Abstract
Introduction
Information on the causes of death among under-five children is key in designing and implementation of appropriate interventions. In Uganda, civil death registration is incomplete which limits the estimation of disease-related mortality burden especially at a local scale. In the absence of routine cause-specific data, we used household surveys to quantify the effects and contribution of main childhood diseases such as malaria, severe or moderate anaemia, severe or moderate malnutrition, diarrhoea and acute respiratory infections (ARIs) on all-cause under-five mortality (U5M) at national and sub-national levels. We related all-cause U5M with risks of childhood diseases after adjusting for geographical disparities in coverages of health interventions, socio-economic, environmental factors and disease co-endemicities.
Methods
Data on U5M, disease prevalence, socio-economic and intervention coverage indicators were obtained from the 2011 Demographic and Health Survey, while data on malaria prevalence were extracted from the 2009 Malaria Indicator Survey. Bayesian geostatistical Weibull proportional hazards models with spatially varying disease effects at sub-national scales were fitted to quantify the associations between childhood diseases and the U5M. Spatial correlation between clusters was incorporated via locational random effects while region-specific random effects with conditional autoregressive prior distributions modeled the geographical variation in the effects of childhood diseases. The models addressed geographical misalignment in the locations of the two surveys. The contribution of childhood diseases to under-five mortality was estimated using population attributable fractions.
Results
The overall U5M rate was 90 deaths per 1000 live births. Large regional variations in U5M rates were observed, lowest in Kampala at 56 and highest in the North-East at 152 per 1000 live births. National malaria parasitemia prevalence was 42%, with Kampala experiencing the lowest of 5% and the Mid-North the highest of 62%. About 27% of Ugandan children aged 6–59 months were severely or moderately anaemic; lowest in South-West (8%) and highest in East-Central (46%). Overall, 17% of children were either severely or moderately malnourished. The percentage of moderately/severely malnourished children varied by region with Kampala having the lowest (8%) and North-East the highest (45%). Nearly a quarter of the children under-five years were reported to have diarrhoea at national level, and this proportion was highest in East-Central (32%) and Mid-Eastern (33%) and lowest in South-West (14%). Overall, ARIs in the two weeks before the survey was 15%; highest in Mid-North (22%) and lowest in Central 1 (9%). At national level, the U5M was associated with prevalence of malaria (hazard ratio (HR) = 1.74; 95% BCI: 1.42, 2.16), severe or moderate anaemia (HR =1.37; 95% BCI: 1.20, 1.75), severe or moderate malnutrition (HR = 1.49; 95% BCI: 1.25, 1.66) and diarrhoea (HR = 1.61; 95% BCI: 1.31, 2.05). The relationship between malaria and U5M was important in the regions of Central 2, East-Central, Mid-North, North-East and West-Nile. Diarrhoea was associated with under-five deaths in Central 2, East-central, Mid-Eastern and Mid-Western. Moderate/severe malnutrition was associated with U5M in East-Central, Mid-Eastern and North-East. Moderate/severe anaemia was associated with deaths in Central 1, Kampala, Mid-North, Mid-Western, North-East, South-West and West-Nile.
At the national level, 97% (PAF = 96.9; 95%BCI: 94.4, 98.0), 91% (PAF = 90.9; 95%BCI: 84.4, 95.3), 89% (PAF = 89.3; 95%BCI: 76.0,93.8) and 93% (PAF = 93.3 95%BCI: 87.7,96.0) of the deaths among children less than five years in Uganda were attributable to malaria, severe/moderate anaemia, severe/moderate malnutrition and diarrhoea respectively. The attribution of malaria was comparable in Central 2, East-Central, Mid-North, North-East and West-Nile while severe/moderate anaemia was more common in all regions except Central 2, East-Central and Mid-Eastern. The attribution of diarrhoea in Central 2, East-Central, Mid-Eastern and Mid-Western was similar. The attribution of severe/moderate malnutrition was common in East-Central, Mid-Eastern and North-East.
Conclusion
In Uganda, the contribution and effects of childhood diseases on U5M vary by region. Majority of the under-five deaths are due to malaria, followed by diarrhoea, severe/moderate anaemia and severe/moderate malnutrition. Thus, strengthening disease-specific interventions especially in the affected regions may be an important strategy to accelerate progress towards the reduction of the U5M as per the SDG target by 2030. In particular, Indoor Residual Spraying, iron supplementation, deworming, exclusive breastfeeding, investment in nutrition and education in nutrition practices, oral rehydration therapy or recommended home fluid, improved sanitation facilities should be improved.
Keywords: DHS, Under-five mortality, Malaria, Anaemia, Malnutrition, Diarrhoea, Respiratory infections, Population attributable fractions, Bayesian geostatistical inference, Uganda
1. Introduction
The under-five mortality (U5M) is one of the numerous health challenges in Uganda, accounting for approximately 4% of the U5M burden in Sub-Saharan Africa (Unicef, 2015). In addition, the indicator is one of the most important among those monitoring many Sustainable Development Goals (SDGs) (World Health Organization, 2015). The burden of U5M in Uganda has reduced over time. For instance, between 2006 and 2011, the under-five mortality rate (U5MR) declined from 137 to 90 deaths per 1000 live births (Uganda Bureau of Statistics (UBOS) and ICF International Inc., 2012; Uganda Bureau of Statistics (UBOS) and Macro International Inc., 2007); indicating that Uganda has made considerable progress in improving the health of the under-fives. Similarly, the leading contributors to under-five morbidity and mortality such as malaria, anaemia, malnutrition, diarrhoea and acute respiratory infections (Ministry of Health, 2013a) have dropped. For example, the prevalences of anaemia decreased from 73% to 49% (Uganda Bureau of Statistics (UBOS) and ICF International Inc., 2012; Uganda Bureau of Statistics (UBOS) and Macro International Inc., 2007).
Despite the huge national improvements, the burden of mortality and childhood diseases is still high and disproportionately distributed among regions. The lowest malnutrition prevalence (20%) was reported in Kampala and the highest (51%) in the South-West (Uganda Bureau of Statistics (UBOS) and ICF International Inc., 2012). Also, wide regional variations in the U5MR occurred during the same time; with the North-East experiencing the highest (152 deaths per 1000 live births) and the lowest (56 deaths per 1000 live births) occurring in Kampala region (Uganda Bureau of Statistics (UBOS) and ICF International Inc., 2012). Whether regional disparities in U5MR are a result of varying childhood diseases across regions needs to be examined.
In Uganda, few studies have assessed the relation between childhood diseases and U5M. Furthermore, scanty literature exists on this relation at a local scale. Most studies that evaluated the above relation have been specific to one community at a time. Recent studies are descriptive and lack statistical evidence. In a community-based cohort of infants conducted in eastern Uganda, anaemia, malaria, diarrhoea and pneumonia were reported as the major single causes of death without estimating their relation to mortality but relying purely on their prevalence (Uganda Bureau of Statistics (UBOS) and ICF Macro, 2010). Studies providing statistical evidence are outdated. Studies in the South-West and North-West Uganda showed that lower anthropometric indicators were associated with higher U5M (Uganda Bureau of Statistics (UBOS) and ICF International Inc., 2015; Uganda Bureau of Statistics, 2017). This study assessed a single cause of childhood mortality and did not use nationally representative data.
Previous studies in Mali (Uganda Bureau of Statistics (UBOS) and ICF, 2018) and Malawi (Nabongo et al., 2014) looked at the relation between child mortality with exposure to malaria risk at national level but they ignored the geographical variation in the burden of childhood diseases that may influence U5M patterns (Vella et al., 1992a), therefore areas affected by the disease burden could not be identified. Furthermore, past studies did not consider exposure to multiple diseases adjusting for several confounders in a single analysis.
In the current study, we assess the association between all-cause U5M and childhood diseases (i.e. malaria, severe or moderate anaemia, severe or moderate malnutrition, diarrhoea and acute respiratory infections) in Uganda at national and sub-national scales, and identify childhood diseases that contribute to U5M by region analyzing the 2011 DHS data and applying Bayesian geostatistical Weibull proportional hazards models. The analysis was adjusted for spatial correlation in U5M and potential confounding effects of socio-demographic characteristics, interventions and environmental/climatic factors. Findings of this study can inform disease control programs to implement disease-specific interventions at a local scale to address U5M in Uganda.
2. Materials and methods
2.1. Study setting
The Republic of Uganda is located in East Africa and lies across the equator. It is a landlocked country that borders Kenya to the East, Tanzania to the South, Rwanda to the South-West, the Democratic Republic of Congo to the West, and South Sudan to the North. The country has an area of 241,039 km2 and a population of about 40 million of which 20% are under-five years of age (Uganda Bureau of Statistics (UBOS) and ICF International Inc., 2012).
