Graphical abstract
Keywords: Alcohol dehydrogenase, Moonlighting proteins, Candida albicans, THP-1 cells, Macrophage differentiation, ERK pathway
Highlights
-
•
ADH of Candida albicans was cloned and purified for the first time.
-
•
ADH triggers THP-1 cells to differentiate into M1 macrophages.
-
•
ERK pathway plays an important role for macrophage differentiation.
-
•
ADH induces the productions of IL-1β, IL-6, and TNF-α by THP-1 cells.
-
•
As an antigen protein, ADH may induce the host innate immune system.
Abstract
Candida albicans proteins located on the cell wall and in the cytoplasm have gained great attention because they are not only involved in cellular metabolism and the maintenance of integrity but also interact with host immune systems. Previous research has reported that enolase from C. albicans exhibits high immunogenicity and effectively protects mice against disseminated candidiasis. In this study, alcohol dehydrogenase (ADH) of C. albicans was cloned and purified for the first time, and this study focused on evaluating its effects on the differentiation of the human monocytic cell line THP-1. The morphological features of THP-1 cells exposed to ADH were similar to those of phorbol-12-myristate acetate-differentiated (PMA-differentiated) macrophages. Functionally, ADH enhanced the adhesion, phagocytosis, and killing capacities of THP-1 cells. A flow cytometric assay demonstrated that ADH-induced THP-1 cells significantly increased CD86 and CD11b expression. The production of IL-1β, IL-6, and TNF-α by cells increased in the presence of ADH. As expected, after pretreatment with a MEK inhibitor (U0126), ADH-induced THP-1 cells exhibited unaltered morphological features, eliminated ERK1/2 phosphorylation, prevented CD86/CD11b upregulation and inhibited pro-inflammatory cytokine increase. Collectively, these results suggest that ADH enables THP-1 cells to differentiate into macrophages via the ERK pathway, and it may play an important role in the immune response against fungal invasion.
Introduction
Candida albicans (C. albicans) is a common opportunistic pathogen of humans that can cause either superficial mucosal infection or systemic disorders with high mortality rates among immunocompromised populations [1]. Given the limited availability of clinical antifungal agents, associated side effects, and increasing drug resistance, there has long been an emphasis on the identification of dominant antigens of the organism that might initiate host immune responses to fungal invasion [2], [3], [4].
Alcohol dehydrogenase (ADH), a ubiquitous enzyme involved in the oxidation reduction reaction, plays an essential role in the growth and metabolisms of C. albicans and is a cell wall-related protein that may interact with host structure proteins (e.g., plasminogens and integrins) [5], [6], [7], [8]. Proteins such as ADH that exhibit dual locations are termed “moonlighting” proteins and have been a subject of great attention [9]. In our previous study, provisional ADH and enolase proteins were the only two antigens recognized by host-specific antibodies (IgG1) from mice immunized with three strains of C. albicans with varying virulence (SC5314, 3630, and 3683). Afterwards, we successfully synthesized enolase from C. albicans and demonstrated that the recombinant enolase effectively protected mice against disseminated candidiasis [10]. Existing studies have reported that ADH is highly immunogenic [6], [11]. However, whether ADH is involved in innate immune systems is rarely reported.
Macrophages are important immune effector cells critical to host prevention of candida infection [12]. When the number of resident macrophages declines due to emigration and cell death, monocytes in the bloodstream stimulated by growth factor and pro-inflammatory cytokines differentiate into macrophages and migrate into tissues to maintain immune system homeostasis and ensure a proper inflammatory response through phagocytosis, antigen presentation, and production of cytokines [13], [14], [15]. Thus, monocyte-to-macrophage differentiation is an important part of the biological process, particularly under conditions such as inflammation [16]. In vitro, THP-1 cells are commonly used for investigation of monocyte and macrophage biology because they exhibit advantages of being easy to culture, stable sensitivity and activity, a homogeneous genetic background, and biological characteristics similar to primary human monocytes [17].
Collectively, in this study, ADH from C. albicans was cloned and purified for the first time, and its effects on the differentiation of THP-1 cells into macrophages, based on cell morphology, functional activity, surface markers, and cytokine production were investigated. ADH was capable of inducing the differentiation of THP-1 cells into macrophages. Furthermore, it is demonstrated that a MEK inhibitor (U0126) inhibited the morphological changes in ADH-induced cells, abrogated ERK1/2 phosphorylation, prevented CD86 and CD11b upregulation and inhibited IL-1β, IL-6, and TNF-α increase, suggesting that ADH-induced THP-1 cells are regulated by the ERK pathway.
Experimental
Cloning, expression, and purification of recombinant ADH
The amino acid sequences of ADH were acquired from NCBI GenBank and analyzed using bioinformatics software. Primers of ADH (forward-GGACATATGATGTCTGTCCCAACTACTC, reverse-TTTCTCGAGTTTGTCGTTGTCCAAGAC; NdeI/XhoI restriction enzyme sites underlined) were designed according to PCRdesign and DNAClub and used to amplify the coding sequence of ADH by polymerase chain reaction (PCR). The methods were described previously [10]. The ADH PCR product was cloned into a prokaryotic vector pET30a (+) (Novagen, Darmstadt, USA) that was subsequently transfected into Escherichia coli BL21/DE3 (LabGene Biotech, Guangzhou, China). ADH expression of recombinant E. coli was induced with isopropyl-β-D-thiogalactopyranoside (IPTG) with continuous horizontal shaking for 5 h. Induced E. coli were collected and disrupted by RIPA lysis buffer (Beyotime, Jiangsu, China) with ultrasonication (350 W, on 4 s, off 6 s) for 120 cycles. The precipitate and supernatant of lysed cells were then harvested. Then, 6*His-tagged recombinant proteins were confirmed by SDS-PAGE. Recombinant ADH was then purified by affinity column chromatography using His Bind Purification Kit (GE Healthcare, Pittsburgh, USA). Endotoxin was removed using ToxinErase™ Endotoxin Removal Kit (GenScript, Nanjing, China) and the concentration of endotoxin was 0.09 unit/mL, as determined by Limulus Amebocyte Lysate assay (GenScript, Nanjing, China). Finally, purified ADH was identified by Western blot and the ADH concentration was determined by RC and DC Protein Assays (BioRad, California, USA).
Culture of THP-1 cells and Candida albicans
THP-1 cells (Cellcook, Guangzhou, China) were cultured in RPMI 1640 (Gibco, New York, USA) supplemented with 10% fetal bovine serum (Gibco, New York, USA), 1% penicillin and streptomycin (Gibco, New York, USA), and 0.05 mM β-mercaptoethanol (Gibco, New York, USA) under standard conditions (95% humidified air of 5% CO2 at 37 °C). C. albicans SC5314 was a gift from Dr. C.S. Farah (University of Queensland, Australia). Yeast was stored at −80 °C in 15% (v/v) glycerol in Sabouraud’s broth (OXOID, Hampshire, UK) and grown overnight at 37 °C with 150 rpm continuous agitation on the experimental day.
