Abstract
We have proposed that arcuate neurons coexpressing kisspeptin, neurokinin B, and dynorphin (KNDy neurons) contribute to hot flushes via projections to neurokinin 3 receptor (NK3R)–expressing neurons in the median preoptic nucleus (MnPO). To characterize the thermoregulatory role of MnPO NK3R neurons in female mice, we ablated these neurons using injections of saporin toxin conjugated to a selective NK3R agonist. Loss of MnPO NK3R neurons increased the core temperature (TCORE) during the light phase, with the frequency distributions indicating a regulated shift in the balance point. The increase in TCORE in the ablated mice occurred despite changes in the ambient temperature and regardless of estrogen status. We next determined whether an acute increase in ambient temperature or higher TCORE would induce Fos in preoptic enhanced green fluorescent protein (EGFP)–immunoreactive neurons in Tacr3-EGFP mice. Fos activation was increased in the MnPO but no induction of Fos was found in NK3R (EGFP-immunoreactive) neurons. Thus, MnPO NK3R neurons are not activated by warm thermosensors in the skin or viscera and are not warm-sensitive neurons. Finally, RNAscope was used to determine whether Tacr3 (NK3R) mRNA was coexpressed with vesicular glutamate transporter 2 or vesicular γ-aminobutyric acid (GABA) transporter mRNA, markers of glutamatergic and GABAergic neurotransmission, respectively. In the MnPO, 94% of NK3R neurons were glutamatergic, but in the adjacent medial preoptic area, 97% of NK3R neurons were GABAergic. Thus, NK3R neurons in the MnPO are glutamatergic and play a role in reducing TCORE but are not activated by warm thermal stimuli (internal or external). These findings suggest that KNDy neurons modulate thermosensory pathways for heat defense indirectly via a subpopulation of glutamatergic MnPO neurons that express NK3R.
Hot flushes occur in most menopausal women and are secondary to estrogen withdrawal (1, 2). Hot flushes are characterized by the activation of heat-dissipation effectors, including cutaneous vasodilation, sweating, and cold-seeking behavior (1–3). We have hypothesized that arcuate neurons coexpressing kisspeptin, neurokinin B, and dynorphin (KNDy neurons) in the human infundibular (arcuate) nucleus are the link between estrogen withdrawal and the occurrence of hot flushes (4, 5). In the hypothalamus of postmenopausal women, KNDy neurons exhibit somatic hypertrophy (4, 6) and express estrogen receptor-α (7), with increased expression of the kisspeptin and neurokinin B (NKB) genes (4, 6). These changes are secondary to estrogen withdrawal, because they have been duplicated by ovariectomy in young monkeys and are reversed with estrogen replacement (6, 8, 9). KNDy neurons play an important role in stimulating pulsatile GnRH secretion (10–13) and LH pulses have been closely timed with hot flushes in women (14–16). Thus, insights into how KNDy neurons influence the central neural circuits controlling heat-dissipation effectors are relevant to understanding the etiology of hot flushes.
We have previously described a neural circuit in which KNDy neurons influence the central thermoregulatory pathways for heat defense in the rat (5). KNDy neurons project to the median preoptic nucleus (MnPO) and medial preoptic area (MPA) (17, 18), two loci in the central heat-defense pathway that express neurokinin 3 receptors (NK3R), the primary receptor for NKB (19, 20). In the rat, microinfusion of a NK3R agonist (senktide) directly into the MnPO increases MnPO Fos expression and markedly reduces the core temperature (TCORE), consistent with activating heat dissipation effectors (20). Systemic injections of senktide also induce MnPO Fos and hypothermia, and this effect is blocked by ablation of NK3R neurons in the MnPO/septal area (21). Finally, ablation of KNDy neurons consistently decreases tail skin vasodilation and partially blocks the effects of estradiol-17β (E2) on thermoregulation (22). Because vasodilation is a cardinal sign of menopausal flushes, these findings strongly support the hypothesis that KNDy neurons contribute to the generation of flushes.
More recently, the mouse has been used as a model to investigate the role of KNDy neurons and NK3R signaling in the generation of flushes. Similar to a hot flush, subcutaneous injections of senktide in the mouse results in tail skin vasodilatation, cold-seeking behavior, and hypothermia (23, 24). The effects of senktide are reduced by E2 treatment of ovariectomized (OVX) mice, suggesting that E2 lowers the sensitivity of thermoregulatory pathways to NK3R activation (23). Moreover, optogenetic activation of KNDy neurons in Kiss1Cre mice induces tail skin vasodilation and hypothermia, accompanied by MnPO Fos expression (25). Tail skin vasodilation and hypothermia is also induced by optogenetic activation of KNDy neuron terminals in the preoptic area (25). Finally, the tail skin vasodilation that is produced by chemogenetic activation of KNDy neurons is blocked by a previous infusion of neurokinin receptor antagonists into the preoptic area and potentiated by ovariectomy (25). These data have confirmed a role for KNDy neurons in the activation of heat dissipation effectors via preoptic NKB signaling in the mouse (25).
Consistent with the rodent studies, clinical data have strongly implicated NKB/NK3R signaling in the generation of hot flushes in women. In a genome-wide association study of >17,000 women, the occurrence of hot flushes was associated with 14 single nucleotide polymorphisms in the TACR3 gene encoding NK3R (26). Thus, genetic polymorphisms in the TACR3 gene could help explain the variable presentation of hot flushes in women. Moreover, intravenous infusion of NKB in young, healthy women induces hot flushes (27). Importantly, the number and severity of hot flushes is markedly reduced by administration of NK3R antagonists (28–31). As a result, there is now intense interest in the potential of NK3R antagonists to provide a targeted treatment of hot flushes that does not rely on estrogen replacement (32, 33).
