Abstract
Polyunsaturated fatty acids (PUFAs) are essential dietary components. They are not only used for energy, but also act as signaling molecules. The delta-6 desaturase (D6D) enzyme, encoded by the FADS2 gene, is one of two rate limiting enzymes that convert the PUFA precursors – α-linolenic (n-3) and linoleic acid (n-6) to their respective metabolites. Alterations in the D6D enzyme activity alters fatty acid profiles and are associated with metabolic and inflammatory diseases including cardiovascular disease and type 2 diabetes. Omega-3 PUFAs, specifically its constituent fatty acids DHA and EPA, are known for their anti-inflammatory ability and are also beneficial in the prevention of skeletal muscle wasting, however the mechanism for muscle preservation is not well understood. Moreover, little is known of the effects of altering the n-6/n-3 ratio in the context of a high-fat diet, which is known to downregulate protein synthesis. Twenty C57BL6 male mice were fed a high-fat lard (HFL, 45% fat (mostly lard), 35% carbohydrate and 20% protein, n-6:n-3 PUFA, 13:1) diet for 6 weeks. Mice were then divided into 4 groups (n = 5 per group): HFL– , high-fat oil– (HFO, 45% fat (mostly Menhaden oil), 35% carbohydrate and 20% protein, n-6:n-3 PUFA, 1:3), HFL+ (HFL diet plus an orally administered FADS2 inhibitor, 100 mg/kg/day), and HFO+ (HFO diet plus an orally administered FADS2 inhibitor, 100 mg/kg/day). After 2 weeks on their respective diets and treatments, animals were sacrificed and gastrocnemius muscle harvested. Protein turnover signaling were analyzed via Western Blot. 4-EBP1 and ribosomal protein S6 expression were measured. A two-way ANOVA revealed no significant change in the phosphorylation of both 4EBP-1 and ribosomal protein S6 with diet or inhibitor. There was a significant reduction in STAT3 phosphorylation with the inhibition of FADS2 (p = 0.03). Additionally, we measured markers of protein degradation through levels of FOXO phosphorylation, ubiquitin, and LC3B expression; there was a trend towards increased phosphorylation of FOXO (p = 0.08) and ubiquitinated proteins (p = 0.05) with FADS2 inhibition. LC3B expression, a marker of autophagy, was significantly higher in the HFL plus FADS2 inhibition group from all other comparisons. Lastly, we analyzed activation of mitochondrial biogenesis which is closely linked with protein synthesis through PGC1-α and Cytochrome-C expression, however no significant differences were associated with either marker across all groups. Collectively, these data suggest that the protective effects of muscle mass by omega-3 fatty acids are from inhibition of protein degradation. Our aim was to determine the role of PUFA metabolites, DHA and EPA, in skeletal muscle protein turnover and assess the effects of n-3s independently. We observed that by inhibiting the FADS2 enzyme, the protective effect of n-3s on protein synthesis and proliferation was lost; concomitantly, protein degradation was increased with FADS2 inhibition regardless of diet.
Keywords: FADS2, Skeletal muscle, Protein turnover, High-fat diet, Omega-3
Highlights
-
•
High fat omega-3 rich diets increase STAT3 signaling in a FADS2 dependent manner.
-
•
Inhibition of FADS2 attenuates the protective effects of omega-3 rich diet.
-
•
Inhibition of FADS2 increases protein degradation regardless of diet.
1. Introduction
The World Health Organization estimates global obesity to have almost doubled to 1.4 billion over the past three decades; concurrently, metabolic and inflammatory disorders such as CVD, diabetes, and sarcopenic obesity are rising. Skeletal muscle mass is involved in numerous metabolic functions, with key roles in whole-body protein metabolism and energy expenditure [1]. Therefore, maintenance of muscle mass is key as an imbalance in protein turnover relates to increased morbidity and disease [2]. Recent evidence has shown polyunsaturated fatty acids (PUFAs), specifically eicosapentaenoic acid (EPA, 20:5n-3) and docosahexaenoic acid (DHA, 22:6n-3), to have beneficial effects on skeletal muscle protein turnover [[3], [4], [5], [6], [7], [8], [9],18,34]. PUFAs are essential dietary components, as they are not endogenously produced by humans. Other than a source of energy, these long-chain fatty acids are the structural components of cell membranes, act as signaling molecules, and regulate enzyme activity, transcription of genes, and membrane fluidity [2,[10], [11], [12]]. The delta-6 desaturase (D6D) enzyme, encoded by the FADS2 gene, is one of two rate limiting enzymes that convert the PUFA precursors – α-linolenic (ALA, 18:3n-3) and linoleic acid (LA, 18:2n-6) to their respective metabolites, EPA/DHA and arachidonic acid (AA, 20:4n-6). Alterations in the D6D enzyme activity alters fatty acid profiles and are associated with metabolic and inflammatory diseases [2,13,14]. A study observing a D6D knockout mouse fed one of four isocaloric diets with differing fat sources (corn oil (LA-enriched), arachidonic acid single cell oil (AA-enriched), flaxseed oil (ALA-enriched), or menhaden fish oil (EPA/DHA) enriched) found the beneficial effects of PUFAs to be both independent of and dependent upon their conversion [15].
Omega-3 PUFAs, specifically EPA and DHA, are known for their anti-inflammatory, anti-cachectic, anti-catabolic, and anabolic properties [7,8,10,16,17]; G. I. [18,19]. EPA, DHA, and AA are all precursors of eicosanoids and docosanoids regulating inflammatory and immune response, yet AA has more of a pro-inflammatory effect while EPA and DHA exert anti-inflammatory responses [13,14]. Preservation of lean muscle mass has been attributed to this anti-inflammatory response in post-operative cancer patients supplementing enteral nutrition with EPA [8]. Both in vitro and in vivo studies report n-3 and EPA to suppress pro-inflammatory cytokine production via inhibition of TNF-α in models of arthritis and muscle atrophy [6,10,17]. The mechanism behind omega-3 PUFAs preventing muscle wasting and preserving muscle mass is less understood. This anti-catabolic response is thought to be mediated by the Akt/FOXO, NF-kB, and ubiquitin proteasome system (UPS) pathways; while the anabolic response is associated with upregulation of the mTOR-p70s6k signaling pathway [3,4,6,20,21]. Yet these effects have not been determined in a high-fat diet which has been shown to decrease protein synthesis by activation of the unfolded protein response and disruption of endoplasmic reticulum homeostasis [22]. Recent evidence points to the manipulation of the n-6/n-3 ratio relative to the total amount of PUFAs to be crucial in affecting metabolic and physiological functioning. The westernized diet has a typical ratio of approximately 20:1 n-6/n-3; this relatively high intake of n-6 to n-3 may exacerbate an already pro-inflammatory state in the context of disease [2,13]. Further, diet-induced obesity has been shown to alter protein synthesis in a murine model [23]. Additionally, the D6D enzyme has a preference for n-3 metabolism but high levels of n-6 can shift its preference toward n-6 conversion [2,14,24]. To our knowledge, our study is novel in that it is the first to assess the effects of FADS2 enzyme inhibition on muscle protein turnover with omega-3 supplementation.
