Abstract
Background
Cryptosporidium is a leading contributor to diarrheal morbidity and mortality in under-5 children worldwide. As there is no vaccine and no effective drug therapy in young children for this infection, preventing infection is critical. We undertook a pilot case-control study to define the extent of person-to-person transmission of cryptosporidiosis within an urban and a rural community in Bangladesh.
Methods
We enrolled 48 case families with a Cryptosporidium-infected child aged 6–18 months. Controls were age- and sex-matched Cryptosporidium-negative children in 12 households. Children and household members were followed for 8 weeks with weekly illness survey and stool testing with quantitative polymerase chain reaction for Cryptosporidium.
Results
In the 24 urban case families, the secondary attack rate was 35.8% (19/53) vs 0% (0/11) in controls (P = .018, χ2 test). In contrast, in the 24 rural case families, the secondary attack rate was 7.8% (5/64) vs 0% (0/21) in controls (P = .19, χ2 test). Genotyping by gp60 demonstrated infection with the same subspecies in 5 families, and evidence of transmission in 2. Serologic response to Cryptosporidium infection was associated with younger age, longer duration of infection, and Cryptosporidium hominis gp60_IbA9G3R2 infection.
Conclusions
In the urban site, the high rate of secondary infection and infection with the same subspecies within families suggests that person-to-person transmission is a major source of Cryptosporidium infection for young children living in this region. Molecular genotyping can be applied to determine transmission of Cryptosporidium in endemic regions. Further work is needed to understand the differences in parasite transmissibility and immunity to different genotypes.
Keywords: Cryptosporidium hominis, Cryptosporidium parvum, Cryptosporidium meleagridis, transmission, diarrhea
In this study of Cryptosporidium transmission, the secondary attack rate of Cryptosporidium was 35.8% in urban households, and family members carried the same Cryptosporidium genotype, suggesting that person-to-person infection may be an important source of infection in children.
Globally, cryptosporidiosis is a leading cause of diarrhea in children, and annually there are 2.9–4.7 million Cryptosporidium-attributable cases in children <2 years of age in sub-Saharan Africa and South Asia [1, 2]. Both diarrheal and subclinical Cryptosporidium infection have been associated with growth faltering and cognitive deficits [3, 4]. Despite the significant public health threat posed by this enteric parasite, there is no effective drug or vaccine for this patient population.
Cryptosporidium is transmitted via fecal–oral spread and outbreaks have been associated with contaminated water supplies [5]. In endemic regions, infection is associated with poor sanitation, poverty, animal rearing, and malnutrition [6]. Recent studies aimed at improving household water and sanitation behaviors in Bangladesh showed reduction in overall diarrhea but did not result in decreased Cryptosporidium infections or reduction in stunted growth, a known consequence of Cryptosporidium [7, 8]. In the absence of effective behavioral and environmental interventions and drug therapies, understanding the factors involved in transmission and acquisition of infection is imperative.
In prior studies, we described Cryptosporidium infection rates as high as 80% during the first 2 years of life [9]. However, the route of transmission remains elusive as Cryptosporidium species can be transmitted through direct or indirect contact (involving contaminated soil, food, or water). Although water contamination is often the cause of Cryptosporidium outbreaks, we hypothesized that in Bangladesh, young children were at greatest risk of infection from household contacts, as >95% of households in this cohort received municipal water, treated by the Dhaka Water and Sewage Authority.
We therefore aimed to understand person-to-person transmission in communities in Bangladesh by tracking the spread of the infection within urban and rural households with young children. The children in these households were of the age range at highest risk for Cryptosporidium infection.
METHODS
Study Design
We designed a case-control study of families in urban and rural Bangladesh (Bangladesh Household Transmission Study), nested within a larger birth cohort study (ClinicalTrials.gov: NCT02764918). Birth cohorts were established at 2 sites in Bangladesh: a periurban slum within Mirpur, and a rural subdistrict located 60 km northwest of Dhaka, in Mirzapur [10]. In the parent study, children were enrolled at birth, and followed by twice-weekly home visits for diarrheal illness until age 2.
Children were monitored for the first Cryptosporidium infection by monthly surveillance stool collection and testing. Children who reached age 6 months without infection were screened on a monthly basis for Cryptosporidium infection using a rapid immunodiagnostic test in the field (Giardia/Cryptosporidium QUIK CHEK, TechLab), and results were confirmed using a Cryptosporidium-specific real-time quantitative polymerase chain reaction (qPCR) assay at the International Centre for Diarrhoeal Disease Research, Bangladesh (icddr,b) Parasitology Laboratory.