Although clinical studies have implicated NK3R signaling in the occurrence of hot flushes, there is limited understanding of how MnPO NK3R neurons influence thermoregulation. In the present study, we examined the thermoregulatory effects of ablating NK3R neurons in the MnPO using focal injections of NK3-saporin (SAP; a conjugate of a selective NK3R agonist and SAP). To determine whether MnPO neurons are a direct component of the thermosensory heat-defense pathway, Fos immunohistochemistry was used to examine whether NK3R neurons are activated by warm cutaneous thermosensors or an increased TCORE. Finally, dual-label fluorescent in situ hybridization was used to determine whether NK3R mRNA is colocalized with vesicular glutamate transporter 2 (VGLUT2) or vesicular γ-aminobutyric acid (GABA) transporter (VGAT) mRNA, markers of glutamatergic or GABAergic neurotransmission, respectively. The goal of these studies was to provide information on how NK3R neurons in the MnPO interface with the central thermoregulatory pathways for heat defense in the mouse.
Materials and Methods
Animals
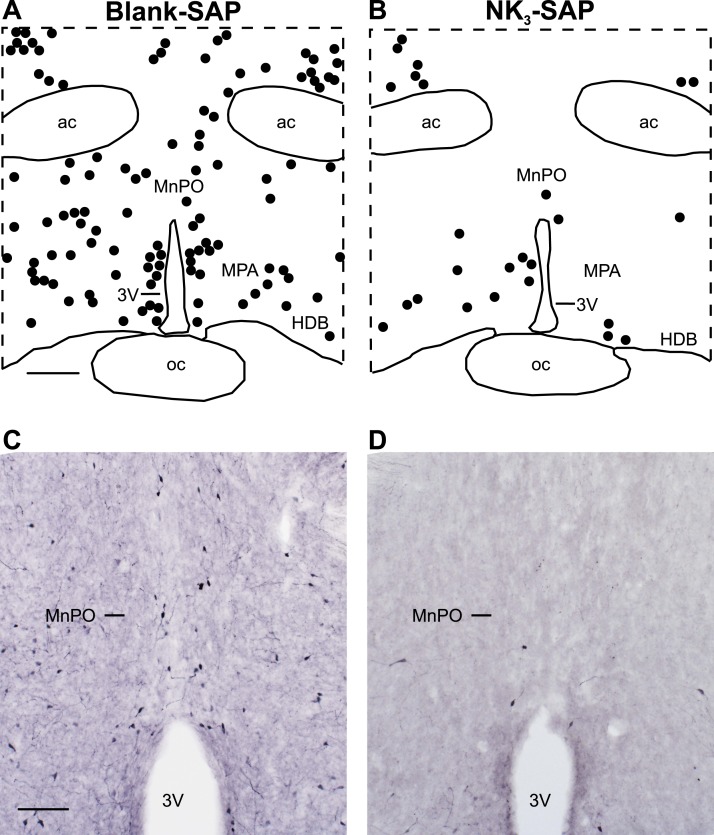
Because the current NK3R antibodies predominantly label cell processes or puncta in the MnPO (20, 21), we used a Tacr3- enhanced green fluorescent protein (EGFP) mouse to visualize NK3R cell bodies. These mice were generated by the Gene Expression Nervous System Atlas Project at Rockefeller University using a bacterial artificial chromosome to insert an EGFP reporter under translational control of the Tacr3 promoter (34). The veracity of Tacr3-EGFP expression was confirmed by comparing EGFP expression in serial sections of the brain and spinal cord to the Allen in situ hybridization atlas and available data (available at: www.gensat.org). In our laboratory, the labeling of EGFP-immunoreactive (ir) cell bodies in the MnPO and other regions closely matched the expression of Tacr3 mRNA by in situ hybridization (described in the results of Experiment 4) and the expression of NK3R mRNA in the Allen Brain Atlas (available at: www.brain-map.org). Further evidence of the validity of this reporter was provided by the loss of EGFP-ir neurons after injecting NK3-SAP, a neurotoxin that is selective for neurons expressing NK3R (35) (Fig. 1).
Figure 1.
Focal injections of NK3-SAP ablated NK3R neurons in the MnPO and adjacent MPA in Tacr3-EGFP mice. Representative (A, B) computer-assisted maps and (C, D) photomicrographs of EGFP-ir neurons in (Left) blank-SAP and (Right) NK3-SAP–injected mice. (A) Scale bar, 250 µm for (A) and (B). (C) Scale bar, 100 µm for (C) and (D). 3V, third ventricle; ac, anterior commissure; HDB, horizontal limb of the diagonal band; oc, optic chiasm.
Heterozygous, male Tacr3-EGFP mice [Stock Tg (Tacr3-EGFP) JM102Gsat/Mmucd; model no. 030693-UCD] were purchased from the Mutant Mouse Regional Resource Center (University of California, Davis, CA). Male Tacr3-EGFP mice were bred with female Hsd:ICR (CD-1) mice (Envigo, Houston, TX) to establish the colony. The litters were genotyped by PCR for EGFP from tail DNA (Mouse Genotype, Escondido, CA). Heterozygous Tacr3-EGFP mice were bred to generate the homozygous Tacr3-EGFP line used in the experiments. To distinguish homozygous from heterozygous mice, quantitative PCR for EGFP DNA was performed by Charles River Laboratories (Wilmington, MA).
The mice were housed in the University of Arizona Animal Care Facility with a 12-hour light, 12-hour dark cycle (lights on at 7:00 am). Water and low phytoestrogen chow (Teklad 2019 Global 19% Protein Extruded Rodent Diet; Envigo) were available ad libitum. The mice were kept in shoebox-style cages with nesting material in sibling groups (two to five mice), except for during the circadian rhythm recordings when they were housed individually. The institutional animal care and use committee at the University of Arizona approved the animal protocols, which followed the National Institutes of Health guidelines.
