Highlights
-
•
An elevated fasting glucose defined 80% of laboratory GDM positive diagnoses.
-
•
Selected glucometers fulfilled ISO 15197:2013 recommended accuracy at fasting of OGTT.
-
•
All glucometers evaluated were inaccurate at one and two hours of OGTT.
Keywords: Gestational diabetes; Screening, primary care; Fasting glucose; Capillary glucose
Abstract
Aims
We investigated the clinical and analytic accuracy of five plasma calibrated glucometers, the use of which is advocated by the World Health Organisation and the International Federation of Gynaecology and Obstetrics, to screen for and diagnose gestational diabetes mellitus (GDM) in low resource settings.
Methods
592 consecutive black African women underwent a 75 g oral glucose tolerance test (OGTT) at 24–28 weeks gestation at an urban South African community health clinic. Capillary glucose was measured by one of five glucometer brands, each paired with a routine laboratory hexokinase method of plasma glucose measurement. The laboratory results served as the gold standard reference test for GDM diagnosis. World Health Organisation GDM diagnostic thresholds were applied to glucometer and laboratory results.
Results
Glucometer and laboratory determined GDM prevalence was 75/592 (12.7%) and 30/592 (5.1%) with an elevated fasting glucose diagnostic in 64/75 (85%) and 24/30 (80%) of cases respectively. The proportion of glucometer results fulfilling ISO 15197:2013 recommended analytic accuracy at fasting, 60, and 120 min of the OGTT was 92.4%, 49.8% and 61.5%, with Bland Altman method revealing a positive glucometer bias of 0.22 mmol/l (−0.69–1.12 mmol/l), 0.96 mmol/l (−0.65–2.56 mmol/l) and 0.73 mmol/l (−0.73–2.19 mmol/l) respectively. Only three of the glucometer brands evaluated fulfilled ISO 15197:2013 analytic accuracy requirements and this was only achieved at fasting. All glucometers tested were inaccurate at one and two hours of the OGTT.
Conclusions
Not all glucometers may be suitable for GDM screening as only three were accurate compared to the reference test and then only at fasting of the OGTT. Importantly, laboratory fasting glucose was diagnostic of GDM in 80% of cases in this study population.
Introduction
The prevalence of Gestational Diabetes Mellitus (GDM) is increasing in parallel with that of type 2 diabetes mellitus. Identifying this usually transient and asymptomatic condition will allow clinical interventions to reduce the perinatal morbidity associated with these high-risk pregnancies [1]. An estimated 16% of pregnancies are affected globally [2]. The World Health Organization (WHO) has adopted the diagnostic criteria for GDM advocated by the International Association of Diabetes in Pregnancy Study Group (IADPSG) [1], [3]. Universal screening by means of a 75 g two-hour oral glucose tolerance test (OGTT) at 24–28 weeks gestation is recommended [1]. Several low-middle income countries have adopted the WHO diagnostic criteria, including the recommendation for universal screening for GDM. In 2017, the Society for Endocrinology, Metabolism, and Diabetes of South Africa (SEMDSA) adopted these guidelines [4]. The South African National Department of Health’s policy is guided by the WHO and SEMDSA recommendations, although universal screening for GDM has not yet been implemented in South Africa. Potential barriers to implementing universal screening in low resourced clinic settings include the limitations of administering the OGTT which is expensive, labor intensive and dependent on calibrated laboratory services. Most Sub-Saharan African countries have minimal or no access to medical laboratory services that meet international quality standards [5]. The density of laboratories in South Africa is comparable with European countries, however, only 18% of these belong to the state-sponsored National Health Laboratory Service (NHLS) which provides services to>80% of the population [5]. The WHO has acknowledged that capillary blood glucose measurement by point of care glucometers is widely used in low resource settings to diagnose diabetes mellitus and that the use of glucometers meeting the current ISO standards (15197:2013) is an acceptable alternative to laboratory glucose measurements [6]. The International Federation of Gynaecology and Obstetrics (FIGO) has endorsed the use of plasma calibrated glucometers to screen for and diagnose GDM in low resource settings [7]. There are no standards for the use of glucometers in the diagnosis of GDM which has lower diagnostic thresholds than dysglycaemia in a non-pregnant adult. Our aims were to evaluate the measurement accuracy of ISO 15197:2013 compliant glucometers relative to a calibrated laboratory reference test and to assess the ability of the glucometers to correctly identify GDM affected women, relative to the laboratory, in the low resource setting of an urban South African community health clinic (CHC).
