Abstract
Background
Vaginal rings (VR) containing antiretroviral (ARV) drugs can be utilized for prevention of human immunodeficiency virus (HIV) with potential for improved adherence compared to daily pills. Combination ARV VRs could improve efficacy.
Methods
MTN-027, a single-blind, randomized, placebo-controlled trial in 48 women, evaluated VRs containing MK-2048 (30 mg) and vicriviroc (VCV, 182 mg), alone or in combination, and placebo used continuously for 28 days. Safety was assessed by recording adverse events. Drug concentrations were quantified in plasma, vaginal fluid, cervical tissue, and rectal fluid. Cervical tissue was utilized for ex vivo HIV inhibition analysis.
Results
There was no difference in related genitourinary adverse events between treatment arms compared to placebo. VCV and MK-2048 released from single or combination VRs both achieved peak concentrations in vaginal fluids, which were substantially higher compared to plasma (200× for VCV, 30× for MK-2048) and rectal fluid. In an ex vivo challenge assay, the antiviral activity of VCV and/or MK-2048 was not correlated with tissue-associated drug concentrations. Most women (77%) were fully adherent to 28 days of continuous VR use and found the VR acceptable.
Conclusions
VCV and/or MK-2048 containing VRs were safe and acceptable. Both VCV and MK-2048 were quantifiable in all matrixes tested with peak compartmental drug concentrations similar for single and combination drug VRs. Tissue-associated VCV and/or MK-2048 did not correlate with inhibition of HIV infection. These data highlight the need to assess adequacy of drug dosing in the VR and measuring genital tissue drug concentrations to develop more precise concentration-response relationships.
Keywords: intravaginal ring, microbicide, HIV prevention, vicriviroc, MK-2048
This study evaluated vaginal rings containing vicriviroc and MK-2048 alone or in combination. The rings were found to be safe and acceptable. Although both drugs were quantifiable in all matrixes tested, HIV inhibition was not demonstrated in cervical tissue.
(See the Major Articles by Chen et al on pages 1144–51 and Liu et al on pages 1129–35.)
Vaginal rings (VRs) have served as an effective delivery method for contraception and hormone replacement [1, 2]. More recently, VRs impregnated with antiretroviral (ARV) compounds have been demonstrated safe and effective in preventing the sexual transmission of human immunodeficiency virus (HIV) to women [3, 4]. Topical microbicides and systemic options for preexposure prophylaxis (PrEP) offer women multiple potential strategies to prevent sexual HIV acquisition [5, 6]. Two phase 3 trials of a dapivirine VR in Africa have demonstrated efficacy (27–31% reduction in HIV incidence) with additional analyses indicating higher efficacy with consistent adherence [3, 4, 7].
The design of a combination microbicide VR is based on strong clinical rationale for combining ARV drugs with different mechanisms of action to increase the breadth of protection against ARV-resistant HIV strains as well as potentially protect against emergence of resistant HIV viral strains. Among phase 3 dapivirine VR clinical trial participants who acquired human immunodeficiency virus type 1 (HIV-1) infection, relevant resistance mutations were not detected at a higher frequency than placebo recipients, but this remains a theoretical concern [3].
Vicriviroc (VCV) is a CCR5-receptor antagonist that is potent against CCR5-tropic viruses, the predominantly transmitted HIV-1 strain [8–10]. MK-2048 is an HIV-1 integrase inhibitor that is highly potent against both wild-type HIV-1 and raltegravir-resistant isolates [11, 12]. Preliminary studies indicate additive antiviral activity of the 2 compounds when tested at equimolar concentrations relative to single drug alone [13]. The combination of 2 potent ARV drugs not currently used for HIV-1 treatment could serve as an ideal HIV prevention modality.
The MTN-027 study evaluated the safety, pharmacokinetics (PK), and pharmacodynamics (PD) of VRs containing VCV and/or MK-2048 compared to placebo.
METHODS
MTN-027 was a multisite, single-blind, 4-arm, randomized, placebo-controlled phase 1 clinical trial conducted at the University of Alabama at Birmingham (Birmingham, AL) and the University of Pittsburgh (Pittsburgh, PA). Each site received institutional review board approval.
All study VRs are smooth, flexible, and have an ethylene vinyl acetate copolymer matrix with an outer diameter of 54 mm and a cross-sectional diameter of 4 mm. The MK-2048 VR contains 30 mg of MK-2048, and the combination VR (MK-2048A) contains 182 mg of VCV and 30 mg of MK-2048; both are off-white and opaque in appearance. The VCV VR contains 182 mg of VCV, and the placebo VR contains no compound; both are clear in appearance.