Because the FADS2, or D6D, enzyme metabolizes n-6 and n-3 PUFAs, we sought to inhibit this process and analyze the effect of downstream metabolites on protein turnover, as well as assess the effect of omega-3s independently and determine its mechanistic effect. Our aim was to determine the role of PUFA metabolites in skeletal muscle protein synthesis, in the presence or absence of FADS2 activity in C57BL6 mice fed a high-fat diet. We hypothesized that by inhibiting the FADS2 enzyme and supplementing with fish oil (menhaden oil), there would be an increase in skeletal muscle anabolic response via upregulation of the mTOR pathway and/or downregulation of Akt/FOXO and ubiquitin proteasome atrophy pathways.
2. Materials and methods
2.1. Animals and experimental design
C57BL/6 male mice were purchased from Envigo. All animals were kept on a 12:12-h light-dark cycle. Animals had ad libitum access to food and water during the course of the study. Mice were fed a high-fat lard (HFL, 45% fat (mostly lard), 35% carbohydrate and 20% protein, n-6:n-3 PUFA, 13:1) diet for 6 weeks. Mice were then divided into 2 groups (n = 10 per group). One group remained on the HFL diet, while the other group was fed a diet high in n-3 PUFAs (HFO, 45% fat (mostly Menhaden oil), 35% carbohydrate and 20% protein, n-6:n-3 PUFA, 1:3). The complete diet composition is listed below in Table 1. Each group was further subdivided with half of the animals receiving an orally administered FADS2 inhibitor (HFO/HFL+, n = 5/group, at 100 mg/kg/day) and half not receiving inhibitor (HFO/HFL−, n = 5/group). After 2 weeks on their respective diets and treatments, animals were sacrificed and gastrocnemius muscle harvested. All animals were fasted for 5 h before euthanasia. Animals were anesthetized with isoflurane and underwent cervical dislocation. Tissues were removed, weighed, and frozen in liquid nitrogen. Tissues were then stored at −80 °C until further analysis. All animal experimentation was approved by the University of Memphis’ Institutional Animal Care and Use Committee.
Table 1.
Composition of high-fat lard and high omega-3 diets.
| HFL | HFO | |
|---|---|---|
| Ingredient | g | g |
| Lard | 177.5 | |
| Menhaden Oil, ARBP-F | 177.5 | |
| Soybean Oil | 25 | 25 |
| Total | 202.5 | 202.5 |
| Saturated (g) | 60.2 | 59.8 |
| Monounsaturated (g) | 67.7 | 41.3 |
| Polyunsaturated (g) | 62.8 | 88.5 |
| Saturated (%) | 31.6 | 31.5 |
| Monounsaturated (%) | 35.5 | 21.8 |
| Polyunsaturated (%) | 32.9 | 46.7 |
| C16, Palmitic | 36.8 | 35.2 |
| C18, Stearic | 19.8 | 6.6 |
| C18:1, Oleic | 64.1 | 22.8 |
| C18:2, Linoleic | 56.2 | 16.1 |
| C18:3, Linolenic, n3 | 4.2 | 4.3 |
| C20, Arachidic | 0.4 | 0.3 |
| C20:4, Arachidonic, n6 | 0.5 | 0.0 |
| C20:5, Eicosapentaenoic, n3 | 0.0 | 23.3 |
| C22:6, Docosahexaenoic, n3 | 0.0 | 29.0 |
| n6 (g) | 57.0 | 17.9 |
| n3 (g) | 4.4 | 66.6 |
| n6/n3 ratio | 13.1 | 0.3 |
Western Blot Analysis. Western blot analysis was performed as previously described in Ref. [25] The gastrocnemius muscle was homogenized in Mueller buffer and protein concentration was measured using the Bradford method [26]. Homogenates were loaded on 10–12% SDS-polyacrylamide gels, ran, and transferred overnight to polyvinylidene difluoride membranes. Primary antibodies for phosphorylated (P)-, 4EBP1, 4EBP1, P-STAT3, STAT3, P-FOXO, FOXO, Ubiquitin, LC3B, Cytochrome-C (Cell Signaling), and PGC-1α (ABCAM) were incubated 1:2,000 for 24 h in -4 degrees Celsius. Secondary antibodies were used at a concentration of 1:5,000 and were incubated for 1–2 h at room temperature. Enhanced chemiluminescence was used to visualize the antibody-antigen interactions and was developed using a Chemidoc system. Blots were analyzed by measuring the integrated optical density of each band using ImageJ software. All Western blots were normalized to Tubulin or the non-phosphorylated control.
Statistical Analysis. All data are represented as means ± SE. A Student t-test was used to determine systemic and baseline differences between HFO- and HFL- treated mice. A two-way ANOVA was used to determine the effects of diet and inhibitor. Bonferroni post hoc analysis was used to examine interactions. Significance was set at p ≤ 0.05.
3. Results
3.1. Muscle mass
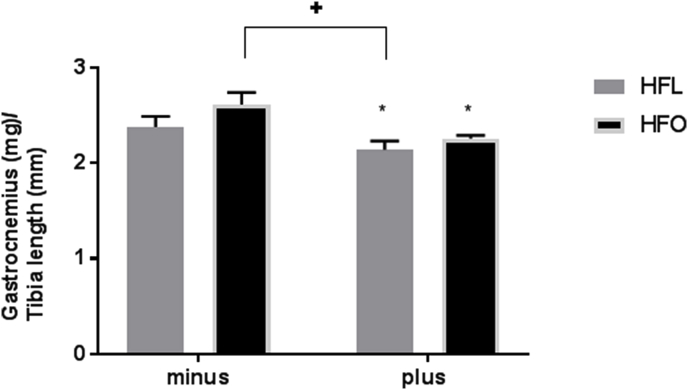
Gastrocnemius muscles, a mixed population of both slow oxidative and fast glycolytic fibers, were measured at the time of sacrifice and mean weights of each group are presented in Fig. 1. There was a significant effect of the FADS2 inhibitor for decreased gastrocnemius weight in mice fed either diet, p = 0.008. These data suggest that inhibition of FADS2 suppresses muscle size independently of supplementation with EPA and DHA.
