Abstract
Study Objectives
This study aims to evaluate the extent to which sleep quality impacts amnestic mild cognitive impairment (aMCI)-related brain regions in a cognitively normal cohort of individuals.
Methods
Seventy-four participants were rigorously evaluated using a battery of cognitive tests and a detailed clinical assessment to verify normal cognitive status. We then screened for sleep quality using the Pittsburgh Sleep Quality Index (PSQI) and depressive symptoms using the Geriatric Depression Scale (GDS). Five subjects were excluded due to mild depression. Overall 38 individuals with mean age 70.7 ± 7 were classified as poor sleepers and 31 with mean age of 69.6 ± 6 years as normal sleepers. Structural MRI and Freesurfer brain parcellation were used to measure aMCI-related brain regions.
Results
Relative to normal sleepers, poor sleepers exhibited significant reductions in cortical and subcortical volumes bilaterally in the hippocampi, as well as in the superior parietal lobules and left amygdala. The effects were strongest in the left superior parietal lobule (p < .015), followed by the hippocampi. Diffuse patterns of cortical thinning were observed in the frontal lobes, but significant effects were concentrated in the right mesial frontal cortex. Lower sleep duration was most correlated with cortical volume and thickness reductions among all subjects.
Conclusions
Atrophy related to poor sleep quality impacted a number of regions implicated in aMCI and Alzheimer’s disease (AD). As such, interventions targeted towards improving sleep quality amongst the elderly may prove an effective tool for modulating the course of aMCI and AD.
Keywords: sleep quality, aMCI, Alzheimer’s disease, hippocampus, cortical atrophy
Statement of Significance
Faced with aging global populations, the medical community has become increasingly interested in developing interventions to slow or prevent age-related dementia disorders like Alzheimer’s disease (AD). Sleep quality has been targeted as a factor that may help modulate the course of amnestic mild cognitive impairment and Alzheimer’s, but the relationship between sleep and dementia disorders is still poorly understood. Our study reports that patterns of cortical and deep gray matter atrophy related to poor sleep quality impact AD-related regions of the cortex even in a population rigorously deemed unaffected by cognitive impairment, psychological disorders, or dementia. The study emphasizes a role for sleep intervention in fighting neurodegeneration of Alzheimer’s-related brain regions.
Introduction
Dementia is becoming a growing public health problem as populations age worldwide. The cost of treating dementia in the US alone was estimated to be between $157 and $215 billion dollars in 2010 [1], and it has been projected that its prevalence will double between 2020 and 2040 [2]. As dementia grows in importance, characterizing the earliest stages of degenerative brain disorders is critical both for clinical risk assessment and the development of therapies to delay progression. Alzheimer’s disease (AD), the most common cause of dementia, generally occurs after 70 years of age, and is often preceded by amnestic mild cognitive impairment (aMCI), a prodromal condition that precedes clinical AD. Clinicians are paying greater attention to these preclinical AD states, particularly among individuals presenting to specialty memory disorder clinics. Considerable attention has also been paid to predicting the course of the disease using many metrics and biomarkers, including cognitive decline, accumulation of β amyloid protein in the brain, and brain atrophy [3–5]. As AD is a multifactorial disease, the processes involved in this condition has been elusive [6], but a growing consensus is emerging regarding the importance of sleep quality in the pathology and progression of AD [7].
Sleep disturbances have long been associated with AD, and may not only present as part of the advanced clinical syndrome, but also early in the course of the disease [8]. Dysfunctions in both slow wave and REM sleep have been characterized in AD patients [9, 10], and it is believed that amyloid β deposits play some role in disrupting sleep-related regions of the brain [7]. A number of cognitive and behavioral traits associated with AD also seem intimately tied to the disruption of circadian rhythm and sleep quality, including the troublesome phenomenon of “sundowning,” [11] where confusion and agitation get worse towards the evening. Given that poor sleep quality negatively impacts cognitive function even in the absence of disease [12], sleep dysfunction has significant potential to worsen the course of clinical AD.
A number of studies have identified sleep quality as a risk factor for both the development of aMCI and its progression to AD [13–16]. This relationship appears to be mediated not only through an increased incidence of dementia contributors in poor sleepers, like cerebrovascular disease [14], and cardiovascular conditions [15], but also directly [16]. Several studies have investigated the mechanism by which poor sleep quality may predispose individuals to aMCI and dementia. It has been suggested that sleep may facilitate β amyloid clearance from the brain [17], or that the anti-apoptotic unfolded protein response (UPR) during sleep deprivation is attenuated in old age [18], but more work is necessary to understand how sleep contributes to neurodegeneration.
There is some evidence that poor sleep quality contributes to patterns of atrophy exhibited in aMCI and dementia. Several meta-analyses have found that atrophy of the hippocampus, amygdala, posterior cingulate, and superior parietal lobule characterize aMCI [19, 20], and may underpin the patterns of memory impairment exhibited in the condition [21]. The atrophy of the hippocampus and other mesial temporal structures has been proposed as a marker for the progression of aMCI to AD [22], and used successfully as a predictor [23–25]. Similarly, a number of studies have noted that sleep quality-related atrophy extensively involves the hippocampus [26–28] and have implicated poor sleep in both diffuse frontal lobe atrophy [29–31], and temporal lobe reductions in cortical thickness [32, 33]. Given the diffuse patterns of atrophy attributed to poor sleep quality and the primary involvement of the hippocampus in both poor sleep and aMCI/AD, it is reasonable that sleep quality is linked to atrophy associated with aMCI.
While previous studies have demonstrated strong associations between poor sleep and dementia, this study focuses on testing the effect of sleep quality in cognitively intact older subjects aged 60–92. We hypothesized that atrophy patterns similar to those in aMCI would be demonstrated in subjects with poorer sleep quality, even in the absence of aMCI symptoms. The presence of atrophy in cognitively normal poor sleepers would suggest that sleep quality influences atrophy patterns subthreshold to symptoms of dementia, and could contribute to or facilitate further neurodegenerative processes. This observation may have important implications for current efforts to slow the progression of aMCI, as identifying and treating poor sleep quality prior to symptoms could provide a critical method to forestall neurodegeneration of regions salient to the development of dementia.
Methods
Criteria for cognitively normal participants
Seventy four older adult participants (23 males, 51 females) from an NIH-funded and IRB approved investigation at the University of Miami Miller School of Medicine were evaluated using a standard clinical assessment protocol consisting of the Clinical Dementia Rating Scale (CDR) [34] and the Mini-Mental Status Examination (MMSE) [35]. Memory and other cognitive complaints were assessed by clinicians who were blind to the neuropsychological test results and had formal training in administering the CDR and MMSE.