The primary objectives were to assess the safety and PK of the VRs in vaginal fluid, plasma and cervical tissue after 28 days of continuous use, followed by 7 days off product. Safety was evaluated as the proportion of women with related genitourinary adverse events (AEs) and proportion of women with any grade 2 or higher AEs [14, 15]. Secondary objectives were to evaluate acceptability and adherence over 28 days of use. Exploratory objectives included evaluation of HIV inhibitory activity in cervical tissue obtained on day 28 using the HIV ex vivo challenge assay, concentrations of VCV and MK-2048 in rectal fluid in a subset of participants, and evaluation of the relationship between participant self-report of adherence with VCV and MK-2048 remnant content in returned VRs.
Eligible women were 18–45 years of age, HIV-negative, sexually abstinent, and using effective contraception. Major exclusion criteria included: receipt of pre- or postexposure HIV prophylaxis in the past 6 months; pregnant or breastfeeding; hepatitis B or C infected; urinary, reproductive tract or sexually transmitted infection requiring treatment; and clinically apparent gynecological abnormalities.
After providing written informed consent and undergoing a screening evaluation, eligible participants were randomized in a 1:1:1:1 ratio to a VR. At the enrollment visit, women were provided instructions for VR self-insertion prior to a pelvic examination to confirm placement. Blood and vaginal fluid were obtained at 1, 2, 4, and 6 hours after VR insertion at enrollment and on day 28 after VR removal for PK assessment. At all other visits (days 1, 2, 3, 7, 14, 21, 29, 30, 31, and 35) blood and vaginal fluid were obtained at a single time point for PK assessment. Participants self-collected a vaginal swab to obtain vaginal fluid using a standardized protocol. The enrollment visit was scheduled to avoid menses in the first 7 days of the study. Tampon use was not allowed 24 hours prior to a study visit. Blood and urine was obtained for safety labs and pregnancy testing, respectively. Cervical tissue biopsies were collected for PK and PD assessment immediately after VR removal; the PK biopsy was frozen at -80° C, and the PD biopsy was transported to the laboratory. The used VRs were assessed for remnant VCV and MK-2048 concentrations. A subset of participants had rectal fluid collected via a sponge at enrollment and day 28. Behavioral assessments were performed via computer assisted self-interviews (CASI) at enrollment, days 7, 14, 21, 28, and at the final visit. Each participant underwent an open-ended in-depth interview at the final study visit.
VCV and MK-2048 concentrations in plasma, vaginal fluid, rectal fluid, and cervical tissue were quantified via validated liquid chromatographic-tandem mass spectrometric assay using human EDTA plasma matrix. The analytical range was 25 pg/mL to 50000 pg/mL and the lower limit of quantitation was 25 pg/mL. Vaginal fluids were extracted from swabs using 1 mL of human EDTA plasma. This plasma-vaginal fluid extract was further diluted (10× to 106×) with human EDTA plasma to achieve final concentrations in the 25 pg/mL to 50000 pg/mL assay range. Rectal fluid was also extracted from the sponge with EDTA plasma. Cervical tissue samples were digested with 1 mL of blank 70% Lithium Heparin plasma: 30% Collagenase 1a 5.0mg/mL solution. Following homogenization, the sample was diluted with human EDTA plasma to achieve a concentration within the analytical range of the assay.
Cervical tissue samples were used for the ex vivo HIV challenge assay [16]. Cervical biopsies were exposed to HIV-1BaL for 2 hours, washed, and placed in culture. On day 1, the biopsies were removed from culture and weighed. Supernatant was collected and replenished on days 4 and 7 of culture. HIV-1 replication was monitored in the supernatant using a p24gag ELISA (Alliance, Perkin-Elmer, Waltham, MA). Data from participants using VCV-only, MK-2048-only, and MK-2048A VRs were combined for the PK/PD analysis. Nondetected VCV and MK-2048 (ng/mL) were imputed at ½ the median tissue weight LLOQ of 50 and 200 for VCV and MK-2048, respectively. The non-detected p24 at days 4, 7, and 11of the ex vivo assay were imputed as 15 pg/mL. All p24 data were adjusted for biopsy weight and cumulative p24 (sum of weight adjusted on days 4, 7, and 11) was log10 transformed and entered into a linear, least squares regression, to model PK endpoints at day 28. A statistically significant negative slope supported the finding of drug-mediated virus suppression.
Remnant drug analysis was performed on VRs removed on day 28. VRs were sanitized, weighed, and cut into 1 mm sections. Each VR section was weighed and transferred to a flask containing 50 mL of tetrahydrofuran and shaken for 4 hours at room temperature. Methanol was added to the tetrahydrofuran mixture, and the supernatants were centrifuged. The VCV and MK-2048 concentrations were determined by reverse phase HPLC analysis using a reference standard comparison.