Study participants, materials and methods
Approvals and permissions
The University of the Witwatersrand Human Research Ethics committee granted approval for this study (M150365). The South African Johannesburg District Department of Health granted permission to conduct the study at a CHC in Soweto (Reference number 2015–16/031). All participants provided written informed consent.
Participants
We conducted this cross-sectional, prospective, pragmatic study between April 2016 and May 2017. Participants were recruited consecutively at their first prenatal visit. Women under 28 weeks gestation and over the age of 18 years with no pre-existing type one or type two diabetes mellitus, attending the antenatal service of a single urban CHC were eligible to participate. Gestational age was assessed by the clinic’s obstetric nurse as part of usual care. Ultrasound and laboratory facilities were not available on site. Data collected from participants included maternal age, parity, gestation at their first prenatal visit and a risk factor assessment for GDM. Risk factors for GDM were those defined by the South African National Department of Health and these include repeated glycosuria, previous GDM, family history of diabetes (first degree relative), poor obstetric history (stillbirth of unknown origin), previous congenital abnormality, history of a high birth weight infant of >4.5 Kg, maternal obesity (body mass index >30 Kg/m2) and women of South Asian descent. In keeping with this clinic’s patient profile, all participants were indigenous African women who are historically considered to be at low risk of GDM. Indigenous Africans form 80% of the South African population. As participants were frequently unable to recall the birth weights from previous pregnancies, descriptions of “small, average or large” were used instead as this was the local standard practice. We recorded the presence of glycosuria (urine dipstick) from the clinic records and measured mid-upper arm circumference, body weight and height.
Materials and methods
Participants returned to the clinic on an appointed morning after an overnight fast for a modified 75 g OGTT at between 24 and 28 weeks' gestation. Study procedures were performed in an office within the usual functioning of the antenatal clinic. Modifications to the OGTT included the pairing of a glucometer test (finger-prick; capillary blood glucose concentration) with a reference laboratory test (venous puncture; plasma glucose concentration) at fasting, 60 and 120 min. Finger-prick blood sampling procedures included: wiping the finger with an alcohol swab, allowing this to dry, puncturing the skin with a single use lancing device, the first blood drop was wiped off with dry cotton-wool and the second blood drop was used for measurement of glucose concentration per time point of the OGTT. Trained research staff operated independently from the community clinic staff. The first author and an experienced diabetes nurse educator supervised all study procedures. Venous blood samples were collected in tubes containing the glycolytic inhibitor sodium fluoride and kept on ice from the time of phlebotomy until delivery to the laboratory at the end of the two-hour OGTT [8]. The time lapse between completion of the OGTT and delivery to the laboratory was approximately one hour. The American NACB (National Academy of Clinical Biochemistry) recommends immediate separation of plasma or alternatively, keeping samples in an ice slurry with the separation of plasma within 30 min [9]. Separation of plasma within 30 min was not possible, but this is not uncommon in a routine clinical setting. Indeed, the NACB and other researchers acknowledge that these procedures are neither widely used nor always practical in routine clinical settings [9], [10], [11]. ISO 15197:2013 compliant glucometers from five manufacturers which are commonly used in South Africa were selected for assessment. These included the Accu-Chek Active (Roche Diagnostics, Germany), used extensively in both the private and state sector; Freestyle Optium Neo (Abbott Diabetes Care Inc., USA), used in the private sector including in a large private hospital group and recently also in the state sector; Glucocheck Classic (TaiDoc Technology Corporation, Taiwan), the preferred glucometer of one of the largest South African medical insurers; One Touch Select Plus Flex (LifeScan Inc., Switzerland), used in the private sector and is marketed as being gold standard ISO 15197:2013 compliant; and Contour Plus (Bayer, Czech Republic), used in the private sector and in the calibration of the Medtronic continuous glucose monitoring system (Medtronic, USA). We followed the ISO 15197:2013 analytic procedures previously described for evaluating the glucometers performance and this included: finger-prick and venous blood sampling were less than five minutes apart; at least 100 participants were tested with each glucometer brand before changing to the next brand which was selected at random; at least three different lots of test strips were used per glucometer brand and at least 10 glucometers per brand were used over a period of at least 10 days [12], [13]. A minimum of 100 paired blood glucose tests would be submitted at each of the time points of the OGTT per glucometer brand. We intended using one glucometer per participant per day, with the glucometer cleaned and reused on another testing day. All other glucometer testing procedures followed the manufacturer’s instructions. All glucometers evaluated used ‘non-coding technology’ and so code chips were not necessary. Glucometer test strips were stored according to manufacturer’s instructions. The ISO 15197:2013 minimum systems accuracy recommendations suggests that ≥95% of glucometer results fall within 0.83 mmol/l of the reference value at blood glucose concentrations <5.56 mmol/l and, that ≥95% of glucometer results fall within 15% of the reference value at blood glucose concentrations ≥ 5.56 mmol/l [12]. In this study, this was assumed as the total allowable error (TAE) between the paired glucometer and the reference laboratory values. The accuracy of the glucometers was evaluated against results from a South African National Accreditation System verified private medical laboratory, which used a hexokinase method to measure plasma glucose concentration (Cobas 6000, Roche Diagnostics, Germany). The hexokinase laboratory method, rather than the glucose oxidase method, is routinely used by both the NHLS and the two largest private sector medical laboratories in South Africa. Although manufacturers may specify a preferred reference test method to evaluate their glucometers performance, according to the ISO 15197:2013 recommendation, any method with verified metrological traceability may be used [12]. The laboratory was off-site and blinded to all but their own results. The WHO 2013 GDM diagnostic thresholds were used to define the test positivity cut-offs for both the glucometers and the reference laboratory results [3]. The laboratory result served as the gold standard reference test and so these values were assumed to be the true result.