Parents of children testing positive for Cryptosporidium were consented for enrollment into the Household Transmission Study. All household members, defined as any individual sleeping under the same roof or eating from the same cooking pot, were consented for enrollment. Written consent was obtained from all participants. Index children testing negative were placed into a pool as potential age- and sex-matched community controls. We enrolled 24 case children and 6 age- and sex-matched controls from each site, and their respective household members.
Surveillance
A baseline demographic survey was collected from each household. Subsequently, all households were visited weekly for 8 weeks, and each subject completed an illness survey querying for diarrhea. A stool specimen was collected from all subjects weekly over 8 weeks. A serum sample was obtained from subjects at weeks 1 and 8.
Laboratory Testing
Stool specimens were transported to the icddr,b laboratory for Cryptosporidium testing using a multiplex qPCR assay [11]. DNA extraction was performed by a modified QiaAmp stool DNA extraction protocol that incorporates a 3-minute bead-beating step to lyse Cryptosporidium oocysts (Qiagen, Valencia, California) [11]. All Cryptosporidium-positive specimens were speciated using the LIB3 assay to distinguish Cryptosporidium hominis from non–C. hominis isolates [12]. The polymorphic region within the gp60 gene was used to genotype Cryptosporidium-positive samples as previously described [9, 13]. Sera was tested for anti-Cryptosporidium immunoglobulin G (IgG), using enzyme-linked immunosorbent assay (ELISA) with whole Cryptosporidium-coated plates (2.5 × 106 oocysts/mL) (Waterborne Inc, New Orleans, Louisiana) [14]. Antihuman horseradish peroxidase conjugate was used, and absorbance was measured on the ELISA reader at 450 nm.
Statistical Analysis
A secondary attack rate was calculated by dividing the number of new infections among household members by the total number of uninfected household members at baseline. A 2-sample t test was used to compare differences in means between continuous variables. The χ2 test was used to compare categorical variables. Multiple linear regression was used to predict association of IgG responses with independent predictors. Statistical analysis was done using Stata version 13.1 software (StataCorp, College Station, Texas).
Ethics Approval
The study was approved by the Research and Ethics Review Committees at the icddr,b and by the Institutional Review Board at the Johns Hopkins University Bloomberg School of Public Health. Parents/guardians of all enrolled children provided written consent.
RESULTS
From August 2015 to February 2017, 60 households were enrolled. This consisted of 24 case families and 6 control families from each site, and included 60 index children and 162 household members. Of the household members, 37% were mothers, 16% were fathers, 28% were siblings, 9% were grandmothers, and the remainder (10%) were grandfathers or other relatives. Of the siblings, 13.8% were aged <5 years.
There were significant differences between the 2 sites (Table 1). The urban site, Mirpur, had a greater percentage of household members testing positive by stool PCR for cryptosporidiosis compared to the rural site, Mirzapur (51% vs 29%; P < .01, t test). Additionally, there was lower maternal education (mean, 4.5 vs 6.4 years; P < .01), more crowding (mean, 4 vs 3 persons/room; P < .01), and higher occurrence of toilet sharing in Mirpur compared to Mirzapur (mean, 3.2 vs 1.1; P = .03).
Table 1.
Characteristics of Households From Mirpur and Mirzapur
| Characteristic | Urban Mirpur | Rural Mirzapur | P Value |
|---|---|---|---|
| No. of case households enrolled | 24 | 24 | |
| No. of household members enrolled | 75 | 87 | |
| No. of children <60 mo of age | 1.2 | 1.2 | .5 |
| % of female case children | 61 | 39 | .12 |
| % of individuals with Cryptosporidium | 51 | 29 | <.01 |
| Years of maternal education, mean (SD) | 4.5 (2.7) | 6.4 (4.0) | <.01 |
| Crowding: No. of persons per room, mean (SD) | 4 (0.7) | 3 (1.0) | <.01 |
| Monthly income (US dollars), mean (SD) | 229 (208) | 241(245) | .86 |
| % of homes with any animal | 38 | 100 | <.01 |
| Cow | 0 | 54.2 | <.01 |
| Goat | 16.7 | 20.8 | <.01 |
| Chicken/duck | 29.1 | 79.2 | <.01 |
| Water source | 100% piped | 100% tube well | |
| % of homes with improved toilet | 79 | 71 | .5 |
| No. of households sharing toilet | 3.2 (4.5) | 1.1 (1.0) | .03 |
Abbreviations: SD, standard deviation; US, United States.
