Abstract
Study Objective
To better understand the inter-individual differences in neurobehavioral impairment in obstructive sleep apnea (OSA) and its treatment with continuous positive airway pressure (CPAP), we examined how changes in sleep electroencephalography (EEG) slow waves were associated with next-day psychomotor vigilance test (PVT) performance.
Methods
Data from 28 OSA subjects (Apnea–Hypopnea Index with 3% desaturation and/or with an associated arousal [AHI3A] > 15/hour; AHI3A = sum of all apneas and hypopneas with 3% O2 desaturation and/or an EEG arousal, divided by total sleep time [TST]), who underwent three full in-lab nocturnal polysomnographies (NPSGs: chronic OSA, CPAP-treated OSA, and acute OSA), and 19 healthy sleepers were assessed. Four 20-minute PVTs were performed after each NPSG along with subjective and objective assessment of sleepiness. Three EEG metrics were calculated: K-complex (KC) Density (#/minute of N2 sleep), change in slow-wave activity in 1-second envelopes surrounding KCs (ΔSWAK), and relative frontal slow-wave activity during non-rapid eye movement (NREM) (%SWA).
Results
CPAP treatment of OSA resulted in a decrease in KC Density (chronic: 3.9 ± 2.2 vs. treated: 2.7 ± 1.1; p < 0.01; mean ± SD) and an increase in ΔSWAK (chronic: 2.6 ± 2.3 vs. treated: 4.1 ± 2.4; p < 0.01) and %SWA (chronic: 20.9 ± 8.8 vs. treated: 26.6 ± 8.6; p < 0.001). Cross-sectionally, lower ΔSWAK values were associated with higher PVT Lapses (chronic: rho = −0.55, p < 0.01; acute: rho = −0.46, p = 0.03). Longitudinally, improvement in PVT Lapses with CPAP was associated with an increase in ΔSWAK (chronic to treated: rho = −0.48, p = 0.02; acute to treated: rho = −0.5, p = 0.03). In contrast, OSA severity or global sleep quality metrics such as arousal index, NREM, REM, or TST were inconsistently associated with PVT Lapses.
Conclusion
Changes in EEG slow waves, in particular ∆SWAK, explain inter-individual differences in PVT performance better than conventional NPSG metrics, suggesting that ΔSWAK is a night-time correlate of next-day vigilance in OSA.
Keywords: OSA, OSA–PAP therapy, slow-wave sleep, sleep-disordered breathing, EEG spectral analysis, K-complex, excessive daytime sleepiness, psychomotor vigilance test
Statement of Significance
A better understanding of the pathophysiology of sleepiness and its manifestation and its link to obstructive sleep apnea (OSA) is essential for clarifying why some patients with OSA show little sleepiness, as well as why some patients have residual sleepiness despite adequate treatment of OSA. In this study, we find that sleep electroencephalography (EEG) markers suggestive of prefrontal cortex dysfunction (slow-wave activity and K-complex characteristics) were more reflective of sleep disruption and its effect on psychomotor vigilance test performance in OSA subjects than OSA severity and sleep macrostructure metrics. This suggests that night-time EEG slow waves may be correlates of OSA-induced dysfunction in connectivity in brain regions recruited during attentional processes.
Introduction
Excessive daytime sleepiness (EDS) is a widely reported and presumed reversible symptom in patients with obstructive sleep apnea (OSA) [1–3]. EDS in OSA patients has been associated with depressive symptoms [4], impaired attention, and reduced vigilance [5–7] and is known to be a risk factor for motor vehicle and work-related accidents [8]. Although EDS is often measured subjectively with a self-reported questionnaire such as the Epworth Sleepiness Scale (ESS) [9] or objectively with the Multiple Sleep Latency Test (MSLT) [10], the psychomotor vigilance test (PVT) [11] is often used as a measurable surrogate for sleepiness [12]. PVT is widely used as a measure of neurobehavioral alertness and sustained attention and has been shown to be sensitive to sleepiness [7, 13, 14]. Sleep fragmentation and intermittent hypoxia, seen in OSA, are causes for EDS. However, the classical OSA severity metrics (i.e. apnea–hypopnea index [AHI]) have been poor predictors of EDS as there is significant inter-individual variability in subjective sleepiness and PVT performance in OSA subjects with equivalent OSA severity. The electroencephalogram (EEG) has been extensively studied for correlations to measures of vigilance, but sleep EEG metrics such as duration of non-rapid eye movement (NREM), REM, or total sleep or metrics of fragmentation have not performed well to explain inter-individual variability. Furthermore, while there have been studies probing wake EEG for correlates of vigilance in healthy sleepers [15, 16], correlations between sleep EEG and daytime function in untreated and treated patients with OSA are not as well studied [17–19].
Among the brain regions implicated in vigilance and in OSA, the prefrontal cortex (PFC) is thought to be among the most sensitive to sleep deprivation or sleep fragmentation and intermittent hypoxia [20–22]. In addition to reversible dysfunction, it has been argued that OSA could cause direct hypoxic, or possibly neuroinflammatory, injury to the thalamocortical neurons, leading to impairment of prefrontal functional integration [23, 24]. It is plausible that the vulnerability of PFC in OSA, manifested as altered sleep EEG activity, could thus be associated with the severity of next-day alterations in alertness or vigilance [25]. Additional evidence implicating dysfunction of PFC in poor vigilance comes from neuroimaging. Studies in healthy adults [26, 27] during PVT testing showed an association between PVT performance and activity in parts of the PFC such as the right dorsolateral PFC. Sleep deprivation studies [28] have also shown that poor performance on sustained attentional tasks is associated with a decrease in activation of the PFC. The PFC has been proposed as the initiation site of cortically generated slow waves (<4 Hz) [29]. It has been shown that EEG slow waves with a low frequency (<1 Hz) are a marker of preserved PFC neuropsychological function in healthy elderly [30], and reduced relative power of EEG slow waves are associated with reduced medial PFC volumes [31, 32]. In addition to slow waves, the K-complex (KC) during NREM sleep has been shown to be cortically generated [33, 34], with a frontal predominance [35]. Several studies [36–39] have reported that KCs have <1 Hz frequency, and as such it can be argued that both KCs and sleep EEG slow waves reflect PFC function. In addition, the slow-wave activity (SWA) and KC features are believed to be markers of the sleep homeostatic process and have been studied in OSA patients at baseline and after treatment with continuous positive airway pressure (CPAP) [40–44]. However, the changes in EEG slow waves with CPAP treatment have not been related to changes in daytime function in OSA.
The primary goal of this study was to determine if night-time EEG slow waves (stage N2 KCs and their corresponding slow-wave features) were associated with next-day PVT performance cross-sectionally in untreated OSA subjects and longitudinally with 3 months of CPAP. In addition, we sought to determine if the night-time EEG slow waves were reflective of effects of “acute” OSA produced by CPAP withdrawal in well-treated OSA subjects.
Methods
The data for the present analyses were obtained from PSGs and daytime PVTs in a subsample of subjects who participated in a larger study relating OSA to daytime functioning. Briefly, the parent study consisted of 124 subjects being evaluated for OSA at the NYU Sleep Disorders Center. Recruitment criteria for the parent study cohort were the following: age above 18 years, complaints of EDS and/or snoring, and a primary diagnosis of OSA and/or upper airway resistance syndrome from an in-laboratory full-night nocturnal polysomnography (NPSG). Criteria for exclusion for the parent study were the following: inability to provide consent, pregnancy, medically unstable conditions, myocardial infarction, congestive heart failure and a change in medications during the trial, recent or confirmed history of alcohol or recreational drug abuse, and inability to perform the PVT due to verbal, auditory, or cognitive impairment. For the present sub-study, we selected the 28 OSA subjects, with an AHI3A >15/hour (AHI3A = AHI with 3% desaturation and/or with an associated arousal), not taking any medications known to affect sleep, who had completed the diagnostic NPSG, repeat NPSG on CPAP, and CPAP withdrawal NPSG. We also recruited 20 healthy subjects from the community (age > 18 years) with no indication of any sleep disorders, i.e. insomnia, narcolepsy, periodic leg movement, etc., based on a sleep physician’s physical examination and clinical interview. These healthy subjects were not taking any medications that could influence EDS at the time of the interview and diagnostic NPSG and reported proper sleep hygiene including 7–9 hours of nightly sleep. These non-OSA healthy sleepers were required to have an AHI3A <10/hour to be included in the present study. All patients and subjects signed informed consent documents and the protocol for the parent study was approved by the NYU IRB. The analysis of data obtained from the parent study was also approved by the Mount Sinai IRB.
Details of the protocol for the parent study are described in our previous work [45]. Briefly, subjects underwent diagnostic NPSG at the NYU Sleep Disorders Center and subjective and objective tests of daytime function were conducted on the following morning (chronic OSA condition). CPAP was titrated using standard clinical guidelines to eliminate sleep-disordered breathing events and inspiratory flow limitation. The validity of the CPAP pressure chosen was confirmed by reviewing the airflow tracing and pressure signals that were recorded continuously for 2 weeks on a custom CPAP machine (Fisher & Paykel Healthcare, Auckland, New Zealand). Following titration, patients used CPAP at the prescribed therapeutic pressure, with adherence (defined as hours of use at pressure) recorded on the device. After 2–3 months, subjects underwent a repeat laboratory NPSG on CPAP and evaluation of daytime function (CPAP-treated OSA condition). Patients then continued CPAP therapy at home and about a month later were instructed to discontinue CPAP for two consecutive nights; the second night was monitored in laboratory with a full NPSG (acute OSA condition). Each PSG was followed by daytime testing.