Demographic and adherence data were analyzed using descriptive statistics. For the primary AE end points, the proportion of participants who experienced at least 1 AE was compared between the 4 study arms using Fisher exact test. For the primary PK end points, descriptive statistics were used to characterize the following PK parameters: area under the concentration-time curve to end of study (AUC(0-t) ), peak concentration (Cmax), time to peak concentration (Tmax), steady state concentration at day 28 (Css28), and terminal decay half-life. AUC(0-t) was calculated using the linear trapezoidal method with the final time point (t) being 1 week after VR removal. AUC(0-t) and Cmax were compared between study arms for each anatomic site using Wilcoxon-Mann-Whitney rank-sum tests.
RESULTS
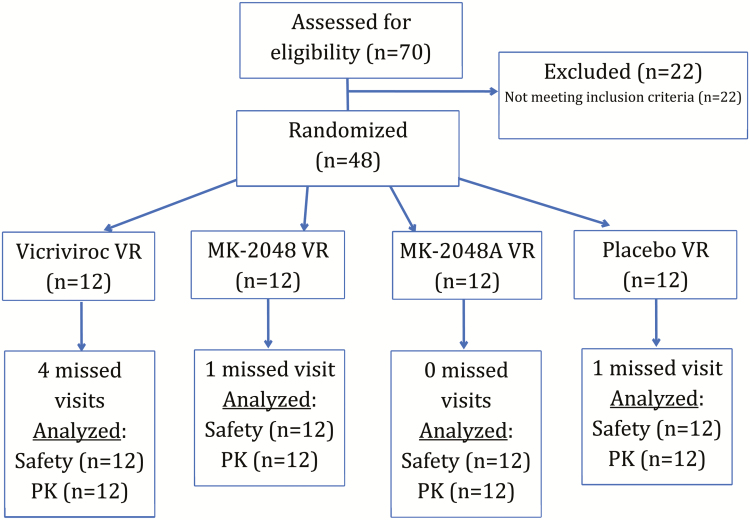
The study was conducted between May 2015 and March 2016 and enrolled 48 women from Birmingham (n = 24) and Pittsburgh (n = 24). Participant demographics and study flow are outlined in Table 1 and Figure 1, respectively. All participants completed the study and contributed to the analysis. One participant had study product held for 2 days for use of a prohibited medication.
Table 1.
Demographics of Study Participants in MTN-027 by Study Arm
| Variable | VCV (n = 12) | MK-2048 (n = 12) | MK-2048A (n = 12) | Placebo (n = 12) |
|---|---|---|---|---|
| Age (years) | 30.4 (6.4) | 29.9 (7.7) | 30.3 (7.9) | 32.0 (6.2) |
| Race | ||||
| Black or AA | 33% (4) | 42% (5) | 25% (3) | 42% (5) |
| White | 67% (8) | 50% (6) | 75% (9) | 58% (7) |
| Married | 25% (3) | 25% (3) | 17% (2) | 42% (5) |
| Live with Partner | 42% (5) | 25% (3) | 33% (4) | 42% (5) |
| Attended college | 83% (10) | 75% (9) | 100% (12) | 100% (12) |
| Earn own income | 92% (11) | 92% (11) | 100% (12) | 100% (12) |
Statistics reported as mean (standard deviation) or percent (number of participants).
Abbreviations: AA, African American; VCV, vicriviroc.
Figure 1.
Flowchart of participants.
Thirteen grade 1 and 2 grade 2 related genitourinary AEs were observed in 9 women. Of the grade 1 AEs, vaginal burning, pruritis, and application site erythema and pain were noted. Both grade 2 related genitourinary AEs were due to vulvovaginal candidiasis. There were no statistically significant differences in the number of participants with related genitourinary AEs or any grade 2 AEs between the placebo arm and treatment arms. There were no grade 3 or higher AEs.