Analysis
Categorical variables are described as frequencies (n) and proportions (%), and continuous variables as means and standard deviations (SD). The TAE between paired glucometer and laboratory values at each time point was calculated. Bland Altman plots were used to evaluate the level of measurement agreement between the glucometer and laboratory results. Sensitivity, specificity and positive and negative predictive values were used to evaluate the ability of the glucometers to distinguish between GDM positive and negative states. The positive likelihood ratios (PLR) and negative likelihood ratios (NLR) served as an overall indicator of the diagnostic accuracy of the glucometers against the reference laboratory test and a PLR ≥ 10 and an NLR ≤ 0.1 were assumed to be favourable associations. McNemar’s test and the Kappa statistic was used to evaluate the clinical agreement between glucometers and the laboratory on GDM diagnoses. The kappa-statistic (k) values are graded as k = 0–0.19, poor; 0.20–0.39, fair; 0.40–0.59, moderate; 0.60–0.79, good; 0.80–1.00, very good regarding the level of agreement between tests. Where appropriate, 95% confidence intervals (95% CI) are reported. A p-value of <0.05 was considered statistically significant. Statistical analysis was performed on STATA software version 15 (Stata Statistical Software: Release 15. College Station, TX: StataCorp LLC, USA).
Results
Participants
592 OGTTs, with paired glucometer and reference laboratory results, were submitted for analysis. The participants’ mean age (SD) was 27.8 years (5.9 years), 173 (29.2%) were pregnant for the first time, 99 (16.8%) had a first-degree family member with diagnosed diabetes, 3 (0.5%) had previous GDM and 43 (7.3%) reported previous large birth weight infants. Participants mean body mass index was 26.8 Kg/m2 (5.8 Kg/m2) and their mid-upper arm circumference was 29.9 cm (4.4 cm). In this study population, 254 of 587 (43.3%) participants had ≥ one risk factor for GDM present. Note that the number of participants for each risk factor varied slightly due to missing values. (Table 1) The laboratory provided results within four hours of receiving samples. Glucometer results were available within five seconds. All glucometers were reused except for the Accu-Chek Active, which we considered unsafe for multiple people use as it was easily visibly soiled with participants blood during testing procedures, creating a potential biohazard. Each Accu-Chek Active meter was used once and then discarded and fewer participants were tested with this glucometer brand. Participants were given their provisional glucometer results on the day of testing subject to confirmation by the gold standard reference laboratory results. Those who were confirmed as GDM positive were referred for clinical intervention.
Table 1.
Participant characteristics.
| Participant characteristic | n | Mean (SD) or, Frequency (%) |
|---|---|---|
| Age (years) | 592 | 27.8 (5.9) |
| Family history of diabetes (first-degree) | 588 | 99 (16.8%) |
| Glycosuria (urine dipstick) ≥ one event | 592 | 6 (1%) |
| Mid-upper arm circumference (cm) | 592 | 29.9 (4.4) |
| Body height (cm) | 588 | 162.9 (7.2) |
| Body weight (Kg) | 592 | 70.6 (15.8) |
| Body mass index (Kg/m2) | 588 | 26.9 (5.8) |
| Obstetric history | ||
| Parity at recruitment | ||
| First pregnancy | 592 | 173 (29.2%) |
| Second pregnancy | 196 (33.1%) | |
| Third pregnancy | 144 (24.3%) | |
| >Three pregnancies | 79 (13.3%) | |
| Gestational age at first visit (weeks) | 592 | 19.1 (5.6) |
| Previous miscarriage | 592 | 65 (11%) |
| Previous large for gestational age birth | 591 | 43 (7.3%) |
| Previous stillbirth | 592 | 32 (5.4%) |
| Previous congenital abnormalities | 591 | 0 (0) |
| Previous GDM | 592 | 3 (0.5%) |
Note: Number (n) of participants for each characteristic varies slightly due to missing values.