Fig. 1.
Gastrocnemius mass to tibia length ratio in mice fed a high fat diet (HFL) with FADS2 inhibition and omega-3 supplementation (HFO). All data are presented as mean ± SEM, n = 5/group. Significance was set at p < 0.05. *signifies a main effect of FADS2 inhibitor. +signifies a difference from HFO minus.
3.2. Muscle inflammation
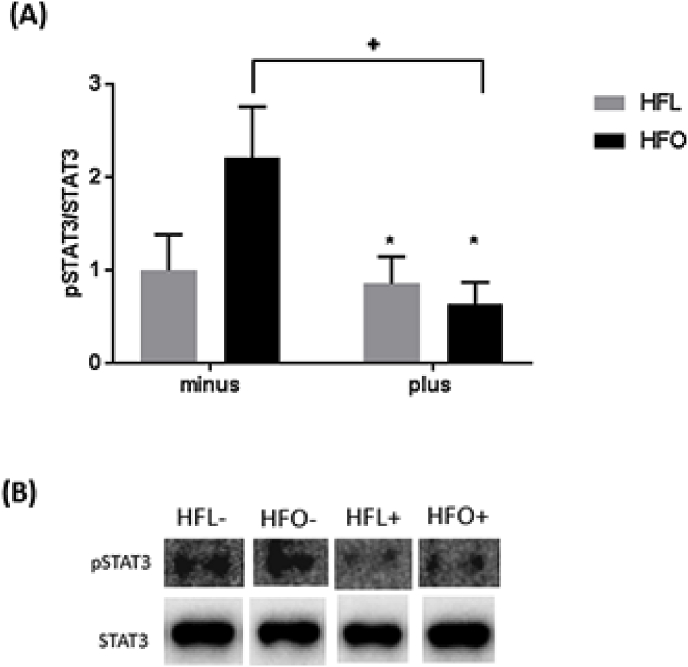
To examine the effect of FADS2 inhibition on markers of inflammation, STAT3 phosphorylation was measured. Skeletal muscle STAT3 phosphorylation in the gastrocnemius was decreased with the inhibition of FADS2 (p = 0.03); however, this effect is primarily due to the decrease in STAT3 phosphorylation in the omega-3 supplemented group with FADS2 inhibition, p = 0.05 (Fig. 2A).
Fig. 2.
Inflammation in gastrocnemius of mice fed a high fat diet (HFL) with FADS2 inhibition and omega-3 supplementation (HFO). A) Ratio of phosphorylated to total STAT3 protein. B) Representative western blot images. All data are presented as mean ± SEM, n = 5/group. Significance was set at p < 0.05. *signifies a main effect of FADS2 inhibitor. +signifies a difference from HFO minus.
3.3. Protein turnover
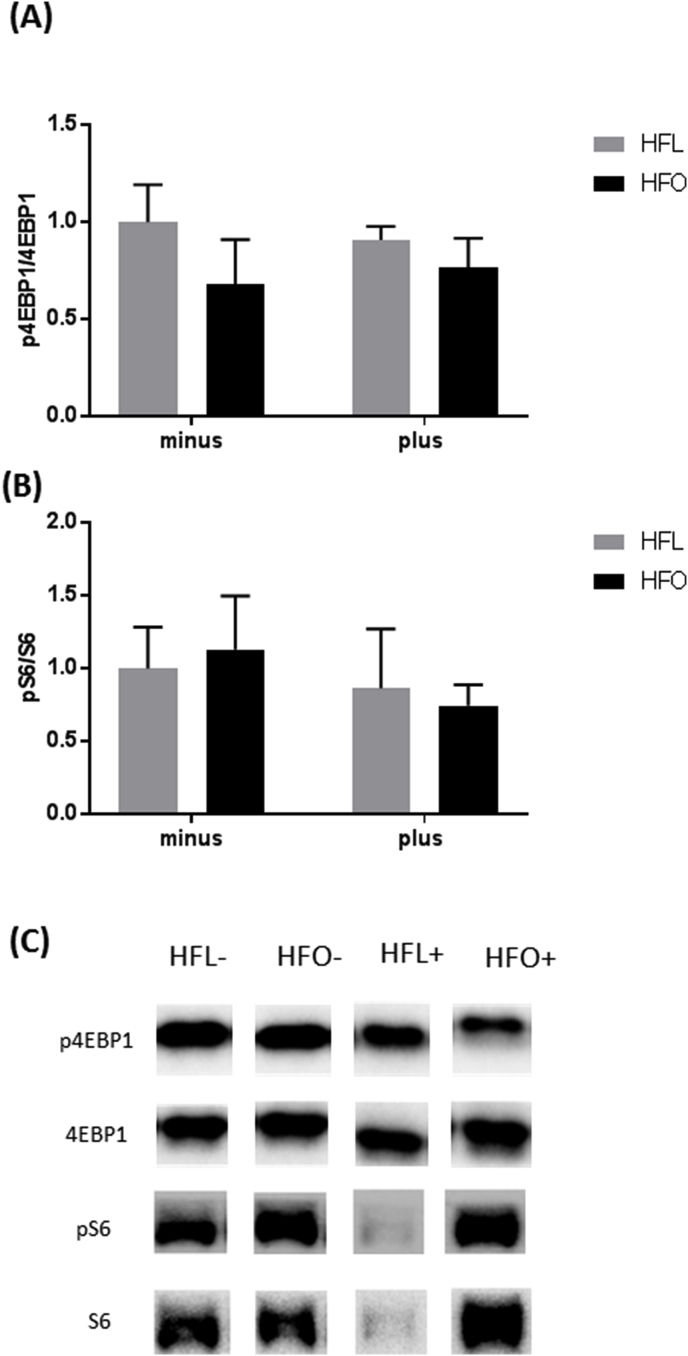
We next examined markers of protein synthesis to determine if fatty acid composition of the diet with or without the FADS2 enzyme inhibition regulated muscle protein turnover. The phosphorylation of both 4EBP-1 and ribosomal protein S6, downstream targets of mTOR, was unaltered with diet or inhibitor (Fig. 3A–B). Although not significant there was a moderate effect of the inhibitor (Cohen's d = 0.78) to decrease the phosphorylation of S6 in the HFO group (Fig. 3B).