All participants were community-dwellers, independent in their activities of daily living, had knowledgeable collateral informants, and did not meet DSM-V criteria for Major Neurocognitive Disorder, active Major Depression, or any other neuropsychiatric disorder. All participants had global CDR scores of 0 after an extensive clinical interview. A standard neuropsychological battery was then administered uniformly across groups independently of the clinical examination. The neuropsychological battery included the Hopkins Verbal Learning Test-Revised (HVLT-R) [36], National Alzheimer’s Coordinating Center (NACC) delayed paragraph recall [37], Category Fluency [38], Block Design of the WAIS-IV [39], and the Trail Making Test (Parts A and B) [40]. All memory and non-memory neuropsychological measures scored within normal limits relative to age and education related norms as determined by an experienced neuropsychologist (this was typically less than 1.0 SD below normative values for all tests).
Depression screening
All participants were administered the Geriatric Depression Scale (GDS) [41] to assess for the prevalence of depressive symptoms in our cohorts. The GDS is a 30-point scale based on self-reported depressive symptoms, under which a score of 0–9 points is considered to be normal, 10–19 points is mild depressive symptoms, and 20–30 points is severe depressive symptoms. The scale has been validated against other indexes of depression in a variety of languages [42, 43], and is designed so that it may be administered even to patients who are ill or exhibit mild cognitive impairment. Screening for depression was an important consideration not only because depression has been tied to atrophy of the hippocampus [44], an aMCI-related region of interest (ROI) for this study, but also because a robust relationship has been found between depression and sleep quality that can complicate analyzing both in tandem [45, 46]. To minimize confounding of the relationship between sleep quality and cortical volumes by depression, this study excluded all participants with GDS scores >9, leaving 69 final subjects for sleep quality and MRI analysis.
Sleep quality assessment
The sleep quality of each subject was self-assessed over a 1-month period using the Pittsburgh Sleep Quality Index (PSQI) [47]. The PSQI has been recognized as an internally consistent and valid measure of self-reported sleep quality among older adults in a variety of languages and clinical circumstances [48, 49]. We established that scores of 5 or above (out of 21) would be counted as self-reported poor sleep quality based on previous studies regarding the diagnostic sensitivity and specificity of the test [47, 49].
MRI data
All participants underwent MRI scanning using a 3T MRI scanner (skyra, Siemens Healthcare). Brain parcellation was obtained using a 3D T1-weighted sequence (MPRAGE) [50] with 1.0 mm isotropic resolution, 2300 ms repetition time, 2.4 ms echo time, 930 ms inversion time, and 9-degree flip angle. The standard Freesurfer (Version 5.3) image analysis suite pipeline was used to evaluate cortical and subcortical regions of interest. Briefly, analysis steps include motion correction and removal of non-brain tissue using a hybrid watershed/surface deformation procedure [51], automated Talairach transformation, segmentation of the subcortical white matter and deep gray matter volumetric structures (including hippocampus, amygdala, caudate, putamen, ventricles) [52, 53], intensity normalization [54], tessellation of the gray matter white matter boundary, automated topology correction [55, 56], and surface deformation following intensity gradients to optimally place the boundaries between gray/white and gray/cerebrospinal fluid regions [57–59]. A number of deformable procedures can be performed for further data processing and analysis including surface inflation [58], registration to a spherical atlas which is based on individual cortical folding patterns to match cortical geometry across subjects [60], parcellation of the cerebral cortex into units with respect to gyral and sulcal structure [61, 62], and creation of a variety of surface-based data including maps of curvature and sulcal depth. This method uses both intensity and continuity information from the entire three dimensional MR volume in segmentation and deformation procedures to produce representations of cortical thickness, calculated as the closest distance from the gray/white boundary to the gray/CSF boundary at each vertex on the tessellated surface [59]. The maps are created using spatial intensity gradients across tissue classes and are therefore not simply reliant on absolute signal intensity.
ROI volumes and thicknesses (where applicable) for this study were extracted from anatomically defined cortical segmentation and parcellation of each subject using the Desikan-Killiany atlas. We selected ROIs by focusing on regions demonstrated to display consistent patterns of aMCI-related cortical atrophy by the meta-analyses of Nickl-Jockschat et al. [19] and Yang et al. [20], and reinforced our selections with the longitudinal aMCI data of Goerlich et al. [21]. These included the hippocampus, amygdala, superior parietal, and posterior cingulate cortices bilaterally. The putamen was selected as a control region because no meta-analysis identified it as an aMCI-dependent region, and studies have reported no or limited differences in putaminal volumes in AD [63, 64], especially compared to other types of dementia [64, 65]. We also evaluated the role of frontal lobe thickness as a marker of sleep quality-related atrophy based on previous sleep studies [29–31]. Regions analyzed included the superior frontal gyri, the orbitofrontal cortices, and the middle frontal gyri, as well as the pars orbitalis and frontal poles bilaterally. The pericalcarine cortex was selected as a control region because previous studies have suggested it is spared by aMCI-related atrophy [66]. Data was collected on regions outside of those previously referenced in sleep-related and aMCI-related atrophy literature, but was not used in any hypothesis-driven analysis.
Statistical analysis
The data was analyzed using SPSS (Version 22). The regional volumes obtained from cortical and subcortical analysis were compared between subjects with normal sleep (PSQI < 5) and poor sleep (PSQI ≥ 5) using the ANOVA test. We accounted for variations in intracranial volume (ICV) between subjects by dividing each subject’s ROI volumes by their respective ICV, effectively feeding the ratio between the volume of the ROI and the ICV into statistical analysis. In efforts to balance type II error rates and statistical power, we performed multiple comparison correction on ROI results using the Benjamini-Hochberg procedure [67] with false discovery rate (FDR) = 0.1. FDR = 0.1 was selected because of the targeted list of regions analyzed and to better identify potential regions where sleep and aMCI may interact for further inquiry. Confidence intervals for significant or close to significant non-ROIs were reported, primarily to inform future hypothesis-driven work.
After analysis of regional differences between poor sleepers and good sleepers, PSQI was treated as a continuous variable and evaluated for potential correlations with ROI volumes. We performed multiple linear regression analysis controlling for age to correlate each ROI with total PSQI score, as well as the following sub-scores: 2 (sleep onset latency), 4a (sleep duration), 5a (inability to sleep within 30 minutes) 5b (nocturnal awakening), 5d (breathing comfortably), 5e (coughing/snoring), and 7 (daytime sleepiness).
Results
Demographic data
No significant differences were observed between the poor and good sleepers groups with regard to age, gender proportions, or years of education. Both groups had more prevalence of women (~70% female). Demographic data are summarized in Table 1.
Table 1.