Experiment 1: to examine the effects of ablating MnPO NK3R neurons on estrogen modulation of body temperature
NK3-SAP (catalog no. IT-63; Advanced Targeting Systems, San Diego, CA) was used to ablate NK3R neurons in the MnPO. NK3-SAP is a conjugate of SAP, a ribosome-inactivating toxin, and [MePhe7]NKB, an NK3R agonist that is 1000 times more selective for NK3R than other neurokinin receptors (36–38). The use of NK3-SAP to selectively ablate NK3R neurons has been previously characterized in the rat (35), a species that has NK3R binding sites identical to those of the mouse (39). Control mice received injections of blank-SAP, an 11-amino acid–scrambled peptide conjugated to SAP (catalog no. IT-21; Advanced Targeting Systems).
We anesthetized 22 female Tacr3-EGFP mice (age, 5.5 to 6.5 months; weight, 33.8 to 67.2 g) with 60 mg/kg ketamine, 12 mg/kg xylazine, and 2 mg/kg acepromazine, and placed them in a stereotaxic device. Topical lidocaine (7 mg/kg) was injected at the scalp incision site. The mice were bilaterally injected with 10 ng NK3-SAP in 100 nL PBS (n = 14) or blank-SAP (n = 8) in the preoptic area adjacent to the MnPO (coordinates, 0.4 mm anterior to the bregma, ±0.25 mm lateral from the midline, and −5.0 mm ventral to the skull surface). The injections were performed using a NanoFil 10-µL syringe and a 33-gauge beveled tip needle attached to an UltraMicroPump with a Micro 4 controller (UMP3; World Precision Instruments, Sarasota, FL). The injections were performed during a 5-minute period (20 nL/min), and the needle was left in place for 5 minutes before removal.
Six to seven days later, the mice were bilaterally OVX under isoflurane anesthesia, and a DSI telemetry transmitter (TA-F10; Data Sciences International, St. Paul, MN) was implanted IP to measure the TCORE and activity. To measure the tail skin temperature (TSKIN), a Star-Oddi Nano-T data storage tag temperature probe (emka TECHNOLOGIES, Falls Church, VA) was placed into a customized Delrin housing (WB Enterprises, Tucson, AZ) and attached to the tail using Loctite 454 prism glue (Fisher Scientific, Pittsburgh, PA), as previously described (23). The tails were monitored daily, and the plastic housing was removed for 12 hours if swelling or erythema was observed. Approximately 3 weeks after stereotaxic surgery (day 28 to 29), the mice were anesthetized with isoflurane and a Silastic capsule was implanted SC (1.57-mm inner diameter, 3.18-mm outer diameter, 20-mm effective length; Dow Corning Corp., Midland, TX) containing 180 µg/mL of E2 dissolved in sesame oil. Our previous studies showed that this regimen of estradiol replacement produces low physiologic levels of serum E2 at 7 and 14 days after implantation (23).
Circadian recordings of TCORE and TSKIN
The circadian temperatures were recorded from the mice during two 3-day intervals (10 to 13 days after OVX and 3 to 6 days after E2 capsule implantation). These experiments were conducted in a dedicated room in the animal facility (relatively free from noise and interruptions) using an ambient temperature (TAMBIENT) of ∼24°C (range, 23.2° to 24.9°C). The individual animals in their home cages were placed on Physiotel receiver boards (Data Sciences International) and TCORE, TSKIN, and activity were recorded every 5 minutes. Although housed separately, the mice still had visual, olfactory, and auditory exposure to the adjacent mice. The TAMBIENT was measured using a C10T converter (Data Sciences International).
Exposure to various TAMBIENT
These experiments were performed 14 to 17 days after OVX and 7 to 9 days after E2 capsule implantation. The mice were brought to the laboratory and placed inside plastic (open to air) grid cages (Nalgene 17.8 × 16.8 × 15.6 cm; Thermo Scientific, Asheville, NC) with ad libitum access to food and water. The cages were placed on Physiotel receiver boards in an environmental chamber (Forma model 2940; Thermo Scientific). To reduce stress, the mice were acclimated to the environmental chamber three times before the experiments. During a 3-day period (from 8:00 am to 2:00 pm), the mice were exposed to different TAMBIENT (18°, 21°, 25°, 28°, 32°, and 35°C) with 50% humidity (2 TAMBIENT/d, randomly selected). The TCORE, TSKIN, TAMBIENT, and activity were recorded every minute for 3 hours.
At 44 to 46 days after stereotaxic surgery, the mice were anesthetized and perfused with 4% paraformaldehyde in phosphate buffer (pH 7.4). The brains were removed, postfixed in 4% paraformaldehyde for 4 hours at 4°C, and cryoprotected in ascending sucrose solutions (10%, 20%, and 30% in 0.1 M of PBS; pH 7.4) during a 3- to 5-day interval. Blocks containing the anterior hypothalamus and septal region were serially sectioned (40 µm, coronal) using a sliding microtome. The sections were stored in cryoprotectant solution at −20°C (40).
Experiment 2: to determine whether an acute increase in TAMBIENT induced Fos in preoptic NK3R neurons
A total of 36 female Tacr3-EGFP mice (age, 2.5 to 3.5 months; weight, 26.6 to 40.8 g) were bilaterally OVX under isoflurane anesthesia. A SubCue data logger (Canadian Analytical Technologies, Calgary, AB, Canada) was implanted IP to record the TCORE. Seven days later, the mice were anesthetized with isoflurane, and a Silastic capsule was implanted SC containing either sesame oil (OVX) or E2 dissolved in sesame oil (OVX + E2), as described.