Differentiation of THP-1 cells
THP-1 cells (2 × 105 cells/mL) were stimulated with 300 nM ADH for 48 h. Additionally, cells treated with 200 nM phorbol-12-myristate acetate (PMA) (Sigma Aldrich, Missouri, USA) served as a positive control, and untreated cells served as a negative control. Cell morphological features were captured by phase contrast microscopy (Olympus, BX63, Tokyo, Japan), and the supernatant was collected and stored at −80 °C for the cytokine assay. Differentiated cells were collected with trypsin/EDTA solution (Gibco, New York, USA) at 37 °C for 5 min, washed twice with phosphate buffer saline (PBS) and used to determine phagocytosis, killing capacity and surface maker expression.
Lactate dehydrogenase (LDH) cytotoxicity assay
THP-1 cells (5 × 104 cells/well) were seeded onto 96-well culture plates in six replicates. Varying concentrations of ADH (150, 300, and 600 nM) were added. Then, cells were incubated for 48 h under standard conditions. Cell cytotoxicity was evaluated using a LDH cytotoxicity assay kit (Beyotime, Jiangsu, China), and 1% Triton X-100-lysed cells (total release of LDH) were used as a positive control. The percentage of cell cytotoxicity was then calculated as follows: (mean OD 490 nm of the experimental group - mean OD 490 nm of negative control)/(mean OD 490 nm of positive control - mean OD 490 nm of negative control) × 100%.
Cell adhesion
Cell adhesion was determined as previously reported with some modifications [14]. THP-1 cells (5 × 104 cells/well) were seeded into a 96-well tissue culture plate in triplicate. Cells were then incubated with 300 nM ADH for 12, 24 and 48 h. Then, unattached cells were removed with PBS and adherent cells were stained with 0.1% crystal violet for 10 min at room temperature (RT). Next, dye was eluted with 95% alcohol, and cell adhesion was measured at OD 595 nm absorbance using a microplate reader (Tecan infinite 200, Zürich, Switzerland). For analysis, 200 nM PMA-induced cells and untreated cells served as positive and negative controls, respectively.
Phagocytosis assay
The phagocytosis assay was conducted as previously described [18]. C. albicans SC5314 cells were fixed with 75% alcohol for 60 min, stained with 1 μM Sytox Green (Life Invitrogen, Carlsbad, USA) at RT in the dark and then washed with PBS to eliminate redundant dye. Differentiated cells were then collected as previously described and co-cultured with labeled yeast at a ratio of 1:5 under standard conditions. After 30 min, cold PBS was used to remove unbound yeasts and stop phagocytosis. The mixture of cells and yeast was then incubated with 6 μg/mL propidium iodide (PI) (Sigma Aldrich, Missouri, USA) at RT in the dark for 5 min. Then, phagocytosis of cells was analyzed by flow cytometry (Beckman Coulter, California, USA). The percentage of phagocytosis was calculated as follows: percentage of only ingestion (mean green fluorescence) + the percentage of ingestion and adhesion (mean green and red fluorescence).
Killing assay
The killing capacity of cells was evaluated as previously described [19]. Briefly, differentiated cells were co-cultured with live C. albicans SC5314 at a ratio of 1:3 under standard conditions. After 90 min, cells were lysed with 1 mL distilled water for 10 min, serially diluted with PBS, plated on yeast extract peptone dextrose (YPD) medium and incubated at 37 °C for 48 h. The same number of yeast alone served as a negative control. Colony forming units (CFU) were calculated, and the percentage of killing was calculated as follows: (1 - CFU of co-cultured cells/CFU of yeast alone) × 100%.
Flow cytometric assay of surface makers
Differentiated cells were collected as described above and incubated for 30 min with phycoerythrin (PE)-conjugated anti-human CD86 and CD11b antibodies (BD Biosciences, New York, USA) at 4 °C. Corresponding isotypes were used as controls. Next, cells were washed with flow cytometer buffer, and fixed with 4% paraformaldehyde for 30 min. Finally, CD86 and CD11b expression was tested using flow cytometry (Beckman Coulter, California, Germany), and data were analyzed with the FlowJo software, version 7.6.1 (Tree Star Inc., San Carlos, USA).
Cytokine production
Supernatants stored at −80 °C were allowed to reach RT. Then, IL-1β, IL-6, IL-10 and TNF-α concentrations were assayed using ELISA kits (R&D systems, Minneapolis, USA) according to manufacturer’s protocols.
Inhibitor studies
THP-1 cells (2 × 105 cells/mL) were pre-incubated with 10 μM inhibitor of MEK 1/2 U0126 (MCE, New Jersey, USA) for 1 h under standard conditions and then stimulated with 300 nM ADH for an additional 48 h. The morphological features were captured by phase contrast microscopy, and surface marker expression and cytokine production were measured as described above.
Western blot for ERK
Following incubation with ADH or U0126 as described above, cells were washed with PBS and lysed in RIPA buffer on ice for 30 min. Cell proteins were then collected by centrifugation and quantified with a BCA kit (Thermo Fisher, Waltham, USA). Next, 30 μg of protein per sample were separated on 12% SDS-polyacrylamide gels and transferred to polyvinylidene difluoride (PVDF) membrane. The membrane was subsequently blocked with 5% (w/v) skim-milk and probed with appropriate dilutions of the following primary antibodies overnight at 4 °C: rabbit anti-phospho-p44/42 MAPK (Thr202/Tyr204) (Cell Signaling, Massachusetts, USA), rabbit anti-p44/42 MAP kinase (137F5) (Cell Signaling, Massachusetts, USA), and mouse anti-GAPDH (Protein Tech Group, Chicago, USA). Next, membranes were incubated with secondary antibodies, including goat anti-rabbit IgG, HPR-linked antibody (Cell Signaling, Massachusetts, USA), and goat anti-mouse IgG, HPR-linked antibody (Emarbio Science &Technology, Beijing, China), for 1 h at RT. Proteins were subsequently detected using an enhanced chemiluminescence (ECL) detection kit (Merck Millipore, Missouri, USA) and visualized with a chemiluminescent imaging system (ImageQuant Las4000mini, Tokyo, Japan). Finally, the results were analyzed using ImageJ software (National Institutes of Health, Maryland, USA).
Statistical analysis
Statistical analyses between groups were conducted using independent t-test using SPSS 13.0. Values of P < 0.05 were considered statistically significant. Data were presented as the means ± SEM of three individual experiments.