Polysomnography
All studies consisted of full in-laboratory NPSG (Sandman sleep system; Embla Systems Inc., Broomfield, CO) performed according to AASM guidelines. Recordings of frontal, central, and occipital EEG, electrooculogram, and submental electromyogram (EMG) were used to monitor sleep metrics. Leg movements were monitored with an anterior tibialis EMG. A unipolar electrocardiogram was used for cardiac monitoring. Oxygen saturation was monitored with a pulse oximeter (Masimo). Chest wall and abdominal movement were monitored with piezoelectric strain gauges. Sleep position was monitored with a multiposition switch. Respiratory airflow was measured using a nasal cannula/pressure transducer system (Protech PTAF2), along with an oral thermistor (diagnostic NPSG only) to detect mouth breathing. During the NPSG on CPAP, flow was recorded directly from the CPAP machine.
Sleep and respiratory event scoring for the NPSG data was carried out according to AASM criteria (AASM Scoring Manual, version 2.5, 2018). From these measures, absolute and relative amounts of each sleep stage, total sleep time (TST), sleep efficiency, and wake after sleep onset (WASO) were extracted. Arousals were scored based on AASM criteria (AASM Scoring Manual, version 2.5, 2018) with an arousal index calculated as number of arousals per hour of sleep. Respiratory events were defined based on AASM criteria (AASM Scoring Manual, version 2.5, 2018). Specifically, apneas were defined relative to baseline as a >90% decrease in airflow for >10 seconds; hypopneas were initially identified from the airflow signal as a visible reduction (typically >30% decrease and accompanied by inspiratory flow limitation) for >10 seconds, and then linked to O2 desaturation and/or EEG arousals to obtain hypopnea with a 3% desaturation and a hypopnea with only an EEG arousal. The AHI3A was calculated as the sum of all apneas and hypopneas with either 3% desaturation and/or an EEG arousal, divided by the TST [46].
Assessment of subjective and objective daytime function
Measurement of daytime function was based on subjective sleepiness and objective performance. The ESS questionnaire was completed with other nightly questionnaires upon the arrival of the patient in the sleep lab prior to lights-out. Four 20-minute PVTs (Ambulatory Monitoring Inc., Ardsley, NY) were performed 2 hours apart, beginning in the morning after each NPSG in chronic OSA, treated OSA, and acute OSA conditions. The primary variable from each PVT trial was the number of lapses (reaction time > 500 ms). We transformed the average number of lapses (PVT Lapses = sqrt[lapses] + sqrt[lapses + 1]) from the four tests and used it as the measure of vigilance (PVT Lapses). Objective daytime sleepiness was assessed using the MSLT. Subjects were given the opportunity to fall asleep during a 20-minute period four times a day at 2-hour intervals, and the tests were administered 30-minute after the PVT. Sleep latency on each 20-minute test was calculated as elapsed time from lights outs to the first epoch scored as sleep and averaged over the four tests.
KC detection
Spontaneous KCs during stage N2 of NREM sleep were detected automatically using a previously validated automated method (DETOKS) [47] written in MATLAB (version R2017a, The MathWorks, Natick, MA). Spontaneous stage N2 KCs were defined as a well-delineated negative sharp wave, immediately followed by a positive sharp wave. The DETOKS method decomposes the EEG signal into low-frequency, transient, and oscillatory components. The low-frequency component is used to detect KCs. The frontal channel (FZ) referenced to linked mastoids was used for the detection of KCs and EEG spectral analyses as described in the subsequent subsections. KCs were discarded if their duration was less than 0.5 seconds. Additionally, a minimum peak-to-peak amplitude of 75 µV was also set for the KCs. A few detected KCs (about 10%) for each study were randomly chosen and visually vetted for parameter tuning of DETOKS. KC Density was defined as the number of stage N2 KCs per minute of stage N2 sleep.
EEG spectral analyses
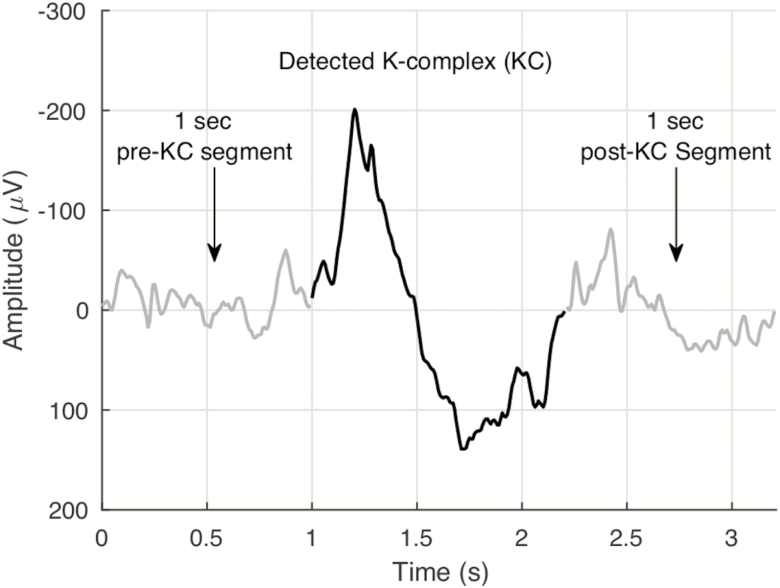
Spectral analyses were done on EEG data (FZ referenced to linked mastoids) during NREM sleep using MATLAB. For the spectral analyses, we used a modified Welch periodogram with overlapping (50%) 10-second segments. The segments were smoothed using the Hamming window. Relative slow-wave activity (%SWA) was calculated by dividing the power within the (1–4 Hz) band by the power across the whole spectrum (1–50 Hz) for the entire NREM period using the above modified Welch periodogram. Power spectral analyses were also conducted on 1-second EEG segments prior to and following each detected KC using MATLAB. Figure 1 illustrates the pre- and post-KC segments. The same Welch periodogram method, but with a Fast Fourier Transform length of 128 samples (50% overlap), as described above, was used to calculate the relative spectral power in the following frequency bands: delta (1–4 Hz), theta (4–8 Hz), alpha (8–12 Hz), sigma (12–15 Hz), beta (15–30 Hz), and gamma (30–50 Hz). For each of the frequency bands, we calculated the percent change in average relative spectral power (pre-KC to post-KC) with ΔSWAK denoting the average percent change in the delta band. Put simply, ΔSWAK calculated the change in SWA (1–4 Hz) surrounding a KC. All EEG analyses, including the detection of KCs, were performed blinded to the study condition.
Figure 1.
An example K-complex and the corresponding 1 second pre- and post-K-complex segments.
Statistical analyses
All statistical analyses were carried out using IBM SPSS 23 and MATLAB. The statistical significance level was set at p <0.05 (two-tailed) for all tests. Normality of data was tested using the Shapiro–Wilk test. Comparisons of sleep and EEG variables of OSA subjects between the three OSA conditions (chronic, treated, and acute) and to values in the healthy sleepers were carried out using t-tests for normal data and Mann–Whitney (or Wilcoxon signed-ranks test for paired data) tests for non-normal data. Listwise deletion was used when comparing groups with unequal sample sizes. All non-normally distributed data are reported as median (interquartile range). Spearman’s rank correlation was used for bivariate and partial correlations in MATLAB and the resulting value of the correlation coefficient (rho) was reported in each case. Cross-sectional associations in the chronic OSA condition were controlled for age, sex, body mass index (BMI), and subjective sleepiness, whereas in the acute OSA condition, they were controlled for age, sex, and BMI only. Longitudinal associations were controlled for age, sex, BMI, and change in ESS.
We used hierarchical regression models to better understand the relationship between OSA severity, sleep EEG, vigilance (PVT Lapses), and objective daytime sleepiness (MSLT) in chronic and acute OSA. The predictors of interest in the cross-sectional regression models were ΔSWAK, KC Density, %SWA, and AHI3A. We also used hierarchical regression models to better understand the relationship between change in OSA severity, change in sleep EEG, and change in vigilance from (1) chronic to treated and (2) acute to treated OSA conditions. Change for each of the variables (OSA severity, sleep EEG, etc.) was defined as the difference from chronic OSA to treated OSA or as the difference from acute OSA to treated OSA, depending on the model. The models for chronic-to-treated OSA were adjusted for covariates first, followed by the addition of the predictors of interest: change in (1) ΔSWAK, (2) KC Density, (3) %SWA, and (4) AHI3A. Covariates used for the regression models were the same as those for the correlations.
Results
The inclusion criteria for the present study resulted in the analyses of 28 subjects (mean age = 47.3 ± 11.3 years; 21 males and 7 females) with chronic OSA (median AHI3A = 72.5/hour) that also underwent a repeat NPSG on CPAP. Of the 28 subjects, 24 underwent an additional repeat NPSG with acute CPAP withdrawal. In addition, EEG data from 19 non-OSA healthy sleepers (mean age = 33.7 ± 7.1 years; 12M/7F; median AHI3A = 3.1/hour) were analyzed. Table 1 summarizes the demographics, sleep architecture, and daytime sleepiness in healthy sleepers and OSA subjects (chronic OSA condition, CPAP-treated OSA condition, and acute OSA condition). Table 2 summarizes the EEG metrics in healthy sleepers and OSA subjects. Figure 2 shows grand mean KC segments from a representative OSA subject in chronic OSA, treated OSA, and acute OSA conditions.
