Abstract
Background
Hypertrophic cardiomyopathy (HCM) is associated with sudden death (SD). Myocardial fibrosis is reportedly correlated with SD.
Objective
We performed a systematic review with meta-analysis, updating the risk markers (RMs) in HCM emphasizing myocardial fibrosis.
Methods
We reviewed HCM studies that addressed severe arrhythmic outcomes and the certain RMs: SD family history, severe ventricular hypertrophy, unexplained syncope, non-sustained ventricular tachycardia (NSVT) on 24-hour Holter monitoring, abnormal blood pressure response to exercise (ABPRE), myocardial fibrosis and left ventricular outflow tract obstruction (LVOTO) in the MEDLINE, LILACS, and SciELO databases. We used relative risks (RRs) as an effect measure and random models for the analysis. The level of significance was set at p < 0.05.
Results
Twenty-one studies were selected (14,901 patients aged 45 ± 16 years; men, 62.8%). Myocardial fibrosis was the major RISK MARKER (RR, 3.43; 95% CI, 1.95-6.03). The other RMs, except for LVOTO, were also predictors: SD family history (RR, 1.75; 95% CI, 1.39-2.20), severe ventricular hypertrophy (RR, 1.86; 95% CI, 1.26-2.74), unexplained syncope (RR, 2.27; 95% CI, 1.69-3.07), NSVT (RR, 2.79; 95% CI, 2.29-3.41), and ABPRE (RR, 1.53; 95% CI, 1.12-2.08).
Conclusions
We confirmed the association of myocardial fibrosis and other RMs with severe arrhythmic outcomes in HCM and emphasize the need for new prediction models in managing these patients.
Keywords: Cardiomyopathy, Hypertrophic, Familial;Endomyocardial Fibrosis; Risk Factors; Death, Sudden,Cardiac; Review; Meta-Analsis
Introduction
Hypertrophic cardiomyopathy (HCM) is an autosomal dominant disease characterized by ventricular hypertrophy in the absence of other conditions that cause heart overload.1-3 It is the most common genetic disease, with a prevalence rate estimated at 1:500, affecting both men and women. Its presentation can vary from asymptomatic to more severe complications, such as sudden death (SD), which has an incidence rate of 1% per year. HCM is mainly responsible for SD in young and competitive athletes.5-7 There is a discussion regarding how we should stratify SD and indicate implantable cardioverter-defibrillator (ICD) for the purpose of primary prevention of this disease. Strategies have been proposed to identify these patients, and classic risk markers (RMs), such as family history of SD, severe ventricular hypertrophy, unexplained syncope, non-sustained ventricular tachycardia (NSVT) on 24-hour Holter monitoring, and abnormal blood pressure response to exercise (ABPRE), have been evaluated in several clinical studies.13 As such, the pathophysiology of SD in HCM is not fully understood. Some factors seem to be involved, including the development of myocardial fibrosis. Studies that investigated myocardial fibrosis using magnetic resonance imaging (MRI) have shown correlations with severe outcomes. In a recent study, Chan et al.14 found that a percentage of fibrosis > 15% of the left ventricular mass was associated with a twofold increase in the risk of SD in patients considered initially at low risk.1 However, the detection of myocardial fibrosiB1 using cardiac MRI continues to generate discussions among experts and is now considered only a risk modifier, as evidenced by the American College of Cardiology Foundation / American Heart Association Task Force on Practice Guidelines.1 The reassessment of RMs, considering the presence of myocardial fibrosis, is fundamental to improve risk stratification. We performed a systematic review and meta-analysis of observational studies that examined RMs in HCM, emphasizing the presence of fibrosis using cardiac MRI, to evaluate their statistical power in predicting SD.
Methods
Study design
Systematic review with meta-analysis of observational studies of the natural history of HCM that reported RMs of SD and severe arrhythmic outcomes.
Search strategy
The search used 3 databases - MEDLINE, LILACS and SciELO - contemplating prospective or retrospective studies conducted between 1980 and 2016, which analyzed the natural history of patients with HCM, regardless of sex or ethnicity. We used the PRISMA statement checklist to conduct the systematic review and meta-analysis. The detailed research adapted for each database was conducted using the following keywords of Medical Subject Heading (MeSH) and DECS: Cardiomyopathy, Hypertrophic, Familial OR "Cardiomyopathy, Hypertrophic" OR "cardiomyopathies" OR cardiomyopathy OR "risk factors" Death OR "defibrillators, implantable" OR "cardioverter defibrillator, implantable". Only the articles published in English, Portuguese, and Spanish were considered for the full-text review.
Selection criteria
We have included only observational studies (prospective or retrospective cohorts) that had a severe arrhythmic outcome equivalent to SD. The studies that also analyzed at least one of the following RMs were included: a) family history of SD, b) severe left ventricular hypertrophy, c) unexplained syncope, d) NSVT on 24-hour Holter monitoring, e) ABPRE, f) presence of left ventricular outflow tract obstruction (LVOTO), and g) presence of myocardial fibrosis on cardiac MRI. The exclusion criteria were (1) studies that were case reports or review articles, (2) studies that did not meet the previously described inclusion criteria, and (3) duplicate studies.