2.2. Data and data sources
2.2.1. Mortality
Data on all-cause U5M were obtained from women birth histories available in the Uganda DHS which was conducted from May to December in 2011 (Uganda Bureau of Statistics (UBOS) and ICF International Inc., 2012). The survey includes a representative sample of 10,086 households selected using a stratified two-stage cluster design. In the first stage, 404 enumeration areas/clusters/locations were chosen. The second stage involved selecting households from a complete listing of households in each cluster. Mortality data were collected on all 7878 children born in the five years preceding the date of the survey. About 8674 women aged between 15 and 49 years who were either routine residents or visitors present in the selected household the night before the survey were interviewed.
2.2.2. Childhood diseases
Data on childhood diseases (severe or moderate malnutrition, severe or moderate anaemia, acute respiratory infections (ARIs), and diarrhoea) and socio-demographic characteristics were extracted from the DHS 2011. The children's nutritional status is reported using three anthropometric indices (i.e. height-for-age, weight-for-height and weight-for-age) based on growth standards defined by World Health Organization (WHO) in 2006 (Vella et al., 1992b). Severely or moderately malnourished children were defined as children with weight-for-age two standard deviations below the median of the WHO reference population. Haemoglobin levels were reported for children aged 6–59 months. We considered anaemic children as those with haemoglobin levels <10 g per deciliter (g/dL). ARIs and diarrhoea data were available from DHS questionnaires, asking mothers whether any of their children under the age of five years had been ill with a cough accompanied by short, rapid breathing or had diarrhoea at any time during the two-week period preceding the survey.
Disease data regarding the dead children were not gathered, making it difficult to assess the disease-mortality relation at an individual level. Therefore, we treated disease prevalence at cluster level as an exposure (Gemperli et al., 2004) linked to the individual level mortality. Disease prevalence was obtained by aggregating the binary disease status of screened children at the cluster level.
Malaria data were not available at the 2011 DHS. This information was collected in the Malaria Indicator survey (MIS) carried out in Uganda by the DHS program in 2009. To predict malaria parasite prevalence at the 2011 DHS clusters, we fitted Bayesian geostatistical models on microscopically confirmed survey data from the 2009 MIS which was carried out during November to December at 170 clusters and included malaria parasite positivity on 3972 children. Bayesian geostatistical models fitted via Markov Chain Monte Carlo (MCMC) simulations are suitable for these data as the DHS collects mortality and morbidity data at neighbouring locations and therefore correlated in space. This is because observations at close geographical proximity are likely to share common exposures and thus affected in a similar way. Ignoring spatial correlation in the data results into imprecise effects of covariates which are essential for determining areas affected mostly by diseases. The flexibility of the Bayesian inferential approach via MCMC simulations provides an appropriate method to deal with over-parametrized models (Kazembe et al., 2007).
2.2.2.1. Disease preventive and curative programs in Uganda
“In Uganda, ITNs and IRS are the main interventions for malaria vector control. These are complemented by other measures to reduce mosquito breeding such as larvicides. The National Malaria Treatment Policy of 2012 stipulates use of ACT for simple or uncomplicated malaria, quinine or artesunate for severe malaria and IPT during pregnancy (Burke et al., 2016). Promotion of ITNs, deworming medication every six months and iron supplementation for children under the age of five are the recommended measures for prevention and treatment of malaria and anaemia in children.
The Uganda National Expanded Program on Immunization (UNEPI) is a national program that mainly targets infants and women of childbearing age. UNEPI ensures that every child and high-risk group is fully vaccinated with high quality and effective vaccines against the target diseases according to recommended strategies (WHO Multicentre Growth Reference Study Group, 2006). The program immunizes the population against several diseases including those related to ARIs such as tuberculosis, diphtheria, whooping cough (pertussis), Haemophilus influenzae and measles. The Ministry of Health has recommended antibiotics for the treatment of ARIs (McGovern and Canning, 2015).
Ministry of Health implements a multi-sectorial approach for diarrheal disease control. Interventions include improvement of water quality, hygiene, and sanitation; provision of ORS or RHF and zinc supplements; and case management. As a preventive measure for rotavirus disease, the Ministry of Health introduced a new vaccine against rotavirus diarrhoea into routine immunization in 2016 (McGovern and Canning, 2015).
Exclusive breastfeeding in the first six months of delivery, breastfeeding coupled with a balanced diet after the six months of birth and education in nutrition practices are the key preventive measures against malnutrition recommended by the Ministry of Health (McGovern and Canning, 2015).”
2.2.3. Socio-demographic factors
Socio-demographic data primarily included maternal (education, literacy, residence, age at birth, early pregnancy termination, number of children born, working status) and child (sex, birth order, birth interval, mode of delivery) characteristics at the individual level. The household asset score was used as a socio-economic proxy.
2.2.4. Environmental and climatic data
Environmental and climatic predictors were extracted from remote sensing sources. Land Surface Temperature (LST), rainfall and Normalized Difference Vegetation Index (NDVI) were averaged during January to December 2009 and January to December 2011. The former climatic summaries were used in the fitting of the malaria parasite data of the 2009 MIS. The latter were considered as predictors in the mortality model based on the 2011 DHS. Land cover types were provided in 17 categories according to the International Global Biosphere Programme (IGBP) classification scheme and re-grouped into three categories, that is, urban, forest and crops. Distance to permanent water bodies was calculated based on the water category of the land cover data. Appendix A contains a list of environmental/climatic data together with their spatio-temporal resolution and data source.
2.2.5. Intervention coverage indicators
Child, maternal and household intervention data, that is, Water, Sanitation and Hygiene (WASH), reproductive health, breastfeeding, vaccinations, micronutrients intake and treatment interventions were obtained from the 2011 DHS. The standard guidelines of the Roll Back Malaria were used to define indicators of malaria interventions (World Health Organization, 2013). These included use and ownership of insecticide treated nets (ITNs) and indoor residual spraying (IRS). All intervention coverage indicators were aggregated at the cluster level because interventions at individual level such as vaccination, ITN use or treatment are not available for dead children. Interventions with coverage of <5% (i.e. zinc; 2%) and those exceeding 95% (i.e. iodized salt; 99%) at the national level were excluded from the analysis due to lack of variation in estimating their relation with mortality. Table A.1 provides details on the intervention indicators used in the study and their corresponding national coverage.
Table A.1.
Intervention indicators and their national coverage, Uganda DHS 2011.
| Intervention | Description | Coverage (%) |
|---|---|---|
| Malaria | ||
| Prop_IRS | Percentage of households sprayed with Indoor Residual Spraying (IRS) in the past 12 months | 7 |
| ITN ownership | ||
| Prop_1ITN | Percentage of households with at least one ITN | 60 |
| Prop_1ITN2 | Percentage of households with at least one ITN for every two people | 28 |
| Prop_ITNA | Percentage of population with access to an ITN within their household (Percentage of the population that could sleep under an ITN, if each ITN in the household were used by up to two people) | 45 |
| ITN use | ||
| Prop_ITNS | Percentage of the population in a household that slept under an ITN the previous night of the survey | 35 |
| Prop_ITN5 | Percentage of children under 5 years in a household who slept under an ITN the previous night of the survey | 43 |
| Prop_ITNU | Percentage of existing ITNs used by the population in a household the previous night of the survey | 35 |
| WASH | ||
| Improved water | Percentage of households with improved source of drinking water | 70 |
| Improved sanitation | Percentage of households using improved sanitation facilities | 16 |
| Prop _wsoap | Percentage of households with soap or detergent and water at hand washing place | 27 |
| Reproductive health | ||
| Family planning | Percentage of married women using any family planning method | 30 |
| ANC provider | Percentage of pregnant mothers receiving ANC from a skilled provider | 95 |
| 4+ ANC visits | Percentage of pregnant women making four or more ANC visits during their entire pregnancy | 48 |
| IPT | Percentage of women who received intermittent preventive treatment for malaria during pregnancy | 27 |
| Skilled delivery | Percentage of births that took place with the assistance of a skilled provider | 58 |
| Postnatal care | Percentage of newborns receiving first postnatal checkup from a skilled provider within two days after delivery | 11 |
| Breastfeeding | ||
| Within one day | Percentage of infants who started breastfeeding within one day of birth | 89 |
| Exclusive | Percentage of infants exclusively breastfed during the first six months after birth | 63 |
| Vaccinations | ||
| Tetanus toxoid | Percentage of last-born child fully protected against neonatal tetanus | 84 |
| BCG | Percentage of children vaccinated against BCG | 94 |
| DPT | Percentage of children with complete vaccination of DPT | 72 |
| Polio | Percentage of children with complete vaccination of polio | 63 |
| Measles | Percentage of children vaccinated against Measles | 76 |
| Micronutrients | ||
| VitaminA_sup | Percentage of children receiving vitamin A supplements in the past 6 months | 57 |
| Iron_sup | Percentage of children receiving Iron supplements in the past 7 days | 7 |
| Iodized salt | Percentage of children living in households with iodized of salt | 99 |
| Treatments | ||
| Antibiotics | Percentage of children with ARIs symptoms who took antibiotics | 47 |
| ORS or RHF | Percentage of children with diarrhoea given fluid from oral rehydration solution (ORS) sachets or recommended home fluids (RHF) | 48 |
| Zinc | Percentage of children with diarrhoea given zinc sulphates | 2 |
| ACTs | Percentage of children with fever during the two weeks prior to the survey and took artemisinin-combination therapy (ACT) | 69 |
| Deworming | Percentage of children given deworming medication in the past 6 months | 50 |
2.3. Statistical analysis
To identify the most important predictors associated with the U5M, Bayesian geostatistical variable selection was used adopting, a stochastic search approach. In particular, a binary indicator was introduced for every disease, health intervention (except ITN coverage measures), land cover and socio-demographic characteristic with values corresponding to the inclusion or exclusion of the variable from the model. We assumed that the indicator arises from a Bernoulli distribution with probability defining the variable-specific inclusion probability in the model. We have chosen a spike and slab prior for the regression coefficients, that is, a mixture of normal prior distributions with a mixing proportion equal to the inclusion probability. The spike component shrinks the regression coefficient to zero when the variable is excluded and the slab assumes a non-informative, normal prior distribution when the covariate has high inclusion probability (i.e. larger than 40%). ITN coverage measures were highly correlated (above 0.85), therefore, only one (or none) measure among those defining ownership and one (or none) among those defining use were selected. Environmental/climatic factors (LST, NDVI, distance to permanent water bodies and rainfall) were included or excluded in the model in a linear or categorical form, introducing indicators with a multinomial prior distribution with three parameters corresponding to the probabilities of exclusion of the variable, inclusion in linear and categorical form respectively. Covariates were categorized based on their quartiles.