Demographics
| Group | Normal sleep | Poor sleep | P value |
|---|---|---|---|
| N | 31 | 38 | n/a |
| Age | 69.6 ± 5.52 | 70.7 ± 7.35 | .54 |
| Median PSQI | 3 | 7 | <.00001a,* |
| % Males | 0.32 | 0.26 | .59b |
| Education (years) | 15 ± 3.61 | 15 ± 3 | .26 |
aMann–Whitney U test.
bChi-square test.
*Significant values.
Cortical volume and thickness analysis
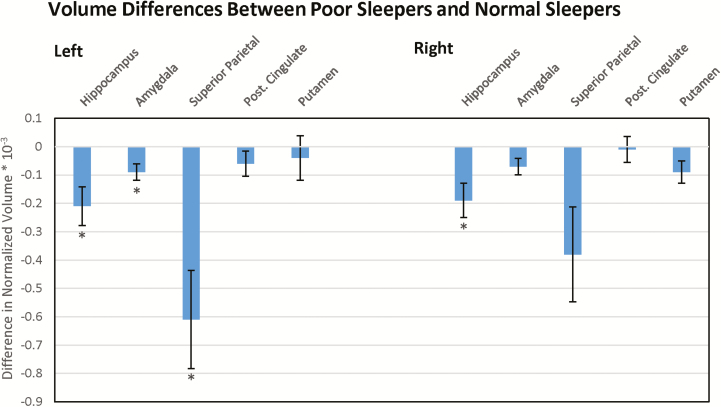
After multiple comparisons correction at FDR = 0.1, poor sleepers exhibited lesser volumes in the left amygdala, hippocampi and parietal lobules bilaterally than controls with normal sleep quality. No values were significant at FDR = 0.05. The non-aMCI related control region, the putamen, did not show significant difference between the two cohorts. These results are demonstrated in Figure 1, which shows differences in volume between the two cohorts at each ROI. Among non-ROIs, 95% confidence intervals for the difference in volumes between poor and normal sleepers were only found to not contain zero for the left insular cortex and left middle temporal gyrus. These results are summarized in Table 2.
Figure 1.
Volume differences between poor sleepers and normal sleepers. Difference in aMCI-related brain volumes between poor sleepers and normal sleepers. Negative values indicate reduced volumes in poor sleepers compared to normal sleepers. * indicate regions of significant difference after multiple comparisons correction at FDR = 0.1; the putamen served as control.
Table 2.
Volumetric results
| ROIs | Poor sleeper mean Vol/ICV (SD) | Normal sleeper mean Vol/ICV (SD) | P value |
|---|---|---|---|
| Left | |||
| Hippocampus | 2.58 (0.387) | 2.79 (0.418) | .036*,** |
| Amygdala | 1.08 (0.172) | 1.17 (0.170) | .045*,** |
| Superior parietal | 8.08 (1.22) | 8.69 (0.769) | .013*,** |
| Post. cingulate | 1.98 (0.240) | 2.04 (0.284) | .205 |
| Putamen | 3.49 (0.489) | 3.45 (0.434) | .747 |
| Right | |||
| Hippocampus | 2.71 (0.387) | 2.90 (0.322) | .025*,** |
| Amygdala | 1.07 (0.174) | 1.14 (0.168) | .06 |
| Superior parietal | 8.18 (1.12) | 8.56 (0.832) | .047*,** |
| Post. cingulate | 2.01 (0.270) | 2.02 (0.266) | .575 |
| Putamen | 3.24 (0.442) | 3.15 (0.365) | .363 |
| Non-ROI regions | Poor sleeper mean Vol/ICV (SD) | Normal sleeper mean Vol/ICV (SD) | 95% CI for mean difference |
| Left insula | 4.20 (0.314) | 4.45 (0.394) | 0.0858–0.414 |
| Left middle temporal | 6.09 (0.917) | 6.54 (0.960) | 0.0108–0.889 |
| Right PCC | 1.36 (0.176) | 1.47 (0.322) | −0.0063–0.226 |
| Right pars orbitalis | 1.64 (0.214) | 1.74 (0.235) | −0.0047–0.205 |
PCC = Pericalcarine Cortex. Mean/SD values are ×10−3. No values were significant at FDR = 0.05.
*Initially significant values. **Significant values after Benjamini-Hochberg multiple comparisons correction with FDR = 0.1.
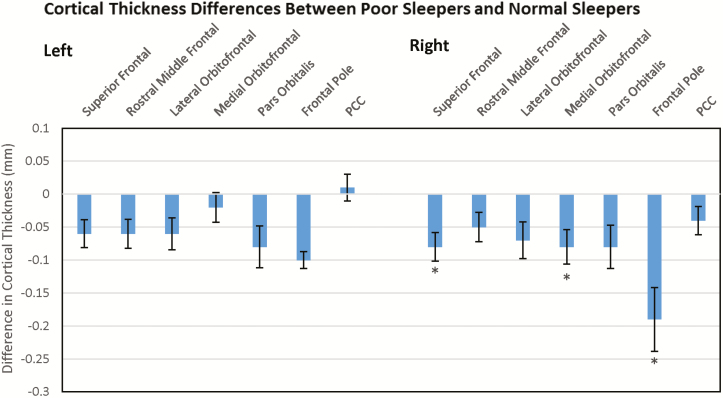
Poor sleepers demonstrated diffuse cortical thinning bilaterally, with the largest area of impact in the right mesial frontal lobe. These results are demonstrated in Figure 2, which shows differences in cortical thickness between the two cohorts at each ROI. Left cortical thinning was initially significant in the superior frontal gyrus, but this relationship dropped out of significance after multiple comparisons correction. Right cortical thinning was initially significant in the superior frontal gyrus, the medial and lateral orbitofrontal cortices, and the frontal pole. Significant effects remained in the superior frontal gyrus, medial orbitofrontal cortex, and frontal pole after correcting for multiple comparisons at FDR = 0.1. Only the effect in the right frontal pole was present at FDR = 0.05. Among non-ROIs, 95% confidence intervals for the difference in thicknesses between poor sleepers and normal sleepers were only found to not contain zero for the insular cortices bilaterally, the right fusiform gyrus, and the left supramarginal gyrus. These results are summarized in Table 3.
Figure 2.
Cortical thickness differences between poor sleepers and normal sleepers. Difference in ROI cortical thicknesses between poor sleepers and normal sleepers. Negative values indicate reduced thicknesses in poor sleepers compared to normal sleepers. * indicate regions of significant difference after multiple comparisons correction at FDR = 0.1; PCC = Pericalcarine Cortex; PCC served as control.
Table 3.
