Abstract
Background
Postmenopausal women have unique sociobiological human immunodeficiency virus (HIV) risks. We evaluated the safety, pharmacokinetics, and acceptability of a microbicide dapivirine (DPV) vaginal ring (VR) versus placebo in postmenopausal women.
Methods
We enrolled 96 HIV-negative postmenopausal US women in a phase 2a double-blind, randomized (3:1) trial of monthly VRs containing 25 mg DPV or placebo used continuously for 12 weeks. We assessed safety by adverse events (AEs). DPV concentrations were quantified in plasma and vaginal fluid. Steady-state concentrations were analyzed at 4, 8, and 12 weeks using repeated measures ANOVA. We assessed acceptability by self-report.
Results
We found no differences in the proportion of women with related grade 2 or higher reproductive system AEs (DPV: 6/72 (8%), placebo: 3/24 (13%), P = .68) or grade 3 or higher AEs (DPV: 4/72 (6%), placebo: 0/24 (0%), P = .57). In the DPV arm, 2/72 (3%) declined to resume product use due to AEs. Median DPV concentrations in plasma (262.0 pg/mL at week 12) and vaginal fluid (40.6 ng/mg at week 12) were constant over 12 weeks and exceeded the in vitro 50% effective concentration by 5000-fold in vaginal fluid by week 4. VR acceptability was high; 84/93 (90%) “very much liked or liked” the VR.
Conclusions
DPV VRs were safe, well tolerated, and acceptable in postmenopausal women. Plasma concentrations were comparable to published data on DPV use in reproductive-age women (median plasma concentration: 264 pg/mL). Given the reassuring safety and pharmacokinetic data, the DPV VR is promising for preexposure prophylaxis in postmenopausal women.
Clinical Trials Registration
Keywords: microbicide, pre-exposure prophylaxis, dapivirine, vaginal rings, menopause
Dapivirine vaginal rings are safe, well tolerated, and acceptable in postmenopausal women with dapivirine concentrations comparable to reproductive-age women. Given the reassuring safety and pharmacokinetic data, dapivirine rings show potential as an option for HIV preexposure prophylaxis in postmenopausal women.
(See the Major Articles by Liu et al on pages 1129–35 and Hoesley et al on pages 1136–43.)
In 2015, 36.7 million people worldwide were living with human immunodeficiency virus (HIV), of whom 2.5 million were women aged 50 and older [1]. In the United States (US), 17% of people diagnosed with HIV in 2014 were aged 50 and older, 27% of whom were women [2, 3]. Older women have unique sociobiological risk factors for HIV acquisition. For example, older women may not be using safe sex practices because they do not need condoms for contraception or may find it embarrassing or difficult to obtain condoms [2, 4]. They may have new sexual partners due to relationship changes, for example, divorced or widowed [5].
Postmenopausal women may be at higher biological risk for HIV due to increased CCR5 expression in the cervix and decreased anti–human immunodeficiency virus type 1 (HIV-1) innate activity [6–8]. Reductions in immune mediators such as secretory leukocyte proteinase inhibitor, human β-defensin-2, MIP-3-α, and tumor necrosis factor-α have been observed in cervicovaginal lavages (CVLs) from postmenopausal women, which may be associated with increased risk for HIV acquisition [9]. Urogenital changes such as vaginal atrophy and vaginal dryness could increase possible trauma during intercourse, further increasing the risk of sexually transmitted infection (STI) acquisition [10]. Thus, it is critical to examine HIV prevention methods in postmenopausal women due to their unique risk factors for HIV acquisition.
Prior clinical trials evaluated microbicides, topically-applied products to prevent sexual acquisition of HIV through preexposure prophylaxis (PrEP), in reproductive-age women. Because lower adherence has been associated with higher rates of HIV acquisition in clinical trials [11–13], vaginal rings (VRs) providing sustained drug release are promising for increased adherence compared to products requiring daily dosing.
Dapivirine (DPV), a non-nucleoside reverse transcriptase inhibitor, has been studied in VR, gel, and film formulations to prevent HIV acquisition [13–22]. With the proven effectiveness of DPV rings in reducing HIV acquisition in reproductive-age women [14, 15], the next step includes examining DPV VR use in postmenopausal women. This study evaluated the safety, pharmacokinetics (PK), and acceptability of DPV rings compared to placebo in postmenopausal US women.
METHODS
Study Design
MTN-024/IPM 031 was a phase 2a multisite, double-blind randomized trial evaluating VRs containing 25 mg DPV or placebo in postmenopausal women. Women used monthly rings continuously over 12 weeks, followed by 1 week off-product. We conducted the study at University of Alabama at Birmingham (Birmingham, AL), Case Western Reserve University (Cleveland, OH) and University of Pittsburgh (Pittsburgh, PA). All sites received local institutional review board approval.
The DPV VR is an off-white, flexible ring (56 mm outer diameter × 7.7 mm cross-sectional diameter) containing 25 mg DPV dispersed in a platinum-catalyzed-cured silicone matrix designed for sustained drug release over 28 days. The placebo VR contained the same inactive components with USP titanium dioxide added as a colorant for blinding.
