Abstract
Herpes simplex virus type 1 and type 2 (HSV-1 & HSV-2) are one of the leading causes of ulcer and blister lesions worldwide. These infections are latent with recurrences but many people may have a seropositive antibody yet remain asymptomatic. Although HSV presenting with hypertrophic lesions have been reported in the literature at urogenital, lung, and conjunctival sites, we describe a case of a mass lesion in the nasal cavity of a 46 year-old female with a history of human immunodeficiency virus (HIV). The patient presented initially with nasal congestion and subsequently developed facial edema. The mass lesion regressed after one month of treatment with valacyclovir.
Keywords: Nasal hypertrophic lesion, Nasal pseudotumor, Herpes simplex virus, Human immunodeficiency virus
Introduction
Based upon a Centers for Disease Control and Prevention CDC report, among people aged 14–49, the prevalence of HSV-1 and HSV-2 are 47.8% and 11.9%, respectively [1]. Skin or mucosal infections can occur at any anatomic site, however the most commonly affected sites are the oral, labial, urogenital area [2], with lung [3] and conjunctival [4,5] involvement being less common. The symptoms of HSV-1 and HSV-2 may be asymptomatic, subtle or non-specific, including: fever, headache, malaise, myalgia, tender lymphadenopathy, localized pain, and pruritus [6].
A retrospective study in Thailand indicated that urogenital hypertrophic manifestations were found in 4.8% of patients with HSV infections―all patients with hypertrophic masses were HIV-coinfected [7]. Several studies have indicated that a low CD4 count or low HIV viral load are not associated with hypertrophic lesions [7,8].
Case presentation
A 46-year-old female, with past medical history significant for HIV, presented to the emergency department with a two-day history of right nasal congestion. She was diagnosed with HIV in 2003 and was non-adherent with her medications. She was previously prescribed fixed combination emtricitabine-tenofovir 1 tablet daily and dolutegravir 1 tablet daily.
Her initial vital signs were stable and the patient denied any sick contacts, fever, chills, cough, sore throat, myalgia, or unintentional weight loss. The physical exam was significant only for hypertrophied turbinates with nasal polyps. Initial ED laboratory values indicated a white blood cell of 3.41 K/μL, neutrophil 21.8%, lymphocyte 51.5%, and monocyte 17.8%. A chest x-ray was unremarkable. The patient was originally diagnosed with sinusitis and treated with amoxicillin-clavulanate 500 mg three times daily and fluticasone propionate spray daily for seven days.
Three weeks later, the patient returned to the emergency department with right-sided maxillary facial edema. The patient completed her course of discharged medications without a significant change in her previous symptoms. On physical exam, she had right-sided non-tender facial edema and a protruding, visible exudative mass in her right inferior turbinate (Fig. 1).
Fig. 1.
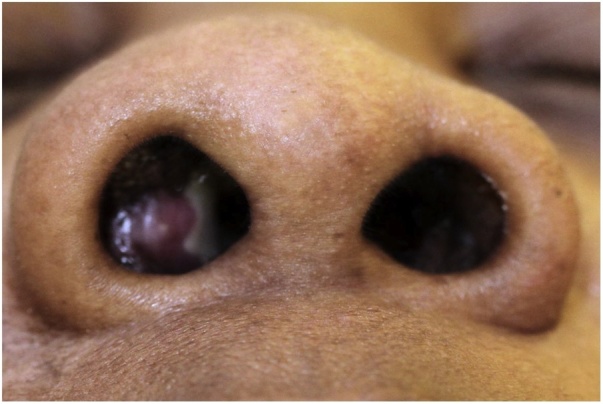
Exudative mass of right inferior turbinate.