Thickness results
| ROIs | Poor mean thickness (mm) | Normal mean thickness (mm) | P value |
|---|---|---|---|
| Left | |||
| Superior frontal | 2.58 (0.124) | 2.64 (0.124) | .046* |
| Rostral middle frontal | 2.23 (0.123) | 2.29 (0.135) | .062 |
| Lateral orbitofrontal | 2.44 (0.155) | 2.50 (0.128) | .1 |
| Medial orbitofrontal | 2.29 (0.140) | 2.31 (0.126) | .45 |
| Pars orbitalis | 2.55 (0.183) | 2.63 (0.191) | .176 |
| Frontal pole | 2.65 (0.188) | 2.75 (0.269) | .101 |
| Pericalcarine cortex | 1.42 (0.120) | 1.41 (0.119) | .120 |
| Right | |||
| Superior frontal | 2.54 (0.136) | 2.62 (0.120) | .018*,** |
| Rostral middle frontal | 2.23 (0.130) | 2.28 (0.132) | .071 |
| Lateral orbitofrontal | 2.50 (0.172) | 2.57 (0.154) | .05 |
| Medial orbitofrontal | 2.33 (0.139) | 2.41 (0.169) | .021*,** |
| Pars orbitalis | 2.58 (0.177) | 2.66 (0.205) | .095 |
| Frontal pole | 2.61 (0.214) | 2.80 (0.341) | .004*,**,*** |
| Pericalcarine cortex | 1.41 (0.133) | 1.45 (0.120) | .116 |
| Non-ROI regions | Poor mean thickness (mm) | Normal mean thickness (mm) | 95% CI for mean difference (mm) |
| Left insula | 2.84 (0.170) | 2.95 (0.137) | 0.0362–0.184 |
| Left supramarginal | 2.32 (0.131) | 2.38 (0.122) | 0.0002–0.120 |
| Right insula | 2.87 (0.202) | 3.00 (0.179) | 0.0395–0.221 |
| Right fusiform | 2.63 (0.145) | 2.72 (0.154) | 0.0201–0.160 |
Mean thickness/standard deviation are in mm.
*Initially significant values. **Significant values after Benjamini-Hochberg multiple comparisons correction with FDR = 0.1. ***Significant values with FDR = 0.05.
PSQI correlational analysis
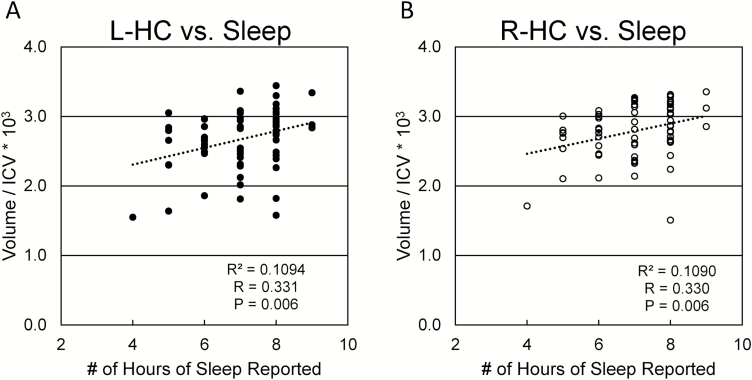
Higher overall PSQI scores were weakly correlated with decreased volumes in the left hippocampus (p < .05, r = −0.229), left amygdala (p < .05, r = −0.254), and right hippocampus (p < .05, r = −0.221), and decreased cortical thickness in the right frontal pole (p < .05, r = −0.24). Sleep durations (item 4a) were positively correlated with higher volumes in the bilateral hippocampi, amygdala, and superior parietal lobules, as well as greater cortical thickness in the bilateral superior frontal and rostral middle frontal cortices, right medial orbitofrontal cortex, and both frontal poles. The relationship between hippocampal volumes and sleep duration are depicted in Figure 3. Increased incidence of nocturnal awakenings were correlated with bilateral decreases in hippocampal volume, and increased daytime sleepiness was correlated with atrophy of the left lateral orbitofrontal cortex. Sleep latency (item 2), reported inability to get to sleep within 30 minutes (5a), inability to breathe comfortably during sleep (5d) and loud snoring or coughing at night (5e) did not correlate significantly with any of the ROIs analyzed. These results are summarized in Table 4.
Figure 3.
Relationship between hippocampal volumes and sleep duration. Relationship between hippocampal volumes and reported sleep duration among all subjects. Subjects who reported less hours of sleep regularly tended to have smaller hippocampal volumes bilaterally. Key: (A) Left hippocampus; (B) Right hippocampus. ICV = intracranial volume.
Table 4.
Correlational analysis results
| Volume ROIs | Total PSQI | Sleep latency | Sleep duration | Nocturnal awakening | Daytime sleepiness |
|---|---|---|---|---|---|
| Left | |||||
| Hippocampus | .040 (−0.229) | ns | .001 (0.361) | .045 (−0.225) | ns |
| Amygdala | .026 (−0.254) | ns | .004 (0.327) | ns | ns |
| Superior Parietal | ns | ns | .002 (0.362) | ns | ns |
| Posterior Cingulate | ns | ns | ns | ns | ns |
| Right | |||||
| Hippocampus | .040 (−0.221) | ns | <.001 (0.365) | .025 (−0.242) | ns |
| Amygdala | ns | ns | .001 (0.356) | ns | ns |
| Superior parietal | ns | ns | .008 (0.310) | ns | ns |
| Posterior cingulate | ns | ns | Ns | ns | ns |
| Thickness ROIs | Total PSQI | Sleep latency | Sleep duration | Nocturnal awakening | Daytime sleepiness |
| Left | |||||
| Superior frontal | ns | ns | .005 (0.327) | ns | ns |
| Rostral middle frontal | ns | ns | .017 (0.286) | ns | ns |
| Lateral orbitofrontal | ns | ns | ns | ns | .015 (−0.279) |
| Medial orbitofrontal | ns | ns | ns | ns | ns |
| Pars orbitalis | ns | ns | ns | ns | ns |
| Frontal pole | ns | ns | .017 (0.286) | ns | ns |
| Right | |||||
| Superior frontal | ns | ns | .002 (0.366) | ns | ns |
| Rostral middle frontal | ns | ns | .041 (0.246) | ns | ns |
| Lateral orbitofrontal | ns | ns | ns | ns | ns |
| Medial orbitofrontal | ns | ns | .019 (0.279) | ns | ns |
| Pars orbitalis | ns | ns | ns | ns | ns |
| Frontal pole | ns | ns | .033 (0.260) | ns | ns |
Significant correlations are presented in the table as “p value (r value).” ns = not significant.
Discussion
Our study of cognitively normal subjects revealed that relative to good sleepers, poor sleepers exhibit reduced volumes in most of the aMCI-related regions studied, with significant reductions in the hippocampi, amygdala, and superior parietal lobes. Volume reductions in these regions correlated most strongly with sleep duration, while hippocampal atrophy was correlated with increased nocturnal awakening frequency and overall PSQI. We also noted a weak (95% CI for mean difference = 0.0108–0.8892) trend towards volume reductions in the left middle temporal lobe, a commonly aMCI affected region adjacent to the hippocampus.