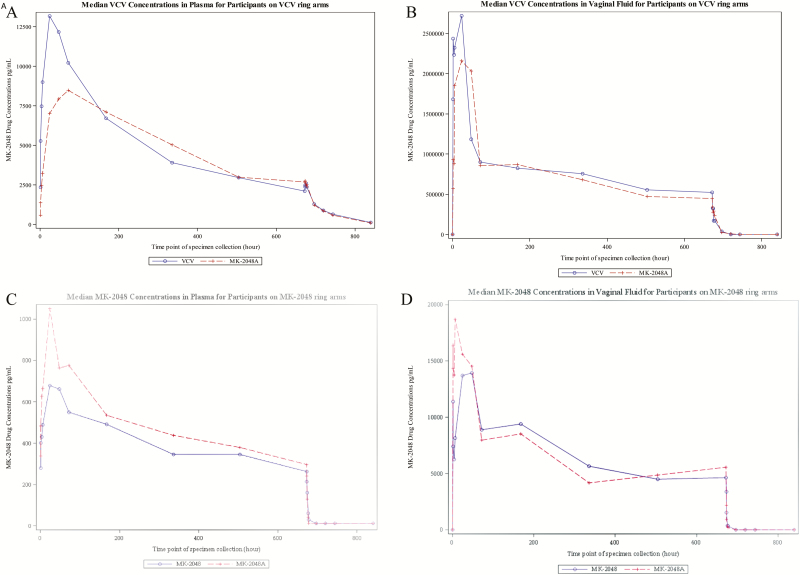
Plasma VCV concentration with the VCV-alone VR achieved peak concentrations more rapidly when compared to the combination VR (MK-2048A) (Tmax median 25 and 59 hours, respectively) followed by a similar decline in concentration after VR removal (median terminal decay half-life of 52 and 50 hours, respectively) (Table 2, Figure 2A). The plasma AUC(0-t) and Cmax of the VCV-aloneVR compared to the MK-2048A combination VR did not differ significantly (P = .63 and P = .11, respectively).
Table 2.
Summary of Pharmacokinetic Parameters at Sampled Anatomic Sites [Median (Interquartile Range)]
| Plasma | Vaginal Fluid | Cervical Tissuec | ||||||
|---|---|---|---|---|---|---|---|---|
| VCV | VCV VR (n = 12) | MK-2048A VR (n = 12) | P-value | VCV VR (n = 12) | MK-2048A VR (n = 12) | P-value | VCV VR (n = 12) | MK-2048A VR (n = 12) |
| AUC (0-t) a | 3864335 (2578547 –5359461) |
3570368 (2748278 –4621145) |
.63 | 600268558 (293962065 –829748742) |
543654441 (234680931–842340205) |
.76 | ||
| C max b | 13008 (9475–20957) |
9555 (6176–12375) |
.11 | 3650711 (1845789–6124672) |
2571520 (1891537–3502415) |
.22 | ||
| C ss 28 b | 1915 (1447–2838) |
6549 (4664–9738) |
488267 (273040–647195) |
803093 (125908–935048) |
13.5 | 15.2 | ||
| T max , hr | 25 (23–50) |
59 (26–77) |
4 (2–14) |
24 (6–28) |
||||
| Half-life^, hr | 52 (48–76) |
50 (39 -61) |
6 (2–29) |
3 (1 -6) |
||||
| MK-2048 | MK-2048 VR (n = 12) | MK-2048A VR (n = 12) | P-value | MK-2048 VR (n = 12) | MK-2048A VR (n = 12) | P-value | MK-2048 VR (n = 12) | MK-2048A VR (n = 12) |
| AUC (0-t) a | 277172 (259460–363548) |
368449 (285376–497953) |
.18 | 5609466 (2505206–8171682) |
5148507 (2453593–9037498) |
.93 | ||
| C max b | 728 (591–903) |
1191 (748–1744) |
.08 | 27398 (18526–40980) |
29336 (20361–35526) |
.84 | ||
| C ss 28 b | 312 (262–368) |
435 (410–547) |
3859 (2560–7818) |
7102 (4799–11711) |
0.50 | 0.19 | ||
| T max , hr | 27 (24–48) |
47 (27–62) |
22 (2–38) |
6 (2–38) |
||||
| Half-life, hr | 3 (2–4) |
2 (2–4) |
2 (1–4) |
2 (1–3) |
||||
P values reflect comparison between the single drug and combination drug vaginal rings using Mann-Whitney U tests with exact significance. AUC, area under the concentration time-curve to 1 week post ring removal; Cmax, peak concentration; Css28, steady state concentration at day 28; Tmax, time to peak concentration ^One participant in the VCV alone arm did not reach half-life. Specimens were not collected for this participant at the final 2 clinic visits.
Abbreviations: VCV, vicriviroc; VR, viral load.
aAUC units are hr-pg/mL for plasma and hr-ng/mL for CVF.
bCmax and Css28 units are pg/mL for plasma, and ng/mL for CVF.
cCervical tissue concentrations are on day 28 (C28) and are presented as median values in ng/mg.
Figure 2.
Median VCV and MK-2048 plasma and vaginal fluid concentrations over time. A, plasma VCV concentrations. B, vaginal fluid VCV concentrations. C, plasma MK-2048 concentrations. D, vaginal fluid MK-2048 concentrations. Abbreviation: VCV, vicriviroc.