Results
Cloning, expression, and purification of ADH
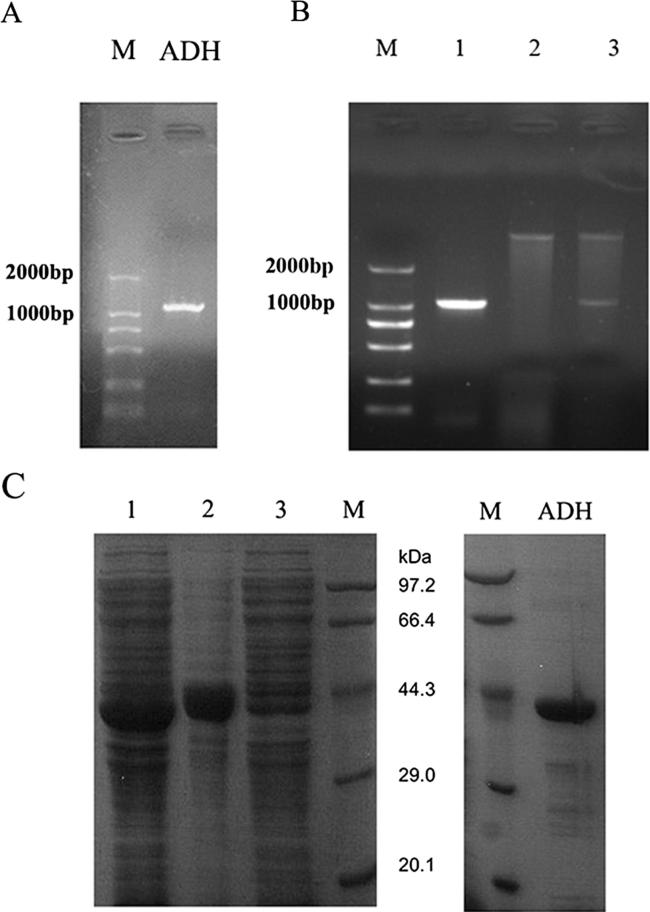
Database (GenBank) and bioinformatics (Expsay) analyses have identified the amino acid sequences of ADH, namely 1047 bp cDNA of ADH1 (XP_717575.1) encoding 348 amino acids with a predicted molecular mass of 36 kDa. As expected, the observed lengths of ADH after PCR amplification were 1047 bp (Fig. 1A). NdeI and XhoI double-digestion of the recombinant plasmid revealed that the ADH gene was correctly cloned in the pET30a vector (Fig. 1B). Additionally, recombinant ADH was detected in the lysis precipitate, and the molecular mass of purified ADH was 36 kDa (Fig. 1C). The concentration of ADH was 0.87 mg/mL.
Fig. 1.
Cloning, Expression, and Purification of ADH. (A) PCR amplification of C. albicans ADH, fragment size 1047 bp. (B) Map of double-digested recombined plasmid of ADH. Lane 1, the product of PCR amplification; Lane 2, NdeI and XhoI double-digested pET30a vector; Lane 3, NdeI and XhoI double-digested recombined plasmid; Lane M, molecular mass standards. (C) Expression and purification of ADH. Left: Lane 1, the whole lysis of E. coli BL21 induced by IPTG; Lane 2, the lysis precipitate; Lane 3, lysis supernatant; and Lane M, protein marker. Right: purified recombinant ADH, 36 kDa.
ADH cytotoxicity and THP-1 cell differentiation
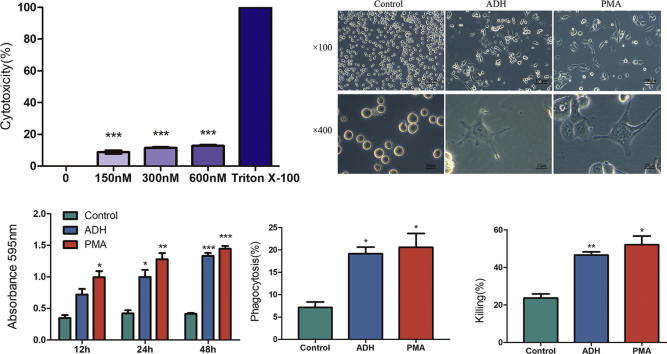
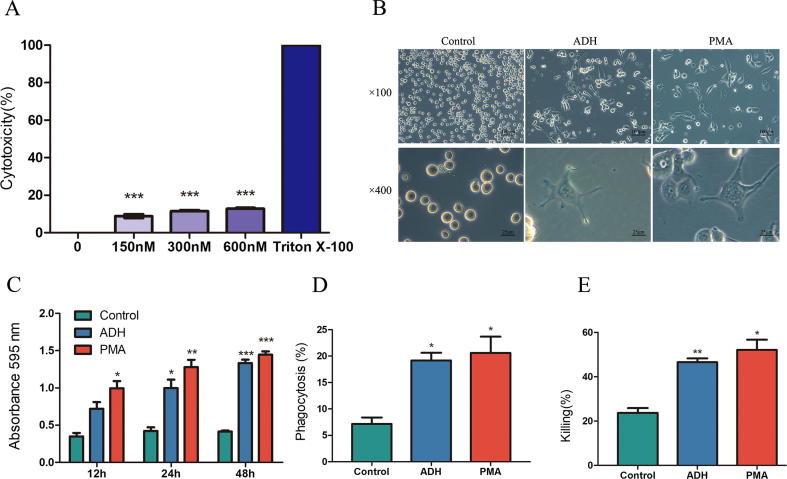
To quantify the cytotoxicity of ADH, LDH assays were performed. LDH levels released following ADH treatment were significantly reduced compared with the positive control (Triton X-100) (Fig. 2A), indicating that ADH exerts no effective cytotoxicity toward THP-1 cells.
Fig. 2.
Cytotoxicity of ADH and ADH-induced THP-1 cells altered the morphology and enhanced the functional capacities of cells. (A) Cytotoxicity of ADH was quantified by LDH assay. Triton X-100 was used as a positive control. (B) THP-1 cells were stimulated with ADH or PMA for 48 h, and untreated cells were used as a negative control. The morphological features were then visualized by phase contrast microscopy at 100× and 400× magnification. (C) Cell adhesion was measured by absorbance at OD 595 nm. (D) Phagocytosis was measured by flow cytometry. (E) The killing capacity of cells was calculated by counting the CFU. Each bar represents the mean ± SEM of three independent experiments performed in six replicates (A), triplicate (C, D, E). ***P < 0.001 vs. positive control (A), *P < 0.05, **P < 0.01, ***P < 0.001 vs. negative control (C, D, E).
To determine whether ADH induces the differentiation of THP-1 cells, the images of differentiated cells were captured by phase contrast microscopy. As shown in Fig. 2B, THP-1 cells attached to the bottom of plates exhibited increased cytoplasmic volume and polygonal shapes compared with the negative control which remained spherical in suspension. Given the similarity to the appearance of PMA-differentiated macrophages, it is noted that ADH stimulated THP-1 cells to differentiate into macrophage-like cells.
ADH enhanced functional capacities of THP-1 cells
Cell adhesion is a widely accepted indicator of monocyte-to-macrophage differentiation [19]. As described above, ADH enabled THP-1 cells to change from a suspended to an attached state. As shown in Fig. 2C, a portion of THP-1 cells attached to the plate in the ADH-treated group at 12 h, whereas PMA-treated cells exhibited a significant increase in cell adhesion compared with the negative control. At the time points of 24 and 48 h, significant increases in the adhesion of ADH-induced cells were noted relative to the negative control (P < 0.05, P < 0.001, respectively), but no significant difference was noted between ADH-treated cells and positive control (PMA-treated cells).