Hippocampal atrophy has been associated with a number of sleep disorders, but findings regarding the relationship between sleep and hippocampal volumes have been mixed. Notably, a number of authors have found no difference in hippocampal volumes between primary insomniacs and good sleepers [68, 69], calling into question whether insomnia plays a primary role in hippocampal atrophy. Another possibility is that hippocampal volumes do not differ absolutely between poor sleepers and good sleepers, but correlate with the severity of insomnia symptoms, as Noh et al. demonstrated with primary insomniacs [70]. Other authors have found hippocampal volume deficits in chronic insomniacs, such as Riemann et al. and Koo et al. [26, 71], and in patients with sleep-disordered breathing, independent of hypoxia [28]. While the relationship between sleep quality and hippocampal volumes remains an open question, our work concurs with these latter studies in suggesting that poor sleep quality and sleep disorders may negatively impact hippocampal volumes, and extends their findings to a rigorously evaluated, cognitively normal population. This reinforces a connection between sleep quality, particularly the sleep duration sub-score of sleep quality, and aMCI/AD-related neurodegeneration of the hippocampus.
Attributions to left superior parietal and middle temporal atrophy in sleep literature are scarcer, but other investigators have previously connected these regions to sleep disturbance. Sexton et al. found heightened rates of temporal and superior parietal atrophy in a longitudinal study of community-dwelling adults with poor sleep quality [33], an observation mirrored across cohorts by this study’s results. Reduced middle temporal volumes have also been found in insomniac patients [72], reinforcing the connection between the region and sleep quality. Other studies have connected temporal lobe reductions in thickness to obstructive sleep apnea (OSA)-related sleep disruption [32, 73], but without evaluating independence from hypoxia. Given the confounding influence of hypoxia, the applicability of these studies to our results is limited, but may still bear some relevance. As a whole, our observations suggest that poor sleep quality and associated sleep disorders may impact aMCI-related temporal and parietal lobe atrophy patterns, but more work is necessary to develop a comprehensive picture of sleep’s influences on these regions.
Although meta-analyses have implicated neurodegeneration of the posterior cingulate in aMCI [19, 20], our poor sleep quality subjects did not show trends towards atrophy in the region. Prior work has suggested that poor sleep quality may affect activity in the posterior cingulate [74], but sleep has not yet been associated with atrophy of the structure. Interestingly, several studies have established that neurofibrillary tangles (NFTs) and tau protein rather than β amyloid predominate in aMCI-related atrophy of the posterior cingulate [75, 76], a relevant factor given the link between sleep and β amyloid clearance [17]. While β amyloid deposits have been shown to occur within the posterior cingulate early in the course of aMCI [77], and even impact the functional connectivity of the structure [78], they have not been shown to correlate with atrophy [79]. This may suggest that sleep quality has a lesser impact on the atrophy of regions that are less β amyloid dependent.
Though the insular cortex is not typically included in the characteristic picture of aMCI-related cortical degeneration, our results suggest that poor sleep quality is linked to bilateral insular thickness reductions, and volumetric reductions in the left insular cortex. As we did not specify the insula as a ROI in this analysis, these results should not be taken with the same confidence, and will need to be investigated in a future study. However, we may propose a tentative basis for insular involvement in sleep quality-related atrophy. The insular cortex is a region intimately involved with the regulation of the autonomic nervous system [80, 81]. Autonomic hyperarousal plays a key pathophysiologic role in poor sleep quality [82, 83], which is consistent with insular volume and thickness reductions in poor sleepers. Because the insular cortex lies in close proximity to the hippocampus, several investigators have also implicated insular degeneration in the pathology of certain AD presentations [84, 85], suggesting that the link between sleep and neurodegeneration of the structure warrants further investigation.
Beyond the insular cortex, the present study found diffuse patterns of cortical thickness reduction, with the majority of significant effects clustered in the right mesial frontal lobe. While not classical for aMCI, frontal lobe atrophy has been associated with insomnia [29–31, 72, 86], and may be related to deficits of slow wave sleep in older adults [87]. These patterns might predispose individuals to possibly deleterious sleep behaviors, but have not been directly correlated with AD; while β amyloid deposition can occur early in the course of aMCI [79], frontal lobe atrophy is typically a later stage marker of AD [88], or associated with relatively uncommon behavioral subtypes of the condition [89, 90]. Future studies might investigate the extent to which insomnia-related frontal atrophy interacts with frontal cortical changes in late Alzheimer’s, but this study’s cognitively normal cohort is not suitable for such inquiry.
Among the relationships observed between sub-scores of the PSQI and ROI volumes/thicknesses, it was perhaps most striking that sleep duration correlated significantly with a number of ROIs, including the bilateral hippocampi, amygdala, and superior parietal lobules, and several frontal lobe regions. This association points to sleep duration as a potential factor for the morphological differences observed between poor and normal sleepers, a particularly interesting observation in light of previous studies of the connection between sleep duration and AD pathology. Given that shorter sleep duration has been previously tied to higher cortical amyloid burden [91], and that sleep has been proposed as a mechanism for β amyloid clearance [17], it is reasonable that reduced sleep duration could modulate cortical atrophy through reduced β amyloid clearance. It is also possible that sleep may influence tau-related neurodegeneration in some way, as increased tau levels have been observed in the cerebrospinal fluid of poor sleepers [92], or that increased oxidative stress and activity associated with sleep loss [93] may contribute to neuronal degeneration and deplete cognitive reserves in AD-related regions. While this correlation does not imply causality, the observed relationship between sleep duration and brain volumes tentatively supports a role for sleep in modulating cortical atrophy, and perhaps warrants more targeted study.
A limitation of this study is that we collected subjective measures of sleep quality, leading to the possibility of imperfect recall or errors confounding our results. We plan to follow the subjects over the next several years and collect longitudinal sleep data that will help verify the long-term validity of our subjective assessments. In addition, while we excluded any subjects with evidence of any cognitive impairment from our study, subjects diagnosed with insomnia and sleep apnea, which have been studied as distinct clinical conditions, were not excluded from the study. As such, we cannot exclude the possibility that either condition impacted the relationship observed between sleep quality and cortical volumes, though our PSQI sub-score analysis does not point to either as a main effect driver. Notably, we found no correlation between several major PSQI sub-scores associated with insomnia (sleep latency, inability to sleep within 30 minutes, and daytime sleepiness) or sleep apnea (breathing comfortably, coughing/snoring at night) and the volumes of the aMCI-dependent ROIs we defined, making it less likely that either condition played a primary role in explaining the results.