The temporal pattern of VCV concentration in vaginal fluid followed a pattern similar to plasma achieving a Tmax median at 4 hours for participants with the VCV-alone VR in contrast to a later peaking Tmax median at 24 hours for the MK-2048A combination VR (Table 2, Figure 2B). Consistent with plasma samples, the AUC(0-t) and Cmax from vaginal fluid samples of the VCV single drug VR compared to the MK-2048A combination VR did not differ significantly (P = .76 and P = .22, respectively). Concentrations for VCV using the day 28 cervical tissue samples were similar between the 2 VR arms (median 13.5 and 15.2 ng/mg, respectively). Cervical tissue VCV concentrations were quantifiable in all participants in the VCV-alone and combination MK-2048A VR arms.
Plasma MK-2048 concentration with the MK-2048-alone VR achieved peak concentrations more rapidly when compared to the combination VR (Tmax median 27 and 47 hours, respectively), followed by a similar decline in concentration after VR removal (median terminal decay half-life of 3 and 2 hours, respectively) (Table 2, Figure 2C). The plasma AUC(0-t) and Cmax of the MK-2048 single drug VR compared to the MK-2048A combination VR did not reach statistical significance (P = .18 and P = .08, respectively).
Vaginal fluid MK-2048 concentrations in the single drug study arm achieved a Tmax median of 22 hours and Cmax median of 27398 ng/mL (Table 2, Figure 2D). This was followed by a rapid fall in concentration after ring removal with median terminal decay half-life of 2 hours. By contrast, vaginal fluid MK-2048 concentrations with the MK-2048A combination VR achieved peak concentration 6 hours after VR placement with median Cmax 29336 pg/mL. This was followed by a 2-hour median terminal half-life after VR removal. The vaginal fluid AUC(0-t) and Cmax of the MK-2048 single drug VR compared to the MK-2048A combination VR was not statistically significant (P = .93 and P = .84, respectively). Concentrations for MK-2048 testing using day 28 cervical tissue samples were similar among the MK-2048 alone and MK-2048A VR arms (median 0.50 and 0.19 ng/mg, respectively). Cervical tissue MK-2048 concentrations were quantifiable in all participants in the MK-2048-alone and MK-2048A combination VR arms.
Thirty of the 48 participants opted into the rectal fluid collection substudy, and 23 of 30 were on an active drug arm. Rectal fluid VCV concentrations from the day 28 samples were quantifiable for all substudy participants on the 2 VCV study product arms (9 in each arm). VCV concentrations did not differ significantly between the VCV single drug and MK-2048A combination VR arms (median 0.07 ng/mg in both arms, P = .96). MK-2048 was detectable in all but 1 of the day 28 rectal fluid samples from participants (5 in MK-2048 single drug arm, 9 in MK-2048A combination arm). The participant with undetectable rectal fluid concentration was in the MK-2048 alone arm. Rectal fluid concentrations were significantly higher in the MK-2048A combination VR arm than in the MK-2048 single drug VR arm (median 0.08 and 0.01 ng/g, respectively) (P = .0075).
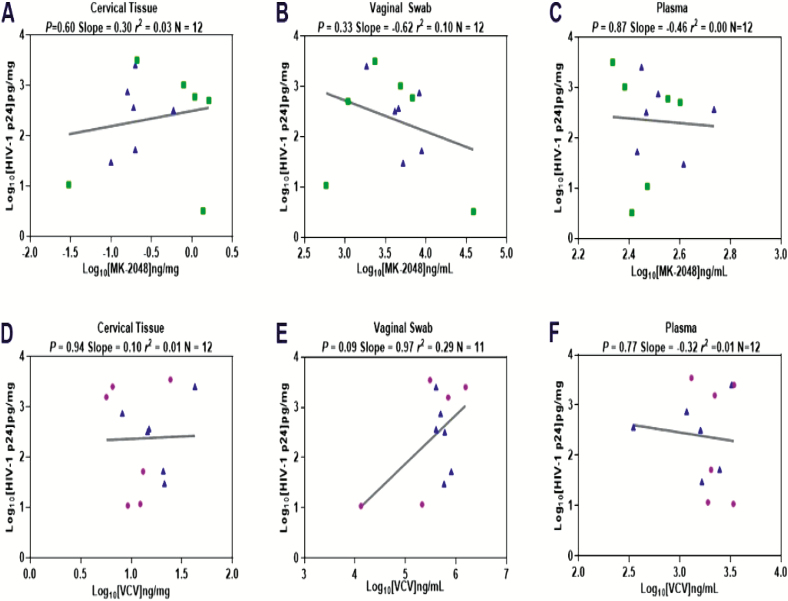
HIV replication as measured by cumulative p24 antigen corrected for tissue weight did not demonstrate a concentration-response relationship between tissue VCV or MK-2048 concentration from VCV, MK-2048, and MK-2048A combination VR users when drug was detectable (Figure 3).
Figure 3.
VCV and MK-2048 related HIV inhibition in cervical tissue, vaginal fluid, and plasma. Abbreviations: HIV, human immunodeficiency virus; VCV, vicriviroc.