Fig. 3.
Protein synthesis signaling in gastrocnemius of mice fed a high fat diet (HFL) with FADS2 inhibition and omega-3 supplementation (HFO). A) Ratio of phosphorylated to total 4EBP1 protein. B) Ratio of phosphorylated to total ribosomal protein S6 C) Representative western blot images. All data are presented as mean ± SEM, n = 5/group. Significance was set at p < 0.05.
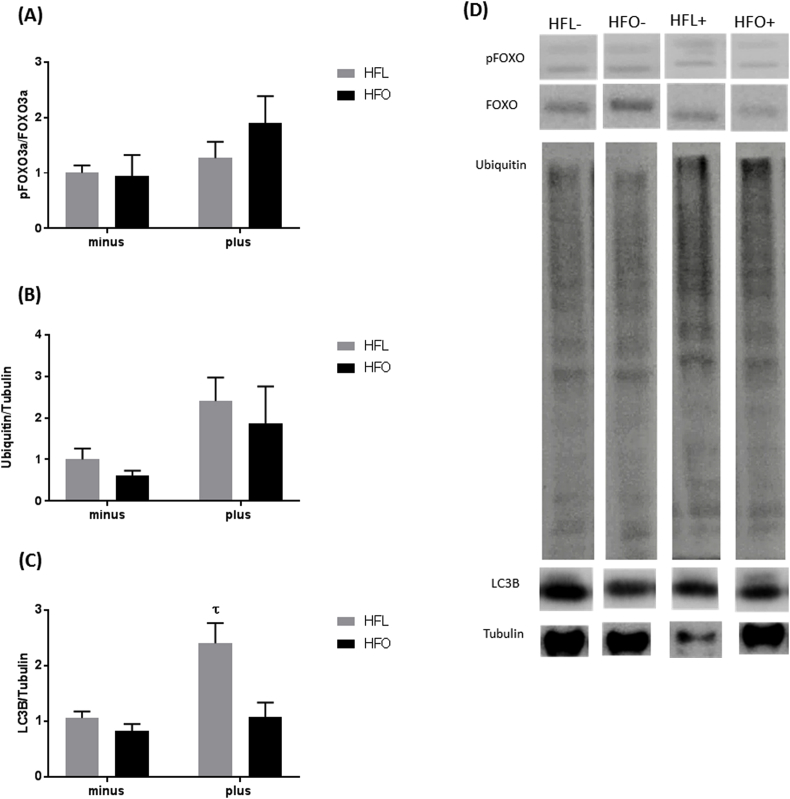
Markers of protein degradation were measured through levels of FOXO phosphorylation, Ubiquitin, and LC3B expression. FOXO can regulate both proteasomal degradation as well as autophagic degradation. There was a trend towards increased phosphorylation of FOXO with FADS2 inhibition, p = 0.08 (Fig. 4A). As a marker of proteasomal degradation protein ubiquitination was measured. There was a trend of increased ubiquitinated proteins with the FADS2 inhibition, p = 0.05 (Fig. 4B). To examine autophagy, LC3B expression was measured. LC3B expression was significantly higher in the HFL plus FADS2 inhibition group from all other comparisons (Fig. 4C). These data suggest a potential role for FADS2 in the regulation of protein degradation processes in muscle.
Fig. 4.
Protein degradation signaling in gastrocnemius of mice fed a high fat diet (HFL) with FADS2 inhibition and omega-3 supplementation (HFO). A) Ratio of phosphorylated to total FOXO3a protein. B) Ubiquitination of proteins normalized to tubulin. C) Total LC3B expression normalized to tubulin D) Representative western blot images. All data are presented as mean ± SEM, n = 5/group. Significance was set at p < 0.05.
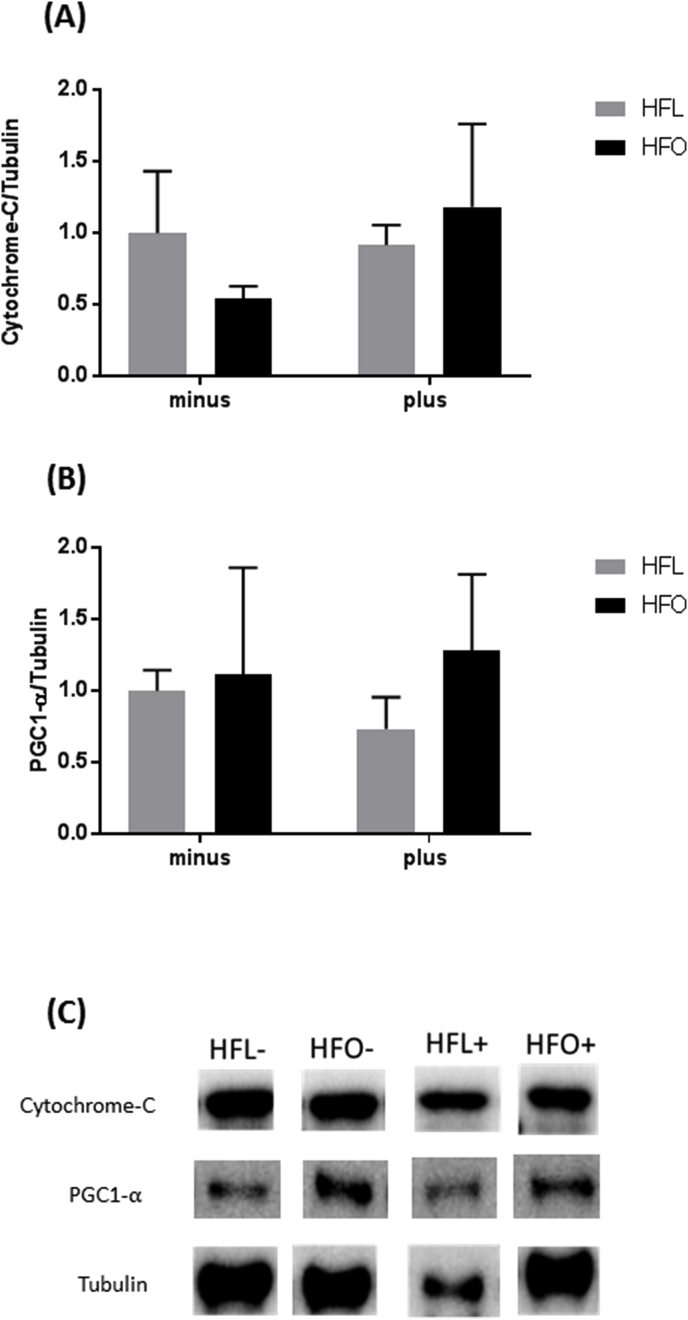
3.4. Mitochondrial biogenesis
We measured markers of mitochondrial biogenesis which is closely linked to protein synthesis. There was no difference in both Cytochrome-C and PGC-1α expression with FADS2 inhibition or diet (Fig. 5A–B). This data suggests that switching from a high fat diet high in omega-6 fatty acids to a high fat diet high in omega 3 fatty acids with or without FADS2 inhibition does not alter signaling for mitochondrial biogenesis after two weeks.
