomarker. PLoS One. 2013;8 doi: 10.1371/journal.pone.0066354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henry M.C., Rogier C., Nzeyimana I., Assi S.B., Dossou-Yovo J., Audibert M., Mathonnat J., Keundjian A., Akodo E., Teuscher T., Carnevale P. Inland valley rice production systems and malaria infection and disease in the savannah of Cote d'Ivoire. Tropical Med. Int. Health. 2003;8:449–458. doi: 10.1046/j.1365-3156.2003.01053.x. [DOI] [PubMed] [Google Scholar]
- Houngbedji C.A., N'dri P.B., Hurlimann E., Yapi R.B., Silue K.D., Soro G., Koudou B.G., Acka C.A., Assi S.B., Vounatsou P., N'goran E.K., Fantodji A., Utzinger J., Raso G. Disparities of Plasmodium falciparum infection, malaria-related morbidity and access to malaria prevention and treatment among school-aged children: a national cross-sectional survey in Cote d'Ivoire. Malar. J. 2015;14:7. doi: 10.1186/1475-2875-14-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keiser J., De Castro M.C., Maltese M.F., Bos R., Tanner M., Singer B.H., Utzinger J. Effect of irrigation and large dams on the burden of malaria on a global and regional scale. Am. J. Trop. Med. Hyg. 2005;72:392–406. [PubMed] [Google Scholar]
- Koudou B.G., Tano Y., Doumbia M., Nsanzabana C., Cisse G., Girardin O., Dao D., N'goran E.K., Vounatsou P., Bordmann G., Keiser J., Tanner M., Utzinger J. Malaria transmission dynamics in central cote d'Ivoire: the influence of changing patterns of irrigated rice agriculture. Med. Vet. Entomol. 2005;19:27–37. doi: 10.1111/j.0269-283X.2005.00530.x. [DOI] [PubMed] [Google Scholar]
- Koudou B.G., Adja A.M., Matthys B., Doumbia M., Cisse G., Kone M., Tanner M., Utzinger J. Agricultural activities and malaria transmission in two eco-epidemiological settings in central Cote d'Ivoire. Bull. Soc. Pathol. Exot. 2007;100:124–126. [PubMed] [Google Scholar]
- Koudou B.G., Ghattas H., Essé C., Nsanzabana C., Rohner F., Utzinger J., Faragher E.B., Tschannen B.A. The use of insecticide-treated nets for reducing malaria morbidity among children aged 6-59 months, in an area of high malaria transmission in central Côte d' Ivoire. Parasit. Vectors. 2010;3:91. doi: 10.1186/1756-3305-3-91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martens P., Hall L. Malaria on the move: human population movement and malaria transmission. Emerg. Infect. Dis. 2000;6:103–109. doi: 10.3201/eid0602.000202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moiroux N., Damien G., Egrot M., Djenontin A., Chandre F., Corbel V., Killeen G., Pennetier C. H u m a n e x p o s u r e t o e a r l y m o r n i n g Anopheles funestus biting behavior and personal protection provided by long-lasting insecticidal nets. PLoS One. 2014;9(8) doi: 10.1371/journal.pone.0104967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mutero C.M., Kabutha C., Kimani V., Kabuage L., Gitau G., Ssennyonga J., Githure J., Muthami L., Kaida A., Musyoka L., Al E. A transdisciplinary perspective on the links between malaria and agroecosystems in Kenya. Acta Trop. 2004;89:171–186. doi: 10.1016/j.actatropica.2003.07.003. [DOI] [PubMed] [Google Scholar]
- Nzeyimana I., Henry M.C., Dossou-Yovo J., Doannio J.M., Diawara L., Carnevale P. The epidemiology of malaria in the southwestern forests of the Ivory Coast (Tai region) Bull. Soc. Pathol. Exot. 2002;95:89–94. [PubMed] [Google Scholar]