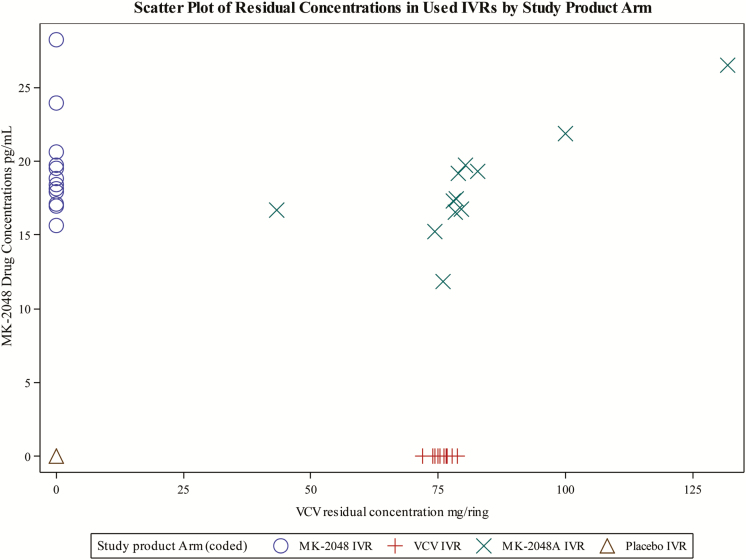
Thirty-seven of 48 (77%) participants enrolled reported being fully adherent to VR use during the 28-day period (ie, the VR was never removed). Of the 11 participants who reported not being fully adherent, 1 received the placebo VR, 1 received MK-2048-aloneVR, 3 received the VCV-alone VR, and 6 received the MK-2048A combination VR. Reasons for nonadherence included menses, tampon use or removal, and urination. In these events, the duration of time the VR was out of the vagina ranged from 1 minute to 21 hours. Remnant drug concentrations in VRs removed after 28 days showed mean residual VCV and MK-2048 concentrations of 76.1 mg and 19.6 mg in the VCV and MK-2048 arms, respectively, representing 41.8% and 65.3% of the loaded doses (Figure 4).
Figure 4.
Remnant VCV and MK-2048 concentrations in used vaginal rings. Abbreviation: VCV, vicriviroc.
During CASI sessions, participants were queried about 4 specific elements: ease of VR use, VR insertion or removal issues, and overall comfort with the VR. Seven of 47 (15%) participants reported at least 1 negative response to these queries over the entire study, including 1 woman in the VCV-alone arm, 2 women in the MK-2048-alone arm, and 4 women in the MK-2048A combination arm. There were no statistically significant differences between treatment and placebo arms based on this combined acceptability measure.
CONCLUSIONS
In this study of combination and single drug VRs containing VCV and/or MK-2048, the VRs were safe and well tolerated over 28 days with good adherence in healthy, sexually abstinent HIV-uninfected women. There were only 15 related genitourinary adverse events (13 grade 1, 2 grade 2) in the study and no statistically significant differences in the number of participants with related genitourinary or any AE between the placebo arm and the treatment arms.
VCV and MK-2048 were detectable in plasma, vaginal fluid, and rectal fluid. When released from the single drug or combination VR, both VCV and MK-2048 achieved peak concentrations in vaginal fluids, which were substantially higher when compared to plasma (200× for VCV, 30× for MK-2048). Both drugs achieved peak concentrations in vaginal fluids rapidly although the median Tmax in vaginal fluid was greater for VCV in the MK-2048A combination VR arm (4 vs 24 hours). Conversely, the median Tmax in vaginal fluid was less for MK-2048 in the MK-2048A combination VR arm (22 vs 6 hours). Assuming the genital tract concentrations achieved are protective against HIV infection, this rapid rise in VCV and MK-2048 concentrations suggests effectiveness within hours of VR placement. The presence of substantially lower drug concentrations in plasma when compared to vaginal fluids suggests less risk for systemic toxicity. In a subset of women, rectal fluid was assessed and both VCV and MK-2048 were detected at low concentrations (median <0.1 ng/mg) suggesting that drug penetration into the rectal compartment may not confer protection [17].