Table 1.
Subject characteristics
| Healthy sleepers (N = 19) | OSA | |||
|---|---|---|---|---|
| Chronic OSA (diagnostic) (N = 28) | Treated OSA(CPAP)(N = 28) | Acute OSA(CPAP withdrawal)(N = 24) | ||
| Demographics | ||||
| Age (years) | 33.7 ± 1.6 | 47.3 ± 11.3a | — | 48.5 ± 2.2a |
| Sex | 12M/7F | 21M/7F | 17M/7F | |
| BMI | 25.6 ± 1.1 | 37.9 ± 1.6a | 37.7 ± 1.6a | |
| AHI3A (/hour) | 4.5 ± 3.5 | 67.9 ± 5.4a | 5.4 ± 0.7b | 59.3 ± 4.5a,c |
| Arousal index (/hour) | 13.3 ± 1.3 | 48.6 ± 4.9a | 12.0 ± 1.2b | 36.4 ± 4.9a,c |
| Adherence (hour) | — | — | 4.9 ± 1.7 | — |
| Sleep architecture | ||||
| TST (min) | 440.4 ± 18.3 | 426.5 ± 14.3 | 445.84 ± 11.5 | 423.8 ± 8.3 |
| WASO (%) | 6.8 (5.1) | 15.2 (13.5)a | 10.1 (7.1) | 13.2 (7.3)a |
| SE (%) | 88.5 (6.7) | 83.1 (12.5) | 88.4 (8.1) | 86.6 (8.8) |
| Stage N1 (%) | 16.6 ± 1.2 | 41.4 ± 3.2a | 12.7 ± 0.9b | 35.7 ± 2.7c,a |
| Stage N2 (%) | 49.1 ± 2.0 | 37.2 ± 2.6a | 48.9 ± 1.9b | 44.8 ± 2.0 |
| SWS (%) | 9.1 (8.1) | 4.2 (13.1) | 13.1 (8.4)b | 2.1 (6.3)c |
| REM (%) | 19.2 ± 1.2 | 14.8 ± 1.3a | 21.5 ± 1.1b | 12.4 ± 1.3a,c |
| Daytime sleepiness | ||||
| PVT Lapses | 1.7 (1.5) | 6.1 (7.1)a | 3.7 (2.3)a,b | 6.5 (7.9)a,c |
| ESS | 5.2 ± 0.5 | 13.0 ± 1.0a | 10.7 ± 1.0a | — |
| Mean MSLT (minute) | 10.5 ± 1.1 | 5.5 ± 0.6a | 7.2 ± 0.6a,b | 5.5 ± 0.6a,c |
Normally distributed variables are reported as mean ± SD, whereas non-normally distributed variables are reported as median (interquartile range). SE, sleep efficiency; SWS, slow-wave sleep.
aCompared to healthy sleepers (p < 0.05).
bCompared to chronic OSA (p < 0.05).
cCompared to treated OSA (p < 0.05).
Table 2.
EEG slow waves and spectral power surrounding stage N2 K-complexes across healthy and OSA (chronic, treated, and acute) groups
| Healthy sleepers (N = 19) | OSA | |||
|---|---|---|---|---|
| Chronic OSA (diagnostic) (N = 28) | Treated OSA (CPAP) (N = 28) | Acute OSA (CPAP withdrawal) (N = 24) | ||
| EEG variables | ||||
| KC Density (#/minute) | 2.9 ± 1.8 | 3.9 ± 2.2 | 2.7 ± 1.1a | 4.0 ± 1.8b |
| %SWA (%) | 27.1 ± 13.3 | 21.0 ± 8.8 | 26.6 ± 8.6a | 16.8 ± 7.0b,c |
| ΔSWAK (%) | 4.1 ± 2.2 | 2.5 ± 2.3c | 4.1 ± 2.4a | 2.8 ± 2.7 |
| Theta (%) | −18.2 ± 10.9 | −18.2 ± 13.2 | −17.2 ± 7.4 | −18.3 ± 10.9 |
| Alpha (%) | −8.2 ± 9.2 | −2.8 ± 15.8 | −5.8 ± 11.5 | −3.9 ± 14.6 |
| Sigma (%) | −15.6 ± 12.5 | −9.2 ± 18.1 | −13.9 ± 13.5 | −9.1 ± 11.9 |
| Beta (%) | −14.3 ± 9.8 | −8.9 ± 13.9 | −15.9 ± 11.5 | −13.6 ± 10.9 |
| Gamma (%) | −12.3 ± 14.5 | −12.1 ± 14.6 | −18.9 ± 14.3 | −17.8 ± 20.3 |
All values are reported as mean ± SD. KC Density: number of stage N2 K-complexes per minute of stage N2 sleep time; %SWA: relative frontal slow wave activity (0.5–4 Hz) during NREM sleep; ΔSWAK: percent change in average slow-wave activity surrounding stage N2 K-complexes.
aCompared to chronic OSA (p < 0.05).
bCompared treated OSA (p < 0.05).
cCompared to healthy sleepers (p < 0.05).
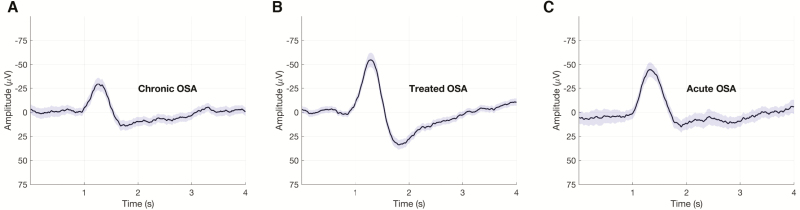
Figure 2.
Changes in K-complex morphology with CPAP treatment. Grand mean K-complex waveforms and the surrounding EEG segments are shown from a representative subject during the (A) chronic OSA (842 segments), (B) treated OSA (614 segments), and (C) acute OSA (714 segments) conditions. Mean of the EEG segments is shown in black and the 95% confidence interval is shaded in purple.
Change in OSA severity, daytime function, and EEG slow waves with CPAP
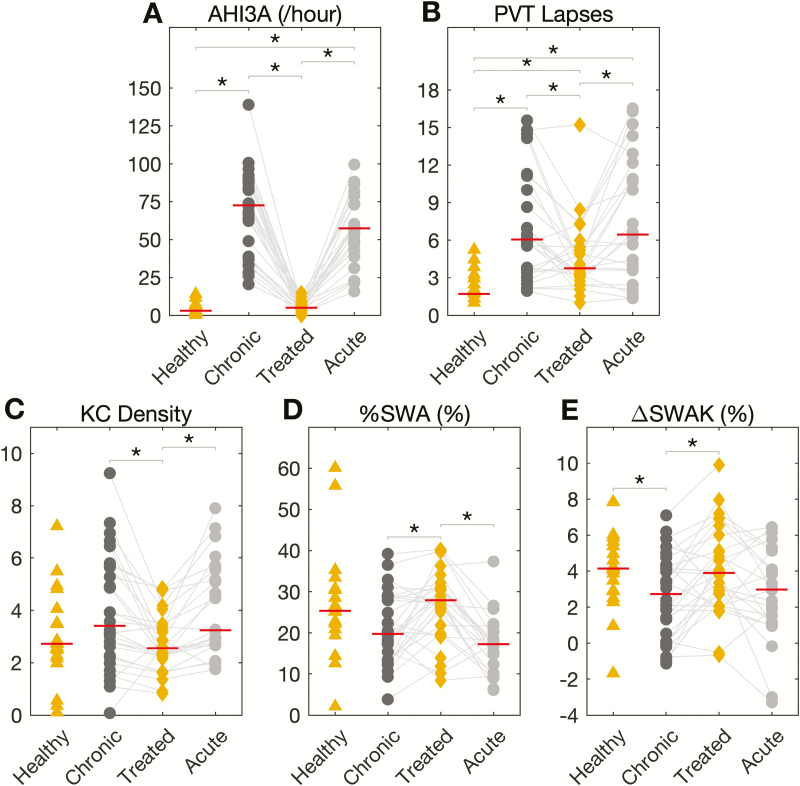
As seen in Table 1 and Figure 3, treatment of chronic OSA with CPAP for about 3 months (mean CPAP adherence = 4.9 ± 1.72 hours/night) was associated with a significant reduction of AHI3A (Figure 3, A), arousal index (Table 1), PVT Lapses (Figure 3, B), and KC Density (Figure 3, C; Table 2). CPAP treatment was also associated with a significant increase in MSLT scores (Table 1), %SWA (Figure 3, D; Table 2), and ΔSWAK (Figure 3, E; Table 2). CPAP treatment of chronic OSA normalized sleep architecture (Table 1). We failed to show a statistically significant decrease in subjective sleepiness (ESS) with CPAP treatment (Table 1). In addition, there were no significant differences in either WASO or TST with CPAP (Table 1). The percent change in average spectral power (theta, alpha, sigma, beta, and gamma bands) surrounding stage N2 KCs was not significantly different with CPAP (Table 2). A comparison of NPSG variables between OSA subjects and healthy sleepers is also shown in Tables 1 and 2.
Figure 3.