One week after capsule implantation, the mice were brought to the laboratory, housed individually inside plastic grid cages with ad libitum food and water, and placed into the environmental chamber with the temperature set at 25°C with 50% humidity. To reduce stress, the mice were acclimated to the environmental chamber twice before the experiment. After ≥2 hours of acclimation, the setting of the TAMBIENT was either left at 25°C or increased to 35°C. The TCORE was recorded every 10 minutes, and the TAMBIENT was recorded every 5 minutes using an IT-18 thermocouple (Physitemp, Clifton, NJ) attached to a TC101A temperature data logger (MadgeTech, Warner, NH). Finally, 90 minutes after the change in TAMBIENT, the mice were anesthetized and perfused with fixative, and the brains were harvested as described. All the procedures were completed by 12:30 pm.
Experiment 3: to determine whether an elevated TCORE induces Fos in preoptic NK3R neurons
To reduce the animal numbers, this experiment was only performed on intact mice. We placed 20 diestrous Tacr3-EGFP mice (age, 4.5 to 5 months; weight, 29.1 to 60.4 g) individually inside plastic grid cages with ad libitum food and water in the environmental chamber. The TAMBIENT was set at 25°C, with 50% humidity. To reduce stress, the mice were acclimated to the environmental chamber twice before the experiment. After 2 hours of acclimation, the TAMBIENT was either left at 25°C (n = 8) or increased to 36°C to increase the TCORE (n = 12). After 2.5 to 3.0 hours, the mice were anesthetized, perfused with fixative, and the brains were harvested, as described. Four of these mice had been previously implanted IP (under isoflurane anesthesia) with DSI telemetry probes to determine the effects of this treatment on TCORE. All procedures were completed by 1:30 pm.
Experiment 4: to determine whether preoptic NK3R neurons are glutamatergic or GABAergic
Eight intact female Tacr3-EGFP mice (age, 5 to 6 months) were anesthetized with isoflurane and decapitated. The brains were rapidly removed, and coronal blocks of the preoptic area and anterior hypothalamus were blocked using a mouse brain matrix. The blocks were snap frozen in isopentane on dry ice and stored at −80°C. Coronal sections (20 µm thick) were cut on a cryostat, mounted on Superfrost Plus slides (Thermo Fisher Scientific, Waltham, MA) and stored at −80°C. The sections were processed for dual-label in situ hybridization using the RNAscope Multiplex Fluorescent Reagent, version 2, Kit (ACD no. 323100; Advanced Cell Diagnostics, Hayward, CA). The probes were complementary to mouse NK3R (ACD no. 481671; Tacr3 gene), mouse VGLUT2 (ACD no. 319171; Slc17A6 gene), and mouse VGAT (ACD no. 319191; Slc32a1 gene). Incubation steps were performed using a humidity tray and slide rack (ACD no. 31002) and the HybEZ™ II hybridization oven (ACD no. 321710). Opal 520 (catalog no. FP1487001KT; Perkin Elmer, Waltham, MA) and cyanine 3 (catalog no. NEL744001KT; Perkin Elmer) were used for visualization with 4′,6-diamidino-2-phenylindole counterstain to visualize the cell nuclei. Positive and negative controls were incorporated into each procedure to verify RNA quality and specific staining.
EGFP and Fos immunohistochemistry
Antibodies
The polyclonal chicken anti-green fluorescent protein (GFP) antibody (41) (lot no. 122FP08) was raised in chicken eggs against recombinant GFP emulsified in Freund adjuvant. This antibody does not label neurons in wild-type mouse tissue. A single band of ~24 kDA was identified on Western blots of whole mouse embryo homogenates (Aves Laboratories, Davis, CA). The polyclonal rabbit anti–c-Fos antibody (42) (lot no. D36405) was directed against amino acids 4 to 17 of the human c-Fos protein. This antibody has been previously verified using Fos knockout mice and preadsorption experiments (43, 44).
Single-label EGFP immunohistochemistry
Sections were rinsed in 0.1 M PBS, pretreated with 0.3% H2O2 in PBS, and incubated in blocking solution (3% normal goat serum and 0.4% Triton-X 100 in PBS). The sections were incubated with the primary GFP antibody (1:5 K in blocking solution) for 48 hours, followed by an overnight incubation with biotinylated goat anti-chicken (45) (1:5 K in blocking solution). The sections were then ABC amplified using a Vectastain Elite ABC kit (46), incubated with biotinyl tyramide (Perkin Elmer, Boston, MA), and again ABC amplified. The sections were rinsed in 0.175 M sodium acetate, and the signal was visualized using nickel-intensified 3,3′-diaminobenzidine (DAB; 0.0025% w/v nickel(II) sulfate, 0.02% w/v DAB, 0.025% v/v H2O2 in 0.175 M sodium acetate). The sections were dehydrated and coverslipped.
Dual-label Fos/EGFP immunohistochemistry
Fos was visualized with nickel-intensified DAB (black; nuclear stain), and EGFP was visualized with tyramide signal-amplified DAB (brown; cytoplasmic stain). The sections were rinsed with PBS and pretreated with 15 mM sodium citrate (pH 8.8; 80°C) and 0.3% H2O2. The sections were incubated with blocking solution (as described), rabbit anti-Fos (1:20 K in blocking solution) for 48 hours, and overnight with biotinylated goat anti-rabbit (47) (1:5 K in blocking solution). The sections were ABC amplified (Vectastain Elite ABC Kit) and visualized using nickel-intensified DAB, as described. The sections were then incubated with the blocking solution, followed by chicken anti-GFP (1:5 K in blocking solution for 48 hours) and overnight treatment with biotinylated goat anti-chicken (1:600 in blocking solution). The sections were then ABC amplified (Vectastain Elite ABC kit), incubated with biotinyl tyramide (Perkin Elmer), and again ABC amplified. The sections were rinsed in 0.05 M Tris buffer (pH 7.2), and the signal was visualized using DAB (0.04% w/v DAB and 0.05% v/v H2O2 in 0.05 M Tris buffer). The sections were dehydrated and coverslipped.
Data analysis
Experiment 1
Quantitative analysis of preoptic NK3R-expressing neurons in mice injected with NK3-SAP or blank-SAP.