In Mirpur, 18.9% (n = 154) of weekly surveillance stool samples tested Cryptosporidium positive. Of the genotyped samples, 87% were C. hominis and 13% were Cryptosporidium parvum. In Mirzapur, 10% of weekly surveillance stools tested Cryptosporidium positive (n = 94). Of these, 92% were Cryptosporidium meleagridis, and 8% C. hominis. During the study period, all 24 episodes of diarrhea recorded and stool specimens collected (21 in Mirpur and 3 in Mirzapur) occurred in index children. Twenty-one of the 24 (87.5%) diarrheal episodes were Cryptosporidium positive (Mirpur, 18; Mirzapur, 3). In Mirpur, 13 of 18 diarrheal stools were successfully speciated and found to be C. hominis. In Mirzapur, 2 of the 3 diarrheal stools were successfully speciated as C. meleagridis.
Across both sites there were no infections in the control families (0/32). In the 48 case families, the rate of secondary infection was 20.5% (24/117) overall. In Mirpur, the secondary attack rate was 35.8% (19/53) vs a rate of 7.5% (5/64) in Mirzapur (P < .001, χ2 test). In 66% (16/24) of case families in Mirpur, multiple family members had concurrent infections with the index child. In 14 of these 24 case families, a second family member who initially tested negative subsequently became positive during the 8-week follow-up period.
Among Mirpur subjects testing positive for Cryptosporidium, children aged ≤2 years had a significantly longer period of shedding (mean, 4.1 weeks) compared to individuals aged >2 years (mean, 1.7 weeks) (P < .001). Additionally, infants had a higher Cryptosporidium parasite burden (lower cycle threshold [Ct]) vs those >2 years of age (mean Ct, 26.3 vs 29.0; P = .08).
GP60 Genotyping Results
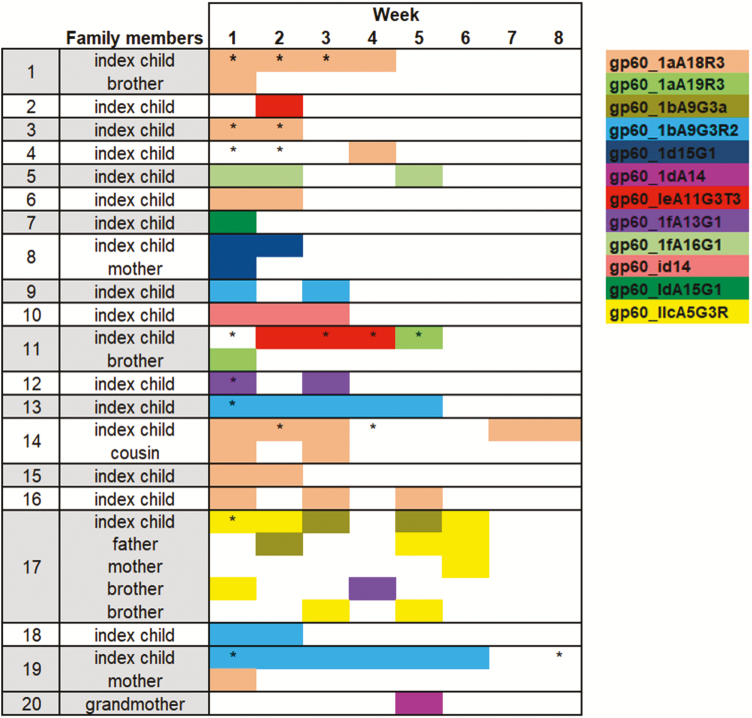
In 8 of 24 Mirpur case families, only the index child tested positive for Cryptosporidium, and in 6 of these the Cryptosporidium isolate was successfully genotyped; the other 2 had low parasite burden and could not be genotyped. Figure 1 depicts the gp60 genotype of these 6 children.
Figure 1.
Cryptosporidium genotypes detected in 20 Mirpur households over the 8-week follow-up period. A box shaded in color indicates that individual tested positive for Cryptosporidium in that week and the genotype was identified. An asterisk (*) indicates the subject had Cryptosporidium-positive diarrhea in that week, and an asterisk without color indicates positive Cryptosporidium detection without identification of genotype. In 6 families, household members were already infected with Cryptosporidium at baseline. Cryptosporidium hominis gp60_1aA18R3 was the most abundant genotype. Families 11 and 17 demonstrate transmission of a novel genotype from a brother and father to the index child. In individuals with multiple genotypes detected in 1 sample, the most abundant genotype is represented here.