GDM prevalence
The number of participants identified as GDM positive as determined by the glucometers and the laboratory were 74 (12.7%) and 30 (5.1%) respectively, being significantly different (p < 0.0001). Overt diabetes mellitus was consistently identified by both test methods in three participants (0.5%). Only one abnormality of the OGTT is necessary for a positive diagnosis of GDM and in this study population, an elevated fasting glucose was diagnostic in 64 of 75 (85%) glucometer positive diagnoses and in 24 of 30 (80%) laboratory positive diagnoses. The remaining GDM positives diagnosed by the glucometer and laboratory had normal fasting glucose but an elevated one and/or two-hour blood glucose in the OGTT. Of the glucometer GDM positive diagnoses, 27 of 75 (36%) were true positive relative to the gold standard reference laboratory test and of these true positives, 21 (77.8%) had an elevated fasting glucose.
Analytic accuracy of the glucometers
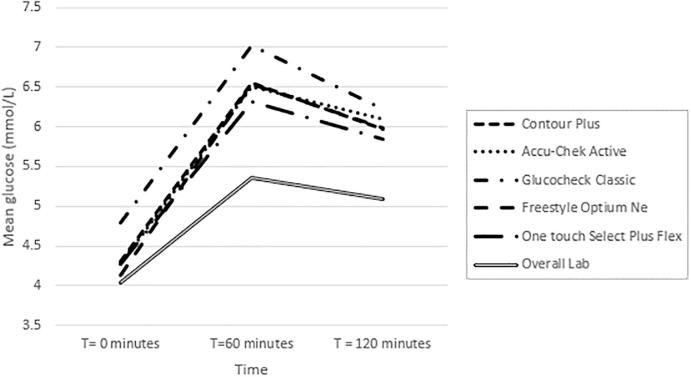
The mean (SD) blood glucose concentration for all glucometer and laboratory results at all time points of the OGTT (1776 observations) was 5.6 mmol/l (1.4 mmol/l) and 5.0 mmol/l (1.3 mmol/l) respectively. The glucometer results were consistently higher than the laboratory. The laboratory determined mean plasma glucose concentration at fasting, 60 and 120 min of the OGTT (592 observations at each time point) was 4.13 mmol/l (0.49 mmol/l), 5.62 mmol/l (1.39 mmol/l) and 5.27 mmol/l (1.21 mmol/l). Mean capillary glucose concentrations per glucometer brand at each time point of the OGTT is displayed in Fig. 1. Only the Contour Plus, Accu-Chek Active and the One Touch Select Plus Flex fulfilled the ISO 15197:2013 recommended TAE compared to the reference laboratory results and this was only achieved at fasting of the OGTT. None of the glucometer brands accuracy were within the TAE at 60 min or at 120 min of the OGTT. Individual glucometer brands performance regarding accuracy at various timepoints of the OGTT is indicated in Table 2. The overall proportion of glucometer results that fulfilled the TAE against the laboratory at fasting, 60 and 120 min of the OGTT were 92.4%, 49.8%, and 61.5% respectively. All glucometer results accuracy deviated the most from laboratory results at 60 min of the OGTT. Results between glucometer brands were also most widely spread at 60 min of the OGTT. Bland Altman plots confirmed that the glucometers results were consistently higher relative to those from the laboratory at all time points, except for the Freestyle Optium Neo which was neutral at fasting. The glucometers had an overall positive systematic bias relative to the laboratory. The overall average difference against the mean between glucometer and laboratory results indicated a glucometer positive bias at fasting, 60 and 120 min of the OGTT of 0.22 mmol/l (−0.69–1.12 mmol/l), 0.96 mmol/l (−0.65–2.56 mmol/l) and 0.73 mmol/l (−0.73–2.19 mmol/l) respectively. Individual glucometer Bland Altman plot results are indicated in Supplementary Fig. 1–5.
Fig. 1.
Mean glucose concentrations as measured by the reference laboratory and each glucometer brand at different time points of the ogtt.
Table 2.