Fig. 5.
Mitochondrial biogenesis signaling in gastrocnemius of mice fed a high fat diet (HFL) with FADS2 inhibition and omega-3 supplementation (HFO). A) Cytochrome C protein expression normalize to tubulin B) PGC-1 alpha protein expression normalized to tubulin. C) Representative western blot images. All data are presented as mean ± SEM, n = 5/group. Significance was set at p < 0.05.
4. Discussion
The beneficial effects of n-3s on skeletal muscle protein turnover are well-known throughout the scientific literature. Studies have shown either supplementing with n-3s or altering the n-6/n-3 ratio to alleviate muscle atrophy, maintain and increase protein synthesis, and inhibit inflammatory markers. However, little is known of these effects within the context of a high-fat diet, which is known to alter protein synthesis. The Westernized diet, high in n-6 to n-3s, is linked to inflammation and chronic disease; n-6s are associated with the production of pro-inflammatory mediators and therefore exacerbate a state of chronic inflammation and contribute to metabolic inflexibility. Additionally, studies have shown that altering the delta-6 desaturase enzyme alters fatty-acid profiles and is associated with metabolic and inflammatory diseases. Therefore, we sought to inhibit the rate-limiting D6D enzyme and observe the effect of downstream metabolites on protein turnover in a high-fat diet. Our aim was to determine the role of PUFA metabolites, DHA and EPA, in skeletal muscle protein turnover and assess the effects of n-3s independently. We observed that by inhibiting the FADS2 enzyme, the protective effect of n-3s on protein synthesis and proliferation was lost; concomitantly, protein degradation was increased with the FADS2 inhibitor regardless of diet.
Other studies have shown that n-3s have a protective and anabolic effect on skeletal muscle mass in both healthy and diseased states [3,7,8,18,27,34]. Our results are consistent with the consensus of n-3s increasing lean muscle mass. We have shown that by inhibiting the FADS2 enzyme, there was a significant reduction in gastrocnemius weight in the HFL-fed mice compared to the HFO-fed mice without the inhibitor (Fig. 1). This data supports body composition findings of an increased fat-free mass change in the HFO – group compared to all other groups (data not shown). Providing the end products EPA/DHA with FADS2 enzyme inhibition was not sufficient to produce the same robust anabolic effect as seen in both diets without the inhibitor. This suggests that the protective effects of skeletal muscle mass are dependent either upon an intermediate metabolite in the downstream metabolism of LA or a combination of metabolites in both LA and EPA/DHA metabolism. According to a study by Palmer et al., arachidonic acid and its upstream derivative dihomo-gamma-linolenic acid (DGLA) stimulated protein synthesis with no effect of EPA and DHA independently [28]. It is known that DGLA is a precursor of eicosanoids and prostaglandins which exert anti-inflammatory and antiproliferative properties in altered cell processes [29,30]. Determining DGLAs role in protein turnover, however, warrants further investigation.
It is well-known that STAT3 upregulates transcriptional control of several genes controlling cellular growth and apoptosis [31]. The activation of STAT3 in skeletal muscle signals differently in a variety of muscle cell types including myofibers, and satellite cells. The chronic activation of STAT3 has been associated with altered growth, while ligand-dependent activation is associated with cell differentiation and growth [32]. STAT3 phosphorylation can be associated with both degradation and cell proliferation. In myofibers, STAT3 activation promotes muscle wasting by inducing MuRF1 and Atrogin 1 expression via upregulation of Myostatin and C/EBPδ or by increasing Caspase 3 expression. Both mechanisms stimulate Ubiquitin Proteasome System activity leading to degradation. However, in muscle stem cells, STAT3 is required for proper myogenic differentiation, yet the mechanism is less understood [33]. Skeletal muscle growth and hypertrophy are dependent upon satellite cell proliferation. Recently, Sun et al. found the cytokine LIF acting on the JAK1-STAT1-STAT3 pathway to regulate myogenic differentiation and proliferation in an injury-induced muscle regeneration model [35]. Studies assessing the JAK/STAT pathway in conjunction with omega-3s are necessary for further investigation. In our study, inhibition of the FADS2 enzyme significantly decreased STAT3 phosphorylation; however, when the FADS2 enzyme was not inhibited in the HFO diet, STAT3 was significantly upregulated. Our data suggests n-3s indeed have an effect on STAT3 phosphorylation. Taken together with our findings of an anabolic response in the HFO – diet by increased gastrocnemius weight(Figs. 1 and 2), STAT3 activation in this study seems to be associated with cell growth and proliferation. There is some evidence in the literature of crosstalk between STAT3 and the mTOR pathway. Studies have shown that mTOR phosphorylates STAT3 at Ser727 which enhances STAT3 transcriptional activity [33,35,36]. However further research in this area is necessary to understand underlying mechanisms.
A well-established mechanism of cell proliferation and protein synthesis lies in the phosphorylation of P13K-Akt and downstream targets 4E-BP1 and S6K1 [37,38]. The anabolic response from n-3s occurs through mechanisms involved in protein synthesis, including upregulation of the Akt-mTOR-p70S6k pathway. In healthy human models, Smith et al. has shown an 8-week supplementation of EPA/DHA to increase mTOR and p70s6k phosphorylation in muscle biopsies during a hyperinsuliemic-hyperaminoacidemic clamp– however found no effect of Akt [27]. Another study found bovine feed enriched with menhaden oil induced greater activation of the Akt-mTOR-S6K1 signaling pathway in the fed steady-state [3]. In our study, protein synthesis signaling was not altered by diet or FADS2 enzyme inhibition. It is well established that a high-fat diet can reduce protein synthesis via disruption of endoplasmic reticulum (ER) homeostasis and activation of the unfolded protein response (UPR). Deldicque et al. showed that administration of palmitic acid to C2C12 muscle cells decreased S6K1 phosphorylation with increased UPR. Additionally, mice on a 20-week high-fat diet (46% fat), comparable to our diet composition, exhibited ER stress with reductions in mTOR pathway activity [22]. While our results did not show a role for omega-3 or FADS2 on protein synthesis, we do provide evidence that n-3s may have an effect on skeletal muscle protein degradation.