Designed as an exploratory analysis, this study employed a liberal FDR threshold of 0.1 that potentially increases the risk of type II error in the results. However, the small, targeted selection of ROIs analyzed mitigated this risk in the experimental design. Moreover, as a cross-sectional analysis, this study also has little ability to assess the directionality of the relationship between self-reported sleep quality and neurodegeneration. However, recent reviews have emphasized the bidirectional influence of neurodegeneration on sleep, wherein poor sleep induces neurodegeneration, and neurodegeneration contributes to poorer quality of sleep [13, 94].
While the specifics of the interplay between sleep and neurodegeneration merit further study, a common denominator is that sleep health interventions amongst the elderly may prove an effective tool to break the sleep-neurodegeneration cycle and delay the onset of dementia.
In conclusion, our results suggest that poor sleep quality contributes to aMCI-related patterns of atrophy even in cognitively normal subjects. A subthreshold level of atrophy could possibly explain the lack of aMCI symptoms. This may be associated with the proposed mechanism of β amyloid clearance from the brain during sleep, as we did not find any effects in the posterior cingulate, where tau-related neurodegeneration is dominant. The observation that sleep duration was the main factor influencing atrophy patterns in poor sleepers further suggests that sleep quality may influence AD pathology. Given these results, improving sleep quality in elderly individuals may be an effective way to protect against neurodegeneration of AD-related regions, potentially slowing the onset and progression of dementia.
Funding
Supported by State of Florida department of health grant #8AZ22 (PI: Alperin), and by Evelyn F. McKnight Brain Institute, University of Miami and NIH grant R01 AG047649 (PI: Loewenstein).
Conflict of interest statement. None declared.
Acknowledgments
We would like to thank the University of Miami Biostatistics Collaboration and Consulting Core (BCCC) for statistical guidance on this project.
References
- 1. Hurd MD, et al. Monetary costs of dementia in the United States. N Engl J Med. 2013;368(14):1326–1334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Ferri CP, et al. ; Alzheimer’s Disease International. Global prevalence of dementia: a Delphi consensus study. Lancet. 2005;366(9503):2112–2117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Razlighi QR, et al. A new algorithm for predicting time to disease endpoints in Alzheimer’s disease patients. J Alzheimers Dis. 2014;38(3):661–668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Braak H, et al. Neuropathological stageing of Alzheimer-related changes. Acta Neuropathol. 1991;82(4):239–259. [DOI] [PubMed] [Google Scholar]
- 5. Atiya M, et al. Structural magnetic resonance imaging in established and prodromal Alzheimer disease: a review. Alzheimer Dis Assoc Disord. 2003;17(3):177–195. [DOI] [PubMed] [Google Scholar]
- 6. Storandt M, et al. Toward a multifactorial model of Alzheimer disease. Neurobiol Aging. 2012;33(10):2262–2271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Ju YE, et al. Sleep and Alzheimer disease pathology–a bidirectional relationship. Nat Rev Neurol. 2014;10(2):115–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Musiek ES, et al. Sleep, circadian rhythms, and the pathogenesis of Alzheimer disease. Exp Mol Med. 2015;47:e148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Loewenstein RJ, et al. Disturbances of sleep and cognitive functioning in patients with dementia. Neurobiol Aging. 1982;3(4):371–377. [DOI] [PubMed] [Google Scholar]
- 10. Prinz PN, et al. Changes in the sleep and waking EEGs of nondemented and demented elderly subjects. J Am Geriatr Soc. 1982;30(2):86–93. [DOI] [PubMed] [Google Scholar]
- 11. Weldemichael DA, Grossberg GT. Circadian rhythm disturbances in patients with Alzheimer’s disease: a review. Int J Alzheimers Dis. 2010;2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Gildner TE, et al. Associations between sleep duration, sleep quality, and cognitive test performance among older adults from six middle income countries: results from the Study on Global Ageing and Adult Health (SAGE). J Clin Sleep Med. 2014;10(6):613–621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Mander BA, et al. Sleep: a novel mechanistic pathway, biomarker, and treatment target in the pathology of Alzheimer’s disease? Trends Neurosci. 2016;39(8):552–566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Elwood PC, et al. Sleep disturbance and daytime sleepiness predict vascular dementia. J Epidemiol Community Health. 2011;65(9):820–824. [DOI] [PubMed] [Google Scholar]
- 15. Landry GJ, et al. Buying time: a rationale for examining the use of circadian rhythm and sleep interventions to delay progression of mild cognitive impairment to Alzheimer’s disease. Front Aging Neurosci. 2014;6:325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Sterniczuk R, et al. Sleep disturbance is associated with incident dementia and mortality. Curr Alzheimer Res. 2013;10(7):767–775. [DOI] [PubMed] [Google Scholar]
- 17. Xie L, et al. Sleep drives metabolite clearance from the adult brain. Science. 2013;342(6156):373–377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Naidoo N, et al. Aging impairs the unfolded protein response to sleep deprivation and leads to proapoptotic signaling. J Neurosci. 2008;28(26):6539–6548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Nickl-Jockschat T, et al. Neuroanatomic changes and their association with cognitive decline in mild cognitive impairment: a meta-analysis. Brain Struct Funct. 2012;217(1):115–125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Yang J, et al. Voxelwise meta-analysis of gray matter anomalies in Alzheimer’s disease and mild cognitive impairment using anatomic likelihood estimation. J Neurol Sci. 2012;316(1–2):21–29. [DOI] [PubMed] [Google Scholar]
- 21. Goerlich KS, et al. Neuroanatomical and neuropsychological markers of amnestic MCI: a three-year longitudinal study in individuals unaware of cognitive decline. Front Aging Neurosci. 2017;9:34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Adaszewski S, et al. ; Alzheimer’s Disease Neuroimaging Initiative. How early can we predict Alzheimer’s disease using computational anatomy? Neurobiol Aging. 2013;34(12):2815–2826. [DOI] [PubMed] [Google Scholar]
- 23. Wolz R, et al. ; Alzheimer’s Disease Neuroimaging Initiative. Measurement of hippocampal atrophy using 4D graph-cut segmentation: application to ADNI. Neuroimage. 2010;52(1):109–118. [DOI] [PubMed] [Google Scholar]