Results of statistical analysis of agreement between the glucometer brands and the reference laboratory at ogtt time points.
| Glucometer brands | T = 0 min | T = 60 min | T = 120 min |
|---|---|---|---|
| Proportion of results within Total Allowable Error: (ISO 15197:2013 defined) between glucometers and the reference laboratory (%) | |||
| Overall (n = 592) | 92.4 | 49.8 | 61.5 |
| Contour Plus (n = 143) | 96.5 | 29.4 | 53.1 |
| Accu-Chek Active (n = 56) | 100 | 58.9 | 67.9 |
| Glucocheck Classic (n = 105) | 76.2 | 30.5 | 35.2 |
| Freestyle Optium Neo (n = 143) | 94.4 | 63.6 | 77.6 |
| One touch Select Plus Flex (n = 145) | 95.2 | 66.9 | 70.3 |
| Bland Altman (95% LoA) mmol/l average difference against the mean between glucometers and the reference laboratory | |||
| Overall (n = 592) | 0.22 (−0.69–1.12) | 0.96 (−0.65–2.56) | 0.73 (−0.73–2.19) |
| Contour Plus (n = 143) | 0.27 (−0.32–0.86) | 1.20 (−0.04–2.44) | 0.88 (−0.18–1.93) |
| Accu-Chek Active (n = 58) | 0.12 (−0.49–0.72) | 0.72(−0.67–2.11) | 0.59 (−0.54–1.71) |
| Glucocheck Classic (n = 105) | 0.57 (−0.52–1.65) | 1.51 (−0.58–3.60) | 1.16 (−0.96–3.29) |
| Freestyle Optium Neo (n = 143) | 0.00 (−0.81– 0.81) | 0.70 (−0.57–1.96) | 0.51 (−0.62–1.64) |
| One Touch Select Plus Flex (n = 145) | 0.17 (−0.74–1.07) | 0.66 (−0.69–2.00) | 0.54 (−0.74–1.82) |
OGTT = oral glucose tolerance test.
Note: Bland Altman difference against the mean: p < 0.001 for all values at all time points of the OGTT, except for the Freestyle Optium Neo which was neutral at fasting.
Clinical accuracy of glucometers
Due to the low prevalence of laboratory identified GDM, this study was underpowered to truly evaluate the clinical accuracy, rather than the analytical accuracy, of the glucometers to identify GDM positive states. However, there are some clinically meaningful observations based on the data available. The One Touch Select Plus Flex failed to identify two participants who had GDM. Of all the glucometer false positive GDM diagnoses, 26/48 (54.2%) were associated with the Glucocheck Classic and one in four participants tested with this glucometer was falsely identified as being GDM positive. (Table 3) The Accu-Chek Active had strong positive and negative likelihood ratios in their ability to identify women affected by GDM relative to the reference laboratory results. However, the Accu-Chek Active failed to identify one woman who had GDM as defined by the reference laboratory. Fewer participants were tested with this meter for reasons indicated previously. Coincidentally, this glucometer was evaluated against results from reference laboratory equipment produced by the same manufacturer. The Kappa statistic was calculated to evaluate the glucometers clinical diagnostic accuracy (binary) relative to the reference laboratory GDM diagnosis (binary). The Kappa statistic (standard error) result for the Contour Plus, Accu-Chek Active, Glucocheck Classic, Freestyle Optium Neo and One Touch Select Plus Flex was 0.72 (0.08), 0.48 (0.13), 0.32 (0.07), 0.71 (0.08) and 0.22 (0.07) respectively. The exact McNemar's significance probability of agreement in GDM positive diagnoses between the glucometers and the reference laboratory were as follows: Contour Plus (p = 0.0625), Accu-Chek Active (p = 1.0000), Glucocheck Classic (p < 0.0001), Freestyle Optium Neo (p = 0.0313) and, One Touch Select Plus Flex (p = 0.0386).
Table 3.
Statistical analysis of agreement in gestational diabetes diagnosis between the glucometers against the hexokinase method laboratory reference.