Changes in OSA severity, vigilance, and EEG slow waves with CPAP. Change in (A) AHI3A (/hour), (B) PVT Lapses (transformed average of lapses from four 20-minute PVTs), (C) KC Density (no. of K-complex/minute of N2 sleep), (D) %SWA (relative frontal slow-wave activity [0.5–4 Hz] during NREM), and (E) ΔSWAK (percent change in average slow-wave activity surrounding stage N2 K-complexes) across the healthy and OSA (chronic, treated and acute) groups. Red solid line indicates the median value. Significant differences between the groups are highlighted with an asterisk. *p < 0.05.
Sleep EEG correlates of vigilance in chronic OSA
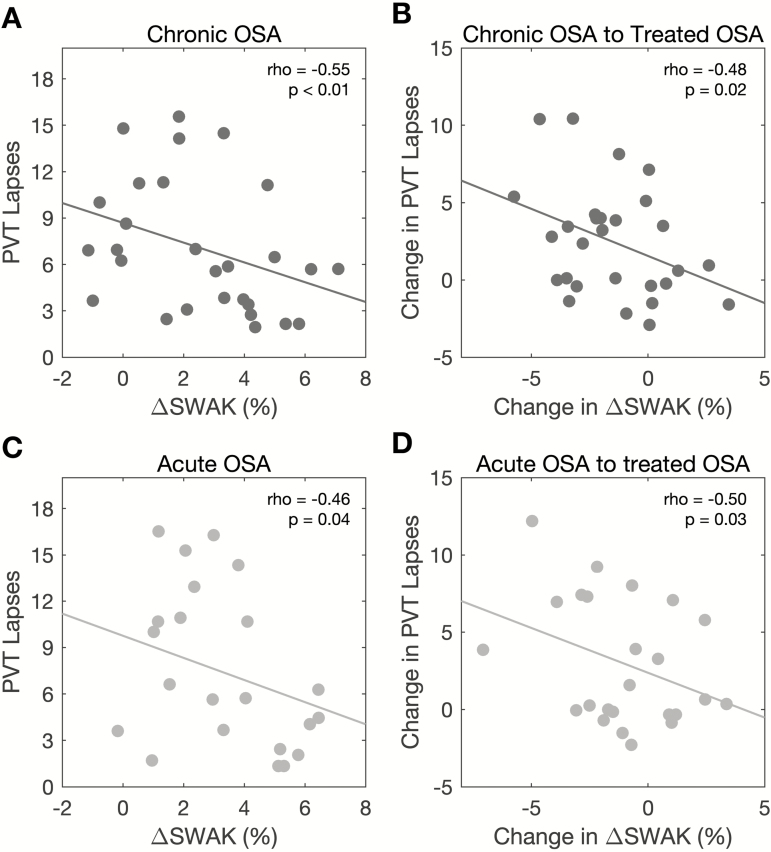
Cross-sectionally in chronic OSA, ΔSWAK was negatively associated with PVT Lapses (Figure 4, A). Mean sleep latency on the MSLT and %SWA were negatively associated with PVT Lapses (Table 3) and although arousal index was positively associated with PVT Lapses, the association was not significant on adjusting for the covariates (adjusted rho = 0.35, p = 0.11). The association between sleep quality measures and daytime outcomes is reported in Table 3. To further evaluate the strength of the association between EEG slow waves and vigilance, we conducted hierarchical regression analyses as shown in Table 4 (Models 1–3). The covariates age, sex, BMI, and subjective sleepiness (ESS) explained 20% of the variance in PVT Lapses, with ESS being a significant predictor of PVT Lapses (Table 4, Model 1). Both ΔSWAK and KC Density were additional significant predictors of PVT Lapses (Table 4, Model 2) above and beyond the effect of the covariates, and improved the predictive power: ΔSWAK improved prediction of PVT Lapses by 13% and KC Density improved prediction by 11%. In contrast, neither %SWA nor AHI3A were significant predictors of PVT Lapses after adjusting for covariates. Table 5 shows the results of the hierarchical regression analyses testing the association of EEG slow waves with objective sleepiness (MSLT) in chronic OSA. KC Density, ΔSWAK, and AHI3A were additional significant predictors of MSLT scores (Table 5, Model 2 and Model 3), and improved the predictive power: KC Density improved prediction by 53%, ΔSWAK by 24%, and AHI3A by 20%.
Figure 4.
Association between slow-wave activity surrounding stage N2 K-complexes (ΔSWAK) and vigilance in OSA. Scatterplots show significant negative association between (A) ΔSWAK (percent change in average slow-wave activity surrounding stage N2 K-complexes) and PVT Lapses in chronic OSA, (B) change in ΔSWAK and change in PVT Lapses (difference in ΔSWAK from chronic to treated OSA), (C) ΔSWAK and PVT Lapses in acute OSA, and (D) change in ΔSWAK and change in PVT Lapses from acute to treated OSA, after adjusting for age, sex, and BMI. Associations shown in (A) and (B) were also adjusted for subjective sleepiness (ESS) and change in subjective sleepiness, respectively.
Table 3.
Partial correlations between nocturnal PSG variables and daytime function in chronic and acute OSA
| Variable | Chronic OSA | Acute OSA | |||
|---|---|---|---|---|---|
| PVT Lapses | MSLT | ESS | PVT Lapses | MSLT | |
| AHI3A | 0.28 | −0.42* | 0.14 | 0.61** | −0.31 |
| TST | 0.17 | 0.23 | 0.07 | −0.19 | 0.2 |
| WASO | −0.34 | −0.06 | 0.26 | −0.26 | 0.09 |
| Stage N2 | −0.39 | 0.67** | −0.12 | −0.58** | 0.36 |
| SWS | −0.33 | 0.12 | 0.09 | −0.21 | −0.09 |
| REM | 0.05 | 0.32 | 0.09 | −0.51* | 0.25 |
| %SWA | −0.34 | 0.34 | −0.35 | −0.42 | 0.17 |
| PVT Lapses | — | −0.53** | 0.28 | — | −0.62** |
| MSLT | −0.53** | — | −0.23 | −0.62** | — |
| ESS | 0.29 | −0.23 | — | — | — |
Values reported are Spearman’s rank correlation coefficients (rho). All partial correlations were adjusted for age, sex, and BMI. The correlations between PSG variables and PVT Lapses (Rows 1–6, Column 2) in chronic OSA were adjusted for ESS as well. Note that ESS was not administered on the acute CPAP withdrawal visit. %SWA: relative frontal slow-wave activity (0.5–4 Hz) during NREM sleep. SWS, slow-wave sleep.
*p < 0.05; **p < 0.01.
Table 4.
Hierarchical regression analyses testing the association between vigilance, EEG slow waves, and OSA severity in chronic and on CPAP-treated OSA (N = 28)
| Dependent variable | Models | Predictors | β | 95% CI for B | p valuea | Adj. R2 | ΔR2 |
|---|---|---|---|---|---|---|---|
| PVT Lapses in chronic OSA | Model 1 | Age | −0.08 | −0.18, 0.13 | 0.71 | 0.20 | — |
| Sex | 0.28 | −1.42, 6.72 | 0.19 | ||||
| BMI | 0.04 | −0.18, 0.22 | 0.85 | ||||
| ESS | 0.43 | 0.04, 0.62 | 0.03 | ||||
| Model 2: Model 1 + one EEG metric | ΔSWAK | −0.41 | −1.38, −0.07 | 0.03 | 0.33 | 0.13* | |
| KC Density | 0.36 | 0.01, 1.38 | 0.047 | 0.31 | 0.11* | ||
| %SWA | −0.30 | −0.34, 0.05 | 0.14 | 0.25 | 0.07 | ||
| Model 3: Model 1 + AHI3A | AHI3A | 0.21 | −0.03, 0.09 | 0.29 | 0.21 | 0.04 | |
| Change in PVT Lapsesb | Model 4 | Age | −0.05 | −0.15, 0.12 | 0.79 | 0.11 | — |
| Sex | 0.29 | −1.21, 6.02 | 0.18 | ||||
| BMI | −0.38 | −0.35, 0.03 | 0.10 | ||||
| Change in ESS | 0.48 | 0.03, 0.55 | 0.03 | ||||
| Model 5: Model 4 + change in one EEG metric | Change in ΔSWAK | −0.50 | −1.41, −0.22 | < 0.01 | 0.32 | 0.20* | |
| Change in KC Density | 0.30 | −0.25, 1.47 | 0.15 | 0.15 | 0.07 | ||
| Change in %SWA | −0.20 | −0.25, 0.10 | 0.37 | 0.10 | 0.03 | ||
| Model 6: Model 4 + change in AHI3A | Change in AHI3A | 0.17 | −0.03, 0.08 | 0.40 | 0.10 | 0.02 |
95% CIs are shown for the unstandardized regression coefficient B and not β. ΔSWAK: change in relative frontal slow-wave activity (1–4 Hz) 1 second pre- to post-stage N2 K-complex; KC Density: number of stage N2 K-complexes per minute of stage N2 sleep time; %SWA: relative frontal slow-wave activity (0.5–4 Hz) during NREM sleep; ΔR2: change in R2 from the model with covariates only. Significant change in R2 is highlighted in bold.
a p value for each predictor.
bChange in PVT Lapses (likewise ESS, EEG metrics, and AHI3A) is defined as PVT Lapses on diagnostic (chronic OSA) minus PVT Lapses on CPAP (treated OSA). Note the 95% CIs are shown for the unstandardized regression coefficient B and not β.