Definitions
The studies included in our meta-analysis often used variable concepts, but fit the definitions listed in Table 1.
Table 1.
Definitions of outcomes and risk markers used in the meta-analysis
| Severe arrhythmic outcomes | SD, aborted SD, documented sustained ventricular tachycardia, or appropriate shock in patients with ICD |
| Family history of SD | Family history of SD in the first-degree relatives of patients |
| Severe left ventricular hypertrophy | Ventricular thickness > 30 mm measured using echocardiography in any left ventricular segment |
| Unexplained syncope | A history of unexplained and transient loss of consciousness with spontaneous recovery |
| NSVT on 24-hour Holter monitoring | ≥ 3 consecutive ventricular extrasystoles with heart rates of ≥ 120 bpm for < 30 seconds |
| LVOTO | Peak gradient of ≥ 30 mmHg in the left ventricular outflow tract detected on echocardiography |
| ABPRE | Increased (< 20 mmHg) or decreased (> 10 mmHg) systolic blood pressure with peak exercise |
| Myocardial fibrosis | Detection of late enhancement on MRI with gadolinium |
SD: sudden death; ICD: implantable cardioverter-defibrillator; NSVT: non-sustained ventricular tachycardia; LVOTO: left ventricular outflow tract obstruction; ABPRE: abnormal blood pressure response to exercise; MRI: magnetic resonance imaging.
Data extraction
The eligibility (using inclusion and exclusion criteria) of each study was systematically analyzed by two reviewers (MIB and SAC), initially by reading the titles and abstracts. Selected articles were read and analyzed in full to assess their eligibility and methodological quality; all references were revised to identify additional studies. Differences in opinion between the two main reviewers were independently resolved by a third reviewer (DV). After this phase, the data were extracted. The information collected from each study included study design, number of patients, demographic data, follow-up, RMs for SD, and severe arrhythmic outcomes. Authors were contacted when any additional information was needed. There was no time restriction for the severe arrhythmic outcomes.
Statistical analysis
The statistical analysis used relative risks (RRs) as a measure of effect with 95% confidence intervals (CIs). The meta-analysis was performed using the DerSimonian and Laird method in case of heterogeneity and the Mantel-Haenszel method in case of homogeneity. The heterogeneity was analyzed using Cochran's Q and I2 Higgins/Thompson tests. The risk of bias was tested using funnel plots and Egger's linear regression test. The software used for the analysis was R 3.4.1. The level of significance was set at p < 0.05.
Ethical and legal aspects
The study protocol was submitted to the Medical Ethics Committee of Pedro Ernesto University Hospital and received a final opinion on November 14, 2013.
Results
Search results
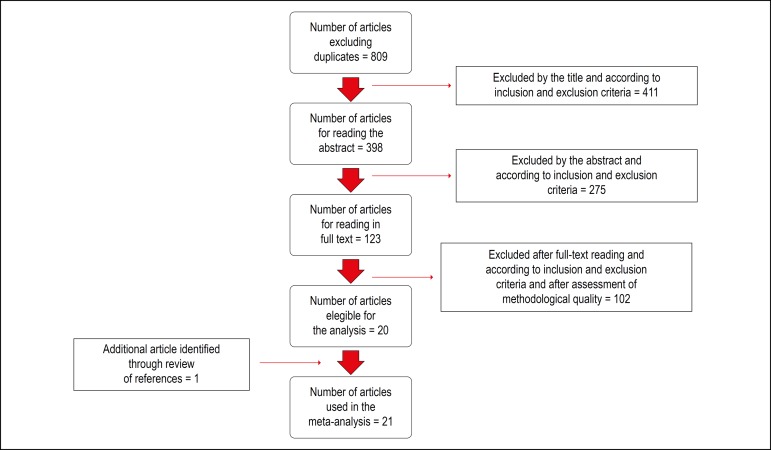
The search strategy identified 809 potentially relevant articles (Figure 1). After reading the titles and abstracts, 123 remained for the eligibility analysis. After detailed evaluation, 103 articles were excluded, and 1 article was added after reviewing the references. Thus, 21 observational studies were selected, including 14 prospective and 7 retrospective studies, comprising 14,901 patients (age, 45 ± 16 years; 62.8% males).11-14,17-33 The main characteristics of the included studies are shown in Table 2.
Figure 1.
Flowchart of the systematic review.
Table 2.