| All Glucometers (95% CI) | Contour Plus (Bayer) (95% CI) | Freestyle Optium Neo (Abbott) (95% CI) | Accu-Chek Active(Roche) (95% CI) | Glucocheck Classic (TaiDoc) (95% CI) | One touch Select Plus Flex (Lifescan) (95% CI) | |
|---|---|---|---|---|---|---|
| Number of participants | 592 | 143 | 143 | 56 | 105 | 145 |
| True Positive (n) | 27 | 7 | 8 | 1 | 9 | 2 |
| False Positive (n) | 48 | 5 | 6 | 1 | 26 | 10 |
| True Negative (n) | 514 | 131 | 129 | 53 | 70 | 131 |
| False Negative (n) | 3 | 0 | 0 | 1 | 0 | 2 |
| Sensitivity (%) | 90 (74; 98) | 100 (59; 100) | 100 (63; 100) | 50 (1; 99) | 100 (66; 100) | 50 (7; 93) |
| Specificity (%) | 91 (89; 94) | 96 (92; 99) | 96 (91; 99) | 98 (90; 100) | 73 (63; 81) | 93 (87; 97) |
| Positive Predictive Value (%) | 36 (25; 48) | 58 (28; 85) | 57 (29; 82) | 50 (1; 99) | 26 (12; 43) | 17 (2; 48) |
| Negative Predictive Value (%) | 99 (98; 100) | 100 (97; 100) | 100 (97; 100) | 98 (90; 100) | 100 (95; 100) | 98 (95; 100) |
| Positive Likelihood Ratio | 10.54 | 27.20 | 22.50 | 27.00 | 3.69 | 7.05 |
| Negative Likelihood ratios | 0.11 | 0.00 | 0.00 | 0.51 | 0.00 | 0.54 |
CI = confidence interval.
Discussion
Prevalence of GDM
The reference laboratory identified a relatively low prevalence of GDM. There is a paucity of data on the prevalence of GDM in Sub-Saharan Africa. A recent South African study by Macaulay et al. reported a 9.1% GDM prevalence in 1906 participants from a similar population but in a tertiary hospital setting and plasma glucose concentrations were determined by a research rather than a routine laboratory service (glucose oxidase method) [14]. Their study population were older (30 years vs 27.8 years), were more overweight (body mass index 29.5 vs 26.9 Kg/m2), with fewer primiparous participants (12.1 vs 29.2%) and a higher proportion of positive laboratory GDM diagnoses based on an elevated fasting glucose (83.3% vs 80%) [14]. A study by Adam and Rheeder reported a GDM prevalence of 14.9% and 26.7% based on glucose concentrations measured by a Roche Accuchek Active glucometer and a routine laboratory service (hexokinase method) respectively. Their laboratory results were 0.40 mmol/l higher than the glucometer results at fasting but 0.7 mmol/l and 0.4 mmol/l lower than the glucometer results at one and two hours of the OGTT respectively. An elevated fasting glucose was diagnostic of GDM in the majority of their laboratory defined positive cases. Their reported laboratory quality controls reflect a bias of 3.65% at a lower glucose concentration, which is not within the ≤2.2% recommended by the NACB [9], [15]. This laboratory inaccuracy, specifically at lower glycaemic concentrations, may have contributed to the relatively higher GDM prevalence reported by Adam and Rheeder. In addition, the socio-demographics of their study sample of 529 women was not well described. This may be significant as South Africans of Asian descent are known to be at high risk of GDM. In our study, a high proportion of GDM positive diagnoses were based on an elevated fasting glucose and this finding was unexpected as this is higher than that reported in the Hyperglycaemia and Adverse Pregnancy Outcome (HAPO) study population. When the IADPSG diagnostic criteria was retrospectively applied to the HAPO study population, 55% (24–74%) of GDM diagnoses were based on an elevated fasting plasma glucose [16]. Sub-Saharan Africa was not included in the HAPO study. Sub-Saharan Africa has a historically undernourished population currently affected by a high prevalence of overweight and obesity and the effect of this on the GDM prevalence is unknown [17], [18].
Clinical and analytical accuracy of glucometers in diagnosing GDM
In this study, the prevalence of GDM as determined by glucometers was significantly higher than the gold standard reference laboratory. Glucometers measured higher blood glucose concentrations than the laboratory at all time points of the OGTT. In a previous report comparing the diagnostic accuracy of a glucometer, routine analysis and an optimized laboratory, glucometer results approximated the optimized laboratory, however, the routine laboratory underdiagnosed GDM positive states [11]. It is known that blood glucose concentration drops by approximately 5–7% per hour ex vivo due to ongoing glycolysis, and sodium fluoride does not necessarily negate this effect within the first four hours [9]. The use of sodium fluoride tubes is included in the WHO recommended procedure for an OGTT [8]. Citrate may be a more effective plasma glucose stabilizer, although, the quality and availability of these tubes may vary and they are not routinely used in South Africa [9], [11]. As glucometer measurements are unaffected by preanalytical factors such as ongoing glycolysis, they may be superior to routine laboratory services, but not to optimised laboratory conditions, in detecting those affected by GDM [11]. The Bland Altman method indicates the glucometer positive bias was within the ISO 15197:2013 recommended technical accuracy of ≤0.83 mmol/l at fasting [12]. All glucometers were inaccurate at one and two hours of the OGTT. It is known that differences between capillary and venous blood glucose are relatively minor at fasting, however, at 60 and 120 min of the OGTT capillary blood glucose concentrations may be 20–25% higher than venous glucose, due to oxygen consumption in tissues [9]. Also, laboratory analyzed plasma glucose of the OGTT are known to have suboptimal reproducibility in diagnosing GDM, particularly at 60 and 120 min of the OGTT [19]. Selected glucometers fulfilled ISO accuracy requirements only at fasting of the OGTT and all were inaccurate at one and two hours of the OGTT. This suggests that glucometers may be unsuitable for the diagnosis of GDM based on results of a complete OGTT.