To assess the association between U5M and childhood diseases at the national and sub-national levels, a Bayesian geostatistical proportional hazards model with a baseline Weibull hazard function was fitted. The model included intervention coverage measures, socio-demographic, climatic and environmental covariates. Climatic/environmental factors were included as proxies of other environmentally driven causes of mortality. Malaria prevalence was not available at the 2011 DHS locations. Instead the Bayesian binomial geostatistical model was fitted on the 2009 MIS data to predict the malaria prevalence. The prediction uncertainty was taken into account as a measurement error in the malaria covariate. Spatial correlation between clusters in both, the survival and the binomial geostatistical models was incorporated on locational random effects modeled by Gaussian processes with an exponential correlation function of the distance between locations. Our model assumed that the relation between childhood diseases and mortality varied across regions by including disease-specific spatially varying coefficients. Region-specific random effects with conditional autoregressive prior distributions modeled geographical variation in the effects of childhood diseases.
The contribution of childhood diseases to under-five mortality was quantified by means of the population attributable fractions (PAF). PAF measures the percentage of under-five deaths attributable to a specific disease. The definition of PAF was used in terms of known prevalence of the disease in the population, p, and the corresponding adjusted odds ratio, aOR, as PAF = p(aOR − 1)/(1 + p(aOR − 1)) (Bbaale, 2015). PAFs were calculated for the whole country and for each region separately following the formula presented above. Mortality can be caused by multiple risk factors, and individual risk factors may interact in their impact on overall risk of mortality. As a result, PAF estimates for individual risk factors often overlap and add up to >100% (Ezeh et al., 2014). Markov Chain Monte Carlo simulation drawing samples from the posterior distribution of the PAF, that is, PAF1, PAF2, … PAFN~p(PAF| data) were used to obtain the 95% BCI for PAFs, with N being the number of simulations. The overall mean and variance of simulated samples are estimates of the posterior mean and variance and were thus used to estimate the 95% BCI for PAFs.
Descriptive data analysis was executed in STATA version 14.0 (Stata Corporation, College Station, TX, USA) and model fit was carried out in OpenBUGS 3.2.3 (Imperial College and Medical Research Council, London, UK). Maps were produced in ArcGIS version 10.5 (ArcGIS version 10.5, Esri, Redlands, CA, USA). The effects of covariates on mortality were summarized by their posterior medians and reported as hazard ratios with their corresponding 95% Bayesian credible interval (95% BCI), which is an equivalent of the confidence interval in the frequentist approach. Effects were regarded statistically important if their credible intervals did not include one. Appendix B describes in details the Bayesian geostatistical methods.
3. Results
3.1. Descriptive data analysis
Table A.2 summarizes childhood disease prevalences and the U5MR estimates by region and at the country level. The overall U5MR was 90 deaths per 1000 live births. Large regional variations in childhood mortality rates per 1000 live births were observed, lowest in Kampala (56) and highest in the North-East (152) deaths.
Table A.2.
U5MR estimates and childhood disease prevalence at national and regional levels, Uganda DHS 2011 and MIS 2009.
| Disease prevalence (%) |
U5MR estimates |
|||||
|---|---|---|---|---|---|---|
| Geographical scale | Malaria | Anaemia | Malnutrition | Diarrhoea | ARIs | per 1000 live births |
| National | 42 | 27 | 17 | 23 | 15 | 90 |
| Region | ||||||
| Central 1 | 39 | 30 | 15 | 22 | 9 | 83 |
| Central 2 | 51 | 32 | 13 | 21 | 12 | 79 |
| East-Central | 56 | 46 | 20 | 32 | 15 | 104 |
| Kampala | 5 | 23 | 8 | 24 | 14 | 56 |
| Mid-Eastern | 37 | 32 | 11 | 33 | 17 | 80 |
| Mid-North | 62 | 13 | 16 | 24 | 22 | 76 |
| Mid-Western | 43 | 16 | 20 | 19 | 17 | 95 |
| North-East | 40 | 35 | 45 | 20 | 20 | 152 |
| South-West | 12 | 8 | 20 | 14 | 11 | 99 |
| West-Nile | 46 | 38 | 23 | 19 | 14 | 100 |
ARIs: Symptoms of acute respiratory infections; Malnutrition: Severe or moderate malnutrition; Anaemia: Severe or moderate anaemia.
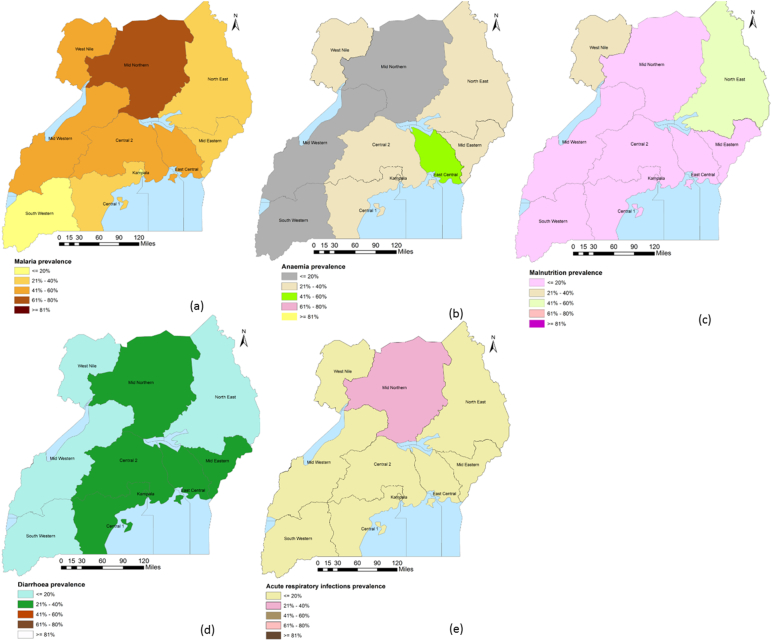
Based on the MIS 2009 data, the microscopy-based national malaria parasitemia prevalence was 42%. There were large regional variations with Kampala experiencing the lowest prevalence (5%) and the Mid-North the highest (62%). About 27% of Ugandan children aged 6–59 months were severely or moderately anaemic; lowest in South-West (8%) and highest in East-Central (46%). Overall, 17% of Ugandan children were either severely or moderately malnourished. The percentage of moderately/severely malnourished children varied by region with Kampala having the lowest (8%) and North-East the highest (45%). Nearly a quarter of the children under-five years were reported to have diarrhoea at national level, and this proportion was highest in East-Central (32%) and Mid-Eastern (33%) and lowest in South-West (14%). Overall ARIs in the two weeks before the survey was 15%, highest in Mid-North (22%) and lowest in Central 1 (9%). Fig. A.1 presents the corresponding geographical distribution of childhood diseases. The variation in childhood diseases in space could be a contributing factor to the observed regional heterogeneities in the U5MR in Uganda.
Fig. A.1.
Geographical distribution of childhood diseases by region, DHS 2011.
The variation in the burden of childhood diseases across regions could be a contributing factor to the existing regional discrepancies in the U5MR in Uganda; (a) Prevalence of malaria, (b) Prevalence of anaemia, (c) Prevalence of malnutrition, (d) Prevalence of diarrhoea, (e) Prevalence of acute ARI.
3.2. Model-based analysis
3.2.1. Bayesian variable selection
Table A.3 presents results from the Bayesian geostatistical variable selection. Socio-demographic and climatic factors, interventions and treatment indicators with posterior inclusion probabilities higher than 40% were incorporated in the final survival model, that is, if a covariate is contained in >40% of the total fitted models was included in the final model. For example, the malaria intervention coverage indicators that were considered in the final model were the proportion of children under 5 years who slept under an insecticide treated nets the previous night of the survey and the proportion of households sprayed with Indoor Residual Spraying. Land Surface Temperature day and Normalized Difference Vegetation Index were included as categorical and linear covariates respectively.