Generally, protein degradation and protein synthesis have an inverse relationship. In skeletal muscle degradation, the ubiquitin proteasome system and autophagy lysosome systems are activated in catabolic conditions, and further modulate one another to balance protein turnover [39]. Regardless of the catabolic condition, atrogenes are upregulated in these pathways. Several studies have found the IGF1-AKT-FOXO signaling pathway to suppress protein degradation while increasing synthesis [40]. A high-fat diet is one factor promoting metabolic inflexibility by altering protein synthesis, yet its mechanism remains elusive [23]. However, PUFAs have been found to modulate Akt/FOXO signaling. You et al. found dietary fish oil (compared to corn oil) increased activation of Akt-p70S6 kinase proteins and suppressed gene expression of muscle-specific E3 ubiquitin ligases, Muscle atrophy F-box and muscle RING finger 1, in soleus muscle atrophy [41]. Other studies assessing the protective effects of dietary fish-oil/EPA on models of muscle atrophy have shown activation of Akt to upregulate protein synthesis or downregulate autophagy markers (via inhibition of FOXO) [4,6].
In our study, there was a trend of increased FOXO3a phosphorylation and ubiquitinated proteins with the FADS2 inhibitor. Concomitantly, FADS2 inhibition also significantly increased LC3B expression in the HFL diet compared to all groups (Fig. 4). In various models of catabolic conditions– cancer, sepsis, starvation, and arthritis– studies have found EPA supplementation to downregulate the activation of the UPS pathway thus attenuating degradation [5,10,19,21]. However, the exact mechanism remains unknown. Here we have inhibited downstream metabolism to assess the independent effects of EPA/DHA and arachidonic acid on protein degradation. We have demonstrated that EPA/DHA independently does not suppress degradation through the UPS, but depends on downstream intermediate metabolites of n-3 and n-6 fatty acids while autophagic degradation is suppressed by EPA/DHA independently of FADS2. Recently, the Atg1 homologue, Unc-51-like kinase 1 (ULK1) has been found to regulate autophagy via interaction with mTOR and its downstream targets 4EBP-1 and ribosomal protein S6. Under nutrient sufficiency, mTOR prevents ULK1 activation; whereas, when mTOR is inhibited, ULK1 is activated and phosphorylates Atg13 and FIP200 to continue autophagy processes [42,43]. Additional evidence has shown ULK1 regulates autophagy protease ATG4B affecting downstream LC3B [44,45]. Further studies are necessary to determine how ULK1 affects the mTOR pathway in skeletal muscle turnover and the direct effects of n-3 and n-6 intermediates on the regulation of autophagy.
To our knowledge, we are the first to investigate the effects of FADS2 enzyme inhibition on protein turnover with omega-3 supplementation. We have shown that by inhibiting the FADS2 enzyme, and thus downstream PUFA metabolism, there is a decrease in gastrocnemius weight. Interestingly, with FADS2 enzyme inhibition with or without omega-3 supplementation, there was no change in protein synthesis. With the enzyme inhibition there were significant reductions in protein degradation. With FADS2 inhibition, there was a trend towards increased ubiquitinated proteins; whereas, this effect was reduced without enzyme inhibition. There was a significant increase in LC3B expression, a marker of autophagy, with FADS2 inhibition, and this effect was completely reversed without the inhibitor. However, we found no significant effect of diet or inhibitor on phosphorylation of FOXO. Limitations to our study include a small sample size and short duration of treatment, which could have caused a lack of robust results. Studies have shown that more than 2-weeks of omega-3 supplementation is needed to see significant effects on protein turnover (H. J. [9,46]. Therefore, we speculate that the protective effects of muscle mass by omega-3 fatty acids are from inhibition of protein degradation; however, further research is needed to determine the mechanistic effect of PUFA metabolites on protein turnover.
Author contributions
Katie Brown- conducted study, collected and processed data, manuscript writing, Ella Baker- conducted study, collected and processed data.
Sunita Sharma- conducted study, collected and processed data, William Hawkins- conducted study, collected and processed data.
Marie van der Merwe- conducted study, collected and processed data, manuscript writing.
Melissa Puppa- conducted study, collected and processed data, manuscript writing.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.bbrep.2019.100622.