*p < 0.05.
Table 5.
Association of objective daytime sleepiness (MSLT) with EEG slow waves and OSA severity in chronic (N = 28) and acute OSA subjects (N = 24)
| Dependent variable | Condition | Models | Predictors | β | 95% CI for B | p valuea | Adj. R2 | ΔR2 |
|---|---|---|---|---|---|---|---|---|
| Mean MSLT (minute) | Chronic OSA | Model 1 | Age | −0.02 | −0.14, 0.10 | 0.68 | −0.11 | — |
| Sex | 0.35 | −2.92, 3.62 | 0.83 | |||||
| BMI | 0.02 | −0.14, 0.18 | 0.77 | |||||
| Model 2: Model 1 + one EEG metric | ΔSWAK | 0.53 | 0.16, 1.17 | 0.01 | 0.13 | 0.24* | ||
| KC Density | −0.76 | −1.41, −0.60 | <0.001 | 0.47 | 0.53* | |||
| %SWA | 0.36 | −0.03, 0.27 | 0.11 | −0.03 | 0.11 | |||
| Model 3: Model 1 + AHI3A | AHI3A | −0.05 | −0.09, −0.009 | 0.02 | 0.09 | 0.20 | ||
| Acute OSA | Model 4 | Age | 0.13 | −0.09, 0.16 | 0.58 | −0.04 | — | |
| Sex | 0.21 | −2.06, 4.62 | 0.43 | |||||
| BMI | −0.28 | −0.29, 0.09 | 0.28 | |||||
| Model 5: Model 4 + one EEG metric | ΔSWAK | 0.6 | 0.23, 1.1 | <0.01 | 0.30 | 0.33** | ||
| KC Density | −0.24 | −1.10, 0.34 | 0.29 | −0.03 | 0.05 | |||
| %SWA | 0.27 | −0.08, 0.29 | 0.23 | −0.01 | 0.07 | |||
| Model 6: Model 4 + AHI3A | AHI3A | −0.29 | −0.09, 0.02 | 0.20 | 0.00 | 0.08 |
95% CIs are shown for the unstandardized regression coefficient B and not β. ΔSWAK: change in relative frontal slow-wave activity (1–4 Hz) 1 second pre-to post-stage N2 K-complex; KC Density: number of stage N2 K-complexes per minute of stage N2 sleep time; %SWA: relative frontal slow-wave activity (0.5–4 Hz) during NREM sleep; ΔR2: change in R2 from the model with covariates only. Significant change in ΔR2 is highlighted in bold.
a p value for each predictor.
*p < 0.05; **p < 0.01.
As seen in Figure 4, B, reduced PVT Lapses with CPAP treatment were negatively associated with change in ΔSWAK from chronic OSA to treated OSA, but not with change in AHI3A (rho = −0.14, p = 0.51), KC Density (rho = −0.11, p = 0.59), or %SWA (rho = 0.03, p = 0.88). Reduced PVT Lapses were not correlated with either improvement in ESS (rho = 0.37, p = 0.07), or CPAP adherence (rho = −0.12, p = 0.58). CPAP adherence (excluding the night of testing) over the 3-month period after chronic OSA was not associated with changes in ΔSWAK (rho = 0.05, p = 0.83), KC Density (rho = 0.27, p = 0.19), or %SWA (rho = 0.08, p = 0.72). Hierarchical regression analyses with PVT Lapses as the dependent variable (Table 4, Models 4–6) reveal that covariates age, sex, BMI, and improvement in subjective sleepiness (ESS) with CPAP, explained 11% of the variance in PVT Lapses. Improvement in subjective sleepiness (ESS) from chronic to treated OSA was associated with reduced PVT Lapses (Table 4, Model 4). Change in ΔSWAK was a significant predictor and improved the predictive power by 20%, above and beyond the effect of covariates (Table 4, Model 5). In contrast, none of the other predictors (change in KC Density or %SWA or AHI3A) were significantly predictive of reduced PVT Lapses (Table 4, Models 5 and 6). Using improvement in mean sleep latency time from MSLT from chronic OSA to treated OSA as the dependent variable, none of the EEG metrics, i.e., change in KC density, %SWA, or ΔSWAK, or change in AHI3A were significant predictors (data not shown).
Change in OSA severity, EEG slow waves, and vigilance in acute OSA
As seen in Table 1, the withdrawal of CPAP (acute OSA) in CPAP-treated subjects resulted in a significant increase in AHI3A (Figure 3, A), arousal index, time spent in sleep stage N1, PVT Lapses (Figure 3, B), and median response times. A significant decrease was also observed in slow wave and REM sleep duration, and mean sleep latency from MSLT (Table 1). The time spent in sleep stage N2 in acute OSA was not significantly different from that in treated OSA (Table 1). The TST and percent WASO in acute OSA were not significantly different from those on CPAP. As seen in Table 2, acute OSA resulted in a significantly higher KC Density (Figure 3, C) and lower %SWA (Figure 3, D) than treated OSA. While ΔSWAK was lower in acute OSA, as compared to treated OSA, we failed to show significance (Figure 3, E; Wilcoxon signed-ranks z = 1.49, p = 0.13).
Sleep EEG correlates of vigilance in acute OSA
As seen in Table 3 and Figure 4, C, cross-sectionally in acute OSA, ΔSWAK was negatively associated with PVT Lapses. While AHI3A was positively associated with PVT Lapses, we failed to show a significant association of either KC Density or %SWA with PVT Lapses. On the other hand, time spent in REM sleep and N2 sleep was associated with PVT Lapses (Table 3). As in chronic OSA, PVT Lapses were negatively associated with MSLT scores (Table 3) in acute OSA. Hierarchical regression analyses, as reported in Table 6 (Models 1–3), reveal that the covariates age, sex, and BMI explain about 3% of the variance in PVT Lapses in acute OSA. ΔSWAK and AHI3A were significant additional predictors of PVT Lapses in acute OSA and improved the predictive power by 20% and 28%, respectively. Table 5 (Models 4–6) shows the results of the hierarchical regression analyses with objective sleepiness (MSLT) as the dependent variable. ΔSWAK was positively correlated with MSLT (rho = 0.63, p < 0.01), above and beyond the effect of the covariates and improved the predictive power by 30% (Table 5, Model 5).
Table 6.
Hierarchical regression analyses testing the association between vigilance, EEG slow waves, and OSA severity on acute OSA (N = 24)
| Dependent variable | Models | Predictors | β | 95% CI for B | p valuea | Adj. R2 | ΔR2 |
|---|---|---|---|---|---|---|---|
| PVT Lapses in acute OSA | Model 1 | Age | −0.30 | −0.35, 0.07 | 0.18 | 0.03 | — |
| Sex | −0.02 | −5.92, 5.42 | 0.93 | ||||
| BMI | 0.24 | −0.17, 0.47 | 0.34 | ||||
| Model 2: Model 1 + one EEG metric | ΔSWAK | −0.46 | −1.63, −0.11 | 0.03 | 0.17 | 0.20* | |
| KC Density | 0.21 | −0.66, 1.80 | 0.34 | 0.03 | 0.04 | ||
| %SWA | −0.39 | −0.58, 0.02 | 0.06 | 0.15 | 0.15 | ||
| Model 3: Model 1 + AHI3A | AHI3A | 0.55 | 0.04, 0.21 | 0.01 | 0.31 | 0.28* | |
| Change in PVT Lapsesb | Model 4 | Age | −0.30 | −0.29, 0.06 | 0.19 | −0.02 | — |
| Sex | −0.06 | −5.22, 4.16 | 0.82 | ||||
| BMI | −0.09 | −0.31, 0.22 | 0.73 | ||||
| Model 5: Model 4 + change in one EEG metric | Change in ΔSWAK | −0.59 | −1.64, −0.29 | <0.01 | 0.27 | 0.29** | |
| Change in KC Density | 0.32 | −0.41, 2.5 | 0.15 | 0.04 | 0.09 | ||
| Change in %SWA | −0.01 | −0.25, 0.24 | 0.97 | −0.07 | 0.00 | ||
| Model 6: Model 4 + change in AHI3A | Change in AHI3A | 0.55 | 0.03, 0.17 | 0.01 | 0.24 | 0.27* |
95% CIs are shown for the unstandardized regression coefficient B and not β. ΔSWAK: change in relative frontal slow-wave activity (1–4 Hz) 1 second pre- to post-stage N2 K-complex; KC Density: number of stage N2 K-complexes per minute of stage N2 sleep time; %SWA: relative frontal slow-wave activity (0.5–4 Hz) during NREM sleep; ΔR2: change in R2 from the model with covariates only. Significant change in ΔR2 is highlighted in bold.
a p value for each predictor.
bChange in PVT Lapses (likewise EEG metrics and AHI3A) is defined as PVT Lapses on CPAP withdrawal (acute OSA) minus PVT Lapses on CPAP (treated OSA)
*p < 0.05; **p < 0.01.
As seen in Figure 4, D, the change in PVT Lapses in acute OSA was negatively associated with change in ΔSWAK from acute OSA to treated OSA, before and after adjusting for age, sex, and BMI. In addition, change in PVT Lapses was associated positively with AHI3A (rho = 0.54, p = 0.01) but not with either KC Density (rho = 0.28, p = 0.37) or %SWA (rho = 0.18, p = 0.43). As seen in Table 6, hierarchical regression analyses show that change in ΔSWAK and change in AHI3A from acute OSA to treated OSA were significant predictors of change in PVT Lapses (Table 6, Models 5 and 6) improving the predictive power by 29% and 27%, respectively. In contrast, changes in KC Density and %SWA were not significant predictors of change in PVT Lapses. Hierarchical regression analyses with change in MSLT scores from acute OSA to treated OSA as the dependent variable did not reveal either change in EEG metrics or change in AHI3A as significant predictors (data not shown).