Table A.3.
Posterior inclusion probabilities of disease prevalence, intervention coverage indicators, socio- demographic and environmental/climatic characteristics.
| Variable | Inclusion probability (%) | Variable | Inclusion probability (%) |
|---|---|---|---|
| Diseases | Treatments | ||
| Malnutrition | 57.6a | Antibiotics | 28.0 |
| Malaria | 63.0a | ORS or RHF | 59.0a |
| Anaemia | 69.6a | ACTs | 15.0 |
| ARIs | 46.4a | Deworming | 46.0a |
| Diarrhoea | 68.2a | Socio-economic and demographic | |
| Malaria | Child | ||
| Prop_IRS | 59.3a | Sex | 86.0a |
| ITN ownership | Birth order | 60.2a | |
| None | 55.0 | Birth intervals | 54.2a |
| Prop_1ITN | 36.7 | Maternal | |
| Prop_1ITN2 | 0.0 | Age at birth | 100.0a |
| Prop_ITNA | 8.3 | Number of children born | 100.0a |
| INT use | Education level | 84.6a | |
| None | 8.7 | Pregnancy terminated | 64.6a |
| Prop_ITNS | 15.0 | Residence (urban vs rural) | 69.0a |
| Prop_ITN5 | 48.0a | Working status | 15.5 |
| Prop_ITNU | 28.3 | Household | |
| WASH | Age of head | 23.4 | |
| Improved water | 30.0 | Wealth index | 68.8a |
| Improved sanitation | 85.0a | #Children under 5 years | 37.0 |
| Prop_wsoap | 13.3 | Environmental/climatic factors | |
| Reproductive health | Land cover | 100.0a | |
| Family planning | 88.0a | LST day | |
| ANC provider | 100.0a | None | 0.0 |
| 4+ ANC visits | 20.0 | Continuous | 0.0 |
| IPT | 54.5a | Categorical | 100.0a |
| Skilled delivery | 49.2a | LST night | |
| Postnatal care | 100.0a | None | 98.0 |
| Breastfeeding | Continuous | 2.0 | |
| Within one day | 24.4 | Categorical | 0.0 |
| Exclusive | 45.2a | NDVI | |
| Vaccinations | None | 5.0 | |
| Tetanus toxoid | 27.0 | Continuous | 95.0a |
| BCG | 2.0 | Categorical | 0.0 |
| DPT | 46.8a | Rainfall | |
| Polio | 30.0 | None | 95.0 |
| Measles | 68.2a | Continuous | 0.5 |
| Micronutrients | Categorical | 0.0 | |
| VitaminA_sup | 31.0 | Distance to water | |
| Iron_sup | 39.0 | None | 100.0 |
| Continuous | 0.0 | ||
| Categorical | 0.0 |
Selected variables with >40% inclusion probability.
3.2.2. Effects and contribution of childhood diseases on U5M
The effects of childhood diseases on U5M are summarized in Table A.4 by their posterior estimates (i.e. estimates of unknown quantities of childhood diseases that maximize their posterior distribution). Results indicate that at the national level, a 100% increase in the prevalence of malaria parasitemia was associated with a 74% increase in the hazard of mortality (HR = 1.74; 95% BCI: 1.42, 2.16). Similarly, a 100% increase in the proportion of children having severe or moderate anaemia, severe or moderate malnutrition and diarrhoea was associated with a 37%, 49% and 61% rise in the hazard of mortality respectively. ARIs were neither associated with mortality at country nor at sub-national level.
Table A.4.
Posterior estimates for the effects of childhood diseases at the national and sub-national scale on U5MR adjusted for socio-economic, demographic and environmental/climatic characteristics.
| Geographical scale | Malariac |
Moderate/severe anaemiac |
Moderate/severe malnutritionc |
Diarrhoeac |
ARIsc |
|---|---|---|---|---|---|
| Hazard ratio (95% BCI) | Hazard ratio (95% BCI) | Hazard ratio (95% BCI) | Hazard ratio (95% BCI) | Hazard ratio (95% BCI) | |
| National | 1.74 (1.42, 2.16)a | 1.37 (1.20, 1.75)a | 1.49 (1.25, 1.66)a | 1.61 (1.31, 2.05)a | 0.99 (0.31, 2.17) |
| Region | |||||
| Central 1 | 1.46 (0.70,2.38) | 1.78 (1.35, 3.86)a | 0.97 (0.63, 1.30) | 1.27 (0.63, 2.85) | 1.02 (0.19, 5.81) |
| Central 2 | 1.72 (1.10, 2.55)a | 1.07 (0.93, 1.52) | 1.08 (0.63, 1.52) | 1.97 (1.16, 3.67)a | 1.12 (0.19, 3.40) |
| East-Central | 2.39 (1.41, 5.74)a | 0.79 (0.62, 1.23) | 2.98 (1.75, 4.21)a | 1.94 (1.21, 4.57)a | 1.21 (0.15, 3.95) |
| Kampala | 1.20 (0.81, 1.97) | 1.87 (1.33, 2.62)a | 1.83 (0.62, 2.64) | 1.07 (0.40, 3.23) | 1.53 (0.17, 7.37) |
| Mid-Eastern | 2.55 (0.80, 3.42) | 0.98 (0.76, 1.53) | 2.58 (1.66, 4.08)a | 2.04 (1.27, 3.58)a | 0.77 (0.10, 4.29) |
| Mid-North | 1.95 (1.27, 3.35)a | 1.28 (1.06, 1.97)a | 0.81 (0.56, 1.19) | 1.75 (0.73, 3.86) | 1.01 (0.15, 2.98) |
| Mid-Western | 1.07 (0.68, 2.23) | 1.16 (1.07, 1.64)a | 1.62 (0.88, 2.18) | 2.17 (1.11, 4.13)a | 0.91 (0.21, 3.35) |
| North-East | 2.20 (1.31, 4.79)a | 1.20 (1.08, 2.23)a | 2.33 (1.33, 3.93)a | 2.38 (0.80, 5.35) | 0.90 (0.20, 5.30) |
| South-West | 0.84 (0.59, 1.80) | 1.77 (1.40, 2.67)a | 1.27 (0.91, 1.69) | 1.52 (0.59, 4.64) | 1.31 (0.22, 4.56) |
| West-Nile | 2.20 (1.06, 2.90)a | 1.87 (1.59, 2.41)a | 1.28 (0.85, 1.80) | 1.54 (0.80, 3.02) | 1.02 (0.10, 3.98) |
| Spatial parameters |
|||||
|---|---|---|---|---|---|
| Variance | Posterior median (95% BCI) | Posterior median (95% BCI) | Posterior median (95% BCI) | Posterior median (95% BCI) | Posterior median (95% BCI) |
| Spatially varyingb | 0.56 (0.48, 0.71) | 0.56 (0.43, 0.60) | 0.68 (0.43, 0.99) | 0.61 (0.38, 0.85) | 0.59 (0.43, 0.76) |
ARIs: Symptoms of acute respiratory infections.
Statistically important effect.
Indicates the degree of variation of disease effects in space.
Disease prevalence was modeled on the scale of 0 to 1, therefore one unit increase in prevalence corresponds to a 100% increase which implies a shift of the current by 100%.
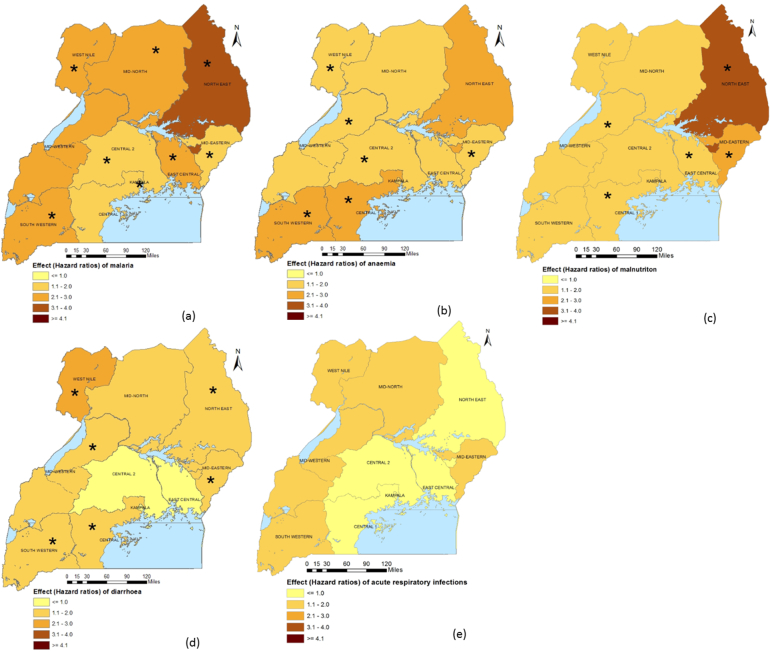
Sub-national analysis shows that malaria was associated with U5M in Central 2, East-Central, Mid-North, North-East and West-Nile regions. In Central 1, Kampala and South-West, only severe/moderate anaemia was associated with mortality. Anaemia also significantly increased hazards of U5M in the Mid-North, Mid-West, North-East and West-Nile regions. The prevalence of moderate/severe malnutrition in East-Central, Mid-Eastern and North-East, and diarrhoea risk in Central 2, East-Central, Mid-eastern and Mid-Western had a statistically important effect on U5M in these areas. There was no important association between the ARIs burden and U5M. Fig. A.2 presents the geographical distribution of spatially varying disease effects that are shown in Table A.4.