The primary objective was to assess the safety of VRs in postmenopausal women over 12 weeks of use. Safety was evaluated as the proportion of women with grade 2 or higher related genital, genitourinary, and reproductive system adverse events (AEs) and proportion of women with any grade 3 or higher AEs, using the Division of AIDS Table for Grading the Severity of Adult and Pediatric Adverse Events and Addendum 1 (Female Genital Grading Table for Use in Microbicide Studies) [16, 17]. A secondary objective was to assess DPV concentrations in plasma, vaginal fluid (VF), and cervical tissue. Acceptability questions (4-point Likert scale) were collected by Computer Assisted Self-Interview at study end. Exploratory objectives included residual DPV levels in returned VRs, pharmacokinetic/pharmacodynamic (PK/PD) correlations using CVL, and flow cytometric analysis of endocervical cells.
Eligible women were aged 45–65, postmenopausal (defined as amenorrheic for the past 12 months or ≥6 months postbilateral oophorectomy), had a follicle-stimulating hormone level of ≥40 mIU/mL, and in general good health. Major exclusion criteria included: CYP3A inducer and/or inhibitor use; chronic or recurrent candidiasis; significant blood chemistry or hematology abnormalities; STI requiring treatment; clinically apparent grade 2 or higher gynecological abnormalities; or severe pelvic organ prolapse. Women diagnosed with an STI at screening could enroll after completing treatment if all symptoms resolved within the 45-day screening period.
After women provided written informed consent and underwent screening evaluations, we randomized eligible participants to a DPV or placebo VR in a 3:1 ratio. The participants self-inserted the ring, followed by a clinical pelvic exam to check ring placement. Participants returned at weeks 4, 8, and 12 for a visual inspection of the vagina and cervix to assess for epithelial changes [18]. Women received new VRs at weeks 4 and 8. We collected used VRs for residual drug levels at weeks 4, 8, and 12. We assessed ring adherence (ring never out) using an interviewer-administered case report form. Women received follow-up phone calls for AE assessment at weeks 1 and 13.
We obtained plasma for PK at weeks 4, 8, and 12 in all participants (N = 96). We collected CVL for PD at enrollment and weeks 4 and 12. In women with a cervix, we collected endocervical cytobrushes for flow cytometry at enrollment and week 12 (Cleveland and Pittsburgh only). At all sites, we collected VF via swab for PK at weeks 4, 8, and 12 for women opting into a PK subset (n = 45). These women could opt into an intensive-PK subset (n = 15), which involved 2 cervical biopsies for tissue PK immediately after ring removal at week 12. For PK testing, net biopsy weights were recorded, and biopsies were immediately snap frozen and stored at ≤−70°C.
Sample Size and Randomization
The sample size of 96 women (DPV: 72; placebo: 24) was based on the size of similar phase 2a studies of vaginal microbicide products. The 3:1 ratio of DPV to placebo allowed for collection of additional safety endpoints in the drug arm while maintaining blinding between arms. PK endpoints at weeks 4, 8, and 12 (approximately 28 days after each ring was inserted) were intended to represent approximate steady-state concentrations. Behavioral, clinical, and laboratory data collection was conducted as in prior DPV ring studies to allow comparison of DPV PK and other endpoints between postmenopausal women and reproductive-age women from prior studies [19, 20]. The PK and intensive-PK subsets used a sparse sampling approach to provide a richer data set of female genital tract sampling while balancing feasible logistical complexity.
The Statistical Data Management Center generated randomization lists for each site. Each list contained 3 permutated blocks of size 8 for assignment to each study arm. Study sites received sequentially numbered, opaque, sealed envelopes containing prescriptions printed with the corresponding randomization number. VRs were supplied in identical overwrappers. Study staff, participants, and pharmacists were blinded to the random assignments of all participants.
Laboratory Methods
We quantified DPV concentrations in plasma, VF, and CVL using validated, previously described, liquid chromatographic-tandem mass spectrometric (LC-MS/MS) methods [21, 22]. The lower limits of quantification (LLOQ) for DPV in plasma and CVL were 20 pg/mL and 2 ng/mL, respectively. The LLOQs for VF and tissue were 0.25 ng/swab and 0.05 ng/sample, respectively. When normalized to fluid or biopsy weights, median LLOQs were 0.007 ng/mg and 0.004 ng/mg, respectively. Residual drug analysis for DPV was performed as previously described [23]. Samples were analyzed by high performance liquid chromatography.
We tested CVL samples for anti-HIV activity using a TZM-bl assay [6, 24, 25]. CVL samples were clarified by centrifugation, and aliquots were made and stored at ≤-70°C until tested. Two dilutions of each CVL were tested in triplicate along with HIV-1BaL. Anti-HIV activity was measured by luminescent assay (BrightGlo; Promega Corp., Madison, WI). Inhibition was determined based on deviations from the HIV-1-only controls and presented as the percent inhibition. DPV concentrations were quantified in CVL samples for PK/PD analysis.
After endocervical cytobrush collection, mucosal immune cells were isolated by placing cytobrushes into RPMI (with 1.125 μg/mL of amphotericin B and 0.5 mg/mL of piperacillin–tazobactam [Sigma-Aldrich, St. Louis, MO]) and delivered directly to the processing lab or shipped overnight for processing. Cervical mononuclear cells were analyzed as previously described [26]. All data were collected with BD FACSDIVA operating system and analyzed using FlowJo (Tree Star Inc. Ashland, OR).