Associations between OSA severity, EEG slow waves, and subjective sleepiness
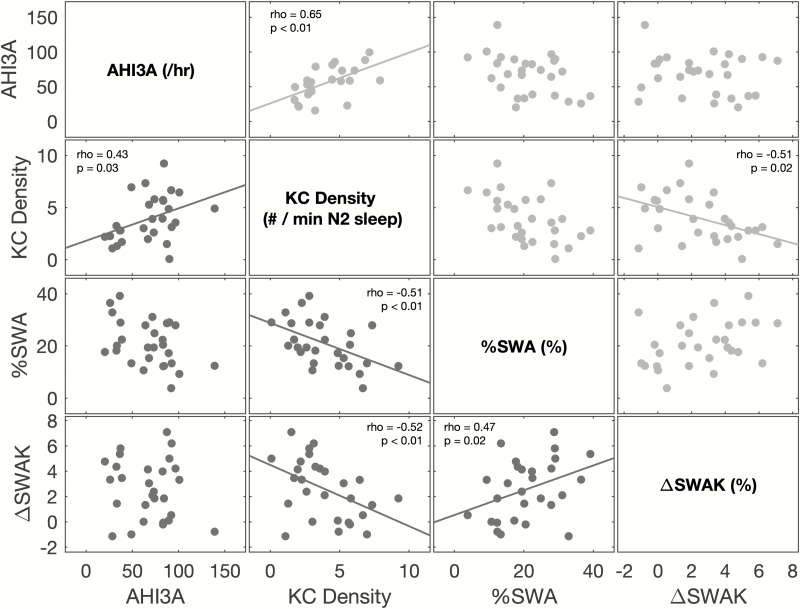
The pairwise partial (adjusted for age, sex, and BMI) correlations between AHI3A, KC Density, %SWA, and ΔSWAK, cross-sectionally in chronic and acute OSA, are shown in Figure 5. KC Density was associated positively with AHI3A and negatively with ΔSWAK in both chronic and acute OSA. KC Density was also negatively associated with %SWA, but only in chronic OSA; we failed to show significant association between KC Density and %SWA in acute OSA. Similarly, ΔSWAK was negatively associated with %SWA only in chronic OSA. Subjective sleepiness (ESS) was not associated with any of the EEG metrics or with AHI3A in chronic OSA (Table 3). Moreover, improvement in subjective sleepiness (ESS) with CPAP was not associated with either change in EEG metrics (KC Density rho = 0.25, p = 0.24; %SWA rho = −0.07, p = 0.73; ΔSWAK rho = −0.12, p = 0.58) or AHI3A (rho = −0.03, p = 0.9).
Figure 5.
Associations between OSA severity and EEG slow waves in chronic and acute OSA. Scatterplots above the diagonal (shaded in light gray) represent the associations in acute OSA, whereas scatterplots below the diagonal (shaded in dark gray) represent the associations in chronic OSA. Significant associations, after adjusting for age, sex, and BMI, are shown with a least square fit line, Spearman’s correlation coefficient (rho) and the corresponding p value.
Discussion
Summary of findings
In this study, we investigated three sleep EEG markers (KC Density, NREM %SWA, and change in SWA [1–4 Hz] surrounding KCs [ΔSWAK]), to investigate the relationship of the nocturnal EEG to daytime function in OSA in order to better understand inter-individual differences in daytime metrics and the effect of OSA treatment. We hypothesized that sleep EEG markers of brain region alterations, especially those reflecting the PFC, correlate with vigilance (a task heavily reliant on PFC) better than conventional NPSG metrics such as OSA severity, REM, NREM, and TST. The results of this study show that ΔSWAK was a significant predictor of vigilance. Our data show that this relationship existed not only cross-sectionally in both chronic and acute OSA conditions, but also longitudinally with CPAP treatment. Furthermore, ΔSWAK was predictive of objective daytime sleepiness (MSLT) cross-sectionally in chronic and acute OSA. In contrast, OSA severity (AHI3A) was inconsistently associated with daytime sleepiness. Treatment of OSA with CPAP resulted in a decrease in KC Density and an increase %SWA and ΔSWAK. Our results suggest that sleep EEG markers of PFC function are more reflective of sleep disruption and its effect on vigilance in OSA subjects than sleep macrostructure variables and thus may mediate the inter-individual susceptibility to a given severity of OSA. We suggest these metrics warrant further mechanistic investigation, as well as exploration of links to other correlates of daytime sleepiness.
Comparison with previous studies
Our previous publication describes the changes in OSA severity, sleep macrostructure, and daytime function with CPAP treatment, as seen in this study [45]. While AHI3A in our data was not correlated with daytime function in chronic OSA, it was a significant predictor of vigilance in acute OSA. In both chronic OSA and acute OSA, AHI3A was not significantly associated with ESS or mean sleep latency from MSLT. CPAP withdrawal (acute OSA) is a model of early OSA and could differ from chronic OSA, where adaptation may have occurred and blunted the relationship to vigilance seen in this study. These results, showing only a poor-to-modest relationship between OSA severity and daytime sleepiness or vigilance, are similar to reports in other studies [48–53]. In studies that do report an association between OSA severity and sleepiness, OSA severity still explains less than 10% of the total variance in PVT Lapses [4] and even less in subjective sleepiness [49]. This unexpectedly poor relationship is the problem we hope to improve with the present further analysis of the sleep EEG.
Consistent with previous studies [54, 55], we showed that increased time spent in stage N2 was associated with increased sleep latency on MSLT in both chronic and acute OSA. We also observe that time spent in stage N2 was associated with PVT Lapses in acute OSA, but not in chronic OSA. As reported in previous studies [4, 56], in our study, hours/night usage of CPAP was not associated with the degree of improvement in PVT performance with CPAP. Consistent with previous studies [12], PVT Lapses in chronic and acute OSA were associated in our data with objectively measured daytime sleepiness (MSLT) but not with subjective daytime sleepiness (ESS) [4]. The findings of this study show that sleep macrostructure variables were inconsistently associated to objective daytime function, for which three explanations are possible: (1) OSA severity and arousal index as currently defined are not effective markers of sleep disruption [57], (2) sleep disruption is mediated by other factors such as adaptation in determining its long-term effects, or (3) sleep fragmentation and hypoxia due to OSA-induced brain injury in susceptible subjects, which is only partly reversible by CPAP, leads to adverse daytime functioning.
In this study, CPAP treatment appeared to be associated with normalization of EEG slow waves and their morphological features, suggesting “improvement” in sleep EEG not identified by conventional metrics. Specifically, CPAP use was associated with reduced KC Density and increased %SWA and ΔSWAK, which are findings consistent with previous studies [44, 58–61]. Conversely, in acute OSA, KC Density, %SWA, and ΔSWAK appeared to return to baseline untreated levels, but did not reach statistical significance, perhaps due to the small size of our study. While several studies in OSA subjects have evaluated sleep EEG slow waves, including other morphological features of KCs, and reported their normalization with CPAP treatment [41, 43, 44, 58, 62], to our knowledge, this is a first study that has assessed these sleep EEG markers in acutely induced OSA with CPAP withdrawal. The previous CPAP withdrawal studies used spectral analyses of wake EEG to study the effects of sleep disruption [63]. Our findings suggest that the changes in sleep EEG metrics in acute OSA may reflect an individual susceptibility to acute effects of CPAP withdrawal.
Several studies [17, 18, 30, 40, 64–68] have suggested that slow frequency (<1 Hz) delta waves are markers of cognitive function and of preserved PFC neuropsychological function, especially in healthy elderly [30]. It is also suggested that EEG delta power during NREM sleep is regulated independently of sleep duration and that after a night of sleep deprivation EEG delta waves are enhanced [67]. However, the effect of sleep fragmentation in OSA on EEG slow waves and neurobehavioral impairment is not well established. Most studies in OSA [42, 58] and in insomnia [66] have reported the effect of sleep disruption on EEG slow-wave measures, but not how these measures then relate to daytime function. In the present study, we observe two categories of delta waves, KC's (<1.5 Hz) and ΔSWAK (1–4Hz), to be associated with objective daytime sleepiness measures (MSLT and PVT Lapses). Further, the association of AHI3A with KC Density but not with ΔSWAK suggests that, as other studies [41, 43] have pointed out, while KC Density may be a surrogate of arousals, other morphological features of KCs such as ΔSWAK may be reflective of the sleep protective mechanism of KCs. Specifically, due to the way ∆SWAK is defined: a high ∆SWAK implies that on average KCs overnight are associated with increase in delta activity in stage N2. It is worth noting that the KCs detected using DETOKS in this study had an average 99% occupied bandwidth of 1.4 Hz by design: DETOKS detects KCs by utilizing a low-pass filter with a user-defined cut-off frequency (set as 1 Hz). In contrast to a previous study [44], we did not find an association of NREM %SWA with PVT Lapses.