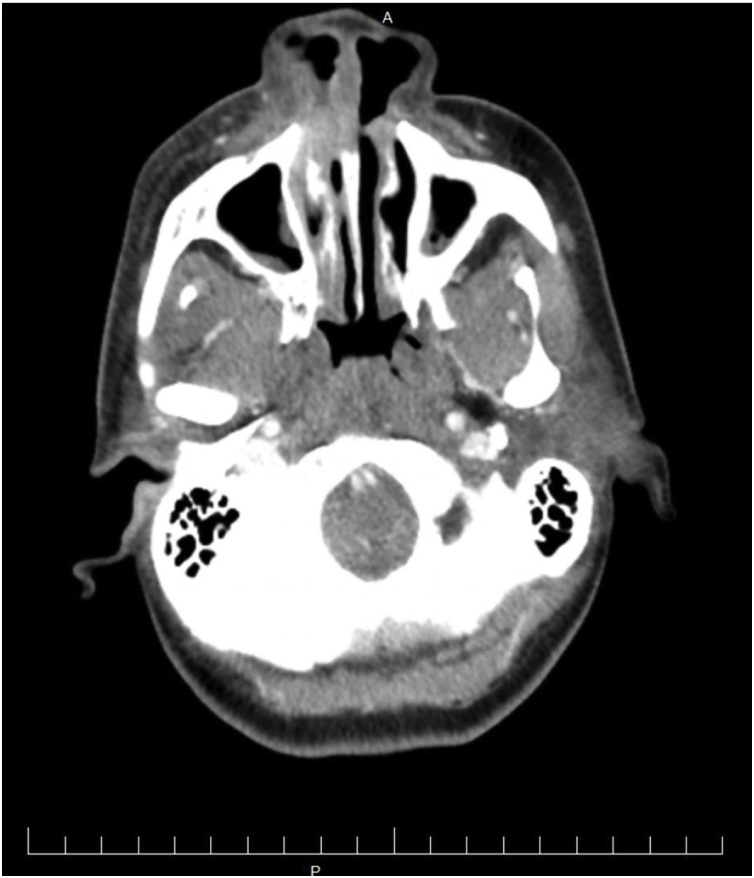
Her vital signs were stable and laboratory studies indicated: white blood cell count 3.33 K/μL, neutrophil count 11.1%, lymphocyte count 66.4%, monocyte count 10.2%, HIV viral load 14,825 copies/mL and an absolute CD4 count of 232/μL(11%). In addition, HSV-1 IgG antibody was 12.30 (negative <0.89) and HSV-2 IgG antibody was 12.40 (negative <0.89). A herpes viral culture was negative. A computed tomography (CT) scan of the head was performed which revealed a soft tissue opacification centered over the right distal nasal cavity with extension into the right naris (Fig. 2).
Fig. 2.
CT scan show soft tissue opacification centered over the right distal nasal cavity.
Fine needle aspiration of the mass failed to aspirate any purulent material. A biopsy indicated a HSV cytopathic effect and an immunohistochemistry stain was positive for HSV-1 and HSV-2 (Fig. 3, Fig. 4). Molecular testing using polymerase chain reaction (PCR) identified HSV-1 and HSV-2 in the tissue specimen. The patient was started on valacyclovir 1 g three times daily for one month. She demonstrated gradual resolution of her symptoms and is currently being followed at our Infectious Diseases Clinic.
Fig. 3.
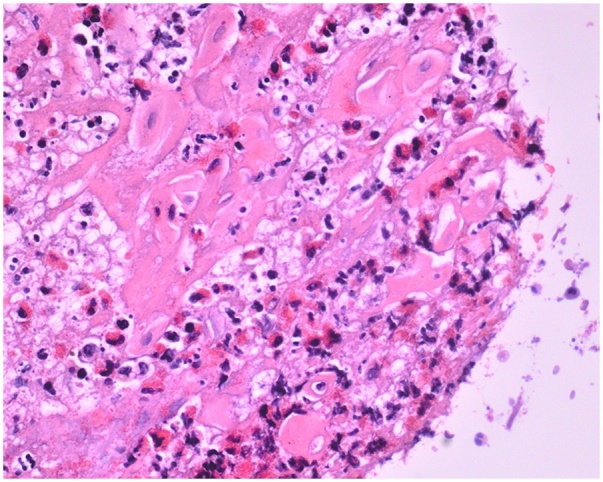
Biopsy shows HSV cytopathic effect with H&E stain.
Fig. 4.
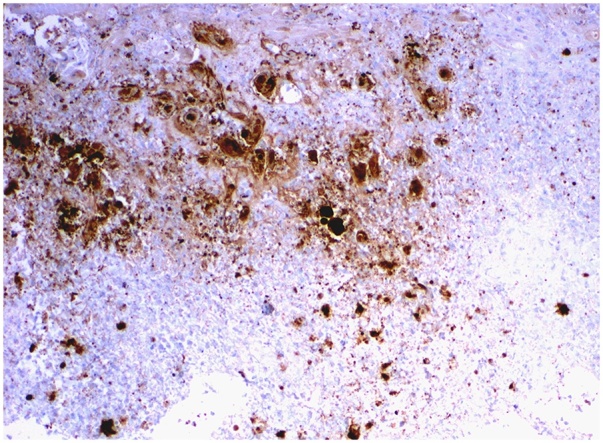
Immunohistochemistry stain is positive for HSV-1 and HSV-2. This Anti-HSV immunohistochemical stain identifies glycoproteins specifically found in HSV infected tissue, but this antibody identifies both HSV-1 and HSV-2, and does not allow the distinction between the two types. This antibody does not cross-react with cytomegalovirus, Epstein-Barr virus, or varicella zoster virus.
Discussion
HSV shedding is increased in people with a concomitant HIV infection. Furthermore, a HIV-infected host has prolonged severity of episodes when compared with a non HIV-infected host. Methods to diagnose HSV include: viral culture, Tzanck smear, serology, and PCR. Although viral culture is the traditional gold standard of diagnosis, the sensitivity is only about 50%–75% and results may not be immediately available [9].