Statistical Analysis
For the primary AE endpoints, we compared the DPV arm to placebo using Fisher’s exact test. We analyzed demographics, ring adherence, acceptability, and DPV concentrations in plasma, VF, and cervical tissue using descriptive statistics. Mean DPV concentrations in plasma and VF across the 3 time points (weeks 4, 8, and 12) were compared using repeated measures analysis of variance. Median concentrations over time were analyzed using the Jonckheere-Terpstra test. We also evaluated predictors of DPV plasma levels using a generalized estimating equation with an exchangeable correlation matrix, adjusting for repeated measures, with visit, race, and/or mean body mass index (BMI) as predictors.
For the CVL PD, we analyzed anti-HIV activity by arm over time (weeks 0, 4, and 12) using a generalized estimating equations method with an exchangeable correlation structure and robust standard error. The CVL PK/PD correlation was assessed by fitting the Hill equation to the anti-HIV activity (arcsine-transformed) and the DPV concentration in CVL from the participants in the active arm (log-transformed).
For the flow cytometry analysis, we compared baseline and week 12 results using the Wilcoxon signed rank test, and between placebo and DPV for each cell subset using the Wilcoxon rank sum test. We excluded 1 participant in the placebo arm for whom paired data were not available (missed time point). P-value adjustment was performed using the false discovery rate adjustment, and significance was assessed at a 10% threshold.
RESULTS
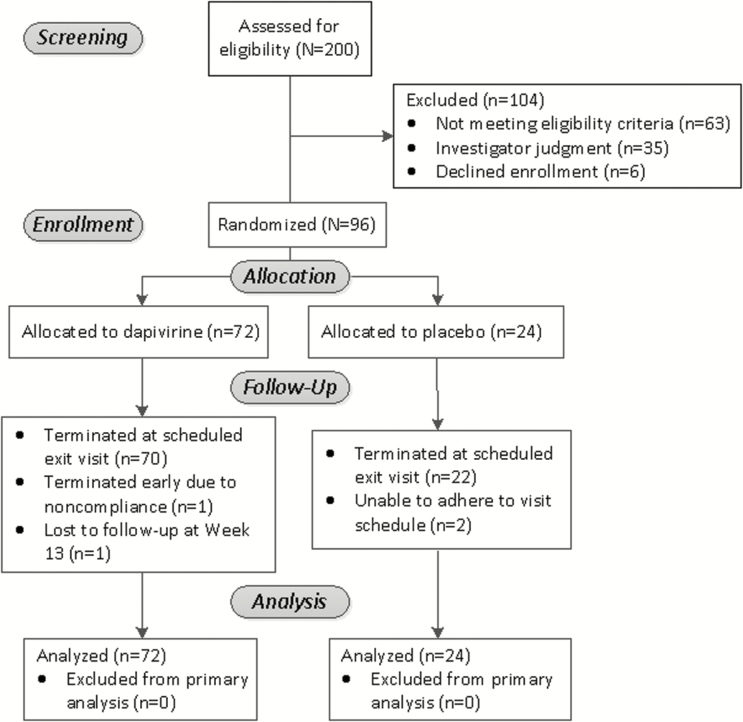
We enrolled 96 women (32 per clinical site) between December 2013 and January 2015. All randomized women were eligible for primary safety outcome analysis (Figure 1). Mean age at enrollment was 56.8 years (standard deviation [SD] 4.1; Table 1). Most women (78/96, 81.2%) underwent spontaneous menopause. For 12 women, age at time of menopause was not evaluable, for example, due to having undergone endometrial ablation or hysterectomy with ovary(ies) in situ. For participants with evaluable menopausal age (n = 84), mean age of menopause was 49.3 (SD 4.8).
Figure 1.
Flowchart of study participants.
Table 1.
Demographics and Study-Related Characteristics of Participants in MTN-024/IPM 031 by Study Arm
| Dapivirine (n = 72) | Placebo (n = 24) | All Arms (N = 96) | |
|---|---|---|---|
| Mean age in years (SD) | 57.2 (4.3) | 55.3 (3.0) | 56.8 (4.1) |
| Age range (min–max) | 46–65 | 50–64 | 46–65 |
| Race | |||
| White | 48 (66.7%) | 15 (62.5%) | 63 (65.6%) |
| Black or African American | 22 (30.6%) | 8 (33.3%) | 30 (31.3%) |
| Other | 2 (2.8%) | 1 (4.2%) | 3 (3.1%) |
| Latina or Hispanic ethnicity | 1 (1.4%) | 0 (0.0%) | 1 (1.0%) |
| Currently married | 27 (37.5%) | 12 (50.0%) | 39 (40.6%) |
| Currently living with partner | 28 (38.9%) | 50 (50.0%) | 40 (41.7%) |
| Any type of sexual activity within 30 days prior to enrollment | 48 (66.7%) | 15 (62.5%) | 63 (65.6%) |
| Age of menopause (years)a | (n = 63) | (n = 21) | (n = 84) |
| <45 years | 8 (12.7%) | 2 (9.5%) | 10 (11.9%) |
| 45–49 years | 21 (33.3%) | 8 (38.1%) | 29 (34.5%) |
| 50–54 years | 27 (42.9%) | 9 (42.9%) | 36 (42.9%) |
| 55+ years | 7 (11.1%) | 2 (9.5%) | 9 (10.7%) |
| Number of participants with completed study visits by week | |||
| Week 1 | 72 (100%) | 24 (100%) | 96 (100%) |
| Week 4 | 72 (100%) | 24 (100%) | 96 (100%) |
| Week 8 | 71 (98.6%) | 24 (100%) | 95 (99.0%) |
| Week 12 | 71 (98.6%) | 22 (91.7%) | 93 (96.9%) |
| Week 13 | 70 (97.2%) | 23 (95.8%) | 93 (96.9%) |
Data are in mean (standard deviation) or n (%) unless otherwise specified.