Transparency document related to this article can be found online at https://doi.org/10.1016/j.bbrep.2019.100622.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
Transparency document
References
- 1.Wolfe R.R. The underappreciated role of muscle in health and disease 1 Ϫ3. Can. J. Appl. Physiol. 2006:475–482. doi: 10.1093/ajcn/84.3.475. August. [DOI] [PubMed] [Google Scholar]
- 2.Jeromson S., Gallagher I., Galloway S., Hamilton D. Omega-3 fatty acids and skeletal muscle health. Mar. Drugs. 2015;13(12):6977–7004. doi: 10.3390/md13116977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gingras A.A., White P.J., Chouinard P.Y., Julien P., Davis T.A., Dombrowski L., Thivierge M.C. Long-chain omega-3 fatty acids regulate bovine whole-body protein metabolism by promoting muscle insulin signalling to the Akt-mTOR-S6K1 pathway and insulin sensitivity. J. Physiol. 2007;579(1):269–284. doi: 10.1113/jphysiol.2006.121079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kamolrat T., Gray S.R. The effect of eicosapentaenoic and docosahexaenoic acid on protein synthesis and breakdown in murine C2C12 myotubes. Biochem. Biophys. Res. Commun. 2013;432(4):593–598. doi: 10.1016/j.bbrc.2013.02.041. [DOI] [PubMed] [Google Scholar]
- 5.Khal J., Tisdale M.J. Downregulation of muscle protein degradation in sepsis by eicosapentaenoic acid (EPA) Biochem. Biophys. Res. Commun. 2008;375(2):238–240. doi: 10.1016/j.bbrc.2008.08.004. [DOI] [PubMed] [Google Scholar]
- 6.Liu Y., Chen F., Odle J., Lin X., Zhu H., Shi H., Yin J. Fish oil increases muscle protein mass and modulates Akt/FOXO, TLR4, and NOD signaling in weanling piglets after lipopolysaccharide challenge. J. Nutr. 2013;143(8):1331–1339. doi: 10.3945/jn.113.176255. [DOI] [PubMed] [Google Scholar]
- 7.Murphy R.A., Mourtzakis M., Chu Q.S.C., Baracos V.E., Reiman T., Mazurak V.C. Nutritional intervention with fish oil provides a benefit over standard of care for weight and skeletal muscle mass in patients with nonsmall cell lung cancer receiving chemotherapy. Cancer. 2011;117(8):1775–1782. doi: 10.1002/cncr.25709. [DOI] [PubMed] [Google Scholar]
- 8.Ryan A.M., Reynolds J.V., Healy L., Byrne M., Moore J., Brannelly N., Flood P. Enteral nutrition enriched with eicosapentaenoic acid (EPA) preserves lean body mass following esophageal cancer surgery: results of a double-blinded randomized controlled trial. Ann. Surg. 2009;249(3):355–363. doi: 10.1097/SLA.0b013e31819a4789. [DOI] [PubMed] [Google Scholar]
- 9.You J., Park M., Song W., Lee Y. 2010. Dietary Fish Oil Alleviates Soleus Atrophy During Immobilization in Association with Akt Signaling to p70s6k and E3 Ubiquitin Ligases in Rats; pp. 310–318. 318. [DOI] [PubMed] [Google Scholar]
- 10.Castillero E., Martín A.I., López-Menduiña M., Villanúa M.A., López-Calderón A. Eicosapentaenoic acid attenuates arthritis-induced muscle wasting acting on atrogin-1 and on myogenic regulatory factors. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2009;297(5):R1322–R1331. doi: 10.1152/ajpregu.00388.2009. [DOI] [PubMed] [Google Scholar]
- 11.Lee J.M., Lee H., Kang S.B., Park W.J. Fatty acid desaturases, polyunsaturated fatty acid regulation, and biotechnological advances. Nutrients. 2016;8(1):1–13. doi: 10.3390/nu8010023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Murphy R.A., Mourtzakis M., Mazurak V.C. N-3 polyunsaturated fatty acids: the potential role for supplementation in cancer. Curr. Opin. Clin. Nutr. Metab. Care. 2012;15(3):246–251. doi: 10.1097/MCO.0b013e328351c32f. [DOI] [PubMed] [Google Scholar]
- 13.Robinson L.E., Buchholz A.C., Mazurak V.C. Inflammation, obesity, and fatty acid metabolism: influence of n -3 polyunsaturated fatty acids on factors contributing to metabolic syndrome. Appl. Physiol. Nutr. Metabol. 2007;32(6):1008–1024. doi: 10.1139/H07-087. [DOI] [PubMed] [Google Scholar]
- 14.Ruxton C.H.S., Reed S.C., Simpson M.J.A., Millington K.J. The health benefits of omega-3 polyunsaturated fatty acids: a review of the evidence. J. Hum. Nutr. Diet. 2004;20(3):275–285. doi: 10.1111/j.1365-277X.2007.00770.x. [DOI] [PubMed] [Google Scholar]
- 15.Monk J.M., Liddle D.M., Cohen D.J.A., Tsang D.H., Hillyer L.M., Abdelmagid S.A., Robinson L.E. The delta 6 desaturase knock out mouse reveals that immunomodulatory effects of essential n-6 and n-3 polyunsaturated fatty acids are both independent of and dependent upon conversion. JNB (J. Nutr. Biochem.) 2016;32:29–38. doi: 10.1016/j.jnutbio.2016.01.004. [DOI] [PubMed] [Google Scholar]
- 16.Beck S.A., Smith K.L., Tisdale M.J. Anticachectic and antitumor effect of eicosapentaenoic acid and its effect on protein turnover. Cancer Res. 1991;51(22):6089–6093. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=1657378 Retrieved from. [PubMed] [Google Scholar]
- 17.Magee P., Pearson S., Allen J. The omega-3 fatty acid, eicosapentaenoic acid (EPA), prevents the damaging effects of tumour necrosis factor (TNF)-alpha during murine skeletal muscle cell differentiation. Lipids Health Dis. 2008;7:1–11. doi: 10.1186/1476-511X-7-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Smith G.I., Atherton P., Reeds D.N., Mohammed B.S., Rankin D., Rennie M.J., Mittendorfer B. 2011. Dietary Omega-3 Fatty Acid Supplementation Increases the Rate of Muscle Protein Synthesis in Older Adults : A Randomized Controlled Trial 1 – 3; pp. 402–412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Whitehouse A.S., Tisdale M.J. Downregulation of ubiquitin-dependent proteolysis by eicosapentaenoic acid in acute starvation. Biochem. Biophys. Res. Commun. 2001;285(3):598–602. doi: 10.1006/bbrc.2001.5209. [DOI] [PubMed] [Google Scholar]
- 20.Wang Y., Lin Q., Zheng P., Zhang J., Huang F. DHA inhibits protein degradation more efficiently than EPA by regulating the PPARγ/NFκB pathway in C2C12 myotubes. BioMed Res. Int. 2013:318981. doi: 10.1155/2013/318981. 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Whitehouse A.S., Smith H.J., Drake J.L., Tisdale M.J. vol. 1. 2001. pp. 3604–3609. (Mechanism of Attenuation of Skeletal Muscle Protein Catabolism in Cancer Cachexia by Eicosapentaenoic Acid Mechanism of Attenuation of Skeletal Muscle Protein Catabolism in Cancer Cachexia by Eicosapentaenoic Acid). [PubMed] [Google Scholar]