In 16 of 24 Mirpur families, the index child and at least 1 family member tested positive for Cryptosporidium. Four of these could not be genotyped and, in 6 additional families, only the index child was successfully genotyped due to low parasite burden in the other family members. However, in 6 of the 16 families, 2 or more individuals in the household were successfully genotyped (Figure 1). Figure 1 depicts the genotypes and weeks of infection in 20 families.
Overall, we detected 12 distinct C. hominis genotypes and 1 C. parvum genotype. Genotype C. hominis gp60_1aA18R3 was the most abundant genotype in this cohort (n = 9), consistent with the 20% prevalence of this genotype described in the parent study [10, 15]. Additionally, we detected a temporal pattern to infection with certain genotypes. Genotype C. hominis gp60_IaA18R3 was present during all 3 years of the study, whereas gp60_IdA15G1 was only detected in 2015, and gp60_IaA19R3 and gp60_IfA13R3 in 2016 (Table 2).
Table 2.
Subjects With Cryptosporidium Genotype and Cryptosporidium Immunoglobulin G Antibody Response
| Date of Enrollment | Family ID | Subject | Age, y | Genotype | Weeks Genotype Detected | Infection in First 4 Weeks | IgG Weeks 8:1 Ratio |
|---|---|---|---|---|---|---|---|
| Jan 2016 | 1 | Index | <1 | Cryptosporidium hominis gp60_IaA18 | 4 | 4 | 1.29 |
| Jan 2016 | 1 | Index | <1 | C. hominis gp60_IaA17 | 2 | 4 | 1.29 |
| Jan 2016 | 1 | Brother | 8 | C. hominis gp60_IaA18 | 1 | 1 | 0.94 |
| Dec 2015 | 2 | Index | 1 | C. hominis gp60_Ie_A11G3T3_V | 1 | 2 | 2.24 |
| Sep 2015 | 3 | Index | 1 | C. hominis gp60_IaA18 | 2 | 4 | 3.47 |
| Sep 2015 | 4 | Index | 1 | C. hominis gp60_IaA17 | 1 | … | 0.79 |
| Sep 2015 | 4 | Index | 1 | C. hominis gp60_IaA18 | 1 | 3 | 0.79 |
| Jan 2016 | 5 | Index | 1 | C. hominis gp60_If_A16G1 | 3 | 4 | 1.34 |
| Sep 2015 | 7 | Index | <1 | C. hominis gp60_IdA15G1 | 1 | 3 | 2.17 |
| Sep 2016 | 8 | Index | <1 | C. hominis gp60_Id15G1 | 2 | 2 | 1.22 |
| Sep 2015 | 8 | Mother | 28 | C. hominis gp60_Id15G1 | 1 | 1 | 1.22 |
| Nov 2015 | 10 | Index | <1 | C. hominis gp60_IdA14 | 3 | 3 | 1.48 |
| Jun 2016 | 11 | Index | 1 | C. hominis gp60_Ie_A11G3T3 | 4 | 4 | 0.95 |
| Jun 2016 | 11 | Brother | 5 | C. hominis gp60_IaA19 | 1 | 1 | … |
| May 2016 | 12 | Index | 1 | C. hominis gp60_IfA13G1 | 2 | 4 | 2.69 |
| Feb 2016 | 13 | Index | 1 | C. hominis gp60_IbA9G3R3_V | 5 | 4 | 4.03 |
| Nov 2015 | 14 | Index | <1 | C. hominis gp60_IaA18 | 5 | 4 | 3.32 |
| Nov 2015 | 14 | Cousin | 2 | C. hominis gp60_IaA18 | 2 | 2 | … |
| Oct 2015 | 16 | Index | <1 | C. hominis gp60_IaA18 | 3 | 4 | 4.89 |
| May 2016 | 17 | Index | 1 | Cryptosporidium parvum gp60_IIcA5G3R2 | 4 | 3 | 1.98 |
| May 2016 | 17 | Father | 32 | C. parvum gp60_IIcA5G3R2 | 3 | 2 | 1.41 |
| May 2016 | 17 | Brother | 5 | C. parvum gp60_IIcA5G3R2 | 2 | 1 | … |
| May 2016 | 17 | Brother | 6 | C. parvum gp60_IIcA5G3R2 | 1 | 3 | … |
| May 2016 | 17 | Mother | 24 | C. parvum gp60_IIcA5G3R2 | 1 | 3 | 1.84 |
| Feb 2016 | 18 | Mother | 30 | Cryptosporidium hominis gp60_IaA18 | 1 | 2 | … |
| Feb 2016 | 19 | Index | <1 | C. hominis gp60_IbA9G3R2 | 6 | 4 | 7.85 |
Included in this table are subjects who had stool samples successfully genotyped and had serum IgG results. Children aged ≤2 years had the most robust increase in IgG from week 1 to week 8. In general, subjects with greater weeks of stool positivity had the most robust response (index children 13, 14, and 19). Cryptosporidium hominis gp60_IaA18 was the most commonly detected in this cohort and was associated with a positive IgG response. Only 1 family had infection with C. parvum gp60_IIcA5G3R2; all individuals tested in this family had a positive serologic response. Index child 11 tested positive for C. hominis gp60_Ie_A11G3T3 for 4 weeks but failed to show a serologic response.