To identify brain landmarks, Nissl stains were performed on every 10th section. EGFP immunohistochemistry was performed on every fifth section between plates 25 and 36 of the mouse brain atlas (48). Additional sections for EGFP immunohistochemistry were matched to the level of plates 28 and 29 for quantitative analysis (48). Labeled cells were mapped using an image-combining computer microscope (Nikon, Tokyo, Japan) outfitted with a motorized stage (LUDL Electronics Products, Hawthorne, NY), a Lucivid miniature CRT, and Neurolucida software (MicroBrightfield, Williston, VT). Tissue boundaries and regions were outlined, the sections were systematically scanned, and the locations of EGFP-ir neurons were manually marked. The numbers of labeled neurons within the defined brain regions were summed for each mouse, and these values were used to calculate the percentage of loss of NK3R neurons. Three NK3-SAP–treated mice were excluded from further analysis because of missed injections (preservation of NK3-containing neurons after injection of NK3-SAP).
For circadian recordings, the data were binned into 3-hour blocks during the 24-hour period. To evaluate the stability of the TCORE over time, a frequency distribution of the TCORE was generated from 6-hour blocks in the light (10:00 am to 4:00 pm) and dark (10:00 pm to 4:00 am) phases (49). For mice exposed to various TAMBIENT, the data were analyzed using the third hour of recording, which allowed for 2 hours of acclimation. The heat loss index (HLI) was calculated by the formula: HLI = (TSKIN − TAMBIENT)/(TCORE − TAMBIENT) (50). The values were calculated for each mouse, and these values were used for the group averages (± SEM). Statistical comparisons were performed using two-way ANOVA and Tukey post hoc analysis with α ≤ 0.05.
Experiments 2 and 3
Quantitative analysis of Fos and EGFP-ir labeling in the preoptic area of Tacr3-EGFP mice.
The sections matched to plate 29 of the mouse atlas (48) were mapped using the computer microscope system as described. The mice were excluded if sectioning artifact had obscured the MnPO. Single- and double-labeled Fos and EGFP-ir neurons were manually marked, and the number of labeled neurons was tallied for each mouse. These values were used to calculate the group averages. The groups were compared using two-way ANOVA (experiment 2) and Tukey post hoc analysis (α ≤ 0.05) or a t test (experiment 3). The TCORE was analyzed using two-way ANOVA with repeated measures and Tukey post hoc analysis with α ≤ 0.05.
Experiment 4
Mapping of neurons expressing NK3R, VGLUT2, or VGAT mRNA.
The sections were mapped at the level of plate 29 of the mouse atlas (48). The fluorescent microscope system included a Nikon Eclipse E1000 fluorescence microscope with an epifluorescent attachment, a motorized stage (LUDL Electronic Products), and a Uniblitz model VMM-D1 shutter driver (Vincent Associates, Rochester, NY). The images were captured with a 20× objective using a Photmetrics Coolsnap FX camera (Roper Technologies, Inc., Sarasota, FL) and MetaMorph imaging software (Molecular Devices, Sunnyvale, CA). The imaging software was used to assemble the images into montages. The preoptic area was then manually scanned through the microscope at 40×, and the locations of single- and dual-labeled Tacr3 mRNA-containing neurons were marked on the montages using MetaMorph imaging tools. For the fluorescent illustrations, Adobe Photoshop was used to adjust for the brightness and contrast.
Results
NK3-SAP injections ablated NK3R neurons in the MnPO
Sections from Tacr3-EGFP mice exhibited dark staining of EGFP-ir neurons and fibers with low background staining. Small scattered neurons were identified in the MnPO, MPA, and bed nucleus of the stria terminalis. Large, multipolar neurons were identified in the diagonal band and septal region. The distribution of EGFP-ir neurons (Fig. 1A) closely matched the labeling of neurons expressing Tacr3 mRNA in the Allen Mouse Brain Atlas (available at: www.brain-map.org) and the expression of Tacr3 mRNA observed with in situ hybridization (experiment 4).
In the Nissl-stained sections, no qualitative difference in cell density was identified between the NK3-SAP and control mice. Except for a needle track, no lesion was identified at the injection sites. Immunohistochemistry for EGFP, however, revealed a substantial loss (77% ± 3%) of NK3R neurons in the MnPO of NK3-SAP–injected mice (Fig. 1). EGFP-ir cell loss also extended along the needle track into the septal region. In the rostral MPA (at the level of the MnPO), NK3-SAP reduced the number of EGFP-ir neurons by 61% ± 8%. Because no EGFP cell loss was present at the caudal levels of MPA (starting at plate 32), most NK3R neurons in the MPA were preserved. No qualitative loss of EGFP was identified in the anterior sections corresponding to plate 25 or 26 or in the posterior sections from plates 32 to 36 (48). The NK3-SAP injections produced no qualitative loss in EGFP-ir neurons in the medial preoptic nucleus, anterior hypothalamus, paraventricular nucleus, or supraoptic nucleus.