- 24. Chupin M, et al. ; Alzheimer’s Disease Neuroimaging Initiative. Fully automatic hippocampus segmentation and classification in Alzheimer’s disease and mild cognitive impairment applied on data from ADNI. Hippocampus. 2009;19(6):579–587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Karow DS, et al. ; Alzheimer’s Disease Neuroimaging Initiative. Relative capability of MR imaging and FDG PET to depict changes associated with prodromal and early Alzheimer disease. Radiology. 2010;256(3):932–942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Koo DL, et al. Changes in subcortical shape and cognitive function in patients with chronic insomnia. Sleep Med. 2017;35:23–26. [DOI] [PubMed] [Google Scholar]
- 27. Joo EY, et al. Hippocampal substructural vulnerability to sleep disturbance and cognitive impairment in patients with chronic primary insomnia: magnetic resonance imaging morphometry. Sleep. 2014;37(7):1189–1198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Sforza E, et al. Hippocampus volume and subjective sleepiness in older people with sleep-disordered breathing: a preliminary report. J Sleep Res. 2016;25(2):190–193. [DOI] [PubMed] [Google Scholar]
- 29. Lim AS, et al. Regional neocortical gray matter structure and sleep fragmentation in older adults. Sleep. 2016;39(1):227–235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Suh S, et al. Cortical thinning and altered cortico-cortical structural covariance of the default mode network in patients with persistent insomnia symptoms. Sleep. 2016;39(1):161–171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Chao LL, et al. Associations between subjective sleep quality and brain volume in Gulf War veterans. Sleep. 2014;37(3):445–452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Macey PM, et al. Obstructive sleep apnea and cortical thickness in females and males. PLoS One. 2018;13(3):e0193854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Sexton CE, et al. Poor sleep quality is associated with increased cortical atrophy in community-dwelling adults. Neurology. 2014;83(11):967–973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Morris JC. The Clinical Dementia Rating (CDR): current version and scoring rules. Neurology. 1993;43(11):2412–2414. [DOI] [PubMed] [Google Scholar]
- 35. Folstein MF, et al. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12(3):189–198. [DOI] [PubMed] [Google Scholar]
- 36. Shapiro AM, et al. Construct and concurrent validity of the Hopkins Verbal Learning Test-revised. Clin Neuropsychol. 1999;13(3):348–358. [DOI] [PubMed] [Google Scholar]
- 37. Beekly DL, et al. ; NIA Alzheimer’s Disease Centers. The National Alzheimer’s Coordinating Center (NACC) database: the uniform data set. Alzheimer Dis Assoc Disord. 2007;21(3):249–258. [DOI] [PubMed] [Google Scholar]
- 38. Lucas JA, et al. Mayo’s older Americans normative studies: category fluency norms. J Clin Exp Neuropsychol. 1998;20(2):194–200. [DOI] [PubMed] [Google Scholar]
- 39. Wechsler D. Wechsler Adult Intelligence Scale. 4th ed. San Antonio, TX: Pearson; 2008. [Google Scholar]
- 40. Reitan R. Validity of the trail making test as an indicator of organic brain damage. Percept Mot Skills. 1958;8:271–276. [Google Scholar]
- 41. Yesavage JA, et al. Development and validation of a geriatric depression screening scale: a preliminary report. J Psychiatr Res. 1982;17(1):37–49. [DOI] [PubMed] [Google Scholar]
- 42. Fernández-San Martín M, et al. Validation of the Spanish version of the geriatric depression scale (GDS) in primary care. Int J Geriatr Psychiatry. 2002;17(3):279–287. [DOI] [PubMed] [Google Scholar]
- 43. Chan AC. Clinical validation of the Geriatric Depression Scale (GDS): Chinese version. J Aging Health. 1996;8(2):238–253. [DOI] [PubMed] [Google Scholar]
- 44. Cole J, et al. Hippocampal atrophy in first episode depression: a meta-analysis of magnetic resonance imaging studies. J Affect Disord. 2011;134(1–3):483–487. [DOI] [PubMed] [Google Scholar]
- 45. Baglioni C, et al. Sleep and emotions: a focus on insomnia. Sleep Med Rev. 2010;14(4):227–238. [DOI] [PubMed] [Google Scholar]
- 46. Lee E, et al. Persistent sleep disturbance: a risk factor for recurrent depression in community-dwelling older adults. Sleep. 2013;36(11):1685–1691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Buysse DJ, et al. The Pittsburgh Sleep Quality Index: a new instrument for psychiatric practice and research. Psychiatry Res. 1989;28(2):193–213. [DOI] [PubMed] [Google Scholar]
- 48. Beaudreau SA, et al. ; Study of Osteoporotic Fractures. Validation of the Pittsburgh sleep quality index and the Epworth sleepiness scale in older black and white women. Sleep Med. 2012;13(1):36–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Salahuddin M, et al. Validation of the Pittsburgh sleep quality index in community dwelling Ethiopian adults. Health Qual Life Outcomes. 2017;15(1):58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Brant-Zawadzki M, et al. MP RAGE: a three-dimensional, T1-weighted, gradient-echo sequence–initial experience in the brain. Radiology. 1992;182(3):769–775. [DOI] [PubMed] [Google Scholar]
- 51. Ségonne F, et al. A hybrid approach to the skull stripping problem in MRI. Neuroimage. 2004;22(3):1060–1075. [DOI] [PubMed] [Google Scholar]
- 52. Fischl B, et al. Whole brain segmentation: automated labeling of neuroanatomical structures in the human brain. Neuron. 2002;33(3):341–355. [DOI] [PubMed] [Google Scholar]
- 53. Fischl B, et al. Sequence-independent segmentation of magnetic resonance images. Neuroimage. 2004;23(suppl 1):S69–S84. [DOI] [PubMed] [Google Scholar]
- 54. Sled JG, et al. A nonparametric method for automatic correction of intensity nonuniformity in MRI data. IEEE Trans Med Imaging. 1998;17(1):87–97. [DOI] [PubMed] [Google Scholar]
- 55. Fischl B, et al. Automated manifold surgery: constructing geometrically accurate and topologically correct models of the human cerebral cortex. IEEE Trans Med Imaging. 2001;20(1):70–80. [DOI] [PubMed] [Google Scholar]
- 56. Ségonne F, et al. Geometrically accurate topology-correction of cortical surfaces using nonseparating loops. IEEE Trans Med Imaging. 2007;26(4):518–529. [DOI] [PubMed] [Google Scholar]
- 57. Dale AM, et al. Improved localizadon of cortical activity by combining EEG and MEG with MRI cortical surface reconstruction: a linear approach. J Cogn Neurosci. 1993;5(2):162–176. [DOI] [PubMed] [Google Scholar]
- 58. Dale AM, et al. Cortical surface-based analysis. I. Segmentation and surface reconstruction. Neuroimage. 1999;9(2):179–194. [DOI] [PubMed] [Google Scholar]
- 59. Fischl B, et al. Measuring the thickness of the human cerebral cortex from magnetic resonance images. Proc Natl Acad Sci USA. 2000;97(20):11050–11055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Fischl B, et al. Cortical surface-based analysis. II: inflation, flattening, and a surface-based coordinate system. Neuroimage. 1999;9(2):195–207. [DOI] [PubMed] [Google Scholar]