Abbreviation: SD, standard deviation.
aParticipants with evaluable menopausal age. For some women, age of menopause could not be evaluated, eg, due to having undergone a hysterectomy with ovary(ies) intact or endometrial ablation.
Visit-specific retention was 97% to 100% (Table 1). Four participants did not complete the study. In the DPV arm, 1 participant missed the week 13 phone call, and 1 participant was withdrawn after week 4 due to noncompliance with study protocol due to repeatedly removing the ring for grade 3 vaginal pain without notifying the study team. In the placebo arm, 1 participant missed the week 12 visit but completed the week 13 call, and 1 participant relocated after week 8.
Safety Assessment
A total of 152 AEs were reported for 60/96 (62.5%) participants, including 117 AEs in 46/72 (63.9%) women in the DPV arm and 35 AEs in 14/24 (58.3%) women in the placebo arm. Most AEs (105/152 [69.1%]) were grade 1 and half (73/152 [48.0%]) were deemed related to study product. We found no difference in the proportion of women with related grade 2 or higher genital, genitourinary, or reproductive system AEs (DPV: 6/72 [8.3%], placebo: 3/24 [12.4%], P = .69) or grade 3 or higher AEs (DPV: 4/72 [5.6%], placebo: 0/24 [0%], P = .57). Three grade 3 AEs were deemed unrelated (vomiting, increased blood pressure, multiple sclerosis relapse), and 1 was deemed related (vaginal pain).
There were 6 protocol-required product holds for 5 women (DPV [n = 3]: vaginal abrasion/cervical friability, vulvovaginitis, cervicovaginal erythema; placebo (n = 2): cervical friability, vaginal erythema), all due to AEs that resolved. Two women in the DPV arm declined to resume study product; 1 participant experienced grade 2 cervical friability and grade 1 vaginal abrasion, and 1 participant experienced grade 2 vulvovaginitis.
Pharmacokinetic Analysis
We found no trend in median DPV concentrations in plasma (P = .53) or VF (P = .80) over 12 weeks (Table 2) although high inter-participant variability was observed. We found a slight increase in mean plasma DPV concentrations from 273.5 pg/mL at week 4 to 298.2 pg/mL at week 12 that could indicate a trend over time (P = .022); no difference in mean VF DPV concentrations (P = .56) was found. The VF DPV concentrations exceeded the in vitro 50% effective concentration (EC50) (2 nM) by more than 5000-fold by week 4 and remained constant through week 12 [25]. When evaluating predictors of DPV plasma levels, neither race nor BMI had a significant effect.
Table 2.
Dapivirine Concentrations in Plasma and Vaginal Fluid
| Median [IQR] | Mean (SD; range) | |
|---|---|---|
| Plasma dapivirine (pg/mL) | ||
| Week 4 (n = 69) | 268.0 [213.0, 325.0] | 273.5 (98.2; 62.2–626.0) |
| Week 8 (n = 70) | 287.5 [217.0, 325.0] | 289.0 (123.8; 53.6–972.0) |
| Week 12 (n = 69) | 262.0 [227.0, 351.0] | 298.2 (143.2; 72.8–1200.0) |
| Vaginal fluid dapivirine (ng/mg) | ||
| Week 4 (n = 33) | 33.7 [26.5, 60.8] | 64.3 (65.9; 4.1–283.2) |
| Week 8 (n = 34) | 45.2 [32.2, 78.0] | 78.5 (92.2; 5.2–397.1) |
| Week 12 (n = 33) | 40.6 [21.9, 81.5] | 72.1 (80.0; 1.0–324.3) |
Abbreviations: IQR, interquartile range; SD, standard deviation.
Dapivirine was detectable in only 5 of the 10 women enrolled in the DPV arm who underwent cervical biopsies. The mean DPV tissue concentration was 2.49 ng/mg (range 0.33–10.10 ng/mg), and the median DPV concentration was 0.60 ng/mg (interquartile range [IQR] 0.51, 0.91) in these 5 women. Median biopsy weights were 36% lower for cervical tissue specimens with undetectable DPV concentrations compared to specimens with detectable DPV (11.5 mg vs. 15.6 mg, P = .42). Within the intensive-PK cohort, lack of DPV quantification in tissue did not correlate with lower plasma (P = .69) or VF (P = .15) drug concentrations though power is limited by the small sample size.
Adherence, Residual DPV Levels in Returned VRs, and Acceptability
Most participants in the DPV arm reported being fully adherent (ring never out) during the study: 81% at week 4 and ≥90% at weeks 8 and 12 (Table 3). The median residual DPV level of returned VRs was 21 mg throughout the study (Table 3). For women with undetectable DPV concentrations in cervical biopsies, residual DPV ring levels ranged from 20.5 to 21.8 mg with no difference in median residual DPV levels in women with detectable vs. undetectable cervical tissue DPV (21.4 vs. 20.9 mg, P = .14). VR acceptability at exit was high with 91% stating they “very much liked or liked” the VR, and 65% indicated that they preferred VR to condoms (Table 3).
Table 3.