Characteristics of the observational studies involving RMs of SD in HCM
| Author / year of publication | Country | N. of patients | Age (years) | % males | Follow-up (months) | Severe arrhythmic outcomes |
|---|---|---|---|---|---|---|
| Elliott et al. 200617 | UK ;1988-2002 | 917 | 43 | 60.4 | 61 | 54 |
| Elliott et al. 200018 | UK; 1988-1998 | 368 | 37 | 64.9 | 43.2 | 22 |
| Gimeno et al. 200913 | UK; 1988 - 2004 | 1380 | 42 | 61.8 | 54 | NI |
| Kofflard et al. 200319 | Netherlands; 1970-1999 | 225 | 41 | 57.7 | 96 | 20 |
| Kofflard et al. 199320 | Netherlands; 1970-1990 | 113 | 38 | 53.09 | 87.6 | 9 |
| Maron B et al. 200721 | Multicentric; 1983-2005 | 383 | 41 | 62.9 | 44.4 | 51 |
| Maron M et al. 200322 | USA and Italy; 1983- 2001 | 1101 | 45 | 59.4 | 75.6 | 71 |
| Michaelides et al. 200923 | Greece; 1999-2001 | 81 | 42 | 70.3 | 63.6 | 8 |
| Monserrat et al. 200312 | UK; 1988-2000 | 531 | 39 | 60.8 | 70 | 32 |
| Rubinshtein et al. 201024 | USA; 2001-2007 | 424 | 55 | 59.1 | 43 | 8 |
| Spirito et al. 200925 | USA and Italy; 1983-2005 | 1511 | 46 | 61.3 | 67.2 | 74 |
| Spirito et al. 200011 | USA and Italy; 1983-1997 | 480 | 47 | 60 | 78 | 23 |
| Syska et al. 201026 | Poland; 1996-2006 | 78 | 36.4 | 47.4 | 55.2 | 13 |
| Chan et al. 201414 | USA and Italy: 2001-2010 | 1293 | 46 | 63 | 39.6 | 37 |
| Spirito et al. 201427 | Multicentric; 1990-2009 | 653 | 44.4 | 70.5 | 63.6 | 24 |
| Magnusson et al. 201628 | Sweden; 1995-2002 | 237 | 52 | 69.2 | 64.8 | 77 |
| Klopotowski et al. 201529 | Poland; 2008-2013 | 328 | 45 | 58.5 | 37 | 14 |
| Mahony et al. 201430 | Multicentric | 3675 | 48 | 63.9 | 68.4 | 198 |
| Debonmaire et al. 201531 | Netherlands and Belgium | 195 | 52 | 61 | 68.4 | 26 |
| Ismail et al. 201432 | UK; 2000-2011 | 711 | 55 | 70.4 | 42 | 22 |
| O´Hanlon et al. 201033 | UK; 2000-2006 | 217 | 53.2 | 70.5 | 37.2 | 12 |
RMs: risk markers; SD: sudden death; HCM: hypertrophic cardiomyopathy; UK: United Kingdom; USA: United States of America; NI: Not informed.
Myocardial fibrosis
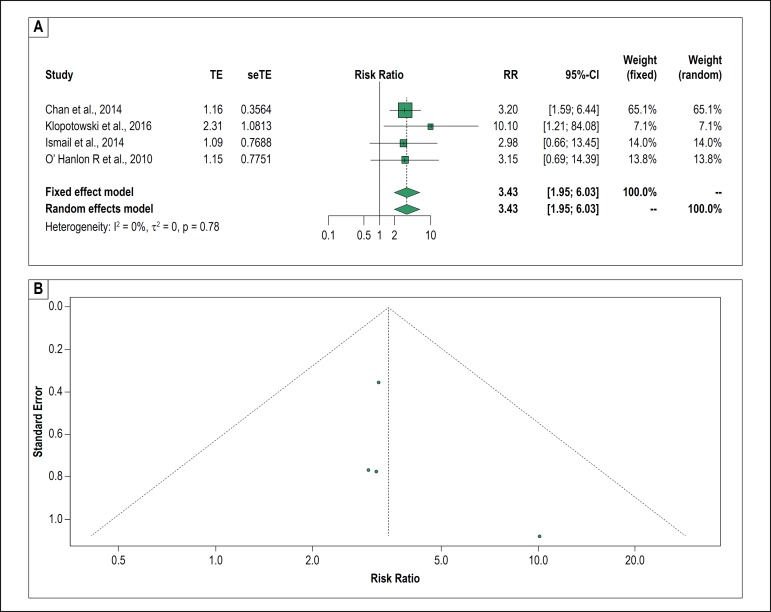
The systematic review selected 5 articles that correlated the presence of fibrosis on cardiac MRI with severe arrhythmic outcomes. One of them was excluded from the meta-analysis because all the patients who had events had myocardial fibrosis using the MRI, making it impossible to calculate the measure of effect.24 Among the four remaining studies involving 2549 patients, the presence of fibrosis was correlated with events equivalent to SD in two studies;14,29 however, this only occurred in the univariate analysis of the other two.32,33 In the meta-analysis, we found a significant probability of severe arrhythmic outcomes in the presence of this variable (RR, 3.43; 95% CI, 1.95-6.03). We highlighted the absence of heterogeneity in the Forest plot and the RR, which was the highest among all the other markers evaluated (Figures 2A and 3). The funnel plot for myocardial fibrosis is shown in figure 2B.