Phagocytosis and killing capacity are two principal functions of macrophages. As shown in Fig. 2D, treatment of THP-1 cells with ADH resulted in significant enhancement of phagocytosis (19.93%) compared with the untreated control group (7.16%) and comparable with positive control (PMA-differentiated cells) (19.98%). Similarly, THP-1 cells stimulated with ADH or PMA exhibited an increased killing capacity of 46.56% and 52.14%, respectively, relative to the negative control (23.73%) (Fig. 2E).
ADH increased macrophage surface markers of THP-1 cells
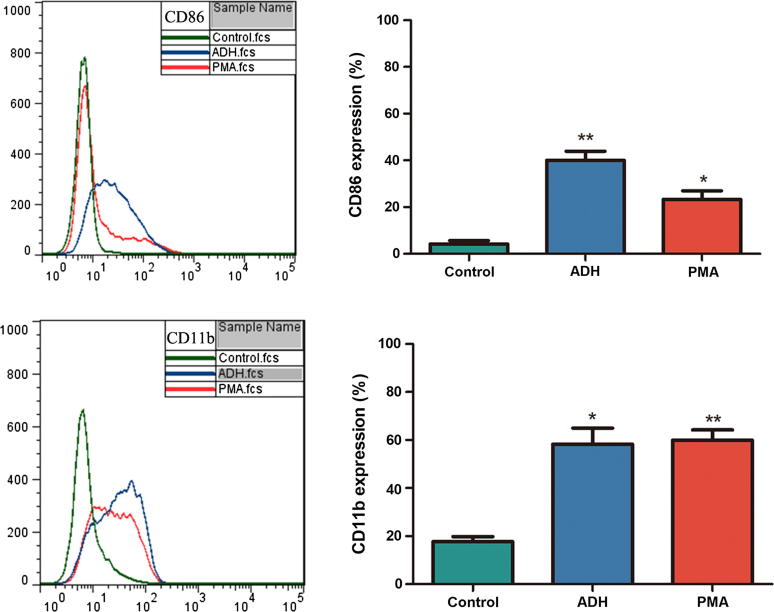
CD86, a co-stimulatory molecule that is the differentiated marker of macrophages [16], [20], was detected by flow cytometry. Compared with untreated cells, THP-1 cells in the presence of ADH significantly increased the expression of CD86 (P < 0.01). Interestingly, ADH-induced cells increased CD86 expression by 1.7-fold compared with PMA-differentiated cells; however, no significant difference was noted. Additionally, the expression of CD11b, another differentiated marker of macrophages, was also significantly increased in the presence of ADH and comparable with positive control (Fig. 3). These results suggested that ADH could differentiate THP-1 cells into macrophages.
Fig. 3.
Macrophage surface markers were detected by flow cytometry analysis. THP-1 cells were stimulated with ADH or PMA for 48 h. Representative histograms of CD86/CD11b expression by flow cytometry. Each bar represents the mean ± SEM of three independent experiments performed in triplicate. *P < 0.05, **P < 0.01 vs. negative control.
ELISA for cytokine production
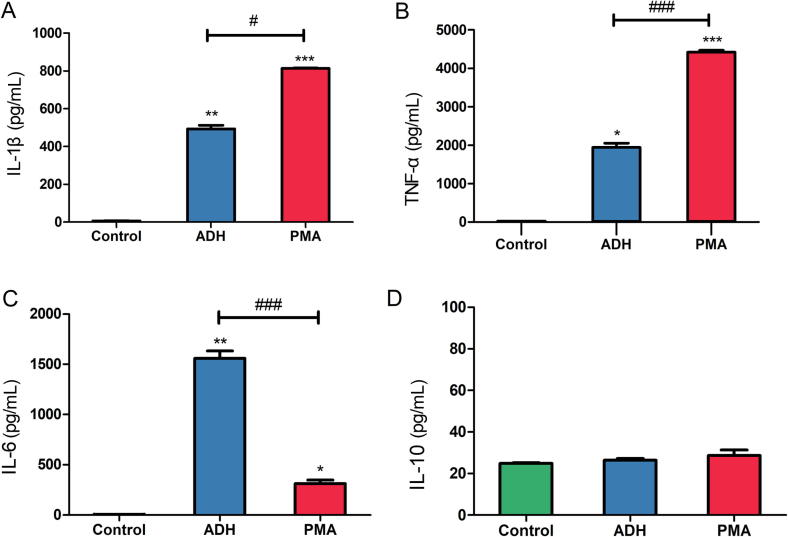
Macrophages are typically divided into M1/classically activated macrophages and M2/alternatively activated macrophages, which possess different cytokine profiles [14], [21]. The above results revealed that ADH-induced THP-1 cells expressed high levels of CD86, which indicate polarization to M1 macrophages [16]. To further ascertain which type of ADH-stimulated cells was driven, the concentrations of IL-1β, IL-6, TNF-α and IL-10 were measured by ELISA. THP-1 cells treated with ADH exhibited high levels of IL-1β and TNF-α (492.76 pg/mL and 1941.06 pg/mL, respectively) compared with untreated cells, whereas cells stimulated with PMA exhibited significant increases in the production of IL-1β (1.6-fold) and TNF-α (2.3-fold) relative to ADH treated cells (Fig. 4A and B). Furthermore, ADH-treated cells secreted 5-fold greater IL-6 levels compared with PMA-differentiated cells (Fig. 4C). As expected, no significant difference in the production of anti-inflammatory cytokine IL-10 was noted among the three groups (Fig. 4D).
Fig. 4.
Cytokine expression profiles were measured by ELISA. THP-1 cells were exposed to ADH or PMA for 48 h. The supernatants were collected, and the concentrations of (A) IL-1β, (B) TNF-α, (C) IL-6, and (D) IL-10 were measured. Each bar represents mean ± SEM from three independent experiments in triplicate. *P < 0.05, **P < 0.01, ***P < 0.001 vs. negative control; #P < 0.05, ###P < 0.001 vs. PMA (positive control).