Fig. A.2.
Geographical distribution of spatially varying childhood disease effects on U5M.
The asterisk (*) implies that the disease is associated with U5M in the respective region; (a) Prevalence of malaria, (b) Prevalence of anaemia, (c) Prevalence of malnutrition, (d) Prevalence of diarrhoea, (e) Prevalence of acute ARI.
Table A.5 shows that among malaria interventions, a 100% increase in the coverage of IRS is associated with a reduction in the mortality hazard by 31%, (HR = 0.69; 95% BCI: 0.61, 0.83). Reproductive health interventions were also important determinants of U5M. In particular, an increasing proportion of married women using any family planning method, proportion of pregnant mothers receiving ANC from a skilled provider and proportion of women receiving intermittent preventive treatment for malaria during pregnancy were associated with lower hazards of mortality. Child interventions considerably influenced mortality. First postnatal checkup from a skilled provider within two days after delivery, exclusive breastfeeding during the first six months after birth, complete vaccination against DPT and measles and, deworming were associated with lower hazard of mortality. Socio-economic and demographic characteristics were statistically important predictors of mortality. An increased mortality hazard was estimated for children born to mothers who once experienced a terminated pregnancy. Since Table A.5 contains variables included in the model as potential confounders of the effects of the primary variables (i.e. childhood diseases), their attributions to U5M were not of principal interest and were therefore not computed.
Table A.5.
Posterior estimates for the effects of socio-economic, demographic and environmental/climatic factors on the U5MR.
| Variable | Hazard ratio (95% BCI) |
|---|---|
| Malariac | |
| Percentage of households sprayed with Indoor Residual Spraying (IRS) in the past 12 months | 0.69 (0.61, 0.83)a |
| Percentage of children under 5 years in a household who slept under an ITN the previous night of the survey | 1.21 (0.85, 1.63) |
| WASHc | |
| Percentage of households using improved sanitation facilities | 0.78 (0.54, 0.87)a |
| Reproductive healthc | |
| Percentage of married women using any family planning method | 0.68 (0.41, 0.79)a |
| Percentage of pregnant mothers receiving ANC from a skilled provider | 0.58 (0.23, 0.84)a |
| Percentage of women who received intermittent preventive treatment for malaria during pregnancy | 0.59 (0.53, 0.73)a |
| Percentage of births that took place with the assistance of a skilled provider | 0.96 (0.80, 1.12) |
| Percentage of newborns receiving first postnatal checkup from a skilled provider within two days after delivery | 0.69 (0.36, 0.67)a |
| Breastfeedingc | |
| Percentage of infants exclusively breastfed during the first six months after birth | 0.54 (0.44, 0.69)a |
| Vaccinationsc | |
| Percentage of children with complete vaccination of DPT | 0.75 (0.63, 0.96)a |
| Percentage of children vaccinated against Measles | 0.71 (0.60, 0.80)a |
| Micronutrientsc | |
| Percentage of children given deworming medication in the past 6 months | 0.40 (0.28, 0.48)a |
| Treatmentsc | |
| Percentage of children with diarrhoea given fluid from oral rehydration solution (ORS) sachets or recommended home fluids (RHF) | 1.13 (0.88, 1.32) |
| Socio-economic and demographic | |
| Child | |
| Sex: Female vs male | |
| Birth order >4 vs 1–4 |
1.19 (1.12, 1.60)a |
| Birth intervals 24–35 vs 1–23 |
0.54 (0.46, 0.81)a |
| 36–47 vs 1–23 | 0.41 (0.35, 0.58)a |
| 48 vs 1–23 | 0.37 (0.24, 0.51)a |
| Maternal | |
| Age at birth 25–29 vs 15–24 |
0.82 (0.63, 1.21) |
| 30–34 vs 15–24 | 0.69 (0.58, 0.91)a |
| 35–49 vs 15–24 | 1.09 (0.81, 1.34) |
| Number of children born | 1.67 (1.57, 1.76)a |
| Pregnancy terminated vs never | 1.31 (1.07, 1.74)a |
| Education level: Primary vs none |
0.87 (0.80, 1.10) |
| Secondary or higher vs none | 0.81 (0.71, 0.92)a |
| Residence Urban vs rural |
0.76 (0.59, 0.91)a |
| Household | |
| Wealth index | 0.84 (0.75, 0.95)a |
| Environmental/Climatic factors | |
| Normalized difference vegetation index | 1.47 (0.60, 2.82) |
| Land surface temperature (day) | |
| 25.7–27.5 vs < 25.7 | 1.11 (0.69, 1.53) |
| 27.6–30.6 vs < 25.7 | 1.18 (0.84, 1.63) |
| 30.6 vs < 25.7 | 0.90 (0.70, 1.31) |
| Land cover | |
| Crops vs forest | 1.14 (0.81, 1.57) |
| Urban vs forest | 0.88 (0.71, 1.42) |
| Spatial parameters | |
| Variance in spatial process | 0.49 (0.40, 0.75) |
| Range (km)b | 3.10 (1.42, 6.40) |
| Other parameters Shape parameterd |
0.43 (0.38, 0.49) |
Statistically significant.
Distance after which spatial correlation becomes <5%.
Covariate takes values on the scale of 0 to 1, therefore one unit increase in coverage corresponds to a 100% increase which implies a shift of the current by 100%.
Shape parameter of the Weibull baseline hazard.
3.3. Contribution of childhood diseases to under-five mortality
PAF estimates (Table A.6) indicate that 97% (PAF = 96.9; 95%BCI: 94.4, 98.0), 91% (PAF = 90.9; 95%BCI: 84.4, 95.3), 89% (PAF = 89.3; 95%BCI: 76.0,93.8) and 93% (PAF = 93.3 95%BCI: 87.7,96.0) of the deaths among children less than five years in Uganda was attributable to malaria, severe/moderate anaemia, severe/moderate malnutrition and diarrhoea respectively. Most deaths in Central 1, Kampala and South-West were attributable to severe/moderate anaemia. In West-Nile, malaria and severe/moderate anaemia were equally responsible for a larger percentage of under-five deaths. In Central 2 and East-Central, malaria and diarrhoea contributed most deaths. In the Mid-North and North-East, malaria accounted for majority of the deaths. In Mid-Eastern and Mid-Western, most deaths were due to diarrhoea.
Table A.6.
Population attributable fraction (PAF) estimates (%) for malaria, Moderate/severe anaemia, Moderate/severe malnutrition, diarrhoea and ARI at the national and regional scale relative to under-five mortality.
| Geographical scale | Malaria |
Moderate/severe anaemia |
Moderate/severe malnutrition |
Diarrhoea |
ARIs |
|---|---|---|---|---|---|
| PAF (95% CI) | PAF (95% CI) | PAF (95% CI) | PAF (95% CI) | PAF (95% CI) | |
| National | 96.9 (94.6,98.0)a | 90.9 (84.4,95.3)a | 89.3 (76.0,93.8)a | 93.3 (87.7,96.0)a | −17.6 (−29.6,40.7) |
| Regions | |||||
| Central 1 | 94.7 (−92.2109.3) | 95.9 (91.3,98.8)a | 92.1 (−87.8112.0) | 85.6 (−114.0,97.6) | 15.3 (−97.7115.9) |
| Central 2 | 97.3 (83.6,98.8)a | 69.1 (−50.6,94.3) | −81.8 (−126.2,67.1) | 95.3 (77.1,98.2)a | 59.0 (−96.6111.5) |
| East-central | 98.7 (95.8,99.6)a | 111.5 (−106.1129.4) | 51.0 (43.8,78.5)a | 96.8 (87.0,99.1)a | 75.9 (−97.8108.5) |
| Kampala | 50.0 (−190,82.9) | 95.2 (88.4,97.4)a | 97.5 (−85.9151.0) | 62.7 (−98.2107.5) | 88.1 (−98.9109.4) |
| Mid-Eastern | 98.9 (−98.3115.6) | −177.8 (−189.0,115.0) | 86.9 (77.9,94.1)a | 97.2 (89.9,98.8)a | 134.4 (−98.2157.0,) |
| Mid-North | 98.3 (94.4,99.3)a | 78.4 (43.8,92.7)a | 94.6 (−75.2116.6) | 94.7 (−98.6118.2) | 18.0 (−97.8105.6) |
| Mid-Western | 75.1 (−68.1107.8) | 71.9 (52.8,91.1)a | 149.0 (−95.9171.4) | 95.7 (67.6,98.3)a | 288.7 (−97.6308.0) |
| North-East | 98.0 (92.5,99.3)a | 87.5 (73.7,97.7)a | 92.5 (83.7,98.6)a | 96.5 (−98.9133.3) | 200.0 (−98.9216.7) |
| South-West | 208.7 (−125.5260.6) | 86.0 (76.2,93.0)a | 98.4 (−93.2125.0) | 87.9 (−121.1,98.1) | 77.3 (−97.5113.2) |
| West Nile | 98.2 (73.4,98.9)a | 97.1 (95.7,98.2)a | 84.4 (−64.8136.8) | 91.1 (−97.5135.7) | 21.9 (−97.7108.6) |
ARIs: Symptoms of acute respiratory infections.