Figure 2.
A. Forest plot of myocardial fibrosis and relative risk of the severe arrhythmic outcomes. B. Funnel plot of myocardial fibrosis to evaluate for publication bias. TE: estimated treatment effect; seTE: standard error of treatment estimate; RR: relative risk; 95%CI: 95% confidence interval.
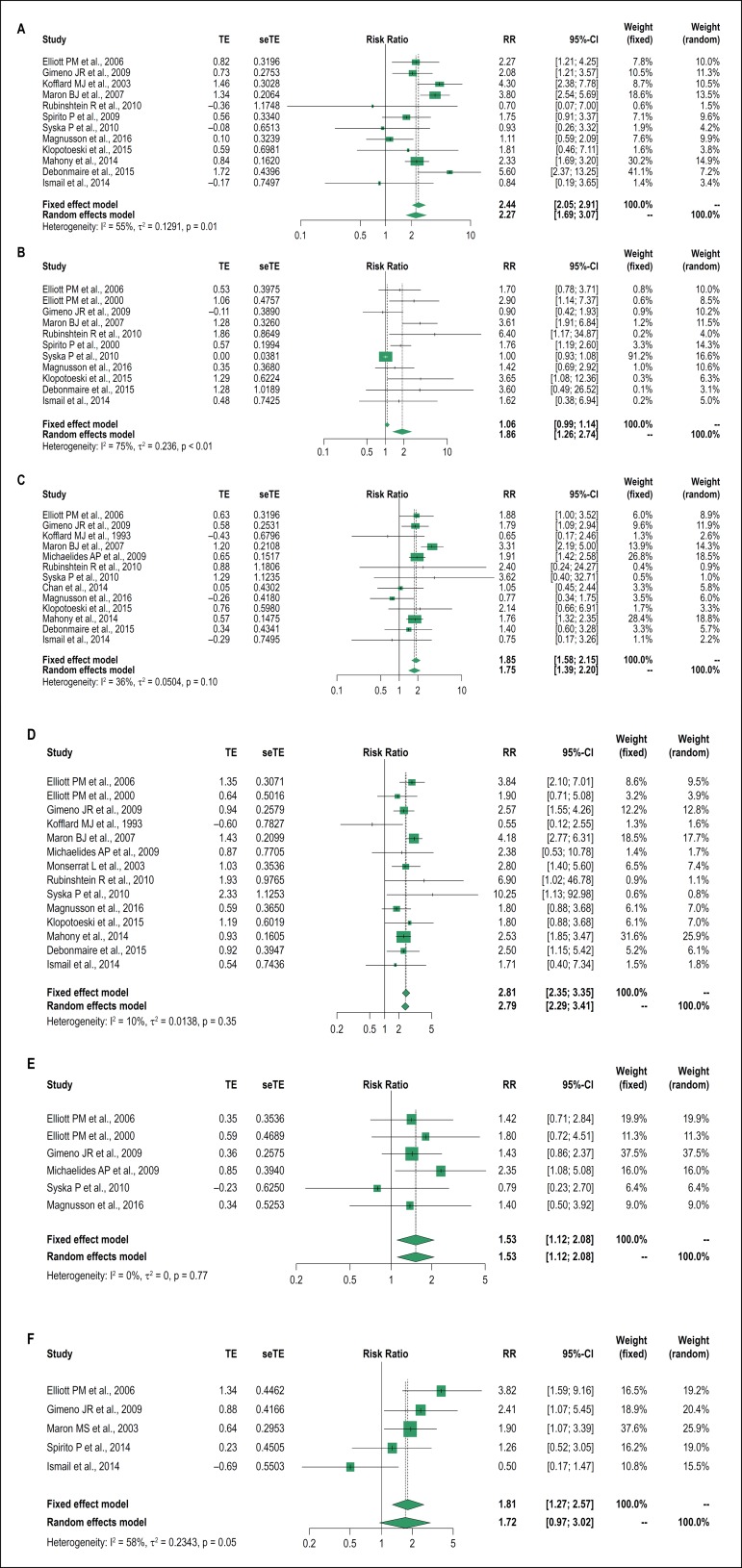
Figure 3.
Forest plot of the risk markers and the relative risk of severe arrhythmic outcomes: A: unexplained syncope; B: severe ventricular hypertrophy; C: family history of sudden death; D: non-sustained ventricular tachycardia; E: abnormal blood pressure response to exercise; F: left ventricular outflow tract obstruction. TE: estimated treatment effect; seTE: standard error of treatment estimate; RR: relative risk; 95%CI: 95% confidence interval.
Meta-analysis of classic RMs
As shown in Figure 3, the following classic RMs demonstrated an association with the outcomes studied: family history of SD (13 studies - 9815 patients; RR, 1.75; 95% CI, 1.39-2.20); severe left ventricular hypertrophy (11 studies - 5501 patients; RR, 1.86; 95% CI, 1.26-2.74); unexplained syncope (12 studies - 10064 patients; RR, 2.27; 95% CI, 1.69-3.07); NSVT on 24-hour Holter monitoring (14 studies - 9421 patients; RR, 2.79; 95% CI, 2.29-3.41); and ABPRE (6 studies - 3061 patients; RR, 1.53; 95% CI, 1.12-2.08).