Significance of our findings
The most important finding of this study is the observation that nocturnal EEG changes correlate to objectively measured daytime function using the PVT in OSA subjects on and off CPAP treatment. Furthermore, we observe that PVT performance in OSA is consistently associated with the ability of a spontaneous stage N2 KC to promote delta waves (∆SWAK). As mentioned in the introduction, while there have been studies that have either probed wake EEG or sleep EEG for obtaining correlates of vigilance [25], the associations have been inconsistent. In contrast, here we observe changes in sleep EEG metrics that are reflective of prefrontal cortical function, to be associated with daytime PVT performance, a task that is known to rely heavily on prefrontal cortical regions. Furthermore, the association was significant on controlling for age, sex, BMI, and subjective sleepiness. However, note that this moderately strong relationship between ΔSWAK, vigilance, and objective daytime sleepiness, which is stronger than traditional NPSG metrics in this study, does not imply that ΔSWAK is the single metric that can fully explain daytime function in OSA subjects. There are several other likely factors—such as genetics, duration of OS, unmeasured metabolic, or neurochemical factors, and perhaps other EEG markers reflective of function in other regions of brain as part of “attention control” network—that may help explain additional variance in neurobehavioral impairment seen in this study.
Limitations
The present study has several limitations which must be kept in mind while interpreting the observed results. First, the low sample size of the present study does not allow for complex analyses such as stratifying sleepy versus non-sleepy subjects using a combination of sleepiness measures [50]. Second, it is known that slow waves and KCs are maximally expressed in frontal regions of the brain [69]. While the present study considered EEG recordings from the frontal channel, to better understand the brain region dysfunction resulting from sleep fragmentation and hypoxia, a high-density EEG (256 electrodes as compared to 20) may provide a better spatial resolution and help evaluate regions other than the PFC. Third, the healthy sleepers, whose data was analyzed in our study, were not matched (age, gender, and BMI) to the OSA subjects and age was a significant predictor in the model relating ΔSWAK and PVT Lapses, as well as in relating change in ΔSWAK to improvement in vigilance. The effect of age on delta waves is reported in many studies [70–72] and thus warrants further investigation of its role in the present study.
Conclusion
The primary intent of this study was to examine night-time EEG markers presumed to reflect prefrontal cortical function in relation to objective daytime function, measured by PVT, to better understand the inter-individual variability in neurobehavioral impairment in OSA on and off treatment. In this study, change in SWA surrounding stage N2 KCs (ΔSWAK) was predictive of PVT performance in OSA subjects cross-sectionally (chronic OSA and acute OSA) as well as longitudinally with CPAP treatment. While, the PVT performance may indicate the ability of a subject to sustain attention, the findings of our study (and the substantial spatial overlap of the brain regions implicated in sustained attention and the brain regions sensitive to sleep fragmentation and hypoxia) suggest that ΔSWAK levels track sleep EEG changes in specific brain regions that are recruited in next-day attentional processes. Thus, we propose that night-time EEG slow waves in OSA subjects may be correlates of OSA-induced structural and/or dysfunctional integrity of brain regions implicated in attention processes. These findings warrant further mechanistic investigation that may help explain patterns of daytime sleepiness in OSA.
Funding
This study was supported by National Institute of Health (NIH) K24HL109156, R01HL081310, R01AG056682, and R21AG059179.
Conflict of interest statement. This was not an industry-supported study. Dr. Rapoport has received research support for grants and clinical trials from Fisher & Paykel Healthcare and consults for Fisher & Paykel Healthcare. In addition, he holds multiple US and foreign patents covering techniques and analysis algorithms for the diagnosis of OSA and techniques for administering CPAP. Several of these have been licensed to Fisher & Paykel Healthcare. Dr. Ayappa has received research support for grants and clinical trials from Fisher & Paykel Healthcare. She also holds multiple US and foreign patents covering techniques and analysis algorithms for the diagnosis of OSA and techniques for administering CPAP. Several of these have been licensed to Fisher & Paykel Healthcare and Advanced Brain Monitoring. The other authors have indicated no financial conflicts of interest.
References
- 1. Rauscher H, et al. Acceptance of CPAP therapy for sleep apnea. Chest. 1991;100(4):1019–1023. [DOI] [PubMed] [Google Scholar]
- 2. Kapur VK, et al. Sleepiness in patients with moderate to severe sleep-disordered breathing. Sleep. 2005;28(4):472–477. [DOI] [PubMed] [Google Scholar]
- 3. Punjabi NM, et al. ; Sleep Heart Health Study Investigators Sleep-disordered breathing, glucose intolerance, and insulin resistance: the Sleep Heart Health Study. Am J Epidemiol. 2004;160(6):521–530. [DOI] [PubMed] [Google Scholar]
- 4. Bhat S, et al. The relationships between improvements in daytime sleepiness, fatigue and depression and psychomotor vigilance task testing with CPAP use in patients with obstructive sleep apnea. Sleep Med. 2018; 49: 81–89. [DOI] [PubMed] [Google Scholar]
- 5. Lindberg E, et al. Role of snoring and daytime sleepiness in occupational accidents. Am J Respir Crit Care Med. 2001;164(11):2031–2035. [DOI] [PubMed] [Google Scholar]
- 6. Li Y, et al. Psychomotor vigilance test and its association with daytime sleepiness and inflammation in sleep apnea: clinical implications. J Clin Sleep Med. 2017;13(9):1049–1056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Yun CH, et al. Daytime sleepiness associated with poor sustained attention in middle and late adulthood. Sleep Med. 2015;16(1):143–151. [DOI] [PubMed] [Google Scholar]
- 8. Sassani A, et al. Reducing motor-vehicle collisions, costs, and fatalities by treating obstructive sleep apnea syndrome. Sleep. 2004;27(3):453–458. [DOI] [PubMed] [Google Scholar]
- 9. Johns MW. A new method for measuring daytime sleepiness: the Epworth Sleepiness Scale. Sleep. 1991;14(6):540–545. [DOI] [PubMed] [Google Scholar]
- 10. Richardson GS, et al. Excessive daytime sleepiness in man: multiple sleep latency measurement in narcoleptic and control subjects. Electroencephalogr Clin Neurophysiol. 1978;45(5):621–627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Dinges DF, et al. Microcomputer analyses of performance on a portable, simple visual RT task during sustained operations. Behav Res Method Instrument Comput. 1985;17(6): 652–655. [Google Scholar]
- 12. Dinges DF, et al. Cumulative sleepiness, mood disturbance, and psychomotor vigilance performance decrements during a week of sleep restricted to 4-5 hours per night. Sleep. 1997;20(4):267–277. [PubMed] [Google Scholar]
- 13. Jewett ME, et al. Dose-response relationship between sleep duration and human psychomotor vigilance and subjective alertness. Sleep. 1999;22(2):171–179. [DOI] [PubMed] [Google Scholar]
- 14. Van Dongen HP, et al. The cumulative cost of additional wakefulness: dose-response effects on neurobehavioral functions and sleep physiology from chronic sleep restriction and total sleep deprivation. Sleep. 2003;26(2):117–126. [DOI] [PubMed] [Google Scholar]
- 15. Strijkstra AM, et al. Subjective sleepiness correlates negatively with global alpha (8-12 Hz) and positively with central frontal theta (4-8 Hz) frequencies in the human resting awake electroencephalogram. Neurosci Lett. 2003;340(1):17–20. [DOI] [PubMed] [Google Scholar]
- 16. Cajochen C, et al. Power density in theta/alpha frequencies of the waking EEG progressively increases during sustained wakefulness. Sleep. 1995;18(10):890–894. [DOI] [PubMed] [Google Scholar]
- 17. D’Rozario AL, et al. A new EEG biomarker of neurobehavioural impairment and sleepiness in sleep apnea patients and controls during extended wakefulness. Clin Neurophysiol. 2013;124(8):1605–1614. [DOI] [PubMed] [Google Scholar]
- 18. Vakulin A, et al. Quantitative sleep EEG and polysomnographic predictors of driving simulator performance in obstructive sleep apnea. Clin Neurophysiol. 2016;127(2):1428–1435. [DOI] [PubMed] [Google Scholar]
- 19. Morisson F, et al. Spectral analysis of wakefulness and REM sleep EEG in patients with sleep apnoea syndrome. Eur Respir J. 1998;11(5):1135–1140. [DOI] [PubMed] [Google Scholar]
- 20. Harrison Y, et al. Sleep loss impairs short and novel language tasks having a prefrontal focus. J Sleep Res. 1998;7(2):95–100. [DOI] [PubMed] [Google Scholar]
- 21. Harrison Y, et al. Prefrontal neuropsychological effects of sleep deprivation in young adults—a model for healthy aging? Sleep. 2000;23(8):1067–1073. [PubMed] [Google Scholar]
- 22. Gozal E, et al. Developmental differences in cortical and hippocampal vulnerability to intermittent hypoxia in the rat. Neurosci Lett. 2001;305(3):197–201. [DOI] [PubMed] [Google Scholar]