Abbreviations: ID, identifier; IgG, immunoglobulin G.
In families with multiple members genotyped, we observed concurrent infection with the same genotype in 4 of 6 families (families 1, 8, 14, 17) and thus we could not determine directionality of the transmission at entry into the study. In the sixth family (family 19), the index child was persistently positive for 6 weeks with genotype gp60_IbA9G3R2, whereas the mother was positive with gp60_IAa18R3 in week 1, and never transmitted this to the child and did not pick up the child’s genotype despite the persistent shedding. In family 11, the index child was persistently positive with genotype gp60_IeA11G3T3_V in weeks 2–4 of the study, then in week 5 became positive with gp60_1aA19, the same genotype that her 5-year-old brother was shedding in week 1.
Family 17 was the only family in which C. parvum was isolated. In this family, in week 1, both the index child and 6-year-old brother were shedding C. parvum IIcA5G3R2. The index child had a concurrent infection with C. hominis gp60_IbA5G3R3. In week 2, the father became infected with C. parvum IIcA5G3R2, and in week 3, a 5-year-old brother also became infected with the same genotype. The younger brother and father remained infected in week 5, and then in week 6, the mother also became infected with the C. parvum genotype.
Serologic Response
Anti-Cryptosporidium IgG was measured at weeks 1 and 8 in 34 individuals in Mirpur (17 children aged ≤2 years, and 17 subjects aged >2 years). There was a greater mean increase in IgG level from week 1 to week 8 in children ≤2 years vs older subjects (mean difference, 0.82 vs 0.32; P = .016). A higher week 1 IgG level was associated with fewer weeks of Cryptosporidium positivity during the follow-up period when adjusting for age (linear regression; β = –0.85 [95% confidence interval {CI}, –1.5 to –.18]).
The serologic response in week 8 was associated with the number of weeks Cryptosporidium was detected in stool during the 8-week period when adjusting for age (β = 0.11 [95% CI, .0–.23]). Genotype C. hominis gp60_IbA9G3R2 was significantly associated with a high IgG response when adjusting for weeks of positivity and sex (linear regression; β = 5.37 [95% CI, 1.1–9.6]). Genotype C. hominis gp60_IaA18 was the most common genotype isolated and appeared to be consistently associated with a positive serologic response (Table 2). Genotype C. hominis gp60_IeA11G3T3 was seen in 1 index child, and despite the child shedding for 4 weeks, there was no serologic response to this genotype.
DISCUSSION
This is the first study to use molecular diagnostics to evaluate and describe person-to-person transmission of cryptosporidiosis to the subspecies level in an endemic region. Our study describes circulating Cryptosporidium genotypes, duration of infection, and development of a serologic response in the setting of subclinical infection. We found significant differences in the rate of household infection and circulating Cryptosporidium species between the urban and rural site.
We describe a high rate of Cryptosporidium infection among persons of all ages in Mirpur households, similar to that reported in a Brazilian study, where 39% of households had a secondary infection in a household contact [16]. However, after applying gp60 genotyping, we were unable to establish directionality of transmission in a majority of cases, due to our study design. We recruited families based on a child already testing positive for Cryptosporidium at the start of the study, so new infections were only studied in relation to the index child. Genotyping results demonstrated that the index child was not transmitting infection to other family members. In this cohort, genotyping revealed 13 distinct subspecies, and we noted multiple species and subspecies of Cryptosporidium circulating within a household; in 6 households, multiple individuals were infected with the same Cryptosporidium subspecies at entry into the study, which could reflect person-to-person transmission. We noted 2 examples of an older household member, a brother and father, transmitting a novel Cryptosporidium infection to the index child.