Steady state concentrations of VCV and MK-2048 in cervical tissue obtained at day 28 were similar in the single drug and combination VR arms, but median VCV concentrations were 20 to 80 times higher than MK-2048 concentrations. Tissue-associated VCV and/or MK-2048 did not inhibit HIV infection in the ex vivo challenge assay in a concentration-dependent manner. The lack of HIV inhibitory activity for MK-2048 cervical tissue samples may correlate with insufficient drug penetration or possible drug transport out of tissue [18]. The estimated in vitro IC50 for M-2048 is 0.14 ng/mg and the MK-2048 steady state concentration in cervical tissue ranged from 0.13 to 1.20 ng/mg, suggesting that not all tissue specimens had an adequate drug concentration for ex vivo HIV inhibition [11]. The median cervical tissue VCV steady state concentration at day 28 was approximately 10-fold above the in vitro IC90, but ex vivo HIV inhibition could not be demonstrated suggesting the possibility of drug loss from tissue during the transport or culturing period, which could have contributed to no apparent PD activity [19]. In another study evaluating VRs containing dapivirine and/or maraviroc, cervical tissues acquired from maraviroc VR users also did not show a concentration-response relationship with HIV replication and very little maraviroc was detected in the tissues [20]. Measuring drug concentrations in tissues as they are processed in the ex vivo challenge assay is warranted to more accurately link to HIV replication [21]. Future studies are needed to determine the optimal drug release and concentration profiles of VCV and MK-2048 needed to achieve protection from HIV acquisition.
The majority (77%) of women reported being fully adherent to use of the VR during the 28-day period. This level of adherence is consistent with what has been described in similar duration Phase 1 studies of candidate microbicide VRs in the United States and Africa [22, 23]. In a postmarket assessment of the NuvaRing®, 20.4% of women reported at least 1 expulsion during any 3-week use period, which is consistent with our findings [24]. The proportion of remnant drug in VRs collected at the end of the study was expected based on prior in vitro analysis of drug release with this VR formulation and suggests the VRs were used by participants as instructed [13].
Because this was a PK and safety study, participants were asked to abstain from sexual activity throughout the study. Despite these restrictions that limit generalizability, study retention and self-reported ring adherence were good. It is unclear whether vaginal intercourse and /or presence of semen will affect PK parameters in vaginal fluids or genital tissue.
In summary, VCV and/or MK-2048 containing VRs were safe and well tolerated in women after 28 days of use. Both VCV and MK-2048 were detectable in plasma, vaginal fluid, cervical tissue, and, to a lesser extent, rectal fluid. Peak compartmental drug concentrations were similar for single drug and combination VRs. Neither VCV, MK-2048, or the combination of the drugs delivered by a VR for 28 days provided dose-dependent inhibition of HIV infection ex vivo in cervical tissue. These data highlight the need to assess the adequacy of drug dosing in the VR and to measure genital tissue drug concentrations to develop more precise concentration-response relationships.
Notes
Acknowledgments. The authors would like to thank all the study participants who participated in this trial.
Funding. The study was designed and implemented by the Microbicide Trials Network (MTN). The MTN is funded by the National Institute of Allergy and Infectious Diseases (UM1AI068633, UM1AI068615, UM1AI106707), with co-funding from the Eunice Kennedy Shriver National Institute of Child Health and Human Development and the National Institute of Mental Health, all components of the U.S. National Institutes of Health. Merck and Co. was a pharmaceutical collaborator on this study.
Potential conflicts of interest. C. S. D., C. S., F. H., J. B., and J .P. have no conflict. A. M. reports grants from the National Institutes of Health (NIH) to FHI 360. B. A. C. is a consultant and has received research funds from Merck and Co and grants from NIH National Institute of Allergy and Infectious Diseases (NIAID). C. J. H. reports grants from NIAID. H. G. reports grants from the NIH Division of AIDS. P. L. A. has received research funds from Gilead Sciences, Inc. and reports grants from NIH through Magee Research in Pitts. J. S. is an employee of Merck and Co. She reports nonfinancial support from National Institute of Health-Division of AIDS. N. R. H. has received statistical consultant fees from Magee-Women’s Research Institute, Pittsburgh, PA. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Mishell DR Jr, Lumkin ME. Contraceptive effect of varying dosages of progestogen in silastic vaginal rings. Fertil Steril 1970; 21:99–103. [DOI] [PubMed] [Google Scholar]
- 2. Kiser PF, Johnson TJ, Clark JT. State of the art in intravaginal ring technology for topical prophylaxis of HIV infection. AIDS Rev 2012; 14:62–77. [PubMed] [Google Scholar]