Percentage of deaths attributable to a disease, for example, for malaria, 97% (PAF = 96.9; 95%BCI: 94.6, 98.0) of deaths among children less than five years in Uganda are attributed to malaria.
4. Discussion
We estimated the effects of childhood diseases in Uganda on all-cause U5M at national and subnational scales taking into account confounding effects of child and maternal interventions, socio-demographic and climatic/environmental factors that have been shown to be significantly related to U5M (World Health Organization, 2013; Bbaale, 2015; Ezeh et al., 2014; Kabagenyi and Rutaremwa, 2013). We found strong geographical variation in the effects of childhood diseases on all-cause U5M across Uganda.
The significant association between malaria and U5M can be explained by the short rainy season during which the survey was conducted in addition to the high malaria prevalence (42%) prevailing in Uganda known as a high malaria burden country in Africa (Kazembe et al., 2006). Rainfall provides suitable conditions for development of malaria parasites within mosquitoes resulting in increased transmission (Nafiu et al., 2016; Colón-González et al., 2016; Gage et al., 2008; Kazembe et al., 2006) and consequently mortality.
The poor WASH practices in Uganda could be responsible for the significant association between diarrhoea and mortality. At the time of the survey, only 14% of households had improved sanitation facilities (Uganda Bureau of Statistics (UBOS) and ICF International Inc., 2012). UN improved sanitation facilities accelerate the transfer of germs by flies and concentrate micro-organisms into food and water sources, which increases diarrhoeal risk and death from the disease (Ehrhardt et al., 2006). Also, other diarrhoea preventive interventions are limited in Uganda. For example, water and soap were present at handwashing places of only 27% of households (Uganda Bureau of Statistics (UBOS) and ICF International Inc., 2012). Hand washing with a detergent ensures that the transmission of germs is restricted, especially among children who are more prone than adults to diarrhoea and other childhood illnesses.
Furthermore, malnutrition is a risk factor for the major causes of U5M (Walker et al., 2013; Brabin et al., 2001) and is a consequence of poor feeding and socioeconomic status (Scott et al., 2014). The inadequate young child feeding practices coupled with poor socioeconomic status in Uganda (Uganda Bureau of Statistics (UBOS) and ICF International Inc., 2012) could have contributed to the significant association between malnutrition and mortality.
Significant relationships between U5M and malaria in Mali and Malawi (Uganda Bureau of Statistics (UBOS) and ICF, 2018; Nabongo et al., 2014), diarrhoea worldwide (Liu et al., 2015), malnutrition in Uganda (Uganda Bureau of Statistics (UBOS) and ICF International Inc., 2015; Uganda Bureau of Statistics, 2017) and anaemia in Sierra Leon, Zaire and Kenya (Liu et al., 2012; Liu et al., 2016) have also been reported.
Sub-national analysis showed that malaria was associated with mortality in Central 2, East-Central, Mid-North, North-East and West-Nile regions. While the Mid-North experienced the highest malaria prevalence, the largest association of the disease with mortality was not observed in this region. This suggests that high disease prevalence may not imply high malaria mortality. Various factors including other diseases (diarrhoea-hookworms, ARI-pneumonia) and other factors than diseases such as other interventions or malaria physiopathology (e.g. immunity) may also play a role in the disease severity and outcomes.
The significant associations between anaemia and mortality in Central 1, Kampala, Mid-North, Mid-Western, North-East, South-West and West-Nile could be partly be related to the high malaria prevalence in these areas (at least 40%) (Uganda Bureau of Statistics (UBOS) and ICF International Inc., 2012). It is also known that anaemia can result from malaria infection (Shankar, 2000), therefore the anaemia relation to mortality in these areas may partly indicate an indirect malaria effect. Given the low prevalence of severe anaemia in these regions (i.e. <10%, (Uganda Bureau of Statistics (UBOS) and ICF International Inc., 2012)), the majority of anaemia-related deaths are associated with moderate anaemia. This implies that even a modest improvement in haemoglobin concentration could reduce the impact of anaemia on U5M (Caulfield et al., 2004a). The most frequently used approach to increase haemoglobin levels and reduce anaemia is universal iron supplementation starting at six months of age (Müller et al., 2003). In Uganda, nearly 85% of the under-fives did not receive iron supplementation (Uganda Bureau of Statistics (UBOS) and ICF International Inc., 2012) and in most regions in which anaemia influenced mortality (Central 1, Kampala, West-Nile, Mid-Western and South-West), iron supplementation was as low as 9% (Uganda Bureau of Statistics (UBOS) and ICF International Inc., 2012). This could have led to iron-deficiency diseases such as anaemia, hence the observed significant associations between anaemia and U5M.
Soil transmission helminthiasis (STH) resulting from parasitic worms may also cause anaemia. Parasitic worms, especially, hookworms are a risk factor of anaemia among children (Caulfield et al., 2004b). Such worms are transmitted by eggs present in human faeces and contaminate the soil in areas where sanitation is poor (Mockenhaupt et al., 2004). Given the poor WASH practices in the country, the Ugandan children are at higher risk of developing STH and STH-associated anaemia. In particular, out of the seven regions with an important association between anaemia and mortality, five have lower coverage of improved sanitation facilities varying from three to 14% which is below the 16% national average (Uganda Bureau of Statistics (UBOS) and ICF International Inc., 2012). The other sources of anaemia in addition to malaria may partly explain why in our study, the generated map of malaria risk did not match with the map of anaemia. Our findings are in line with previous studies carried out in Uganda (Rice et al., 2000) showing that malaria is not the only contributing factor of anaemia among children under five years.
The significant associations between severe or moderate malnutrition and mortality in East-Central, Mid-Eastern and North-East can be explained by the high malnutrition prevalence in the areas. Out of the three affected regions, two (East-Central and North-East) had malnutrition prevalence higher than the national average. East-Central and North-East regions are characterized by frequent dry spells and lack of agricultural extension services. This affects production and productivity resulting in poor food access and utilization, hence malnutrition (Ssempiira, 2018). Surprisingly, malnutrition is associated with mortality in regions with high food production such as the Mid-Eastern. Poor infant and young child feeding practices may partly be responsible for the persistent malnutrition. For example, nearly half of the under-fives in each of the regions where malnutrition was associated with mortality, were not exclusively breast fed in the first six months after delivery (Uganda Bureau of Statistics (UBOS) and ICF International Inc., 2012).
The positive association between diarrhoea risk and U5M in Central 2, East-Central, Mid-Eastern and Mid-Western can be attributed to the almost stagnant coverage of interventions since 2006 (Uganda Bureau of Statistics (UBOS) and ICF International Inc., 2012; Uganda Bureau of Statistics (UBOS) and Macro International Inc., 2007) proven to reduce diarrhoea-deaths in areas where they are widely used (Ssempiira et al., 2017a). For instance, utmost, only 14% of households in each of the four affected regions had improved sanitation facilities and, health care seeking for diarrhoea was poor, with about half of children having the disease in each of the four high risk regions not receiving ORS or RHF (Uganda Bureau of Statistics (UBOS) and ICF International Inc., 2012).
Even though ARIs is among the ten leading causes of death in children under five years in Uganda (Ssempiira et al., 2017b), the disease was not associated with U5M. Universal coverage of the pentavalent (Haemophilus Influenzae type B disease) vaccine could have contributed to this finding (Ssempiira et al., 2017b).
The contribution of a disease on the overall mortality rate of >100% can be explained by the interplay of diseases in influencing U5M. Malnutrition is a risk factor for anaemia and malaria-associated morbidity and mortality (Scott et al., 2014; Ezzati et al., 2004; Gera et al., 2007; Christofides et al., 2006). Many of the children who die from malaria also have malarial anaemia (Walker et al., 2013). The adverse effect of malnutrition on diarrhoea and malaria has been described (Karagiannis-Voules et al., 2015; Gyorkos and Gilbert, 2014; Uganda Integrated Food Security Phase Classification technical working group, 2017). This implies that controlling one disease alone is not enough to significantly curb under-five morbidity and mortality as the causes are interconnected. Thus, the comprehension of the interaction of childhood diseases is important for the understanding of under-five morbidity and mortality and, for the development of effective interventions. Hence, health personnel should be trained to distinguish and treat major childhood diseases in the presence of other illnesses among children. This alongside malaria, malnutrition, diarrhoea and anaemia control measures may reduce the U5M burden related to such diseases.