Though we were unable to establish directionality of transmission in most households, we noted a high rate of new infections among family members in Mirpur, but not in rural Mirzapur. The low rate of secondary infections in Mirzapur could be explained by differences in predominant species at the different sites. We previously reported C. meleagridis in 90% of samples collected from Mirzapur, whereas C. hominis was the most common species in Mirpur. In the present study, 92% of speciated samples from Mirzapur were C. meleagridis [9, 10]. Avian species have been identified as the natural reservoir for C. meleagridis, and zoonotic transmission has been described from domesticated chickens to humans [17]. In our study, 6 of the 8 families with C. meleagridis infection kept chickens at home, suggesting zoonotic transmission. In contrast, most infections in the urban site were attributable to C. hominis, for which humans are the only natural host. Thus, the differences in rates of infection may be directly due to different transmission modes of predominant Cryptosporidium species in these 2 different regions. Additionally, it is known that the infectious dose of Cryptosporidium can vary from 10 to 1000 oocysts, depending on the species and strain [18, 19]. Thus, it is possible that in Mirpur, the higher secondary attack rate is a direct result of a lower infectious dose of C. hominis vs that of C. meleagridis, which was predominant in the rural site.
Cryptosporidium hominis gp60_1aA18R3 was the most frequently isolated genotype, and mostly noted in concurrently infected individuals in the same household. It is difficult to say whether this genotype is more prevalent because it is environmentally ubiquitous, or because it is more easily transmitted, either due to low infectious dose or other pathogenic characteristics of the parasite.
In 2 households that were completely genotyped, we observed that infection started in 1 individual and then spread to other individuals, which we propose is evidence for person-to-person transmission. In 1 family, we documented transmission from an older sibling to the index child. And in the second family, the only family with C. parvum infection, we documented transmission from children to parents and then to other children. Notably, we did not document any other cases of child-to-adult transmission, presumably because adults had acquired immunity. We have previously documented that C. hominis is the dominant circulating species in Mirpur, and likely most older subjects have been exposed to C. hominis previously in life; in contrast, C. parvum has previously been documented in only 2% of infections in this area [9, 10]. One possibility is that the C. parvum genotype currently isolated was newer to this area and, potentially, even adults had not previously developed immunity. This would explain why the C. parvum IIcA5G3R2 genotype was so easily transmitted between individuals of all ages in this household.
We found that the first Cryptosporidium infection may be the most important in stimulating a serologic response, as children with their first infection had the most robust increase in IgG over the 8-week follow-up period vs older subjects. This is consistent with a study from India, which showed that IgG response peaked 9 weeks after a Cryptosporidium diarrheal episode in children [20]. However, our findings are novel as we detected a serologic response even in subclinical, nondiarrheal infections [21, 22]. We also noted that higher IgG level at baseline was associated with fewer weeks of Cryptosporidium positivity, implying that elevated IgG may be correlated with an effective immune response. In subjects older than 2 years, the average duration of shedding was significantly lower compared with younger children. This likely reflects greater immunity; even if older individuals become infected, they may be better able to control infection and have less shedding of the parasite, as reflected by the lower parasite burden we found in older individuals.
Prior studies have noted immunologic cross-reactivity with infection from different Cryptosporidium species. One study in Bangladesh demonstrated serologic response to a C. parvum gp15 antigen in children with symptomatic C. hominis disease [23]. In our study, we used whole C. parvum oocyst as the antigen in the ELISA, and even though most infections were due to C. hominis, we found a robust IgG response in children infected with C. hominis subtypes. Cryptosporidium hominis gp60_IbA9G3R2 was associated with a particularly strong response. Conversely, in a case with C. hominis gp60_IeA11G3T3, despite persistent infection for 4 weeks, the child failed to demonstrate a serologic response. Our results suggest that there may be species- and subspecies-level cross-reactivity in immunogenic responses in children, which warrants further study.