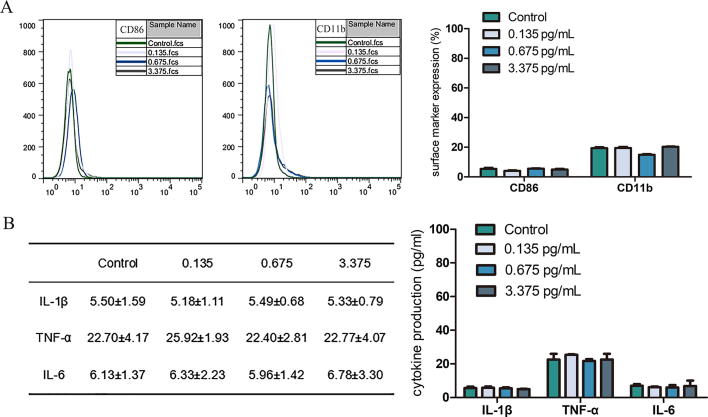
Additionally, the concentration of endotoxin in original recombinant ADH (0.87 mg/mL) was 0.09 unit/mL which is equal to 10.8 pg/mL according to calculate conversions [22], therefore 300 nM ADH contains 0.135 pg/mL LPS. The effective concentrations of LPS to stimulate monocytes/macrophages commonly range from 100 ng/mL to 20 μg/mL [14], [23], [24], [25], [26], [27], [28]. Although the concentration of LPS was extremely low in this study, flow cytometric assay and ELISA were performed on LPS-treated THP-1 cells in order to completely exclude the influences of LPS which is considered an inflammatory activator and able to increase monocytes surface markers expression and pro-inflammatory cytokines production [28]. As expected, LPS at these low concentrations had no effects on THP-1 cells (Fig. 5). From above results, it is suggested that ADH may differentiate THP-1 cells into the M1 polarization state.
Fig. 5.
Effect of LPS was measured by Flow cytometric assay and ELISA. THP-1 cells were exposed to a range of LPS concentrations (0.135 pg/mL, 0.675 pg/mL, and 3.375 pg/mL) for 48 h. Surface markers expression (A) and cytokines production (B) of LPS-treated THP-1 cells were measured. Each bar/value represents mean ± SEM from three independent experiments in triplicate.
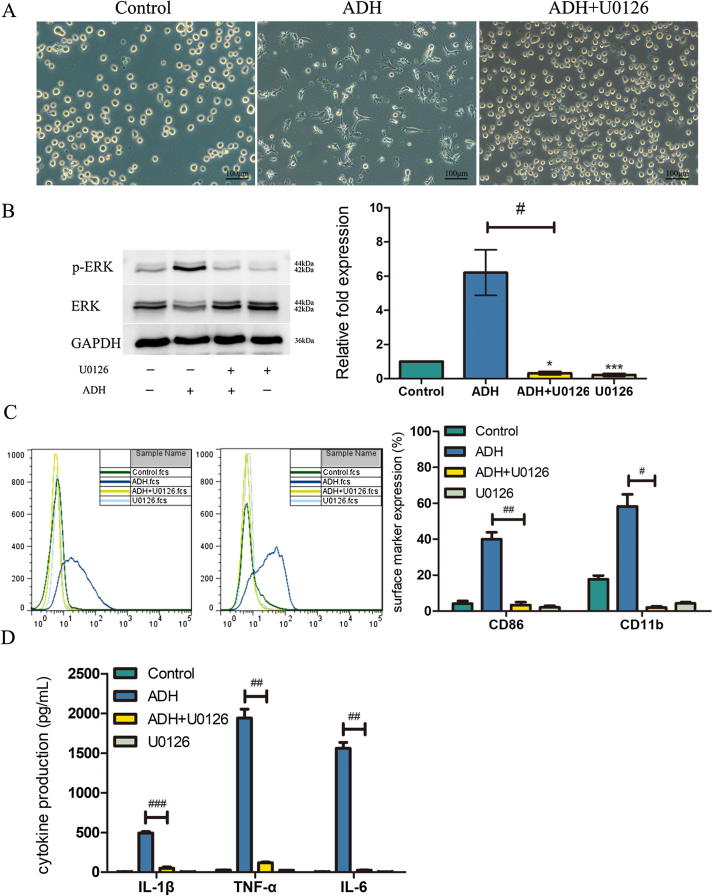
ERK pathway regulated ADH-induced differentiation and production of pro-inflammation cytokines
ERK1/2 is a key growth and differentiation signaling pathway [29]. However, whether the ERK pathway mediates ADH-induced THP-1 cell differentiation remains unclear. To investigate this possibility, THP-1 cells were pretreated with U0126. As shown in Fig. 6A, the cells were maintained in suspension and there were no obvious morphological changes. THP-1 cells stimulated with ADH expressed high levels of phosphorylated ERK1/2, and the expression was significantly eliminated by U0126 pretreatment (Fig. 6B). Furthermore, CD86 and CD11b expression in ADH-induced cells was not upregulated in the presence of U0126 (Fig. 6C). IL-1β, TNF-α and IL-6 levels in ADH-treated THP-1 cells pretreated with U0126 were not increased (Fig. 6D). As expected, untreated cells or cells treated with U0126 alone exhibited minimal expression of surface markers and cytokines. Taken together, these results suggest that the ERK pathway regulates ADH-induced THP-1 differentiation and the secretion of pro-inflammatory cytokines.
Fig. 6.
Effect of a MEK inhibitor on ADH-induced THP-1 cell differentiation and pro-inflammation cytokine production. (A) Cell morphology was visualized by phase contrast microscopy at a magnification of 100×. (B) U0126 abrogated the phosphorylation of ERK1/2 in the presence of ADH. The phosphorylated ERK1/2 band intensities were quantified by normalization to total ERK protein. The fold increase in phosphorylated ERK1/2 was expressed relative to negative control, which was considered to be 1. The results obtained from three independent experiments and representative image are presented. (C) U0126 prevented the upregulation of CD86/CD11b. The figure is representative of three independent experiments. (D) U0126 eliminated the production of pro-inflammation cytokines. Each bar represents the mean ± SEM of three independent experiments performed in triplicate (C, D). *P < 0.05, **P < 0.01, ***P < 0.001 vs. negative control; #P < 0.05, ##P < 0.01, ###P < 0.001 vs. ADH-treated cells pretreated with U0126.
Discussion
Monocyte-to-macrophage differentiation is an important step during the onset of immune responses. In vitro, the human monocytic leukemia cell line THP-1 has been widely used for the investigation of monocyte and macrophage function, activity, and differentiation. As the most effective stimulating agent, PMA induces the differentiation of THP-1 cells, which represents a simplified macrophage model [17]. Thus, PMA was regarded as a positive control in this study. At present, recombinant ADH proteins from Saccharomyces cerevisiae [30] and Candida maltose [31] have been cloned and characterized by others. Here, ADH from Candida albicans was cloned and purified for the first time, and this study focuses on investigating its effects on macrophage differentiation.
The differentiation of monocytes into macrophages is reported as changes in morphology and metabolism, increasing functional capability, expression of macrophage surface markers, and secretion of cytokines [14], [32]. Here, this report demonstrated that ADH stimulated THP-1 cells to differentiate into macrophage-like cells. The increasing ability for adhesion, phagocytosis, and killing of C. albicans by ADH-treated cells occurs via macrophage-like capabilities, which are comparable to the positive control. Furthermore, macrophage-typical surface markers CD86 and CD11b were significantly upregulated by ADH. THP-1 cells exposed to ADH exhibited an approximately 2-fold increase in the expression of CD86 relative to PMA-differentiated macrophages. However, no significant differences were noted, suggesting that the capabilities of antigen presentation of ADH-induced cells are stronger than those of PMA-differentiated macrophages, which might result from the immunogenicity of ADH itself.