Adherence to and Acceptability of the Vaginal Ring by Study Arm
| DPV (n = 72) | Placebo (n = 24) | All Arms (N = 96) | |
|---|---|---|---|
| Adherence by self-report | |||
| Week 4 | 58 (80.6%) | 22 (91.7%) | 80 (83.3%) |
| Week 8 | 67 (93.1%) | 22 (91.7%) | 89 (92.7%) |
| Week 12 | 65 (90.3%) | 21 (87.5%) | 86 (89.6%) |
| Median residual DPV levels in VR [IQR] | NA | NA | |
| Week 4 | 21.2 mg [20.8, 21.6] | ||
| Week 8 | 21.1 mg [20.7, 21.5] | ||
| Week 12 | 21.0 mg [20.6, 21.4] | ||
| Overall acceptability of the VRa,b | n = 71 | n = 22 | n = 93 |
| Dislike very much | 0 | 0 | ... |
| Dislike | 6 (8.5%) | 3 (14.6%) | 9 (9.7%) |
| Like | 38 (53.5%) | 12 (54.6%) | 50 (53.8%) |
| Like very much | 27 (38.0%) | 7 (31.8%) | 34 (36.6%) |
| Preference for VR vs condomsb | n = 69 | n = 22 | n = 91 |
| Prefers VR | 42 (60.9%) | 17 (77.3%) | 59 (64.8%) |
| Prefers condoms | 6 (8.7%) | 2 (9.1%) | 8 (8.8%) |
| Likes both | 19 (27.5%) | 3 (13.6%) | 22 (24.2%) |
| Likes neither | 2 (2.9%) | 0 (0%) | 2 (2.2%) |
| Comfort with VRb | n = 72 | n = 22 | n = 94 |
| VR is very easy /easy to use | 71 (98.6%) | 22 (100%) | 93 (98.9%) |
| Is very comfortable /comfortable with VR inside every day | 70 (97.2%) | 21 (95.5%) | 91 (96.8%) |
| Agreed that VR is easy to insert | 62 (86.1%) | 18 (81.8%) | 80 (85.1%) |
| Agreed that VR is easy to remove | 58 (80.6%) | 17 (77.3%) | 75 (79.8%) |
Abbreviations: DPV, dapivirine; IQR, interquartile range; NA, not applicable; VR, vaginal ring.
aMissing 1 answer.
bAsked by computer assisted self-interview at week 12.
Anti-HIV Activity in CVL
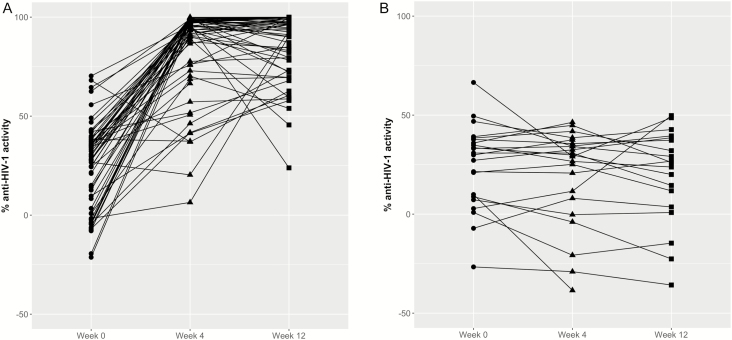
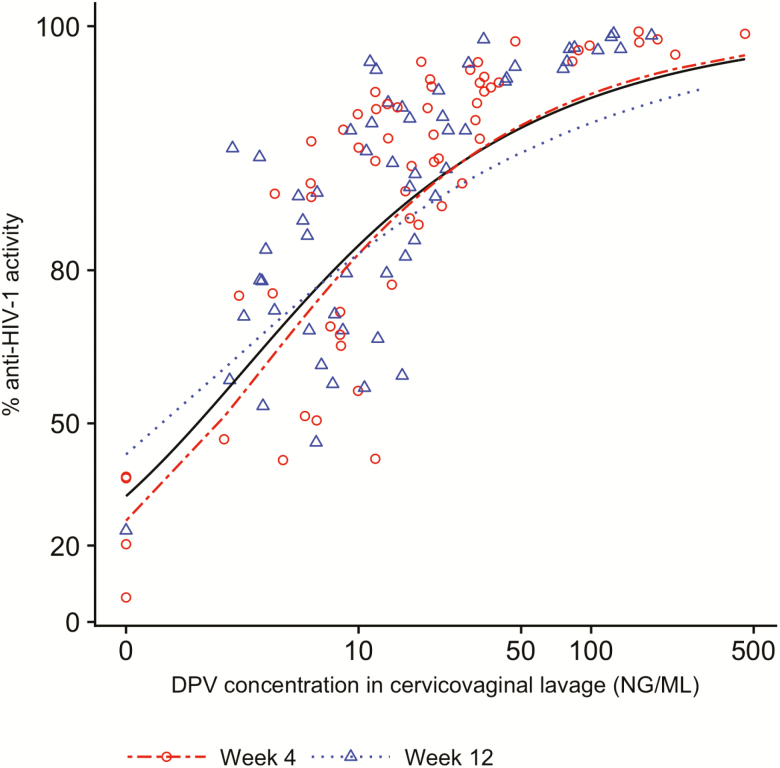
We found a statistically significant difference in anti-HIV activity in CVL between the DPV and placebo ring arms at week 4 (P < .0001) and week 12 (P < .0001) (Figure 2). The mean % anti-HIV activity at weeks 4 and 12 were 76.2 (SD 11.1) and 86.7 (SD 11.7), respectively. PK/PD correlations from CVF showed a statistically significant concentration-response relationship with Hill slope and EC50 parameter estimates of 1.03 (95% confidence interval [CI] 0.79—1.31) and 2.03 ng/mL (95% CI, 1.59—2.50 ng/mL), respectively (pooled weeks 4 and 12 data, Figure 3).