The main limitation of our study is that disease data for the dead children were not available and therefore we were not able to estimate the disease-related mortality using disease information at individual level. Instead, we treated the disease prevalence at the cluster level as an exposure and quantified the associations with U5M, adjusting for birth-related factors at the individual level, maternal and household characteristics as well as coverage of interventions at cluster level. Our results are therefore prone to ecological fallacy; however, they inform about geographical distribution of the effects of childhood diseases on U5M in Uganda. The methodology presented in this paper can be applied to other countries with dysfunctional civil registration systems, and be used as a tool for providing information for decision making in programming of interventions at sub-national scales to address U5MR.
5. Conclusion
In Uganda, the majority of deaths among children under-five years are due to malaria, followed by diarrhoea, severe/moderate anaemia and severe/moderate malnutrition. Thus, improving disease-specific interventions especially in the affected regions may reduce under-five mortality.
Disease-specific interventions should be strengthened specifically in the affected regions. Interventions related to malaria, in particular, IRS should be reinforced in Central 2, East-Central, Mid-North, North-East and West-Nile. The coverage of iron supplementation and deworming should be increased especially during pregnancy and infancy in Central 1, Kampala, Mid-North, Mid-Western, North-East, South-West and West-Nile to lessen anaemia-mortality. Balanced exclusive breastfeeding within the first six months of delivery, investment in nutrition and education in nutrition practices mainly in more malnourished regions (East-Central, Mid-Eastern and North-East) would alleviate deaths related to malnutrition. Scaling up coverage of diarrhoea interventions, such as ORS or RHF and improved sanitation facilities in Central 2, East-Central, Mid-Eastern and Mid-Western, and educating the population on the benefits of hygienic practices will prevent diarrhoea.
Abbreviations
- ACT
Artemisinin-combination therapy
- ANC
Antenatal care
- ARIs
Acute respiratory infections
- BCG
Bacillus Calmette Guerin
- CA
California
- DHS
Demographic and Health Survey
- DPT
Diphtheria, pertussis and tetanus
- g/dL
grams per deciliter
- IGBP
International Global Biosphere Programme
- IPT
Intermittent preventive treatment
- IRS
Indoor Residual Spraying
- ITN
Insecticide Treated Net
- LST
Land Surface Temperature
- MIS
Malaria Indicator Survey
- NDVI
Normalized Difference Vegetation Index
- ORS or RHF
Oral rehydration solution or recommended home fluids
- SDG
Sustainable Development Goals
- TX
Texas
- UK
United Kingdom
- US
United States
- USAID
United States Agency for International Development
- U5MR
Under-five mortality rate
- WASH
Water, Sanitation and Hygiene
- WHO
World Health Organization
Acknowledgments
Acknowledgements
The authors are thankful to the Uganda Bureau of Statistics, Makerere University School of Public Health and DHS MEASURE for making the data available.
Declarations
Declarations of interest
None.
Authors' contributions
All authors take responsibility for the structure and content of the paper. BBN conceptualized the research, managed and analyzed the data, developed the methodology and implemented it in software, interpreted results and wrote the first draft of the manuscript. Author JS participated in manuscript editing. Authors FEM and SK formulated research goals and objectives, and also participated in the process of acquisition of project financial support. PV was the lead author who conceived the research, formulated research goals and objectives, acquired project financial support, led methodology development, model fitting and result interpretation and manuscript writing. All authors read and approved the submitted manuscript.
Funding
This research was supported by the Swiss Programme for Research on Global Issues for Development project (Grant number: IZ01Z0-147286) and the European Research Council advanced grant project (Grant number: 323180).
Ethical approval and consent to participate
In this research article secondary data that was made available to us by the Uganda Bureau of Statistics (UBOS) and the DHS Program (www.dhsprogram.com) was used. According to survey reports, ethical approval and consent to participate was obtained by the above bodies from the Institutional Review Board of International Consulting Firm (ICF) of Calverton, Maryland, USA, and from Makerere University School of Biomedical Sciences Higher Degrees Research and Ethics committee (SBS-HDREC) and the Uganda National Council for Science and Technology (UNCST). Information on ethical approval and consent to participate is published in the 2011 DHS (Uganda Bureau of Statistics (UBOS) and ICF International Inc., 2012) and 2009 MIS reports (Victora et al., 1993).
Consent for publication
Not applicable.
Competing risks
The authors declare that they have no competing interests.
Availability of data
The data that support findings of this article are available at the DHS MEASURE website (www.dhsprogram.com) with request for access and following instructions at https://dhsprogram.com/data/available-datasets.cfm
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.parepi.2019.e00089.
Contributor Information
Betty Bukenya Nambuusi, Email: betty.nambuusi@swisstph.ch.
Julius Ssempiira, Email: julius.ssempiira@swisstph.ch.
Fredrick E. Makumbi, Email: fmakumbi@musph.ac.ug.
Simon Kasasa, Email: skasasa@musph.ac.ug.
Penelope Vounatsou, Email: penelope.vounatsou@swisstph.ch.
Appendix A. Supplementary data
Bayesian geostatistical modelling.
References
- Bbaale E. Immunization status and child survival in Uganda. Afr. J. Econ. Rev. 2015;3:1–20. [Google Scholar]
- Brabin B., Premji Z., Verhoeff F. An analysis of anemia and child mortality2. J. Nutr. 2001;131:636S–648S. doi: 10.1093/jn/131.2.636S. [DOI] [PubMed] [Google Scholar]
- Burke M., Heft-Neal S., Bendavid E. Sources of variation in under-5 mortality across sub-Saharan Africa: a spatial analysis. Lancet Glob. Health. 2016;4:e936–e945. doi: 10.1016/S2214-109X(16)30212-1. [DOI] [PubMed] [Google Scholar]
- Caulfield L.E., Richard S.A., Black R. Undernutrition as an underlying cause of malaria morbidity and mortality in children less than five years old. Am. J. Trop. Med. Hyg. 2004;71:55–63. [PubMed] [Google Scholar]
- Caulfield L.E., de Onis M., Blössner M., Black R.E. Undernutrition as an underlying cause of child deaths associated with diarrhea, pneumonia, malaria, and measles. Am. J. Clin. Nutr. 2004;80:193–198. doi: 10.1093/ajcn/80.1.193. [DOI] [PubMed] [Google Scholar]
- Christofides A., Asante K.P., Schauer C., Sharieff W., Owusu-Agyei S., Zlotkin S. Multi-micronutrient sprinkles including a low dose of iron provided as microencapsulated ferrous fumarate improves haematologic indices in anaemic children: a randomized clinical trial. Matern. Child Nutr. 2006;2:169–180. doi: 10.1111/j.1740-8709.2006.00060.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colón-González F.J., Tompkins A.M., Biondi R., Bizimana J.P., Namanya D.B. Assessing the effects of air temperature and rainfall on malaria incidence: An epidemiological study across Rwanda and Uganda. 2016;1(11) doi: 10.4081/gh.2016.379. [DOI] [PubMed] [Google Scholar]
- Ehrhardt S., Burchard G.D., Mantel C., Cramer J.P., Kaiser S., Kubo M. Malaria, anemia, and malnutrition in African children—defining intervention priorities. J. Infect. Dis. 2006;194:108–114. doi: 10.1086/504688. [DOI] [PubMed] [Google Scholar]
- Ezeh O.K., Agho K.E., Dibley M.J., Hall J., Page A.N. The impact of water and sanitation on childhood mortality in Nigeria: evidence from demographic and health surveys, 2003–2013. Int. J. Environ. Res. Public Health. 2014;11:9256–9272. doi: 10.3390/ijerph110909256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ezzati M., Lopez A.D., Rodgers A.A., Murray C.J.L. World Health Organization; Geneva: 2004. Comparative quantification of health risks: Global and regional burden of disease attributable to selected major risk factors.http://www.who.int/iris/handle/10665/42770 edited by Majid Ezzati … et al. [Google Scholar]
- Gage K.L., Burkot T.R., Eisen R.J., Hayes E.B. Climate and vectorborne diseases. Am. J. Prev. Med. 2008;35:436–450. doi: 10.1016/j.amepre.2008.08.030. [DOI] [PubMed] [Google Scholar]
- Gemperli A., Vounatsou P., Kleinschmidt I., Bagayoko M., Lengeler C., Smith T. Spatial patterns of infant mortality in Mali: the effect of malaria endemicity. Am. J. Epidemiol. 2004;159:64–72. doi: 10.1093/aje/kwh001. [DOI] [PubMed] [Google Scholar]