Selection of glucometers suitable for GDM screening and diagnosis
Multiple glucometers were selected for assessment as we anticipated inconsistencies in performance between brands. There are independent reports of suboptimal glucometer fulfilment of ISO recommendations post-marketing [13]. Although the FIGO has endorsed the use of handheld plasma calibrated glucometers in low resource settings that are remote from laboratory services, they acknowledge there are no standards for their use in the screening and diagnosis of GDM [7]. This study was a calibration exercise between glucometers, the use of which is endorsed by the WHO and FIGO, and a local calibrated laboratory, in the setting of a low resource urban CHC rather than an optimal research setting. The ISO 1597:2013 recommendations for glucometer accuracy describe voluntary, manufacturer conducted, premarketing evaluation of the minimal accuracy requirements of over the counter glucometers [12]. The validation of glucometers level of compliance with ISO 15197:2013 recommendations would require a controlled research setting which was beyond the scope of this study. Independent ongoing surveillance of glucometer performance is necessary to ensure their safe use in screening for GDM.
Limitations
All our participants were indigenous African women and these results may not be generalizable beyond this population. This pragmatic study was conducted in the setting of a low resource community clinic, which may equate to routine care and it was not possible to process venous samples immediately. This may have resulted in a lower level of glucose measured by the laboratory with a relative underestimation of GDM prevalence by this reference test. The low prevalence of GDM in this study population limits the generalisability of using glucometers for screening purposes.
Conclusion
The future use of plasma calibrated glucometers rather than laboratory services is inevitable and even desirable in low resource community clinic settings. However, only selected ISO 15197:2013 compliant glucometers may be suitable for the screening of GDM and then, only based on an elevated fasting blood glucose and not for a complete OGTT. This study provides evidence of a pragmatic and valid approach for the use of selected glucometers to determine fasting blood glucose concentration in pregnant women and may be essential to rolling out universal screening for GDM in low resource settings.
Acknowledgments
Acknowledgements
The Johannesburg District South African Department of Health granted us permission to conduct research at a CHC in Soweto. We are grateful for the support of the patients and community clinic staff. The sustained efforts of our research assistant Ms Nandi Mtshali were essential to the completion of the study on schedule.
Contributors
Methodology: LMD; EJB; SAN. Investigation: LMD. Formal analysis: LMD; EJB; CJVR; SAN. Writing: LMD. Reviewing and editing: EJB; CJVR; SAN. All authors have approved the final article.
Declarations of interest
None.
Role of the funding source
Neither the glucometer manufacturers nor the laboratory had any role in the study design, data analysis and interpretation of results, in the writing of the report or in the decision to submit the article for publication. The work reported herein was made possible through funding by the South African Medical Research Council through its Division of Research Capacity Development under the SAMRC CLINICIAN RESEARCHER (M.D. PHD) SCHOLARSHIP PROGRAMME from funding received from the South African National Treasury. The contents hereof is the sole responsibility of the authors and do not necessarily represent the official views of the SAMRC or the funders. SAN is supported by the Department of Science and Technology and National Research Foundation Centre of Excellence in Human Development at the University of the Witwatersrand, Johannesburg, South Africa.