Macrophages are classified into two states: M1 and M2. M1 produces pro-inflammatory cytokines and expresses pattern recognition receptors (PRRs) that respond to pathogen-associated molecular patterns (PAMPs), which play a role in microbial and viral infections and tumor regression. M2 expresses anti-inflammatory factors and is involved in parasite infection, tissue modeling, immune regulation, allergies, and tumor progression [33], [34]. In this study, both ADH and PMA induce macrophage differentiation of THP-1 cells; however, some differences in the levels of cytokine production were noted between two inducers. As a chemical reagent, PMA is commonly used as a model of macrophage differentiation [17]. In this study, PMA induced the relatively low level of IL-6 produced by cells. Similarly, Pujari [14] also reported that THP-1 cells induced with a fungus lectin produced high concentrations of pro-inflammatory cytokines (IL-1β, IL-6, TNF-α) compared with PMA-treated cells. Obviously, further experiments should be carried out to elucidate these results although it may come from the immunostimulatory activities of antigen protein. Furthermore, ADH-induced cells tended to differentiate into M1-like macrophages as they upregulated the expression of CD86 and produced high levels of pro-inflammatory cytokines (IL-1β, IL-6, and TNF-α). However, these cells rarely express IL-10. This result was in contrast to Reales-Calderon’s research [35], in which macrophages that interacted with C. albicans promoted an M1-to-M2 switch in polarization that might contribute to Candida pathogenicity. In fact, macrophages possess great plasticity and versatility [36], and different stimuli or environments can initiate a unique macrophage phenotype [37], which may explain the discrepancy among the three different inducers (PMA, yeast and protein). On the other hand, as an antigen protein, ADH plays a protective role in humoral immunity [38], [39]. Therefore, the M1 polarization of ADH-treated THP-1 cells may further invoke adaptive immune system to provide protection against Candida invasion.
The ERK1/2 pathway participates in cell growth and differentiation [29]. Given our hypothesis that the ERK pathway may be involved in the differentiation of THP-1 cells following the exposure to ADH, we found that ADH-induced THP-1 cells exhibited no morphological changes upon pretreatment with the MEK inhibitor U0126. The upregulated levels of phosphorylated ERK1/2 of THP-1 cells were eliminated by U0126. In addition, the expression of surface markers and pro-inflammatory cytokines was prevented by the inhibitor. These results are consistent with those of previous reports [40], [41] and highlight the pivotal role of ERK in macrophage development.
As shown above, the ERK pathway mediates ADH-induced THP-1 differentiation. However, the details regarding the ADH signaling pathway are yet to be elucidated. In vivo, integrins of monocytes that are the principal cell adhesion receptors can attach to extracellular matrix and control differentiation [42]. Klotz [7] hypothesized that C. albicans displays integrin-like receptors and reported that human α5β1 and αvβ3 integrins could react with ADH of C. albicans. These findings indicate that ADH might act as an integrin-like receptor that can activate monocyte surface integrins to mediate monocyte differentiation.
In antifungal innate immunity, the interactions between the PRRs and PAMPs lead to increasing phagocytosis and killing and the expression of cytokines and chemokines [43]. Our findings demonstrated that ADH enhanced the functional capacities of THP-1 cells and increased cytokine production. As a member of “moonlighting” proteins, such as ADH, C. albicans enolase is a heat-shock cognate protein that could stimulate innate immune responses through Toll-like receptors [44], [45], [46]. Thus, ADH may stimulate monocyte differentiation or/and cytokine production through the interaction of PAMP and PRRs.
THP-1 cells exposed to ADH exhibit similar functions as PMA-differentiated macrophages under the same experimental conditions, such as adhesion, phagocytosis and killing capacity; expression of surface markers; and cytokine production. Furthermore, the ERK pathway also participates in PMA-induced THP-1 differentiation [47]. Previous work reported that PMA could activate protein kinase C (PKC) and induce monocyte differentiation and cytokine production [48]. Therefore, we hypothesized that similar mechanisms of monocyte activation might exist between ADH and PMA.
Additionally, it should be necessary to realize the limitations of this study. First, it’s well-known that ADH is a “moonlighting” protein that locates at cell walls and cytoplasm [9], thus the use of soluble ADH is not sufficient to clarify the roles of ADH in the interactions between host and yeast although it could be secreted out of cells as an enzyme [49]. Use of C. albicans over-expressed ADH protein may be one of options in further study. Second, more details of ERK downstream regulators (RSK and MSK family [50]), and other pathways such as NF-κB, NF1 and Bcl-2 [14], [51] should be investigated since ADH induced higher levels of M1 marker (CD86) expression and inflammatory cytokine secretion (IL-6) in THP-1 cells than did PMA and ADH may partially play a role as inflammatory agent in monocytes stimulation. Last, selecting of an inflammatory agent as one of positive controls may help to elucidate the effects of ADH on THP-1 cells differentiation better, although PMA is often used as an activator in the macrophage differentiation model.
Conclusions
In this study, ADH from C. albicans was synthesized for the first time and enabled THP-1 cells to differentiate into macrophage-like cells via the ERK pathway. These results suggest that, ADH, as an antigen protein, may play an important role in the host to initiate the innate immune system against fungal invasion. The work may shed a light on the development of new drugs and vaccine targets in clinic against Candida infection. Accordingly, further studies about the immune effect of ADH on other innate immune cells (macrophages, neutrophils, dendritic cells) and humoral immune response, such as the specific antibody induced in ADH-immunized mice should be carried out soon.
Conflict of interest
The authors have declared no conflict of interest.
Compliance with Ethics Requirements
This article does not contain any studies with human or animal subjects.
Acknowledgements
This work was supported by the National Natural Science of Foundation of China (No. 81170969).
Footnotes
Peer review under responsibility of Cairo University.
Contributor Information
Xiaohuan Zhang, Email: zhxhuan@mail.sysu.edu.cn.
Yan Hu, Email: huyan2@mail.sysu.edu.cn.