- 3. Nel A, van Niekerk N, Kapiga S, et al. ; Ring Study Team Safety and efficacy of a dapivirine vaginal ring for HIV prevention in women. N Engl J Med 2016; 375:2133–43. [DOI] [PubMed] [Google Scholar]
- 4. Baeten JM, Palanee-Phillips T, Brown ER, et al. ; MTN-020–ASPIRE Study Team Use of a vaginal ring containing dapivirine for HIV-1 prevention in women. N Engl J Med 2016; 375:2121–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Van Damme L, Corneli A, Ahmed K, et al. ; FEM-PrEP Study Group Preexposure prophylaxis for HIV infection among African women. N Engl J Med 2012; 367:411–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Montgomery ET, van der Straten A, Chitukuta M, et al. ; MTN-020/ASPIRE Study Acceptability and use of a dapivirine vaginal ring in a phase III trial. AIDS 2017; 31:1159–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Brown E, Palanee-Philips T, Marzinke M, et al. Residual dapivirine ring levels indicate higher adherence to vaginal ring is associated with HIV-1 protection. In: AIDS 2016; Durban, South Africa. [Google Scholar]
- 8. Merck & Co. Inc. Investigator’s Brochure: Vicriviroc (SCH 417690) Maleate. 17 August 2010. [Google Scholar]
- 9. Crawford KW, Li C, Keung A, et al. ; ACTG A5211 Study Team Pharmacokinetic/pharmacodynamic modeling of the antiretroviral activity of the CCR5 antagonist Vicriviroc in treatment experienced HIV-infected subjects (ACTG protocol 5211). J Acquir Immune Defic Syndr 2010; 53:598–605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Putcharoen O, Lee SH, Henrich TJ, et al. HIV-1 clinical isolates resistant to CCR5 antagonists exhibit delayed entry kinetics that are corrected in the presence of drug. J Virol 2012; 86:1119–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Merck & Co. Inc. Investigator’s Brochure: MK-2048. 15 November 2005. [Google Scholar]
- 12. Van Wesenbeeck L, Rondelez E, Feyaerts M, et al. Cross-resistance profile determination of two second-generation HIV-1 integrase inhibitors using a panel of recombinant viruses derived from raltegravir-treated clinical isolates. Antimicrob Agents Chemother 2011; 55:321–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Merck & Co., Inc. Investigator’s Brochure. MK-2048A (Combination intravaginal ring containing vicriviroc and MK-2048). August 2014. [Google Scholar]
- 14. Division of AIDS Table for Grading the Severity of Adult and Pediatric Events Version 1.0 Dec. 2004. Clarification August 2009. Available at: http://rsc.tech-res.com/docs/default-source/safety/division-of-aids-(daids)-table-for-grading-the-severity-of-adult-and-pediatric-adverse-events-corrected-v-2-1.pdf?sfvrsn=2. Accessed 24 August 2018.
- 15. Division of AIDS Table for Grading the Severity of Adult and Pediatric Adverse Events Addendum 1 Female Genital Grading Table for Use in Microbicide Studies Available at: http://rsc.tech-res.com/docs/default-source/safety/addendum_1_female_genital_grading_table_v1_nov_2007.pdf?sfvrsn=8. Accessed 24 August 2018.
- 16. Dezzutti CS, Uranker K, Bunge KE, Richardson-Harman N, Macio I, Hillier SL. HIV-1 infection of female genital tract tissue for use in prevention studies. J Acquir Immune Defic Syndr 2013; 63:548–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Cottrell ML, Prince HM, Allmon A, et al. Cervicovaginal and rectal fluid as a surrogate marker of antiretroviral tissue concentration: implications for clinical trial design. J Acquir Immune Defic Syndr 2016; 72:498–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Zhou T, Hu M, Cost M, Poloyac S, Rohan L. Short communication: expression of transporters and metabolizing enzymes in the female lower genital tract: implications for microbicide research. AIDS Res Hum Retroviruses 2013; 29:1496–503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Lu Y, Fuchs EJ, Hendrix CW, Bumpus NN. CYP3A5 genotype impacts maraviroc concentrations in healthy volunteers. Drug Metab Dispos 2014; 42:1796–802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Chen BA, Panther L, Marzinke MA, et al. Phase 1 safety, pharmacokinetics, and pharmacodynamics of dapivirine and maraviroc vaginal rings: a double-blind randomized trial. J Acquir Immune Defic Syndr 2015; 70:242–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Dezzutti CS, Richardson-Harman N, Rohan LC, et al. ; Microbicide Trials Network, MTN-013IPM 026 Protocol Team Pharmacodynamic correlations using fresh and cryopreserved tissue following use of vaginal rings containing dapivirine and/or maraviroc in a randomized, placebo controlled trial. Medicine (Baltimore) 2016; 95:e4174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. van der Straten A, Panther L, Laborde N, et al. Adherence and acceptability of a multidrug vaginal ring for HIV prevention in a phase I study in the United States. AIDS Behav 2016; 20:2644–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Nel A, Bekker LG, Bukusi E, et al. Safety, acceptability and adherence of dapivirine vaginal ring in a microbicide clinical trial conducted in multiple countries in Sub-Saharan Africa. PLoS One 2016; 11:e0147743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Creinin MD, Meyn LA, Borgatta L, et al. Multicenter comparison of the contraceptive ring and patch: a randomized controlled trial. Obstet Gynecol 2008; 111:267–77. [DOI] [PubMed] [Google Scholar]