During this period of intense surveillance, we detected 24 diarrheal episodes, 21 of which were Cryptosporidium associated, and all occurred in index children. This is consistent with our prior study, which found high rates of nondiarrheal Cryptosporidium infection in Bangladesh [9]. There was no association between diarrheal infection and transmission. This highlights the fact that in households with small children, multiple individuals have subclinical Cryptosporidium infection, and even though they lack symptoms, they may be silently shedding the parasite and spreading infection.
Limitations of this study include the sample size, as this was a pilot study. We successfully demonstrated that adults and children within this community could be intensively and longitudinally studied, and our findings can now be followed up in a larger study. Second, we were unable to genotype all Cryptosporidium-positive stool samples, likely due to the low parasite burden in some stool specimens, primarily in adults. Last, we lacked sampling and testing of environmental samples, such as drinking water and soil, which could have helped to establish environmental burden.
Our study offers unique insight into the endemic transmission of cryptosporidiosis, a disease without vaccine or effective therapy in children. Cryptosporidiosis is known to be spread by the fecal–oral route, and our findings of higher prevalence in areas with more overcrowding and toilet sharing are consistent with this paradigm. The recent WASH Benefits Bangladesh Trial, a cluster randomized study of clean water, handwashing, and sanitation intervention, did not find any decrease in cryptosporidiosis in children receiving improved water and sanitation [8]. Another Indian study also failed to show reduction in cryptosporidiosis with clean drinking water [24]. Coupled with these results, our findings suggest that even if water and sanitation interventions achieve high adherence, children still acquire cryptosporidiosis, likely from other infected individuals. Humans are the only natural host for anthroponotic species of Cryptosporidium, so further work is needed to understand determinants of transmissibility of the parasite, including whether age and acquired immunity impact oocyst shedding and infectivity. Ultimately, tackling cryptosporidiosis in endemic regions will require an improved understanding of routes of transmission to young children, as well as concerted efforts at identifying environmental reservoirs of the parasites, and understanding differences in pathogenicity of different species and subspecies.
Notes
Acknowledgments. We thank the children and families of Mirpur and Mirzapur for their participation in this study.
Financial support. This work was supported by the Bill & Melinda Gates Foundation (grant number OPP1127192 to P. K.); the National Institutes of Health (grant numbers K23 AI108790 to P. K. and R01 AI043596 to W. A. P.); and the Sherrilyn and Ken Fisher Center for Environmental Infectious Diseases Discovery Program (to P. D.).
Potential conflicts of interest. W. A. P. has received personal fees from TechLab, Inc. All other authors report no potential conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Kotloff KL, Nataro JP, Blackwelder WC, et al. Burden and aetiology of diarrhoeal disease in infants and young children in developing countries (the Global Enteric Multicenter Study, GEMS): a prospective, case-control study. Lancet 2013; 382:209–22. [DOI] [PubMed] [Google Scholar]
- 2. Sow SO, Muhsen K, Nasrin D, et al. The burden of Cryptosporidium diarrheal disease among children <24 months of age in moderate/high mortality regions of sub-Saharan Africa and South Asia, utilizing data from the Global Enteric Multicenter Study (GEMS). PLoS Negl Trop Dis 2016; 10:e0004729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Checkley W, Gilman RH, Epstein LD, et al. Asymptomatic and symptomatic cryptosporidiosis: their acute effect on weight gain in Peruvian children. Am J Epidemiol 1997; 145:156–63. [DOI] [PubMed] [Google Scholar]
- 4. Guerrant DI, Moore SR, Lima AA, Patrick PD, Schorling JB, Guerrant RL. Association of early childhood diarrhea and cryptosporidiosis with impaired physical fitness and cognitive function four–seven years later in a poor urban community in northeast Brazil. Am J Trop Med Hyg 1999; 61:707–13. [DOI] [PubMed] [Google Scholar]
- 5. Chalmers RM, Elwin K, Thomas AL, Guy EC, Mason B. Long-term Cryptosporidium typing reveals the aetiology and species-specific epidemiology of human cryptosporidiosis in England and Wales, 2000 to 2003. Euro Surveill 2009;14. pii:19086. [DOI] [PubMed] [Google Scholar]