- Pluess B., Mueller I., Levi D., King G., Smith T.A., Lengeler C. Malaria–a major health problem within an oil palm plantation around Popondetta. Papua New Guinea. Malar J. 2009;8:56. doi: 10.1186/1475-2875-8-56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poinsignon A., Cornelie S., Mestres-Simon M., Lanfrancotti A., Rossignol M., Boulanger D., Cisse B., Sokhna C., Arca B., Simondon F., Remoue F. Novel peptide marker corresponding to salivary protein gSG6 potentially identifies exposure to Anopheles bites. PLoS One. 2008;3 doi: 10.1371/journal.pone.0002472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poinsignon A., Cornelie S., Ba F., Boulanger D., Sow C., Rossignol M., Sokhna C., Cisse B., Simondon F., Remoue F. Human IgG response to a salivary peptide, gSG6-P1, as a new immuno-epidemiological tool for evaluating low-level exposure to Anopheles bites. Malar. J. 2009;8:198. doi: 10.1186/1475-2875-8-198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poinsignon A., Samb B., Doucoure S., Drame P.M., Sarr J.B., Sow C., Cornelie S., Maiga S., Thiam C., Rogerie F., Guindo S., Hermann E., Simondon F., Dia I., Riveau G., Konate L., Remoue F. First attempt to validate the gSG6-P1 salivary peptide as an immuno-epidemiological tool for evaluating human exposure to Anopheles funestus bites. Tropical Med. Int. Health. 2010;15:1198–1203. doi: 10.1111/j.1365-3156.2010.02611.x. [DOI] [PubMed] [Google Scholar]
- Sagna A., Poinsignon A., Remoue F. Arthropod Vector: Controller of Disease Transmission. Vol. 2. 2017. Chapter 12 –epidemiological applications of assessing mosquito exposure in a malaria-endemic area; pp. 209–229. [Google Scholar]
- Sagna A.B., Gaayeb L., Sarr J.B., Senghor S., Poinsignon A., Boutouaba-Combe S., Schacht A.M., Hermann E., Faye N., Remoue F., Riveau G. Plasmodium falciparum infection during dry season: IgG responses to Anopheles gambiae salivary gSG6-P1 peptide as sensitive biomarker for malaria risk in northern Senegal. Malar. J. 2013;12:301. doi: 10.1186/1475-2875-12-301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sagna A.B., Sarr J.B., Gaayeb L., Drame P.M., Ndiath M.O., Senghor S., Sow C.S., Poinsignon A., Seck M., Hermann E., Schacht A.M., Faye N., Sokhna C., Remoue F., Riveau G. gSG6-P1 salivary biomarker discriminates micro-geographical heterogeneity of human exposure to Anopheles bites in low and seasonal malaria areas. Parasit. Vectors. 2013;6:68. doi: 10.1186/1756-3305-6-68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somboon P., Aramrattana A., Lines J., Webber R. Entomological and epidemiological investigations of malaria transmission in relation to population movements in forest areas of north-west Thailand. Southeast Asian J. Trop. Med. Public Health. 1998;29:3–9. [PubMed] [Google Scholar]
- Tanga M.C., Ngundu W.I., Tchouassi P.D. Daily survival and human blood index of major malaria vectors associated with oil palm cultivation in Cameroon and their role in malaria transmission. Tropical Med. Int. Health. 2011;16:447–457. doi: 10.1111/j.1365-3156.2011.02726.x. [DOI] [PubMed] [Google Scholar]
- WHO . World Health Organization; Geneva: 2017. World Malaria Report. [Google Scholar]
- Yasuoka J., Levins R. Impact of deforestation and agricultural development on anopheline ecology and malaria epidemiology. Am. J. Trop. Med. Hyg. 2007;76:450–460. [PubMed] [Google Scholar]
- Ya-Umphan P., Cerqueira D., Parker D.M., Cottrell G., Poinsignon A., Remoue F., Brengues C., Chareonviriyaphap T., Nosten F., Corbel V. Use of an Anopheles salivary biomarker to assess malaria transmission risk along the Thailand-Myanmar border. J. Infect. Dis. 2017;215:396–404. doi: 10.1093/infdis/jiw543. [DOI] [PMC free article] [PubMed] [Google Scholar]