In our analysis, the only RISK MARKER that showed no correlation with serious arrhythmic outcomes was LVOTO (5 studies - 4762 patients; RR, 1.72; 95% CI, 0.97-3.02). This may have been influenced by the small number of studies involved in the combined analysis. It should be emphasized that the inclusion of this marker in the stratification of SD has always been a matter of debate.
When we evaluated the heterogeneity in all the RMs, we observed that only severe left ventricular hypertrophy was significant (I2 = 75%, p < 0.01), although we emphasize that the random effect model used in the meta-analysis already had mitigated this aspect. The Egger´s test also points to a publication bias for this RISK MARKER (p = 0.002)
We did not observe any publication bias by Egger's test or by the funnel plot for the other classic RMs.
Based on these results, it seems plausible that all classic RMs can still be used in SD stratification in HCM, except for LVOTO.
Discussion
This systematic review and meta-analysis shows the importance of a broad approach in SD risk stratification in patients with HCM, including myocardial fibrosis assessment.
The evaluation of patients with HCM may include multiple complementary examinations in an attempt to predict SD. Obviously, this has several effects, including economic burdening. Thus, knowing how to select the more important RMs is essential. Although primary prevention has been the object of research in several studies in the last decades, attempting to predict which patients with HCM have a higher risk of SD remains challenging. Therefore, systematic reviews and meta-analyses of such a controversial topic becomes important. The presence of the RMs studied here may define the need for ICD placement, considering that it is the only safe and effective tool in preventing SD.36 Some RMs have been reported to be more relevant, such as family history of SD, which was highlighted in the study by Dimitrow et al.,10 however, its low positive predictive values is a limitation. In the event of syncope, it is only indicative of the risk when unexplained. Thus, all RMs have their limitations.
Strategies using the sum of classic RMs were not feasible. In the multicenter registry performed by Maron et al.8 in patients with HCM who were treated with ICD placement, it was observed that 35% who received an appropriate shock had only 1 RM. These data were reinforced by a recent meta-analysis of patients with HCM and ICD who had 1.8 RISK MARKERS for SD on average, with a rate of 3.3% appropriate shocks per year. We also emphasize that the analysis did not include studies that had myocardial fibrosis as an RM.
Among the more recently studied markers aiming to establish correlations with an increased risk of SD in HCM, the most important was myocardial fibrosis. The mechanism suggested for this predisposition is that the presence of myocardial fibrosis could be a substrate for ventricular reentry areas. A classic study demonstrated that this finding correlates with the presence of NSVT in the 24-hour Holter monitoring. Shiozaki et al.39 in a recent national experience with 26 patients with HCM and ICD, assessed myocardial fibrosis by another method, the contrast-enhanced computed tomography, and found a higher rate of appropriate shocks in patients who had a fibrosis mass ≥ 18 g. Most of the studies that evaluated myocardial fibrosis in this population used cardiac MRI, and these were the experiences we analyzed with the focus on outcomes associated with MS.
Putting the results into context, this meta-analysis of observational studies reports a statistically significant association between myocardial fibrosis detected on cardiac MRI and outcomes equivalent to SD. Although we have assessed few articles, this is the most important finding in this study, showing the highest RR among all RMs studied with a very reliable CI. And even if the funnel plot has revealed discrete asymmetry suggesting a publication bias for myocardial fibrosis, it is important to note that the small number of articles does not allow one to conclude this assertion.
A meta-analysis published by Briasoulis et al.,40 addressing only myocardial fibrosis, found similar results. However, one article used in the analyses did not allow a precise calculation of an effect measure because the group without fibrosis did not have any event.24 Our option was to remove it, because we understood this would compromise the statistical analysis. It stands out that this meta-analysis included the article of Klopotowski et al.29 with 328 patients and updated other RMs.
We consider that this finding is of much clinical relevance, as the latest guidelines on the subject do not address the presence of fibrosis as an RM. In its latest document regarding the disease, the European Society of Cardiology based the indication of ICD placement on using a risk calculator (HCM-Risk SCD) created to provide more accurate stratifications.2 Based on a cohort, the derived model used the parameters of age, maximum ventricular thickness, LVOTO, left atrial diameter, family history of SD, presence of NSVT, and unexplained syncope.30 Subsequent studies showed conflicting results regarding the calculator., Perhaps, the fact that it does not assess fibrosis may be a limitation.
Regarding the other findings, we observed that all classic RMs correlated with the occurrence of the outcomes studied, except for LVOTO. In contrast to what has been observed in a previously published meta-analysis, our findings do not indicate that LVOTO may be associated with severe arrhythmic outcomes. This was probably because of the smaller number of patients used in our analysis and the inclusion of two recent studies of which results do not indicate the association between this marker and SD.