- 23. Rosenzweig I, et al. Changes in neurocognitive architecture in patients with obstructive sleep apnea treated with continuous positive airway pressure. EBioMedicine. 2016;7:221–229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Rosenzweig I, et al. The impact of sleep and hypoxia on the brain: potential mechanisms for the effects of obstructive sleep apnea. Curr Opin Pulm Med. 2014;20(6):565–571. [DOI] [PubMed] [Google Scholar]
- 25. D’Rozario AL, et al. Quantitative electroencephalogram measures in adult obstructive sleep apnea—potential biomarkers of neurobehavioural functioning. Sleep Med Rev. 2017;36:29–42. [DOI] [PubMed] [Google Scholar]
- 26. Gui D, et al. Resting spontaneous activity in the default mode network predicts performance decline during prolonged attention workload. Neuroimage. 2015;120:323–330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Drummond SP, et al. The neural basis of the psychomotor vigilance task. Sleep. 2005;28(9):1059–1068. [PubMed] [Google Scholar]
- 28. Tomasi D, et al. Impairment of attentional networks after 1 night of sleep deprivation. Cereb Cortex. 2009;19(1):233–240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Murphy M, et al. Source modeling sleep slow waves. Proc Natl Acad Sci USA. 2009;106(5):1608–1613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Anderson C, et al. Prefrontal cortex: links between low frequency delta EEG in sleep and neuropsychological performance in healthy, older people. Psychophysiology. 2003;40(3):349–357. [DOI] [PubMed] [Google Scholar]
- 31. Varga AW, et al. Effects of aging on slow-wave sleep dynamics and human spatial navigational memory consolidation. Neurobiol Aging. 2016;42:142–149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Mander BA, et al. Prefrontal atrophy, disrupted NREM slow waves and impaired hippocampal-dependent memory in aging. Nat Neurosci. 2013;16(3):357–364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Wennberg R. Intracranial cortical localization of the human K-complex. Clin Neurophysiol. 2010;121(8):1176–1186. [DOI] [PubMed] [Google Scholar]
- 34. De Gennaro L, et al. The fall of sleep K-complex in Alzheimer disease. Sci Rep. 2017;7:39688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Cote KA, et al. Scalp topography of the auditory evoked K-complex in stage 2 and slow wave sleep. J Sleep Res. 1999;8(4):263–272. [DOI] [PubMed] [Google Scholar]
- 36. Steriade M, et al. The slow (< 1 Hz) oscillation in reticular thalamic and thalamocortical neurons: scenario of sleep rhythm generation in interacting thalamic and neocortical networks. J Neurosci. 1993;13(8):3284–3299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Steriade M, et al. Electrophysiological properties of intralaminar thalamocortical cells discharging rhythmic (approximately 40 HZ) spike-bursts at approximately 1000 HZ during waking and rapid eye movement sleep. Neuroscience. 1993;56(1):1–9. [DOI] [PubMed] [Google Scholar]
- 38. Steriade M, et al. Thalamocortical oscillations in the sleeping and aroused brain. Science. 1993;262(5134):679–685. [DOI] [PubMed] [Google Scholar]
- 39. Steriade M, et al. A novel slow (< 1 Hz) oscillation of neocortical neurons in vivo: depolarizing and hyperpolarizing components. J Neurosci. 1993;13(8):3252–3265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Dijk DJ, et al. Effects of SWS deprivation on subsequent EEG power density and spontaneous sleep duration. Electroencephalogr Clin Neurophysiol. 1989;72(4):312–320. [DOI] [PubMed] [Google Scholar]
- 41. Sun L, et al. K-complex morphological features in male obstructive sleep apnea-hypopnea syndrome patients. Respir Physiol Neurobiol. 2018;248:10–16. [DOI] [PubMed] [Google Scholar]
- 42. Tapia IE, et al. Respiratory cortical processing to inspiratory resistances during wakefulness in children with the obstructive sleep apnea syndrome. J Appl Physiol (1985). 2015;118(4):400–407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Parapatics S, et al. K-complex amplitude as a marker of sleep homeostasis in obstructive sleep apnea syndrome and healthy controls. Somnologie. 2015;19:22–29. [Google Scholar]
- 44. Heinzer R, et al. Slow-wave activity in sleep apnea patients before and after continuous positive airway pressure treatment: contribution to daytime sleepiness. Chest. 2001;119(6):1807–1813. [DOI] [PubMed] [Google Scholar]
- 45. Young LR, et al. Response to CPAP withdrawal in patients with mild versus severe obstructive sleep apnea/hypopnea syndrome. Sleep. 2013;36(3):405–412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Force T. Sleep-related breathing disorders in adults: recommendations for syndrome definition and measurement techniques in clinical research. The Report of an American Academy of Sleep Medicine Task Force. Sleep. 1999;22:667–689. [PubMed] [Google Scholar]
- 47. Parekh A, et al. Detection of K-complexes and sleep spindles (DETOKS) using sparse optimization. J Neurosci Methods. 2015;251:37–46. [DOI] [PubMed] [Google Scholar]
- 48. Guilleminault C, et al. Determinants of daytime sleepiness in obstructive sleep apnea. Chest. 1988;94(1):32–37. [DOI] [PubMed] [Google Scholar]
- 49. Budhiraja R, et al. Predictors of sleepiness in obstructive sleep apnoea at baseline and after 6 months of continuous positive airway pressure therapy. Eur Respir J. 2017;50(5):1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Prasad B, et al. Determinants of sleepiness in obstructive sleep apnea. Sleep. 2018;41(2): 1–9. doi:10.1093/sleep/zsx199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Uysal A, et al. Nocturnal hypoxemia biomarker predicts sleepiness in patients with severe obstructive sleep apnea. Sleep Breath. 2014;18(1):77–84. [DOI] [PubMed] [Google Scholar]
- 52. Quan SF, et al. The association between obstructive sleep apnea and neurocognitive performance–the Apnea Positive Pressure Long-term Efficacy Study (APPLES). Sleep. 2011;34(3):303–314B. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Kotterba S, et al. Neuropsychological investigations and event-related potentials in obstructive sleep apnea syndrome before and during CPAP-therapy. J Neurol Sci. 1998;159(1):45–50. [DOI] [PubMed] [Google Scholar]
- 54. Miliauskas S, et al. [Obstructive sleep apnea: factors important for severe daytime sleepiness]. Medicina (Kaunas). 2003;39(3):232–236. [PubMed] [Google Scholar]
- 55. Oksenberg A, et al. Severe obstructive sleep apnea: sleepy versus nonsleepy patients. Laryngoscope. 2010;120(3):643–648. [DOI] [PubMed] [Google Scholar]
- 56. Jackson ML, et al. Neurobehavioral impairment and CPAP treatment response in mild-moderate obstructive sleep apneas. J Clin Sleep Med. 2018;14(1):47–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Rapoport DM. On beyond Zebra (and the Apnea-Hypopnea Index) in obstructive sleep apnea. Am J Respir Crit Care Med. 2018;197(9):1104–1106. [DOI] [PubMed] [Google Scholar]
- 58. Nguyen CD, et al. Mild airflow limitation during N2 sleep increases K-complex frequency and slows electroencephalographic activity. Sleep. 2016;39(3): 541–550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Nicholas CL, et al. Increased production of evoked and spontaneous K-complexes following a night of fragmented sleep. Sleep. 2002;25(8):882–887. [PubMed] [Google Scholar]
- 60. Curcio G, et al. Effect of total sleep deprivation on the landmarks of stage 2 sleep. Clin Neurophysiol. 2003;114(12):2279–2285. [DOI] [PubMed] [Google Scholar]
- 61. Ondze B, et al. Sleep architecture, slow wave activity and sleep spindles in mild sleep disordered breathing. Clin Neurophysiol. 2003;114(5):867–874. [DOI] [PubMed] [Google Scholar]
- 62. Saunamäki T, et al. CPAP treatment partly normalizes sleep spindle features in obstructive sleep apnea. Sleep Disord. 2017;2017:2962479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Filtness AJ, et al. One night’s CPAP withdrawal in otherwise compliant OSA patients: marked driving impairment but good awareness of increased sleepiness. Sleep Breath. 2012;16(3):865–871. [DOI] [PubMed] [Google Scholar]
- 64. Halász P, et al. Are microarousals preceded by electroencephalographic slow wave synchronization precursors of confusional awakenings? Sleep. 1985;8(3):231–238. [DOI] [PubMed] [Google Scholar]
- 65. Bastien CH, et al. EEG characteristics prior to and following the evoked K-Complex. Can J Exp Psychol. 2000;54(4):255–265. [DOI] [PubMed] [Google Scholar]
- 66. Forget D, et al. The role of the spontaneous and evoked k-complex in good-sleeper controls and in individuals with insomnia. Sleep. 2011;34(9):1251–1260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Davis CJ, et al. Delta wave power: an independent sleep phenotype or epiphenomenon? J Clin Sleep Med. 2011;7(5 Suppl):S16–S18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Lee SD, et al. Improvement of EEG slowing in OSAS after CPAP treatment. J Psychosom Res. 2012;73(2):126–131. [DOI] [PubMed] [Google Scholar]
- 69. McCormick L, et al. Topographical distribution of spindles and K-complexes in normal subjects. Sleep. 1997;20(11):939–941. [DOI] [PubMed] [Google Scholar]
- 70. Chinoy ED, et al. Age-related changes in slow wave activity rise time and NREM sleep EEG with and without Zolpidem in healthy young and older adults. Sleep Med. 2014;15(9):1037–1045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Colrain IM, et al. Sleep evoked delta frequency responses show a linear decline in amplitude across the adult lifespan. Neurobiol Aging. 2010;31(5):874–883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Crowley K, et al. The effects of normal aging on sleep spindle and K-complex production. Clin Neurophysiol. 2002;113(10):1615–1622. [DOI] [PubMed] [Google Scholar]