For patients with HSV-associated hypertrophic mass lesions, the visible mass takes time to grow. Surgical removal may be appropriate for these patients if they lack a response to medication, or in order to get a high-quality sample for diagnosis. Antiviral treatment such as acyclovir, valacyclovir, and ganciclovir are the mainstay of treatment, while immunomodulators such as topical imiquimod, oral leflunomide, and oral thalidomide have demonstrated clinical improvement in several cases [5,7,8,10].
Conclusion
In summary, this case illustrates a nasal hypertrophic lesion caused by HSV-1 and HSV-2. Mass lesions are a rare presentation of HSV in HIV-infected patients. Clinicians should be aware that biopsy of the mass lesion is the easiest way to reach a diagnosis and to rule out malignancy or an abscess caused by a bacterial infection. HSV PCR testing of a sample should be sent when an immunocompromised patient has a nasal lesion. However, resection of the mass may not be necessary to accelerate treatment. Oral antiviral therapy is effective to decrease the size of the mass lesion slowly, as clinical symptoms improved in this case. Further research is needed to develop the treatment duration and prevention of recurrent mass lesions.
Conflicts of interest
The author declares that there are no conflicts of interest regarding the publication of this article.
CRediT authorship contribution statement
Chia-Yu Chiu: Writing - original draft. Gurchetan Randhawa: Writing - original draft. Khaled Nada: Visualization, Supervision. Ewa Tomczak: Visualization, Supervision. Addi Feinstein: Writing - review & editing, Conceptualization. Karen Hennessey: Conceptualization.
References
- 1.McQuillan G., Kruszon-Moran D., Flagg E.W., Paulose-Ram R. Prevalence of herpes simplex virus type 1 and type 2 in persons aged 14–49: United States, 2015–2016. NCHS Data Brief. 2018;(February (304)):1–8. [PubMed] [Google Scholar]
- 2.Mosunjac M., Park J., Wang W., Tadros T., Siddiqui M., Bagirov M. Genital and perianal herpes simplex simulating neoplasia in patients with AIDS. AIDS Patient Care STDS. 2009;23(March (3)):153–158. doi: 10.1089/apc.2008.0143. [DOI] [PubMed] [Google Scholar]
- 3.Dantas G.C., Shoji H., Hoelz C., Funari M.B., Szarf G. Herpes simplex lesion mimicking left upper lobe bronchial tumour. Thorax. 2018;73(January (1)):94–95. doi: 10.1136/thoraxjnl-2017-210026. [DOI] [PubMed] [Google Scholar]
- 4.Mitra A., Ramin E. Herpes simplex virus and human papillomavirus coinfections in hyperimmunoglobulin E syndrome presenting as a conjunctival mass lesion. Case Rep Med. 2017;2017:1650841. doi: 10.1155/2017/1650841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Vora G.K., Marr B., Cummings T.J., Mruthyunjaya P. Conjunctival pseudotumor caused by herpes simplex virus infection. JAMA Ophthalmol. 2015;133(January (1)):105–107. doi: 10.1001/jamaophthalmol.2014.3316. [DOI] [PubMed] [Google Scholar]
- 6.Bernstein D.I., Bellamy A.R., Hook E.W., 3rd, Levin M.J., Wald A., Ewell M.G. Epidemiology, clinical presentation, and antibody response to primary infection with herpes simplex virus type 1 and type 2 in young women. Clin Infect Dis. 2013;56(February (3)):344–351. doi: 10.1093/cid/cis891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Leeyaphan C., Surawan T.M., Chirachanakul P. Clinical characteristics of hypertrophic herpes simplex genitalis and treatment outcomes of imiquimod: a retrospective observational study. Int J Infect Dis. 2015;33(April):165–170. doi: 10.1016/j.ijid.2015.02.002. [DOI] [PubMed] [Google Scholar]
- 8.Sbidian E., Battistella M., Legoff J., Lafaurie M., Bezier M., Agbalika F. Recalcitrant pseudotumoral anogenital herpes simplex virus type 2 in HIV-infected patients: evidence for predominant B-lymphoplasmocytic infiltration and immunomodulators as effective therapeutic strategy. Clin Infect Dis. 2013;57(December (11)):1648–1655. doi: 10.1093/cid/cit592. [DOI] [PubMed] [Google Scholar]
- 9.Singh A., Preiksaitis J., Ferenczy A. The laboratory diagnosis of herpes simplex virus infections. Can J Infect Dis Med Microbiol. 2005;16(March (2)):92–98. doi: 10.1155/2005/318294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Roger M.R., Anstead G.M. Leflunomide in the treatment of a pseudotumoral genital herpes simplex virus infection in an HIV patient. Case Rep Infect Dis. 2017;2017:1589356. doi: 10.1155/2017/1589356. [DOI] [PMC free article] [PubMed] [Google Scholar]