References
- 1.Qin Y., Zhang L., Xu Z., Zhang J., Jiang Y.Y., Cao Y. Innate immune cell response upon Candida albicans infection. Virulence. 2016;7:512–526. doi: 10.1080/21505594.2016.1138201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brown G.D., Denning D.W., Gow N.A., Levitz S.M., Netea M.G., White T.C. Hidden killers: human fungal infections. Sci Transl Med. 2012;4:165rv13. doi: 10.1126/scitranslmed.3004404. [DOI] [PubMed] [Google Scholar]
- 3.Cannon R.D., Lamping E., Holmes A.R., Niimi K., Baret P.V., Keniya M.V. Efflux-mediated antifungal drug resistance. Clin Microbiol Rev. 2009;22:291–321. doi: 10.1128/CMR.00051-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gow N.A., Hube B. Importance of the Candida albicans cell wall during commensalism and infection. Curr Opin Microbiol. 2012;15:406–412. doi: 10.1016/j.mib.2012.04.005. [DOI] [PubMed] [Google Scholar]
- 5.Clark D.P. The fermentation pathways of Escherichia coli. FEMS Microbiol Rev. 1989;5:223–234. doi: 10.1016/0168-6445(89)90033-8. [DOI] [PubMed] [Google Scholar]
- 6.Kelly J., Kavanagh K. Proteomic analysis of proteins released from growth-arrested Candida albicans following exposure to caspofungin. Med Mycol. 2010;48:598–605. doi: 10.3109/13693780903405782. [DOI] [PubMed] [Google Scholar]
- 7.Klotz S.A., Pendrak M.L., Hein R.C. Antibodies to alpha5beta1 and alpha(v)beta3 integrins react with Candida albicans alcohol dehydrogenase. Microbiology. 2001;147:3159–3164. doi: 10.1099/00221287-147-11-3159. [DOI] [PubMed] [Google Scholar]
- 8.Reid M.F., Fewson C.A. Molecular characterization of microbial alcohol dehydrogenases. Crit Rev Microbiol. 1994;20:13–56. doi: 10.3109/10408419409113545. [DOI] [PubMed] [Google Scholar]
- 9.Chaffin W.L. Candida albicans cell wall proteins. Microbiol Mol Biol Rev. 2008;72:495–544. doi: 10.1128/MMBR.00032-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Li W., Hu X., Zhang X., Ge Y., Zhao S., Hu Y. Immunisation with the glycolytic enzyme enolase confers effective protection against Candida albicans infection in mice. Vaccine. 2011;29:5526–5533. doi: 10.1016/j.vaccine.2011.05.030. [DOI] [PubMed] [Google Scholar]
- 11.Pitarch A., Diez-Orejas R., Molero G., Pardo M., Sanchez M., Gil C. Analysis of the serologic response to systemic Candida albicans infection in a murine model. Proteomics. 2001;1:550–559. doi: 10.1002/1615-9861(200104)1:4<550::AID-PROT550>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- 12.Krysan D.J., Sutterwala F.S., Wellington M. Catching fire: Candida albicans, macrophages, and pyroptosis. PLoS Pathog. 2014;10:e1004139. doi: 10.1371/journal.ppat.1004139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Italiani P., Boraschi D. From monocytes to M1/M2 macrophages: phenotypical vs. Functional Differentiation. Front Immunol. 2014;5:514. doi: 10.3389/fimmu.2014.00514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pujari R., Kumar N., Ballal S., Eligar S.M., Anupama S., Bhat G. Rhizoctonia bataticola lectin (RBL) induces phenotypic and functional characteristics of macrophages in THP-1 cells and human monocytes. Immunol Lett. 2015;163:163–172. doi: 10.1016/j.imlet.2014.12.005. [DOI] [PubMed] [Google Scholar]
- 15.Shi C., Pamer E.G. Monocyte recruitment during infection and inflammation. Nat Rev Immunol. 2011;11:762–774. doi: 10.1038/nri3070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Smith S.R., Schaaf K., Rajabalee N., Wagner F., Duverger A., Kutsch O. The phosphatase PPM1A controls monocyte-to-macrophage differentiation. Sci Rep. 2018;8:902. doi: 10.1038/s41598-017-18832-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chanput W., Mes J.J., Wichers H.J. THP-1 cell line: an in vitro cell model for immune modulation approach. Int Immunopharmacol. 2014;23:37–45. doi: 10.1016/j.intimp.2014.08.002. [DOI] [PubMed] [Google Scholar]
- 18.Carneiro C., Vaz C., Carvalho-Pereira J., Pais C., Sampaio P. A new method for yeast phagocytosis analysis by flow cytometry. J Microbiol Methods. 2014;101:56–62. doi: 10.1016/j.mimet.2014.03.013. [DOI] [PubMed] [Google Scholar]
- 19.Jeon J.W., Jung J.G., Shin E.C., Choi H.I., Kim H.Y., Cho M.L. Soluble CD93 induces differentiation of monocytes and enhances TLR responses. J Immunol. 2010;185:4921–4927. doi: 10.4049/jimmunol.0904011. [DOI] [PubMed] [Google Scholar]
- 20.Fahy N., de Vries-van Melle M.L., Lehmann J., Wei W., Grotenhuis N., Farrell E. Human osteoarthritic synovium impacts chondrogenic differentiation of mesenchymal stem cells via macrophage polarisation state. Osteoarthritis Cartilage. 2014;22:1167–1175. doi: 10.1016/j.joca.2014.05.021. [DOI] [PubMed] [Google Scholar]
- 21.Mantovani A., Sozzani S., Locati M., Allavena P., Sica A. Macrophage polarization: tumor-associated macrophages as a paradigm for polarized M2 mononuclear phagocytes. Trends Immunol. 2002;23:549–555. doi: 10.1016/s1471-4906(02)02302-5. [DOI] [PubMed] [Google Scholar]
- 22.Magalhaes P.O., Lopes A.M., Mazzola P.G., Rangel-Yagui C., Penna T.C., Pessoa A., Jr. Methods of endotoxin removal from biological preparations: a review. J Pharm Pharm Sci. 2007;10:388–404. [PubMed] [Google Scholar]
- 23.Budai M.M., Varga A., Milesz S., Tozser J., Benko S. Aloe vera downregulates LPS-induced inflammatory cytokine production and expression of NLRP3 inflammasome in human macrophages. Mol Immunol. 2013;56:471–479. doi: 10.1016/j.molimm.2013.05.005. [DOI] [PubMed] [Google Scholar]
- 24.Chanput W., Mes J., Vreeburg R.A., Savelkoul H.F., Wichers H.J. Transcription profiles of LPS-stimulated THP-1 monocytes and macrophages: a tool to study inflammation modulating effects of food-derived compounds. Food Funct. 2010;1:254–261. doi: 10.1039/c0fo00113a. [DOI] [PubMed] [Google Scholar]
- 25.Kew V.G., Wills M.R., Reeves M.B. LPS promotes a monocyte phenotype permissive for human cytomegalovirus immediate-early gene expression upon infection but not reactivation from latency. Sci Rep. 2017;7:810. doi: 10.1038/s41598-017-00999-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mytych J., Romerowicz-Misielak M., Koziorowski M. Long-term culture with lipopolysaccharide induces dose-dependent cytostatic and cytotoxic effects in THP-1 monocytes. Toxicol In Vitro. 2017;42:1–9. doi: 10.1016/j.tiv.2017.03.009. [DOI] [PubMed] [Google Scholar]