Figure 2.
Anti-HIV activity in cervicovaginal lavage by study arm. A, dapivirine; B, placebo. Abbreviation: HIV, human immunodeficiency virus.
Figure 3.
Pharmacokinetic and pharmacodynamic correlations from cervicovaginal lavage. The fitted curves are the arcsine of % anti-HIV-1 activity vs log10 of drug concentrations. Week 4: Hill slope 1.12, EC50 2.5 ng/mL. Week 12: Hill slope 0.80, EC50 1.44 ng/mL. Overall: Hill slope 1.03, EC50 2.03 ng/mL. Abbbreviation: DPV, dapivirine.
Flow Cytometry Analysis
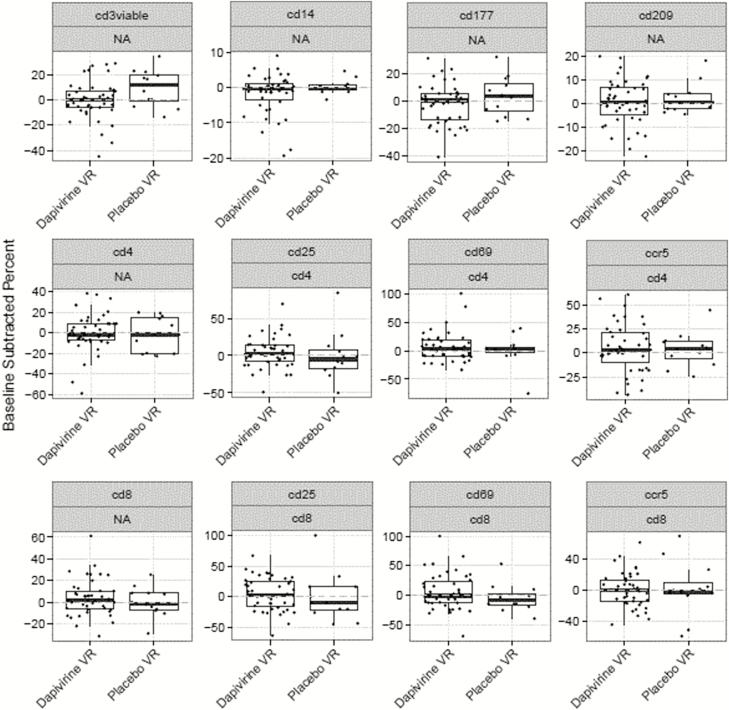
We collected endocervical specimens for flow cytometry in 43 and 13 women in the DPV and placebo arms, respectively. Figure 4 depicts the distribution of the difference in percent cells between week 12 and baseline by study arm, faceted by cell subset and parent. We found no difference for each cell subset between week 12 and baseline in the DPV arm or across arms. CD3+ viable cells showed a significant difference of 8% between the 2 visits in the placebo arm, but this was not statistically significant when adjusting for multiple testing.
Figure 4.
Distribution of the difference in percent cells between week 12 and baseline (week 0) in each treatment arm, faceted by cell subset and parent. Abbreviations: NA, not applicable; VR, vaginal ring.
DISCUSSION
DPV VRs were safe, well tolerated, well used, and acceptable in postmenopausal women. CVL after ring use demonstrated a concentration-response relationship in ex vivo anti-HIV activity. Reassuringly, product use was not associated with any significant change in T-cell phenotype. Specifically, there was no increase in T-cell activation or expression of the CCR5 HIV coreceptor, cell phenotype changes that might be associated with increased risk of HIV infection. With the proven effectiveness of DPV VRs in reducing HIV-1 acquisition in reproductive-age women [14, 15], these findings are promising for DPV ring use for HIV prevention in postmenopausal women.
Although most AEs were mild or moderate, 2 women chose not to continue VR use due to reproductive system AEs. Because participants likely had hypoestrogenic genital tract changes such as vaginal atrophy due to menopause, a multipurpose prevention product such as a vaginal microbicide combined with vaginal estrogen to treat vaginal atrophy could potentially improve product tolerance.
A slight increase in mean plasma DPV concentrations over time was observed. However, there were no temporal differences with regards to median plasma DPV concentrations or mean and median VF DPV concentrations; thus, this finding may be due to outliers in the plasma DPV samples. Despite the wide variability, the mean plasma DPV concentration in this study (273 pg/mL at 28 days) was comparable to the means found in reproductive-age women from prior studies (217.5 to 260 pg/mL at 28 days) [19, 20]. The median plasma DPV concentration of 268.0 pg/mL was also similar to the median DPV concentration in reproductive-age women (264 pg/mL) [15].
In comparison to reproductive-age women where DPV concentrations were quantifiable in all DPV users [20, 27], only half of the cervical tissue specimens in this study had DPV concentrations above LLOQ. However, median biopsy weights were 36% lower for cervical tissue specimens with undetectable DPV; thus, the undetectable DPV concentrations in cervical tissue may be due to small biopsy specimens. Because DPV was detectable in both plasma and VF in women with undetectable DPV cervical tissue concentrations and residual ring DPV content were comparable in women with and without detectable DPV cervical tissue concentrations, clinicians may have been more tentative in obtaining cervical biopsies in postmenopausal women, resulting in smaller specimens that were below LLOQ.