The methods that investigated possible publication bias only found significant result for severe left ventricular hypertrophy. However, the clinical relevance of this risk marker has already been documented in several studies and emphasized in the last guidelines.1,2,11,18,43
The limitations of our study include: (1) the inclusion and exclusion criteria, diagnostic methods, and definitions varied discreetly among the different studies; (2) the data of the patients from the same institution may have overlapped, although this did not occur in most of the analyses; (3) the absence of randomized trials may also be considered a relative limitation, but it is important to remember that the systematic review with meta-analysis has the capacity of minimizing this problem, bringing information from observational studies to a higher level of evidence; (4) myocardial fibrosis was analyzed in a few studies and in a binary manner, not quantitatively, although the latter has been gaining attention in recent publications.14 Although the study of Chan et al.,14 which was used in this meta-analysis, provided quantitative information, we used only the RR for the presence or absence of fibrosis.14 Another relevant issue for discussion, although not addressed in this study, which can be considered a limitation, is the cost of cardiac MRI. No cost-effectiveness analysis aimed at the investigation of fibrosis using MRI in patients with HCM has been conducted yet.
Despite these limitations, we rely on the findings of our study because of its methodology, consistency of results (absence of heterogeneity in most analyses), and especially the close and well-known association between myocardial fibrosis and arrhythmias. And with the purpose of studying this association, it is important to emphasize that we chose to include only studies that evaluated outcomes equivalent to SD.
Conclusions
In summary, although it is very difficult to make clinical decisions of great relevance to patients based solely on information from observational studies, it is important to weigh the risks and benefits of ICDs with patients and their families. Nevertheless, this meta-analysis of observational studies emphasizes the importance of cardiac MRI in the detection of myocardial fibrosis for the risk stratification of SD in HCM and confirms the role of traditional RMs, with a doubtful role for LVOTO. Thus, new clinical prediction models using myocardial fibrosis should be considered as a primary prevention strategy for SD in these patients in the future.
Footnotes
Sources of Funding
There were no external funding sources for this study.
Study Association
This article is part of the thesis of Doctoral submitted by Marcelo Imbroinise Bittencourt, from Universidade do Estado do Rio de Janeiro.
Ethics approval and consent to participate
This study was approved by the Ethics Committee of the Hospital Universitário Pedro Ernesto under the protocol number 457893. All the procedures in this study were in accordance with the 1975 Helsinki Declaration, updated in 2013. Informed consent was obtained from all participants included in the study.
Author contributions
Conception and design of the research and analysis and interpretation of the data: Bittencourt MI, Cader SA, Araújo DV, Mourilhe-Rocha R; acquisition of data: Bittencourt MI, Cader AS; statistical analysis: Bittencourt MI, Araújo DV; writing of the manuscript: Bittencourt MI, Cader SA, Mourilhe-Rocha R; critical revision of the manuscript for intellectual content: Bittencourt MI, Cader SA, Araújo DV, Salles ALF, Albuquerque FN, Spineti PPM, Albuquerque DC, Mourilhe-Rocha R.
Potential Conflict of Interest
No potential conflict of interest relevant to this article was reported.
References
- 1.Chan RH, Maron BJ, Olivotto I, Pencina MJ, Assenza GE, Haas T, et al. Prognostic value of quantitative contrast-enhanced cardiovascular magnetic resonance for the evaluation of sudden death risk in patients with hypertrophic cardiomyopathy. Circulation. 2014;130(6):484–495. doi: 10.1161/CIRCULATIONAHA.113.007094. [DOI] [PubMed] [Google Scholar]
- 2.Bittencourt MI, Cader SA, Araújo DV, Salles ALF, Albuquerque FN, Spineti PPM, et al. Sudden death in hypertrophic cardiomyopathy. Int J Cardiovasc Sci. 2016;29(6):504–511. [Google Scholar]