Ablation of MnPO NK3R neurons produced a regulated increase in TCORE during the light phase but did not block the effects of E2
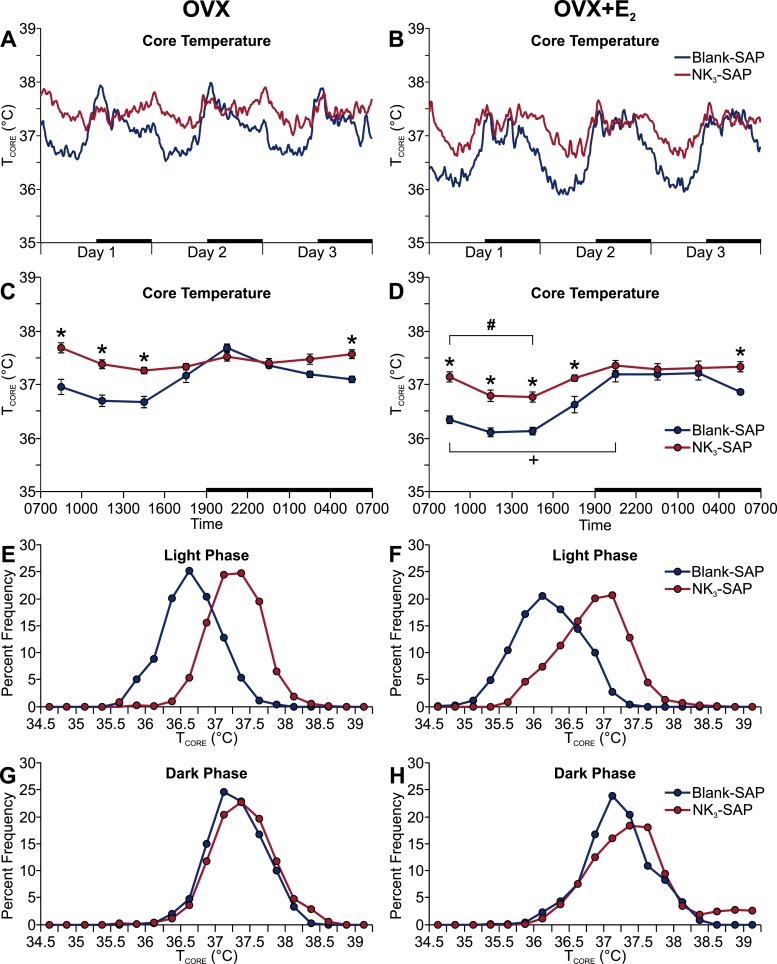
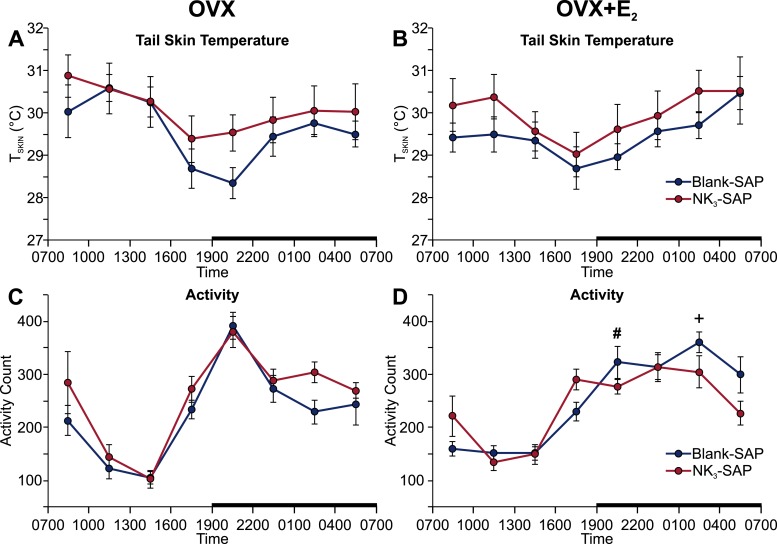
The TCORE was significantly elevated in NK3-SAP mice (compared with the blank-SAP controls) during the light phase in both OVX and OVX plus E2 groups (Fig. 2A–2D). NK3-SAP mice also exhibited a higher TCORE at the end of the dark phase (Fig. 2C and 2D). During the light phase, the NK3-SAP mice exhibited a frequency distribution that was virtually identical to that of the control mice but had shifted to a higher TCORE (Fig. 2E and 2F). These data indicate a regulated shift in the balance point of TCORE with similar thermal stability (49). Similar to our previous studies in the mouse (23), E2 significantly reduced TCORE during the light phase in both NK3-SAP and control mice (Fig. 2C and 2D). In contrast to the effects on TCORE, no effect was found for NK3R neuron ablation on TSKIN or activity (Fig. 3). Although the circadian rhythms of TCORE were blunted in NK3-SAP mice (Fig. 2A–2D), the circadian activity rhythms were preserved (Fig. 3C and 3D).
Figure 2.
Ablation of NK3R neurons in the MnPO increased the average TCORE during the light phase in both (Left) OVX and (Right) OVX plus E2 treated mice. (A, B) Average TCORE lines were generated using a moving average of five data points. (C, D) TCORE data were binned into 3-hour blocks (beginning at 7:00 am) for statistical comparisons. (A–D) Black bars on the x-axes denote the dark phase. (E–H) Frequency distributions of TCORE during 6-hour intervals of the (E, F) light phase (10:00 am to 4:00 pm) and (G, H) dark phase (10:00 pm to 4:00 am). n = 8 to 9 mice per group. *P < 0.01, blank-SAP vs NK3-SAP; +P < 0.01, OVX vs OVX + E2, within blank-SAP; #P < 0.01, OVX vs OVX + E2, within NK3-SAP.
Figure 3.
Ablation of NK3R neurons in the MnPO did not alter circadian rhythms of (A, B) TSKIN or (C, D) activity in (Left) OVX or (Right) OVX + E2 mice. The data were binned into 3-hour blocks (beginning at 7:00 am) for statistical comparisons. Black bars on the x-axes denote the dark phase. n = 7 to 9 mice per group. +P < 0.01, OVX vs OVX + E2, within blank-SAP; #P < 0.05, OVX vs OVX + E2, within NK3-SAP.