Alternatively, there may be differences in drug permeability or transporters in the vaginas of postmenopausal women resulting in different plasma:tissue concentrations of DPV. For example, postmenopausal women have been found to have decreased ABCB1 (P-glycoprotein) expression in the vagina compared to premenopausal women [28]. Differences in drug transporter expression by phase of estrous cycle have been seen in a mouse model [29]. Changes in uptake or efflux drug transporters may have resulted in our findings of decreased DPV concentrations in cervical tissue; however, the drug transporters involved in DPV distribution are not yet known [30, 31]. Further studies are needed to characterize the drug transporters involved in DPV distribution and biological differences in the postmenopausal genital tract.
Although these responses may be subject to social desirability bias, high rates of adherence to ring use were reported by participants, which were corroborated by residual drug levels comparable to other 25 mg DPV ring studies [14, 15, 27]. Given the reassuring safety and PK data from this study as well as high acceptability and adherence to ring use, the DPV ring is a promising option for PrEP in postmenopausal women.
Notes
Author contributions. The MTN-024/IPM 031 study team includes the following:
Protocol Chair: B. A. Chen, MD, MPH
Birmingham, AL, USA: C. J. Hoesley, MD
Pittsburgh, PA, USA: B. A. Chen, MD, MPH
Cleveland, OH, USA: R. A. Salata, MD
Study Implementation Team: FHI 360
Statistical and Data Center: Statistical Center for HIV/AIDS Research and Prevention
Laboratory: MTN Laboratory Center
Vaginal rings were supplied by IPM.
Acknowledgments. In memory of Charlene Dezzutti, whose expertise and support will be greatly missed. We also gratefully acknowledge the contributions of the study participants.
Disclaimer. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health (NIH).
Funding. MTN-024/IPM 031 was conducted by the Microbicide Trials Network (MTN). MTN is funded by the National Institute of Allergy and Infectious Diseases under award numbers UM1AI068633, UM1AI068615, UM1AI106707, with cofunding from the Eunice Kennedy Shriver National Institute of Child Health and Human Development and the National Institute of Mental Health, all components of the US National Institutes of Health. The vaginal rings used in this study were supplied by the IND sponsor, International Partnership for Microbicides (IPM).
Potential conflicts of interest. A. N. is employed by International Partnership for Microbicides, the supplier of both the placebo and dapivirine vaginal rings. A. V. D. S. reports money from NIH. C. H. has received research support from Gilead Sciences managed through Johns Hopkins University, board membership to Population Council and UCLA. Consultancy fees from Viiv/GSK—past relationship, not active. Grants from NIH, Gates Foundation, and patents for IP not related to this paper. B. C. reports consultancy fees, travel expenses, and grants from Merck. I. M. is the Chief Medical Officer of AELIX Therapeutics and the Chief Scientific Officer of Orion Biotechnology has also received funding from Novicol Health Sciences and ABIVAX. All other authors: No reported conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
Presented in part: Poster presentation at Conference on Retroviruses and Opportunistic Infections 2016, Boston, MA, February 21–25, 2016. Abstract 872 and 873.
Contributor Information
MTN-024/IPM 031 Protocol Team for the Microbicide Trials Network:
B A Chen, C J Hoesley, B A Chen, and R A Salata
References
- 1. UNAIDS. AIDSinfo: Number of people living with HIV Available at: http://aidsinfo.unaids.org/. Accessed 21 September 2016.
- 2. Centers for Disease Control and Prevention. HIV among people aged 50 and over Updated 14 September 2016. Available at: http://www.cdc.gov/hiv/group/age/olderamericans/index.html. Accessed 21 September 2016.