- 6. Mondal D, Minak J, Alam M, et al. Contribution of enteric infection, altered intestinal barrier function, and maternal malnutrition to infant malnutrition in Bangladesh. Clin Infect Dis 2012; 54:185–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Luby SP, Rahman M, Arnold BF, et al. Effects of water quality, sanitation, handwashing, and nutritional interventions on diarrhoea and child growth in rural Bangladesh: a cluster randomised controlled trial. Lancet Glob Health 2018; 6:e302–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Lin A. Effects of interventions on protozoan infections in Bangladesh [abstract 170] . In: American Society of Tropical Medicine and Hygiene conference, Baltimore, MD, 8 November 2017. [Google Scholar]
- 9. Korpe PS, Haque R, Gilchrist C, et al. Natural history of cryptosporidiosis in a longitudinal study of slum-dwelling Bangladeshi children: association with severe malnutrition. PLoS Negl Trop Dis 2016; 10:e0004564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Steiner KL, Ahmed S, Gilchrist C et al. Species of Cryptosporidia causing subclinical infection associated with growth faltering in rural and urban Bangladesh—a birth cohort study [manuscript published online ahead of print 20 April 2018]. Clin Infect Dis 2018. doi: 10.1093/cid/ciy310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Liu J, Kabir F, Manneh J, et al. Development and assessment of molecular diagnostic tests for 15 enteropathogens causing childhood diarrhoea: a multicentre study. Lancet Infect Dis 2014; 14:716–24. [DOI] [PubMed] [Google Scholar]
- 12. Hadfield SJ, Robinson G, Elwin K, Chalmers RM. Detection and differentiation of Cryptosporidium spp. in human clinical samples by use of real-time PCR. J Clin Microbiol 2011; 49:918–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Alves M, Xiao L, Sulaiman I, Lal AA, Matos O, Antunes F. Subgenotype analysis of Cryptosporidium isolates from humans, cattle, and zoo ruminants in Portugal. J Clin Microbiol 2003; 41:2744–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Korpe PS, Liu Y, Siddique A, et al. Breast milk parasite-specific antibodies and protection from amebiasis and cryptosporidiosis in Bangladeshi infants: a prospective cohort study. Clin Infect Dis 2013; 56:988–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Gilchrist CA, Cotton JA, Burkey C, et al. Genetic diversity of Cryptosporidium hominis in a Bangladeshi community as revealed by whole-genome sequencing. J Infect Dis 2018; 218:259–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Newman RD, Zu SX, Wuhib T, Lima AA, Guerrant RL, Sears CL. Household epidemiology of Cryptosporidium parvum infection in an urban community in northeast Brazil. Ann Intern Med 1994; 120:500–5. [DOI] [PubMed] [Google Scholar]
- 17. Silverlås C, Mattsson JG, Insulander M, Lebbad M. Zoonotic transmission of Cryptosporidium meleagridis on an organic Swedish farm. Int J Parasitol 2012; 42:963–7. [DOI] [PubMed] [Google Scholar]
- 18. Okhuysen PC, Chappell CL, Crabb JH, Sterling CR, DuPont HL. Virulence of three distinct Cryptosporidium parvum isolates for healthy adults. J Infect Dis 1999; 180:1275–81. [DOI] [PubMed] [Google Scholar]
- 19. DuPont HL, Chappell CL, Sterling CR, Okhuysen PC, Rose JB, Jakubowski W. The infectivity of Cryptosporidium parvum in healthy volunteers. N Engl J Med 1995; 332:855–9. [DOI] [PubMed] [Google Scholar]
- 20. Ajjampur SS, Sarkar R, Allison G, et al. Serum IgG response to Cryptosporidium immunodominant antigen gp15 and polymorphic antigen gp40 in children with cryptosporidiosis in South India. Clin Vaccine Immunol 2011; 18:633–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. McDonald AC, MacKenzie WR, Addiss DG et al. Cryptosporidium parvum–specific antibody responses among children residing in Milwaukee during the 1993 waterborne outbreak. J Infect Dis 2001; 183:1373–9. [DOI] [PubMed] [Google Scholar]
- 22. Priest JW, Bern C, Xiao L, et al. Longitudinal analysis of Cryptosporidium species-specific immunoglobulin G antibody responses in Peruvian children. Clin Vaccine Immunol 2006; 13:123–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Allison GM, Rogers KA, Borad A, et al. Antibody responses to the immunodominant Cryptosporidium gp15 antigen and gp15 polymorphisms in a case-control study of cryptosporidiosis in children in Bangladesh. Am J Trop Med Hyg 2011; 85:97–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Sarkar R, Ajjampur SS, Prabakaran AD, et al. Cryptosporidiosis among children in an endemic semiurban community in southern India: does a protected drinking water source decrease infection?Clin Infect Dis 2013; 57:398–406. [DOI] [PMC free article] [PubMed] [Google Scholar]