- 22.Deldicque L., Cani P.D., Philp A., Raymackers J.-M., Meakin P.J., Ashford M.L.J., Baar K. The unfolded protein response is activated in skeletal muscle by high-fat feeding: potential role in the downregulation of protein synthesis. AJP: Endocrinol. Metabol. 2010;299(5):E695–E705. doi: 10.1152/ajpendo.00038.2010. [DOI] [PubMed] [Google Scholar]
- 23.Anderson S.R., Gilge D.A., Steiber A.L., Previs S.F. Diet-induced obesity alters protein synthesis: tissue-specific effects in fasted versus fed mice. Metab., Clin. Exp. 2008;57(3):347–354. doi: 10.1016/j.metabol.2007.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Su H., Zhou D., Pan Y.-X., Wang X., Nakamura M.T. Compensatory induction of Fads1 gene expression in heterozygous Fads2-null mice and by diet with a high n-6/n-3 PUFA ratio. JLR (J. Lipid Res.) 2016;57(11):1995–2004. doi: 10.1194/jlr.M064956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Puppa M.J., White J.P., Velázquez K.T., Baltgalvis K.A., Sato S., Baynes J.W., Carson J.A. The effect of exercise on IL-6-induced cachexia in the ApcMin/+mouse. J. Cachexia, Sarcopenia Muscle. 2012;3(2):117–137. doi: 10.1007/s13539-011-0047-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bradford, Marion M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- 27.Smith Gordon I., Atherton Philip, Reeds Dominic N., Mohammed Selma B., Rankin Debbie, Rennie Michael J., Mittendorfer B. vol. 121. 2012. pp. 267–278. (Omega-3 Polyunsaturated Fatty Acids Augment the Muscle Protein Anabolic Response to Hyperaminoacidemia-Hyperinsulinemia in Healthy Young and Middle Aged Men and Women). (6) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Palmer Robert, M., Wahle Klaus, W.J. Protein synthesis and degradation in isolated muscle Effect of n3 and n6 fatty acids. Biochemistry. 1987;242:615–618. doi: 10.1042/bj2420615. http://umm.edu/health/medical/altmed/supplement/omega3-fatty-acids Retrieved from. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fan Y.Y., Chapkin R.S. Importance of dietary g -linolenic acid in human health and nutrition. J. Nutr. 1998;128:1411–1414. doi: 10.1093/jn/128.9.1411. June 1998. [DOI] [PubMed] [Google Scholar]
- 30.Yazawa H., Iwahashi H., Kamisaka Y., Kimura K., Aki T., Ono K., Uemura H. Heterologous production of dihomo-γ-linolenic acid in Saccharomyces cerevisiae. Appl. Environ. Microbiol. 2007;73(21):6965–6971. doi: 10.1128/AEM.01008-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Qi Q.-R., Yang Z.-M. Regulation and function of signal transducer and activator of transcription 3. World J. Biol. Chem. 2014;5(2):231–239. doi: 10.4331/wjbc.v5.i2.231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bromberg J.F., Wrzeszczynska M.H., Devgan G., Zhao Y., Pestell R.G., Albanese C., Darnell J.E. Stat3 as an oncogene. Cell. 1999;98(3):295–303. doi: 10.1016/S0092-8674(00)81959-5. [DOI] [PubMed] [Google Scholar]
- 33.Sala D., Sacco A. STAT3 signaling as a potential target to treat muscle-wasting diseases. Curr. Opin. Clin. Nutr. Metab. Care. 2017;19(3):171–176. doi: 10.1097/MCO.0000000000000273.STAT3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Smith G.I., Julliand S., Reeds D.N., Sinacore D.R., Klein S., Mittendorfer B. Fish oil – derived n – 3 PUFA therapy increases muscle mass and function in healthy older adults 1. Am. J. Clin. Nutr. 2015:115–122. doi: 10.3945/ajcn.114.105833.INTRODUCTION. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sun L., Ma K., Wang H., Xiao F., Gao Y., Zhang W., Wu Z. JAK1-STAT1-STAT3, a key pathway promoting proliferation and preventing premature differentiation of myoblasts. JCB (J. Cell Biol.) 2007;179(1):129–138. doi: 10.1083/jcb.200703184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yokogami K., Wakisaka S., Avruch J., Reeves S.A. Serine phosphorylation and maximal activation of STAT3 during CNTF signaling is mediated by the rapamycin target mTOR. Curr. Biol. 2000;10(1):47–50. doi: 10.1016/S0960-9822(99)00268-7. [DOI] [PubMed] [Google Scholar]
- 37.Hemmings B.A., Restuccia D.F., Sassone-corsi P., Morrison D.K., Bootman M.D., Ingham P.W., Restuccia D.F. 2012. PI3K-PKB/Akt Pathway PI3K-PKB/Akt Pathway; pp. 1–4. [DOI] [Google Scholar]
- 38.Rosner M., Hanneder M., Siegel N., Valli A., Fuchs C., Hengstschläger M. The mTOR pathway and its role in human genetic diseases. Mutat. Res. Rev. Mutat. Res. 2008;659(3):284–292. doi: 10.1016/j.mrrev.2008.06.001. [DOI] [PubMed] [Google Scholar]
- 39.Mammucari C., Schiaffino S., Sandri M. Downstream of Akt: FoxO3 and mTOR in the regulation of autophagy in skeletal muscle. Autophagy. 2008;4(4):524–526. doi: 10.4161/auto.5905. [DOI] [PubMed] [Google Scholar]
- 40.Schiaffino S., Dyar K.A., Ciciliot S., Blaauw B., Sandri M. Mechanisms regulating skeletal muscle growth and atrophy. FEBS J. 2013;280(17):4294–4314. doi: 10.1111/febs.12253. [DOI] [PubMed] [Google Scholar]
- 41.You J.S., Park M.N., Lee Y.S. Dietary fish oil inhibits the early stage of recovery of atrophied soleus muscle in rats via Akt-p70s6k signaling and PGF2α. JNB (J. Nutr. Biochem.) 2010;21(10):929–934. doi: 10.1016/j.jnutbio.2009.07.007. [DOI] [PubMed] [Google Scholar]
- 42.He C., Klionsky D.J. Regulation mechanisms and signalling pathways of autophagy. Annu. Rev. Genet. 2009;43(68):67–93. doi: 10.1146/annurev-genet-102808-114910. (Regulation) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kim Joungmok, Kundu Mondira, Viollet Benoit, Guan K.-L. vol. 6. 2013. pp. 132–141. (AMPK and mTOR Regulate Autophagy through Direct Phosphorylation of Ulk1). 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pengo N., Agrotis A., Prak K., Jones J., Ketteler R. A reversible phospho-switch mediated by ULK1 regulates the activity of autophagy protease ATG4B. Nat. Commun. 2017;8(1):1–10. doi: 10.1038/s41467-017-00303-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zachari M., Ganley I.G. The mammalian ULK1 complex and autophagy initiation. Essays Biochem. 2017;61(6):585–596. doi: 10.1042/EBC20170021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Smith H.J., Greenberg N.A., Tisdale M.J. Effect of eicosapentaenoic acid, protein and amino acids on protein synthesis and degradation in skeletal muscle of cachectic mice. Br. J. Canc. 2004;91(2):408–412. doi: 10.1038/sj.bjc.6601981. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.