- 3.Moher D, Liberati A, Tetzlaff J, Altman DG, PRISMA Group Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Ann Intern Med. 2009;151(4):264–269. doi: 10.7326/0003-4819-151-4-200908180-00135. [DOI] [PubMed] [Google Scholar]
- 4.Elliott PM, Gimeno JR, Tomé MT, Shah J, Ward D, Thaman R, et al. Left ventricular outflow tract obstruction and sudden death risk in patients with hypertrophic cardiomyopathy. Eur Heart J. 2006;27(16):1933–1941. doi: 10.1093/eurheartj/ehl041. [DOI] [PubMed] [Google Scholar]
- 5.Elliott PM, Poloniecki J, Dickie S, Sharma S, Monserrat L, Varnava A, et al. Sudden death in hypertrophic cardiomyopathy: identification of high risk patients. J Am Coll Cardiol. 2000;36(7):2212–2218. doi: 10.1016/s0735-1097(00)01003-2. [DOI] [PubMed] [Google Scholar]
- 6.Kofflard MJ, Ten Cate FJ, van der Lee C, van Domburg RT. Hypertrophic cardiomyopathy in a large community-based population: clinical outcome and identification of risk factors for sudden cardiac death and clinical deterioration. J Am Coll Cardiol. 2003;41(6):987–993. doi: 10.1016/s0735-1097(02)03004-8. [DOI] [PubMed] [Google Scholar]
- 7.Kofflard MJ, Waldstein DJ, Vos J, ten Cate FJ. Prognosis in hypertrophic cardiomyopathy observed in a large clinic population. Am J Cardiol. 1993;72(12):939–943. doi: 10.1016/0002-9149(93)91111-t. [DOI] [PubMed] [Google Scholar]
- 8.Maron BJ, Spirito P, Shen WK, Haas TS, Formisano F, Link MS, et al. Implantable cardioverter-defibrillators and prevention of sudden cardiac death in hypertrophic cardiomyopathy. JAMA. 2007;298(4):405–412. doi: 10.1001/jama.298.4.405. [DOI] [PubMed] [Google Scholar]
- 9.Maron MS, Olivotto I, Betocchi S, Casey SA, Lesser JR, Losi MA, et al. Effect of left ventricular outflow tract obstruction on clinical outcome in hypertrophic cardiomyopathy. N Engl J Med. 2003;348(4):295–303. doi: 10.1056/NEJMoa021332. [DOI] [PubMed] [Google Scholar]
- 10.Michaelides AP, Stamatopoulos I, Antoniades C, Anastasakis A, Kotsiopoulou C, Theopistou A, et al. ST segment "hump" during exercise testing and the risk of sudden cardiac death in patients with hypertrophic cardiomyopathy. Ann Noninvasive Electrocardiol. 2009;14(2):158–164. doi: 10.1111/j.1542-474X.2009.00291.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rubinshtein R, Glockner JF, Ommen SR, Araoz PA, Ackerman MJ, Sorajja P, et al. Characteristics and clinical significance of late gadolinium enhancement by contrast-enhanced magnetic resonance imaging in patients with hypertrophic cardiomyopathy. Circ Heart Fail. 2010;3(1):51–58. doi: 10.1161/CIRCHEARTFAILURE.109.854026. [DOI] [PubMed] [Google Scholar]
- 12.Spirito P, Autore C, Rapezzi C, Bernabò P, Badagliacca R, Maron MS, et al. Syncope and risk of sudden death in hypertrophic cardiomyopathy. Circulation. 2009;119(13):1703–1710. doi: 10.1161/CIRCULATIONAHA.108.798314. [DOI] [PubMed] [Google Scholar]
- 13.Syska P, Przybylski A, Chojnowska L, Lewandowski M, Sterlinski M, Maciag A, et al. Implantable cardioverter-defibrillator in patients with hypertrophic cardiomyopathy: efficacy and complications of the therapy in long-term follow-up. J Cardiovasc Electrophysiol. 2010;21(8):883–889. doi: 10.1111/j.1540-8167.2009.01716.x. [DOI] [PubMed] [Google Scholar]
- 14.Spirito P, Autore C, Formisano F, Assenza GE, Biagini E, Haas TS, et al. Risk of sudden death and outcome in patients with hypertrophic cardiomyopathy with benign presentation and without risk factors. Am J Cardiol. 2014;113(9):1550–1555. doi: 10.1016/j.amjcard.2014.01.435. [DOI] [PubMed] [Google Scholar]
- 15.Magnusson P, Gadler F, Liv P, Mörner S. Risk markers and appropriate implantable defibrillator therapy in hypertrophic cardiomyopathy. Pacing Clin Electrophysiol. 2016;39(3):291–301. doi: 10.1111/pace.12801. [DOI] [PubMed] [Google Scholar]
- 16.Klopotowski M, Kukula K, Malek LA, Spiewak M, Polanska-Skrzypczyk M, Jamiolkowski J, et al. The value of cardiac magnetic resonance and distribution of late gadolinium enhancement for risk stratification of sudden cardiac death in patients with hypertrophic cardiomyopathy. J Cardiol. 2016;68(1):49–56. doi: 10.1016/j.jjcc.2015.07.020. [DOI] [PubMed] [Google Scholar]
- 17.O'Mahony C, Jichi F, Pavlou M, Monserrat L, Anastasakis A, Rapezzi C, et al. A novel clinical risk prediction model for sudden cardiac death in hypertrophic cardiomyopathy (HCM risk-SCD) Eur Heart J. 2014;35(30):2010–2020. doi: 10.1093/eurheartj/eht439. [DOI] [PubMed] [Google Scholar]