TCORE was elevated in MnPO NK3R-ablated mice exposed to a range of TAMBIENT
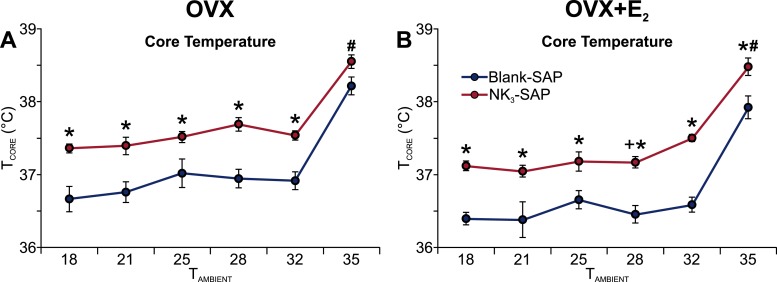
The average TCORE of NK3-SAP mice was higher than the TCORE of blank-SAP controls at multiple TAMBIENT, ranging from 18° to 32°C (Fig. 4A and 4B). At the TAMBIENT of 35°C, the TCORE was significantly elevated in OVX plus E2 NK3-SAP mice (compared with the OVX plus E2 blank-SAP mice). In both NK3-SAP and control mice, E2 treatment significantly reduced the TCORE at the TAMBIENT of 28°C (Fig. 4A and 4B). In all groups, the TCORE was significantly higher in the mice exposed to a TAMBIENT of 35°C (Fig. 4A and 4B). At all TAMBIENT, the TSKIN and HLI of the NK3-SAP mice were similar to those of the control mice (data not shown).
Figure 4.
Ablation of preoptic NK3R neurons resulted in a higher TCORE at multiple TAMBIENT. (A, B) Average TCORE in (Left) OVX or (Right) OVX + E2 mice at various TAMBIENT. n = 7 to 11 mice per group. *P = 0.01, blank-SAP vs NK3-SAP; +P < 0.05, OVX vs OVX + E2 (applies to both NK3-SAP and blank-SAP mice); #P < 0.01, elevated at TAMBIENT of 35°C compared with all other TAMBIENT.
The body weights between the NK3-SAP and blank-SAP mice were not significantly different at any time after stereotaxic surgery. At 14 days after stereotaxic surgery, the blank-SAP mice weighed 39.8 ± 2.3 g (n = 8), and the NK3-SAP mice weighed 37.6 ± 1.7 g (n = 11). On day 45, the blank-SAP and NK3-SAP mice weighed 40.2 ± 1.6 g and 37.6 ± 1.1g, respectively. No correlation was observed within the groups between body weight and TCORE at any TAMBIENT.
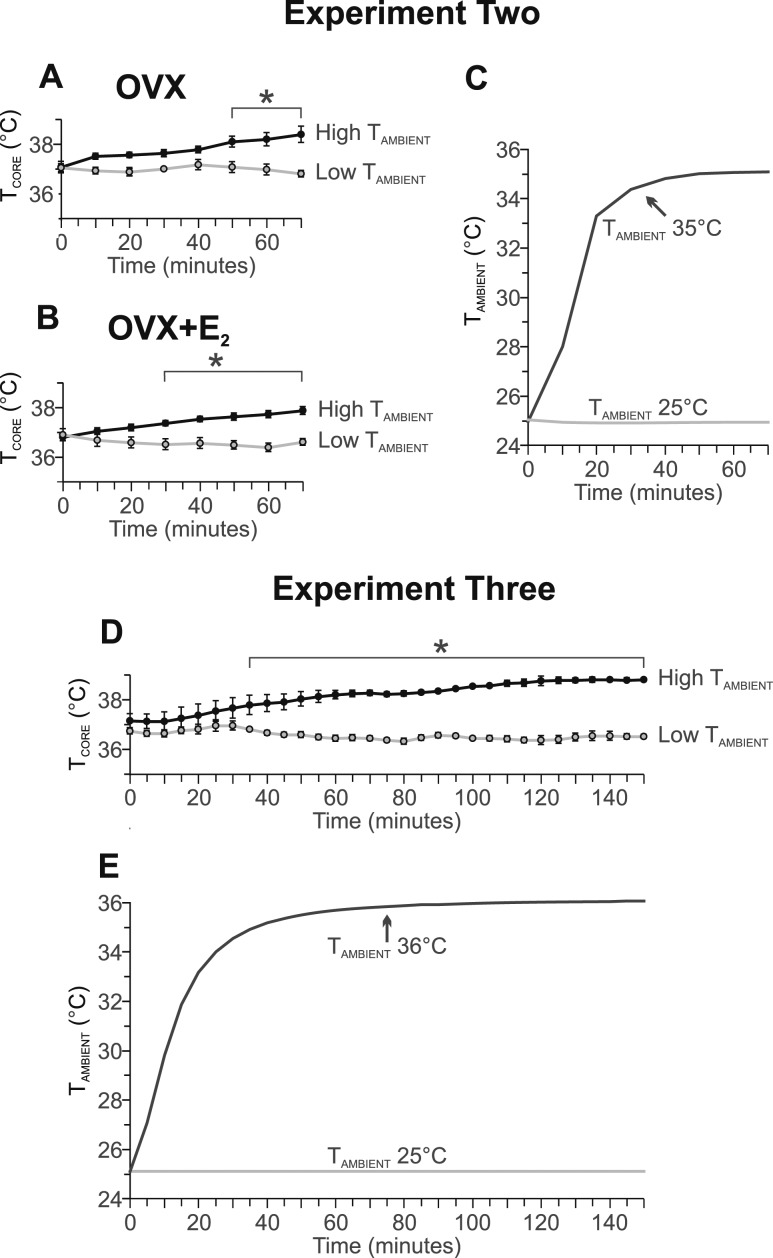
Experiment 2: an acute increase in TAMBIENT increased Fos in the MnPO but not in NK3R neurons
Increasing the temperature setting of the environmental chamber resulted in an increase in TAMBIENT from 25°C to 30°C within 10 minutes and to 35°C by 30 minutes (Fig. 5C). At 50 minutes after increasing the TAMBIENT, the TCORE of the OVX mice was significantly elevated (Fig. 5A). At 30 minutes after increasing the TAMBIENT, the TCORE was elevated in the OVX plus E2 mice (Fig. 5B).