- 3. Centers for Disease Control and Prevention. Diagnoses of HIV infection among adults aged 50 years and older in the United States and dependent areas, 2010–2014. HIV Surveillance Supplemental Report 2016; 21(2). Available at: http://www.cdc.gov/hiv/library/reports/surveillance/. Accessed 21 September 2016. [Google Scholar]
- 4. Lusti-Narasimhan M, Beard JR. Sexual health in older women. Bull World Health Organ 2013; 91:707–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Sherman CA, Harvey SM, Noell J. “Are they still having sex?” STIs and unintended pregnancy among mid-life women. J Women Aging 2005; 17:41–55. [DOI] [PubMed] [Google Scholar]
- 6. Chappell CA, Isaacs CE, Xu W, et al. The effect of menopause on the innate antiviral activity of cervicovaginal lavage. Am J Obstet Gynecol 2015; 213:204 e1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Meditz AL, Moreau KL, MaWhinney S, et al. CCR5 expression is elevated on endocervical CD4+ T cells in healthy postmenopausal women. J Acquir Immune Defic Syndr 2012; 59:221–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Thurman AR, Yousefieh N, Chandra N, et al. Comparison of mucosal markers of human immunodeficiency virus susceptibility in healthy premenopausal versus postmenopausal women. AIDS Res Hum Retroviruses 2017; 33:807–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Jais M, Younes N, Chapman S, Cu-Uvin S, Ghosh M. Reduced levels of genital tract immune biomarkers in postmenopausal women: implications for HIV acquisition. Am J Obstet Gynecol 2016; 215:324.e1–e10. [DOI] [PubMed] [Google Scholar]
- 10. Ghosh M, Rodriguez-Garcia M, Wira CR. Immunobiology of genital tract trauma: endocrine regulation of HIV acquisition in women following sexual assault or genital tract mutilation. Am J Reprod Immunol 2013; 69(Suppl 1):51–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Abdool Karim Q, Abdool Karim SS, Frohlich JA, et al. Effectiveness and safety of tenofovir gel, an antiretroviral microbicide, for the prevention of HIV infection in women.[Erratum appears in Science. 2011 Jul 29;333(6042):524]. Science 2010; 329:1168–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Van Damme L, Corneli A, Ahmed K, et al. ; FEM-PrEP Study Group Preexposure prophylaxis for HIV infection among African women. N Engl J Med 2012; 367:411–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. van der Straten A, Van Damme L, Haberer JE, Bangsberg DR. Unraveling the divergent results of pre-exposure prophylaxis trials for HIV prevention. AIDS 2012; 26:F13–9. [DOI] [PubMed] [Google Scholar]
- 14. Baeten JM, Palanee-Phillips T, Brown ER, et al. ; MTN-020–ASPIRE Study Team Use of a vaginal ring containing dapivirine for HIV-1 prevention in women. N Engl J Med 2016; 375:2121–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Nel A, van Niekerk N, Kapiga S, et al. ; Ring Study Team Safety and efficacy of a dapivirine vaginal ring for HIV prevention in women. N Engl J Med 2016; 375:2133–43. [DOI] [PubMed] [Google Scholar]
- 16. Beksinska M, Smit J, Joanis C, Potter W. New female condoms in the pipeline. Reprod Health Matters 2012; 20:188–96. [DOI] [PubMed] [Google Scholar]
- 17. Chatterjee K, Markham Shaw C. Media portrayals of the female condom. J Health Commun 2012; 17:1138–50. [DOI] [PubMed] [Google Scholar]
- 18.World Health Organization/CONRAD. Manual for the standardization of colposcopy for the evaluation of vaginal products, update 2004. Geneva: World Health Organization, 2004. [Google Scholar]
- 19. Nel A, Haazen W, Nuttall J, Romano J, Rosenberg Z, van Niekerk N. A safety and pharmacokinetic trial assessing delivery of dapivirine from a vaginal ring in healthy women. AIDS 2014; 28:1479–87. [DOI] [PubMed] [Google Scholar]
- 20. Nel AM, Haazen W, Nuttall JP, et al. Pharmacokinetics and safety assessment of anti-HIV dapivirine vaginal microbicide rings with multiple dosing. J AIDS Clin Res 2014; 5:1–10. [Google Scholar]
- 21. Parsons TL, Emory JF, Seserko LA, Aung WS, Marzinke MA. Dual quantification of dapivirine and maraviroc in cervicovaginal secretions from ophthalmic tear strips and polyester-based swabs via liquid chromatographic-tandem mass spectrometric (LC-MS/MS) analysis. J Pharm Biomed Anal 2014; 98:407–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Seserko LA, Emory JF, Hendrix CW, Marzinke MA. The development and validation of an UHPLC-MS/MS method for the rapid quantification of the antiretroviral agent dapivirine in human plasma. Bioanalysis 2013; 5:2771–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Lyndgaard LB, Spångberg R, Gilmour C, Lyndgaard CB, van den Berg F. A process analytical approach for quality control of dapivirine in HIV preventive vaginal rings by Raman spectroscopy. J Raman Spectrosc 2014; 45:149–56. [Google Scholar]
- 24. Dezzutti CS, Rohan LC, Wang L, et al. Reformulated tenofovir gel for use as a dual compartment microbicide. J Antimicrob Chemother 2012; 67:2139–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Dezzutti CS, Yandura S, Wang L, et al. Pharmacodynamic activity of dapivirine and maraviroc single entity and combination topical gels for HIV-1 prevention. Pharm Res 2015; 32:3768–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Herold BC, Chen BA, Salata RA, et al. Impact of sex on the pharmacokinetics and pharmacodynamics of 1% tenofovir gel. Clin Infect Dis 2016; 62:375–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Chen BA, Panther L, Marzinke MA, et al. Phase 1 safety, pharmacokinetics, and pharmacodynamics of dapivirine and maraviroc vaginal rings: a double-blind randomized trial. J Acquir Immune Defic Syndr 2015; 70:242–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Nicol MR, Fedoriw Y, Mathews M, et al. Expression of six drug transporters in vaginal, cervical, and colorectal tissues: implications for drug disposition in HIV prevention. J Clin Pharmacol 2014; 54:574–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Zhou T, Hu M, Pearlman A, Rohan LC. Expression, regulation, and function of drug transporters in cervicovaginal tissues of a mouse model used for microbicide testing. Biochem Pharmacol 2016; 116:162–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Hijazi K, Cuppone AM, Smith K, et al. Expression of genes for drug transporters in the human female genital tract and modulatory effect of antiretroviral drugs. PLoS One 2015; 10:e0131405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. das Neves J, Martins JP, Sarmento B. Will dapivirine redeem the promises of anti-HIV microbicides? Overview of product design and clinical testing. Adv Drug Deliv Rev 2016; 103:20–32. [DOI] [PubMed] [Google Scholar]