- 18.Debonnaire P, Katsanos S, Joyce E, VAN DEN Brink OV, Atsma DE, Schalij MJ, et al. QRS fragmentation and QTc duration relate to malignant ventricular tachyarrhythmias and sudden cardiac death in patients with hypertrophic cardiomyopathy. J Cardiovasc Electrophysiol. 2015;26(5):547–555. doi: 10.1111/jce.12629. [DOI] [PubMed] [Google Scholar]
- 19.Ismail TF, Jabbour A, Gulati A, Mallorie A, Raza S, Cowling TE, et al. Role of late gadolinium enhancement cardiovascular magnetic resonance in the risk stratification of hypertrophic cardiomyopathy. Heart. 2014;100(23):1851–1858. doi: 10.1136/heartjnl-2013-305471. [DOI] [PubMed] [Google Scholar]
- 20.O'Hanlon R, Grasso A, Roughton M, Moon JC, Clark S, Wage R, et al. Prognostic significance of myocardial fibrosis in hypertrophic cardiomyopathy. J Am Coll Cardiol. 2010;56(11):867–874. doi: 10.1016/j.jacc.2010.05.010. [DOI] [PubMed] [Google Scholar]
- 21.Almquist AK, Montgomery JV, Haas TS, Maron BJ. Cardioverter-defibrillator implantation in high-risk patients with hypertrophic cardiomyopathy. Heart Rhythm. 2005;2(8):814–819. doi: 10.1016/j.hrthm.2005.05.008. [DOI] [PubMed] [Google Scholar]
- 22.Jayatilleke I, Doolan A, Ingles J, McGuire M, Booth V, Richmond DR, et al. Long-term follow-up of implantable cardioverter defibrillator therapy for hypertrophic cardiomyopathy. Am J Cardiol. 2004;93(9):1192–1194. doi: 10.1016/j.amjcard.2004.01.057. [DOI] [PubMed] [Google Scholar]
- 23.Maron BJ, Spirito P. Implantable defibrillators and prevention of sudden death in hypertrophic cardiomyopathy. J Cardiovasc Electrophysiol. 2008;19(10):1118–1126. doi: 10.1111/j.1540-8167.2008.01147.x. [DOI] [PubMed] [Google Scholar]
- 24.Schinkel AF, Vriesendorp PA, Sijbrands EJ, Jordaens LJ, ten Cate FJ, Michels M. Outcome and complications after implantable cardioverter defibrillator therapy in hypertrophic cardiomyopathy: systematic review and meta-analysis. Circ Heart Fail. 2012;5(5):552–559. doi: 10.1161/CIRCHEARTFAILURE.112.969626. [DOI] [PubMed] [Google Scholar]
- 25.Adabag AS, Maron BJ, Appelbaum E, Harrigan CJ, Buros JL, Gibson CM, et al. Occurrence and frequency of arrhythmias in hypertrophic cardiomyopathy in relation to delayed enhancement on cardiovascular magnetic resonance. J Am Coll Cardiol. 2008;51(14):1369–1374. doi: 10.1016/j.jacc.2007.11.071. [DOI] [PubMed] [Google Scholar]
- 26.Shiozaki AA, Senra T, Arteaga E, Martinelli Filho M, Pita CG, Ávila LF, et al. Myocardial fibrosis detected by cardiac CT predicts ventricular fibrillation/ventricular tachycardia events in patients with hypertrophic cardiomyopathy. J Cardiovasc Comput Tomogr. 2013;7(3):173–181. doi: 10.1016/j.jcct.2013.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Briasoulis A, Mallikethi-Reddy S, Palla M, Alesh I, Afonso L. Myocardial fibrosis on cardiac magnetic resonance and cardiac outcomes in hypertrophic cardiomyopathy: a meta-analysis. Heart. 2015;101(17):1406–1411. doi: 10.1136/heartjnl-2015-307682. [DOI] [PubMed] [Google Scholar]
- 28.Vriesendorp PA, Schinkel AF, Liebregts M, Theuns DA, van Cleemput J, Ten Cate FJ, et al. Validation of the 2014 European Society of Cardiology guidelines risk prediction model for the primary prevention of sudden cardiac death in hypertrophic cardiomyopathy. Circ Arrhythm Electrophysiol. 2015;8(4):829–835. doi: 10.1161/CIRCEP.114.002553. [DOI] [PubMed] [Google Scholar]
- 29.Maron BJ, Casey SA, Chan RH, Garberich RF, Rowin EJ, Maron MS. Independent assessment of the European Society of Cardiology sudden death risk model for hypertrophic cardiomyopathy. Am J Cardiol. 2015;116(5):757–764. doi: 10.1016/j.amjcard.2015.05.047. [DOI] [PubMed] [Google Scholar]
- 30.Christiaans I, van Engelen K, van Langen IM, Birnie E, Bonsel GJ, Elliott PM, et al. Risk stratification for sudden cardiac death in hypertrophic cardiomyopathy: systematic review of clinical risk markers. Europace. 2010;12(3):313–321. doi: 10.1093/europace/eup431. [DOI] [PubMed] [Google Scholar]
- 31.Oliveira DC, Assunção FB, Santos AA, Nacif MS. Cardiac magnetic resonance and computed tomography in hypertrophic cardiomyopathy: an update. Arq Bras Cardiol. 2016;107(2):163–172. doi: 10.5935/abc.20160081. [DOI] [PMC free article] [PubMed] [Google Scholar]