- 27.Feng Y.H., Zhu Y.N., Liu J., Ren Y.X., Xu J.Y., Yang Y.F. Differential regulation of resveratrol on lipopolysacchride-stimulated human macrophages with or without IFN-gamma pre-priming. Int Immunopharmacol. 2004;4:713–720. doi: 10.1016/j.intimp.2004.02.006. [DOI] [PubMed] [Google Scholar]
- 28.Kim B.G., Song Y., Lee M.G., Ku J.M., Jin S.J., Hong J.W. Macrophages from mice administered rhus verniciflua stokes extract show selective anti-inflammatory activity. Nutrients. 2018;10:1926. doi: 10.3390/nu10121926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Richardson E.T., Shukla S., Nagy N., Boom W.H., Beck R.C., Zhou L. ERK signaling is essential for macrophage development. PLoS One. 2015;10:e0140064. doi: 10.1371/journal.pone.0140064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Leskovac V., Trivic S., Pericin D. The three zinc-containing alcohol dehydrogenases from baker's yeast, Saccharomyces cerevisiae. FEMS Yeast Res. 2002;2:481–494. doi: 10.1111/j.1567-1364.2002.tb00116.x. [DOI] [PubMed] [Google Scholar]
- 31.Lin Y., He P., Wang Q., Lu D., Li Z., Wu C. The alcohol dehydrogenase system in the xylose-fermenting yeast Candida maltosa. PLoS One. 2010;5:e11752. doi: 10.1371/journal.pone.0011752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jakubzick C.V., Randolph G.J., Henson P.M. Monocyte differentiation and antigen-presenting functions. Nat Rev Immunol. 2017;17:349–362. doi: 10.1038/nri.2017.28. [DOI] [PubMed] [Google Scholar]
- 33.Chanput W., Mes J.J., Savelkoul H.F., Wichers H.J. Characterization of polarized THP-1 macrophages and polarizing ability of LPS and food compounds. Food Funct. 2013;4:266–276. doi: 10.1039/c2fo30156c. [DOI] [PubMed] [Google Scholar]
- 34.Nakanishi Y., Nakatsuji M., Seno H., Ishizu S., Akitake-Kawano R., Kanda K. COX-2 inhibition alters the phenotype of tumor-associated macrophages from M2 to M1 in ApcMin/+ mouse polyps. Carcinogenesis. 2011;32:1333–1339. doi: 10.1093/carcin/bgr128. [DOI] [PubMed] [Google Scholar]
- 35.Reales-Calderon J.A., Aguilera-Montilla N., Corbi A.L., Molero G., Gil C. Proteomic characterization of human proinflammatory M1 and anti-inflammatory M2 macrophages and their response to Candida albicans. Proteomics. 2014;14:1503–1518. doi: 10.1002/pmic.201300508. [DOI] [PubMed] [Google Scholar]
- 36.Wynn T.A., Chawla A., Pollard J.W. Macrophage biology in development, homeostasis and disease. Nature. 2013;496:445–455. doi: 10.1038/nature12034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mosser D.M., Edwards J.P. Exploring the full spectrum of macrophage activation. Nat Rev Immunol. 2008;8:958–969. doi: 10.1038/nri2448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fernandez-Arenas E., Molero G., Nombela C., Diez-Orejas R., Gil C. Contribution of the antibodies response induced by a low virulent Candida albicans strain in protection against systemic candidiasis. Proteomics. 2004;4:1204–1215. doi: 10.1002/pmic.200300678. [DOI] [PubMed] [Google Scholar]
- 39.Fernandez-Arenas E., Molero G., Nombela C., Diez-Orejas R., Gil C. Low virulent strains of Candida albicans: unravelling the antigens for a future vaccine. Proteomics. 2004;4:3007–3020. doi: 10.1002/pmic.200400929. [DOI] [PubMed] [Google Scholar]
- 40.Kurihara Y., Furue M. Interferon-gamma enhances phorbol myristate acetate-induced cell attachment and tumor necrosis factor production via the NF-kappaB pathway in THP-1 human monocytic cells. Mol Med Rep. 2013;7:1739–1744. doi: 10.3892/mmr.2013.1419. [DOI] [PubMed] [Google Scholar]
- 41.Son Y., Kim B.Y., Park Y.C., Eo S.K., Cho H.R., Kim K. PI3K and ERK signaling pathways are involved in differentiation of monocytic cells induced by 27-hydroxycholesterol. Kor. J Physiol Pharmacol. 2017;21:301–308. doi: 10.4196/kjpp.2017.21.3.301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Shi C., Simon D.I. Integrin signals, transcription factors, and monocyte differentiation. Trends Cardiovasc Med. 2006;16:146–152. doi: 10.1016/j.tcm.2006.03.002. [DOI] [PubMed] [Google Scholar]
- 43.Becker K.L., Ifrim D.C., Quintin J., Netea M.G., van de Veerdonk F.L. Antifungal innate immunity: recognition and inflammatory networks. Semin Immunopathol. 2015;37:107–116. doi: 10.1007/s00281-014-0467-z. [DOI] [PubMed] [Google Scholar]
- 44.Franklyn K.M., Warmington J.R. The expression of Candida albicans enolase is not heat shock inducible. FEMS Microbiol Lett. 1994;118:219–225. doi: 10.1111/j.1574-6968.1994.tb06831.x. [DOI] [PubMed] [Google Scholar]
- 45.Gancedo C., Flores C.L. Moonlighting proteins in yeasts. Microbiol Mol Biol Rev. 2008;72:197–210. doi: 10.1128/MMBR.00036-07. table of contents. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tamura Y., Torigoe T., Kukita K., Saito K., Okuya K., Kutomi G. Heat-shock proteins as endogenous ligands building a bridge between innate and adaptive immunity. Immunotherapy. 2012;4:841–852. doi: 10.2217/imt.12.75. [DOI] [PubMed] [Google Scholar]
- 47.Tsai C.S., Lin Y.W., Huang C.Y., Shih C.M., Tsai Y.T., Tsao N.W. Thrombomodulin regulates monocye differentiation via PKCdelta and ERK1/2 pathway in vitro and in atherosclerotic artery. Sci Rep. 2016;6:38421. doi: 10.1038/srep38421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Liu W.S., Heckman C.A. The sevenfold way of PKC regulation. Cell Signal. 1998;10:529–542. doi: 10.1016/s0898-6568(98)00012-6. [DOI] [PubMed] [Google Scholar]
- 49.Chaffin W.L., Lopez-Ribot J.L., Casanova M., Gozalbo D., Martinez J.P. Cell wall and secreted proteins of Candida albicans: identification, function, and expression. Microbiol Mol Biol Rev. 1998;62:130–180. doi: 10.1128/mmbr.62.1.130-180.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Frodin M., Gammeltoft S. Role and regulation of 90 kDa ribosomal S6 kinase (RSK) in signal transduction. Mol Cell Endocrinol. 1999;151:65–77. doi: 10.1016/s0303-7207(99)00061-1. [DOI] [PubMed] [Google Scholar]
- 51.Sun X.X., Zhang S.S., Dai C.Y., Peng J., Pan Q., Xu L.F. LukS-PV-Regulated MicroRNA-125a-3p Promotes THP-1 Macrophages Differentiation and Apoptosis by Down-Regulating NF1 and Bcl-2. Cell Physiol Biochem. 2017;44:1093–1105. doi: 10.1159/000485415. [DOI] [PubMed] [Google Scholar]