- 61. Desikan RS, et al. An automated labeling system for subdividing the human cerebral cortex on MRI scans into gyral based regions of interest. Neuroimage. 2006;31(3):968–980. [DOI] [PubMed] [Google Scholar]
- 62. Fischl B, et al. Automatically parcellating the human cerebral cortex. Cereb Cortex. 2004;14(1):11–22. [DOI] [PubMed] [Google Scholar]
- 63. Cousins DA, et al. Atrophy of the putamen in dementia with Lewy bodies but not Alzheimer’s disease: an MRI study. Neurology. 2003;61(9):1191–1195. [DOI] [PubMed] [Google Scholar]
- 64. Looi JC, et al. Putaminal volume in frontotemporal lobar degeneration and Alzheimer disease: differential volumes in dementia subtypes and controls. AJNR Am J Neuroradiol. 2009;30(8):1552–1560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Mueller AD, et al. Sleep deprivation can inhibit adult hippocampal neurogenesis independent of adrenal stress hormones. Am J Physiol Regul Integr Comp Physiol. 2008;294(5):R1693–R1703. [DOI] [PubMed] [Google Scholar]
- 66. McDonald CR, et al. ; Alzheimer’s Disease Neuroimaging Initiative. Regional rates of neocortical atrophy from normal aging to early Alzheimer disease. Neurology. 2009;73(6):457–465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Benjamini Y, et al. Controlling the false discovery rate in behavior genetics research. Behav Brain Res. 2001;125(1-2):279–284. [DOI] [PubMed] [Google Scholar]
- 68. Spiegelhalder K, et al. Insomnia does not appear to be associated with substantial structural brain changes. Sleep. 2013;36(5):731–737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Winkelman JW, et al. Lack of hippocampal volume differences in primary insomnia and good sleeper controls: an MRI volumetric study at 3 Tesla. Sleep Med. 2010;11(6):576–582. [DOI] [PubMed] [Google Scholar]
- 70. Noh HJ, et al. The relationship between hippocampal volume and cognition in patients with chronic primary insomnia. J Clin Neurol. 2012;8(2):130–138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Riemann D, et al. Chronic insomnia and MRI-measured hippocampal volumes: a pilot study. Sleep. 2007;30(8):955–958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Joo EY, et al. Brain gray matter deficits in patients with chronic primary insomnia. Sleep. 2013;36(7):999–1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Joo EY, et al. Reduced brain gray matter concentration in patients with obstructive sleep apnea syndrome. Sleep. 2010;33(2):235–241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Kay DB, et al. Sleep-wake differences in relative regional cerebral metabolic rate for glucose among patients with insomnia compared with good sleepers. Sleep. 2016;39(10):1779–1794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Maarouf CL, et al. Biochemical assessment of precuneus and posterior cingulate gyrus in the context of brain aging and Alzheimer’s disease. PLoS One. 2014;9(8):e105784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Shima K, et al. Posterior cingulate atrophy and metabolic decline in early stage Alzheimer’s disease. Neurobiol Aging. 2012;33(9):2006–2017. [DOI] [PubMed] [Google Scholar]
- 77. Sepulcre J, et al. In vivo characterization of the early states of the amyloid-beta network. Brain. 2013;136(Pt 7):2239–2252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Sperling RA, et al. Amyloid deposition is associated with impaired default network function in older persons without dementia. Neuron. 2009;63(2):178–188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Palmqvist S, et al. Earliest accumulation of β-amyloid occurs within the default-mode network and concurrently affects brain connectivity. Nat Commun. 2017;8(1):1214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Critchley HD. Neural mechanisms of autonomic, affective, and cognitive integration. J Comp Neurol. 2005;493(1): 154–166. [DOI] [PubMed] [Google Scholar]
- 81. Oppenheimer SM, et al. Cardiovascular effects of human insular cortex stimulation. Neurology. 1992;42(9):1727–1732. [DOI] [PubMed] [Google Scholar]
- 82. Bonnet MH. Hyperarousal and insomnia. Sleep Med Rev. 2010;14(1):33. [DOI] [PubMed] [Google Scholar]
- 83. Roth T, et al. Insomnia: pathophysiology and implications for treatment. Sleep Med Rev. 2007;11(1):71–79. [DOI] [PubMed] [Google Scholar]
- 84. Moon Y, et al. Regional atrophy of the insular cortex is associated with neuropsychiatric symptoms in Alzheimer’s disease patients. Eur Neurol. 2014;71(5–6):223–229. [DOI] [PubMed] [Google Scholar]
- 85. Foundas AL, et al. Atrophy of the hippocampus, parietal cortex, and insula in Alzheimer’s disease: a volumetric magnetic resonance imaging study. Neuropsychiatry Neuropsychol Behav Neurol. 1997;10(2):81–89. [PubMed] [Google Scholar]
- 86. Altena E, et al. Reduced orbitofrontal and parietal gray matter in chronic insomnia: a voxel-based morphometric study. Biol Psychiatry. 2010;67(2):182–185. [DOI] [PubMed] [Google Scholar]
- 87. Mander BA, et al. Prefrontal atrophy, disrupted NREM slow waves and impaired hippocampal-dependent memory in aging. Nat Neurosci. 2013;16(3):357–364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. McDonald CR, et al. ; Alzheimer’s Disease Neuroimaging Initiative. Relationship between regional atrophy rates and cognitive decline in mild cognitive impairment. Neurobiol Aging. 2012;33(2):242–253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Blennerhassett R, et al. Distribution of pathology in frontal variant Alzheimer’s disease. J Alzheimers Dis. 2014;39(1):63–70. [DOI] [PubMed] [Google Scholar]
- 90. Dickerson BC, et al. ; Alzheimer’s Disease Neuroimaging Initiative. Dysexecutive versus amnesic phenotypes of very mild Alzheimer’s disease are associated with distinct clinical, genetic and cortical thinning characteristics. J Neurol Neurosurg Psychiatry. 2011;82(1):45–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Spira AP, et al. Self-reported sleep and β-amyloid deposition in community-dwelling older adults. JAMA Neurol. 2013;70(12):1537–1543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Liguori C, et al. Orexinergic system dysregulation, sleep impairment, and cognitive decline in Alzheimer disease. JAMA Neurol. 2014;71(12):1498–1505. [DOI] [PubMed] [Google Scholar]
- 93. Villafuerte G, et al. Sleep deprivation and oxidative stress in animal models: a systematic review. Oxid Med Cell Longev. 2015;2015:234952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Holth J, et al. Sleep in Alzheimer’s disease - beyond amyloid. Neurobiol Sleep Circadian Rhythms. 2017;2:4–14. [DOI] [PMC free article] [PubMed] [Google Scholar]