- Gera T., Sachdev H.P.S., Nestel P., Sachdev S.S. Effect of iron supplementation on haemoglobin response in children: systematic review of randomised controlled trials. J. Pediatr. Gastroenterol. Nutr. 2007;44:468–486. doi: 10.1097/01.mpg.0000243440.85452.38. [DOI] [PubMed] [Google Scholar]
- Gyorkos T.W., Gilbert N.L. Blood drain: soil-transmitted helminths and anemia in pregnant women. PLoS Negl. Trop. Dis. 2014;8 doi: 10.1371/journal.pntd.0002912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kabagenyi A., Rutaremwa G. The effect of household characteristics on child mortality in Uganda. Am. J. Sociol. Res. 2013;3:1–5. [Google Scholar]
- Karagiannis-Voules D.-A., Biedermann P., Ekpo U.F., Garba A., Langer E., Mathieu E. Spatial and temporal distribution of soil-transmitted helminth infection in sub-Saharan Africa: a systematic review and geostatistical meta-analysis. Lancet Infect. Dis. 2015;15:74–84. doi: 10.1016/S1473-3099(14)71004-7. [DOI] [PubMed] [Google Scholar]
- Kazembe L.N., Kleinschmidt I., Holtz T.H., Sharp B.L. Spatial analysis and mapping of malaria risk in Malawi using point-referenced prevalence of infection data. Int. J. Health Geogr. 2006;5 doi: 10.1186/1476-072X-5-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kazembe L.N., Appleton C.C., Kleinschmidt I. Spatial analysis of the relationship between early childhood mortality and malaria endemicity in Malawi. Geospat. Health. 2007;2:41–50. doi: 10.4081/gh.2007.253. [DOI] [PubMed] [Google Scholar]
- Liu L., Johnson H.L., Cousens S., Perin J., Scott S., Lawn J.E. Global, regional, and national causes of child mortality: an updated systematic analysis for 2010 with time trends since 2000. Lancet. 2012;379:2151–2161. doi: 10.1016/S0140-6736(12)60560-1. [DOI] [PubMed] [Google Scholar]
- Liu L., Oza S., Hogan D., Perin J., Rudan I., Lawn J.E. Global, regional, and national causes of child mortality in 2000–13, with projections to inform post-2015 priorities: an updated systematic analysis. Lancet. 2015;385:430–440. doi: 10.1016/S0140-6736(14)61698-6. [DOI] [PubMed] [Google Scholar]
- Liu L., Oza S., Hogan D., Chu Y., Perin J., Zhu J. Global, regional, and national causes of under-5 mortality in 2000–15: an updated systematic analysis with implications for the Sustainable Development Goals. Lancet. 2016;388:3027–3035. doi: 10.1016/S0140-6736(16)31593-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGovern M.E., Canning D. Vaccination and all-cause child mortality from 1985 to 2011: global evidence from the Demographic and Health Surveys. Am. J. Epidemiol. 2015;182:791–798. doi: 10.1093/aje/kwv125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ministry of Health . vol. 2. Ministry of Health; Kampala: 2013. Midterm Analytical Review of Performance of the Health Sector Strategic and Investment Plan 2010/11–2014/15. [Google Scholar]
- Mockenhaupt F.P., Ehrhardt S., Burkhardt J., Bosomtwe S.Y., Laryea S., Anemana S.D. Manifestation and outcome of severe malaria in children in northern Ghana. Am. J. Trop. Med. Hyg. 2004;71:167–172. [PubMed] [Google Scholar]
- Müller O., Traoré C., Jahn A., Becher H. Severe anaemia in west African children: malaria or malnutrition? Lancet. 2003;361:86–87. doi: 10.1016/S0140-6736(03)12154-X. [DOI] [PubMed] [Google Scholar]
- Nabongo P., Verver S., Nangobi E., Mutunzi R., Wajja A., Mayanja-Kizza H. Two year mortality and associated factors in a cohort of children from rural Uganda. BMC Public Health. 2014;14 doi: 10.1186/1471-2458-14-314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nafiu L.A., Okello M., Adiukwu R.N. Determinants of under-five mortality in Abim district, Uganda. Pac. J. Sci. Technol. 2016;17:223–228. [Google Scholar]
- Rice A.L., Sacco L., Hyder A., Black R. Malnutrition as an underlying cause of childhood deaths associated with infectious diseases in developing countries. Bull. World Health Organ. 2000;78:1207–1221. [PMC free article] [PubMed] [Google Scholar]
- Scott S.P., Chen-Edinboro L.P., Caulfield L.E., Murray-Kolb L.E. The impact of anemia on child mortality: an updated review. Nutrients. 2014;6:5915–5932. doi: 10.3390/nu6125915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shankar A.H. Nutritional modulation of malaria morbidity and mortality. J. Infect. Dis. 2000;182:S37–S53. doi: 10.1086/315906. [DOI] [PubMed] [Google Scholar]
- Ssempiira J. University of Basel; Switzerland: 2018. Bayesian Spatio-temporal Modelling of Malaria Surveillance in Uganda. Doctoral dissertation. [Google Scholar]
- Ssempiira J., Nambuusi B., Kissa J., Agaba B., Makumbi F., Kasasa S. Geostatistical modelling of malaria indicator survey data to assess the effects of interventions on the geographical distribution of malaria prevalence in children less than 5 years in Uganda. PLoS One. 2017;12 doi: 10.1371/journal.pone.0174948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ssempiira J., Nambuusi B., Kissa J., Agaba B., Makumbi F., Kasasa S. The contribution of malaria control interventions on spatio-temporal changes of parasitaemia risk in Uganda during 2009–2014. Parasit. Vectors. 2017;10 doi: 10.1186/s13071-017-2393-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uganda Bureau of Statistics . Uganda Bureau of Statistics; Kampala: 2017. Uganda National Household Survey 2016/17. [Google Scholar]
- Uganda Bureau of Statistics (UBOS) and ICF . UBOS and ICF International; Kampala, Uganda and Rockville, Maryland, USA: 2018. Uganda Demographic and Health Survey 2016. [Google Scholar]
- Uganda Bureau of Statistics (UBOS), ICF International Inc . ICF International Inc; Kampala, Uganda: UBOS and Calverton, Maryland: 2012. Uganda Demographic and Health Survey 2011. [Google Scholar]
- Uganda Bureau of Statistics (UBOS), ICF International Inc . UBOS and ICF International; Kampala, Uganda, and Rockville, Maryland, USA: 2015. Uganda Malaria Indicator Survey 2014–15. [Google Scholar]
- Uganda Bureau of Statistics (UBOS), ICF Macro . UBOS and ICF Macro; Calverton, Maryland, USA: 2010. Uganda Malaria Indicator Survey 2009. [Google Scholar]
- Uganda Bureau of Statistics (UBOS), Macro International Inc . UBOS and Macro International Inc; Calverton, Maryland, USA: 2007. Uganda Demographic and Health Survey 2006. [Google Scholar]
- Uganda Integrated Food Security Phase Classification technical working group . Uganda Integrated Food Security Phase Classification; Kampala: 2017. Report of the Integrated Food Security Phase Classification: Analysis for Uganda Prepared Integrated Food Security Phase Classification. [Google Scholar]
- Unicef . Unicef; New York: 2015. Levels & Trends in Child Mortality: Report 2015. Estimates Developed by the UN Inter-agency Group for Child Mortality Estimation. [Google Scholar]
- Vella V., Tomkins A., Nidku J., Marshall T. Determinants of child mortality in south-west Uganda. J. Biosoc. Sci. 1992;24:103–112. doi: 10.1017/s0021932000006842. [DOI] [PubMed] [Google Scholar]
- Vella V., Tomkins A., Borghesi A., Migliori G.B., Adriko B.C., Crevatin E. Determinants of child nutrition and mortality in north-west Uganda. Bull. World Health Organ. 1992;70:637–643. [PMC free article] [PubMed] [Google Scholar]
- Victora C.G., Huttly S.R., Fuchs S.C., Barros F.C., Garenne M., Leroy O. International differences in clinical patterns of diarrhoeal deaths: a comparison of children from Brazil, Senegal, Bangladesh, and India. J. Diarrhoeal Dis. Res. 1993;11:25–29. [PubMed] [Google Scholar]
- Walker C.L.F., Rudan I., Liu L., Nair H., Theodoratou E., Bhutta Z.A. Global burden of childhood pneumonia and diarrhoea. Lancet. 2013;381:1405–1416. doi: 10.1016/S0140-6736(13)60222-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHO Multicentre Growth Reference Study Group . World Health Organization; Geneva: 2006. WHO Child Growth Standards: Length/Height-for-age, Weight-for-age, Weight-for-length, Weight-for-height and Body Mass Index-for-age: Methods and Development. [Google Scholar]
- World Health Organization . World Health Organization; Geneva: 2013. Household Survey Indicators for Malaria Control. [Google Scholar]
- World Health Organization . World Health Organization; Geneva: 2015. Health in 2015: From MDGs, Millennium Development Goals to SDGs, Sustainable Development Goals. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Bayesian geostatistical modelling.
Data Availability Statement
The data that support findings of this article are available at the DHS MEASURE website (www.dhsprogram.com) with request for access and following instructions at https://dhsprogram.com/data/available-datasets.cfm