Glossary
- CI
Confidence Interval
- CHC
Community Health Clinic
- FIGO
International Federation of Gynaecology and Obstetrics
- GDM
Gestational Diabetes Mellitus
- IADPSG
International Association of Diabetes in Pregnancy Study Group
- MUAC
Mid-Upper Arm Circumference (≥33 cm is associated with obesity)
- NACB
National Academy of Clinical Biochemistry
- NHLS
National Health Laboratory Service (South Africa)
- NLR
Negative Likelihood Ratios
- OGTT
Oral Glucose Tolerance Test
- PLR
Positive Likelihood Ratios
- SEMDSA
Society for Endocrinology, Metabolism, and Diabetes of South Africa
- TAE
Total Allowable Error
- WHO
World Health Organization
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jcte.2018.12.003.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- 1.Metzger B.E. International association of diabetes and pregnancy study groups recommendations on the diagnosis and classification of hyperglycemia in pregnancy. Diabetes Care. 2010;33:676–682. doi: 10.2337/dc09-1848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ogurtsova K. IDF Diabetes Atlas: Global estimates for the prevalence of diabetes for 2015 and 2040. Diabetes Res Clin Pract. 2017;128:40–50. doi: 10.1016/j.diabres.2017.03.024. [DOI] [PubMed] [Google Scholar]
- 3.World Health Organization Diagnostic criteria and classification of hyperglycaemia first detected in pregnancy. World Heal Organ. 2013;1–63 [PubMed] [Google Scholar]
- 4.The 2017 SEMDSA Guideline for the Management of Type 2 Diabetes. JEMDSA 22, S1–S192, 2017.
- 5.Schroeder L.F., Amukele T. Medical laboratories in sub-Saharan Africa that meet international quality standards. Am J Clin Pathol. 2014;141:791–795. doi: 10.1309/AJCPQ5KTKAGSSCFN. [DOI] [PubMed] [Google Scholar]
- 6.World Health Organization. Global Report on Diabetes 2016. Isbn 978, 88; 2016.
- 7.Hod M. The international federation of gynecology and obstetrics (FIGO) initiative on gestational diabetes mellitus: a pragmatic guide for diagnosis, management, and care. Int J Gynecol Obstet. 2015;131:S173–S211. doi: 10.1016/S0020-7292(15)30033-3. [DOI] [PubMed] [Google Scholar]
- 8.WHO. Definition and Diagnosis of Diabetes Mellitus and Intermediate Hyperglycemia. Who2 50; 2006. Doi: ISBN 92 4 159493 4.
- 9.Sacks D.B. Guidelines and recommendations for laboratory analysis in the diagnosis and management of diabetes mellitus. Diabetes Care. 2011;34 doi: 10.2337/dc11-9998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mansell E., Lunt H., Docherty P. Laboratory diagnosis of gestational diabetes: An in silico investigation into the effects of pre-analytical processing on the diagnostic sensitivity and specificity of the oral glucose tolerance test. Clin Biochem. 2017;50:506–512. doi: 10.1016/j.clinbiochem.2017.01.012. [DOI] [PubMed] [Google Scholar]
- 11.van den Berg S.A.A. Pregnancy diabetes: a comparison of diagnostic protocols based on point-of-care, routine and optimized laboratory conditions. Sci Rep. 2015;5:16302. doi: 10.1038/srep16302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Freckmann G. Analytical performance requirements for systems for self-monitoring of blood glucose with focus on system accuracy. J Diabetes Sci Technol. 2015;9:885–894. doi: 10.1177/1932296815580160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Freckmann G. System accuracy evaluation of 43 blood glucose monitoring systems for self-monitoring of blood glucose according to DIN EN ISO 15197. J Diabetes Sci Technol. 2012;6:1060–1075. doi: 10.1177/193229681200600510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Macaulay S., Ngobeni M., Dunger D.B., Norris S.A. The prevalence of gestational diabetes mellitus amongst black South African women is a public health concern. Diabetes Res Clin Pract. 2018;139:278–287. doi: 10.1016/j.diabres.2018.03.012. [DOI] [PubMed] [Google Scholar]
- 15.Adam S., Rheeder P. Evaluating the utility of a point-of-care glucometer for the diagnosis of gestational diabetes. Int J Gynaecol Obstet. 2017;12(10) doi: 10.1002/ijgo.12399. [DOI] [PubMed] [Google Scholar]
- 16.Sacks D.A. for the HAPO study cooperative research group. Frequency of gestational diabetes mellitus at collaborating centers based on IADPSG consensus. Diabetes Care. 2012;35:526–528. doi: 10.2337/dc11-1641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kengne A.P. Trends in obesity and diabetes across Africa from 1980 to 2014: an analysis of pooled population-based studies. Int J Epidemiol. 2017;46:1421–1432. doi: 10.1093/ije/dyx078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Norris S.A. Size at birth, weight gain in infancy and childhood, and adult diabetes risk in five low- or middle-income country birth cohorts. Diabetes Care. 2012;35:72–79. doi: 10.2337/dc11-0456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gambino R. Glucose: a simple molecule that is not simple to quantify. Clin Chem. 2007;53:2040–2041. doi: 10.1373/clinchem.2007.094466. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.