Figure 5.
Graphs showing the (A, B, D) average TCORE and (C, E) TAMBIENT in experiments (A–C) 2 and (D, E) 3. At time 0, the thermostat setting of the environmental chamber remained at 25°C or was increased to 35°C (experiment 2) or 36°C (experiment 3). Experiment 2, n = 8 to 9 mice per group; experiment 3, n = 4 mice per group. *P < 0.01, TCORE in mice exposed to the higher TAMBIENT compared with the low TAMBIENT of 25°C.
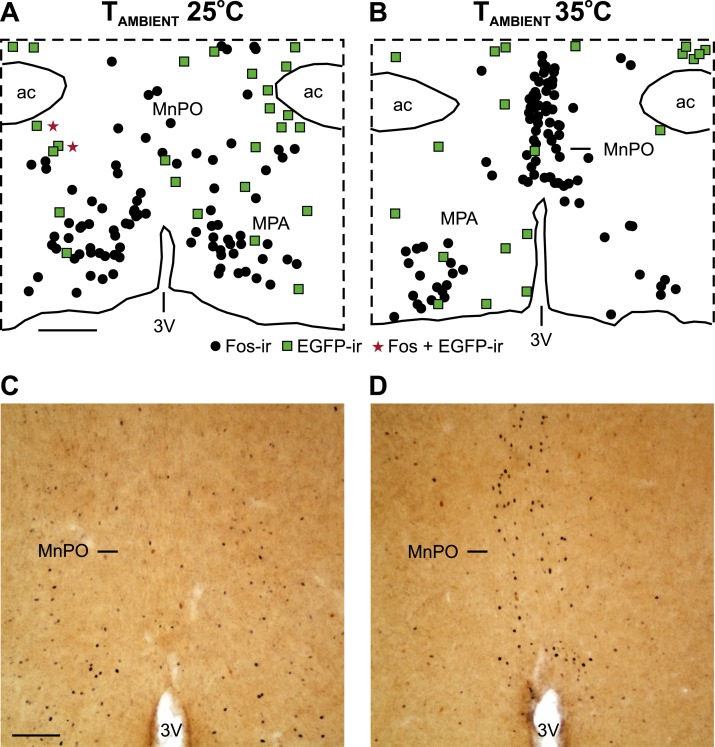
In the OVX mice, the acute increase in the TAMBIENT resulted in a fivefold increase in the number of Fos-ir neurons in the MnPO (Fig. 6; Table 1). In the OVX plus E2 mice, the number of Fos-ir cells in the MnPO was also increased; however, this difference was not statistically significant (Table 1). At the TAMBIENT of 35°C, the OVX mice exhibited substantially more Fos-ir cells in the MnPO than did the OVX plus E2 mice (Table 1). The higher TAMBIENT did not increase the number of Fos-ir neurons in the adjacent MPA.
Figure 6.
An acute increase in TAMBIENT activated Fos in the MnPO but not in preoptic NK3R neurons. (A, B) Maps of NK3R (EGFP-ir neurons, green squares), Fos-ir neurons (black circles), and dual labeled neurons (red stars) in a representative OVX mouse exposed to TAMBIENT of (A) 25°C and (B) 35°C. (C, D) Photomicrographs showing the increase in Fos-ir neurons in the MnPO (black; nuclear stain) at a TAMBIENT of 35°C. EGFP-ir neurons not expressing Fos can also be seen (brown; cytoplasmic stain). (A) Scale bar, 250 µm for (A) and (B). (C) Scale bar, 100 µm for (C) and (D). 3V, third ventricle; ac, anterior commissure.
Table 1.
Effect of Acute Increase in TAMBIENT on Average ± SEM Number of Fos, EGFP, and Dual-Labeled Fos/EGFP-ir Cells in OVX and OVX Plus E2Tacr3-EGFP Mice
| Variable | TAMBIENT 25°C |
TAMBIENT 35°C |
||
|---|---|---|---|---|
| OVX (n = 7) | OVX + E2 (n = 4) | OVX (n = 8) | OVX + E2 (n = 6) | |
| MnPO | ||||
| Fos | 6.9 ± 2.1 | 4.3 ± 1.4 | 37.9 ± 10.2a | 11.5 ± 4.0b |
| EGFP | 8.0 ± 2.0 | 10.8 ± 3.8 | 5.6 ± 2.7 | 7.5 ± 2.2 |
| Fos/EGFP | 0 | 0 | 0 | 0 |
| MPA | ||||
| Fos | 58.3 ± 11.5 | 33.5 ± 7.8 | 54.7 ± 14.6 | 22.3 ± 5.9 |
| EGFP | 29.7 ± 14.1 | 23.8 ± 3.7 | 18.6 ± 4.7 | 17.5 ± 4.8 |
| Fos/EGFP | 0.4 ± 0.3 | 0.3 ± 0.2 | 0.3 ± 0.2 | 0 |
P < 0.005, TAMBIENT of 25°C vs 35°C within OVX mice.
P < 0.05, TAMBIENT of 35°C, OVX vs OVX + E2 mice.
The high TAMBIENT did not induce Fos in EGFP-ir neurons in either the MnPO or MPA (Fig. 6; Table 1). The number of EGFP-ir neurons was similar across the treatment groups (Table 1). In most of the mice, no colocalization of Fos in EGFP neurons had occurred. In the other mice, only one to two EGFP/Fos neurons were identified.
Experiment 3: a higher TCORE increased Fos in the MnPO and MPA but not in NK3R neurons
Increasing the temperature setting of the environmental chamber from 25°C to 36°C resulted in an increase in the TAMBIENT to 35°C within 30 minutes (Fig. 5E). The high TAMBIENT induced a substantial elevation in the TCORE by 35 minutes, which remained elevated for the duration of the recording (Fig. 5D).
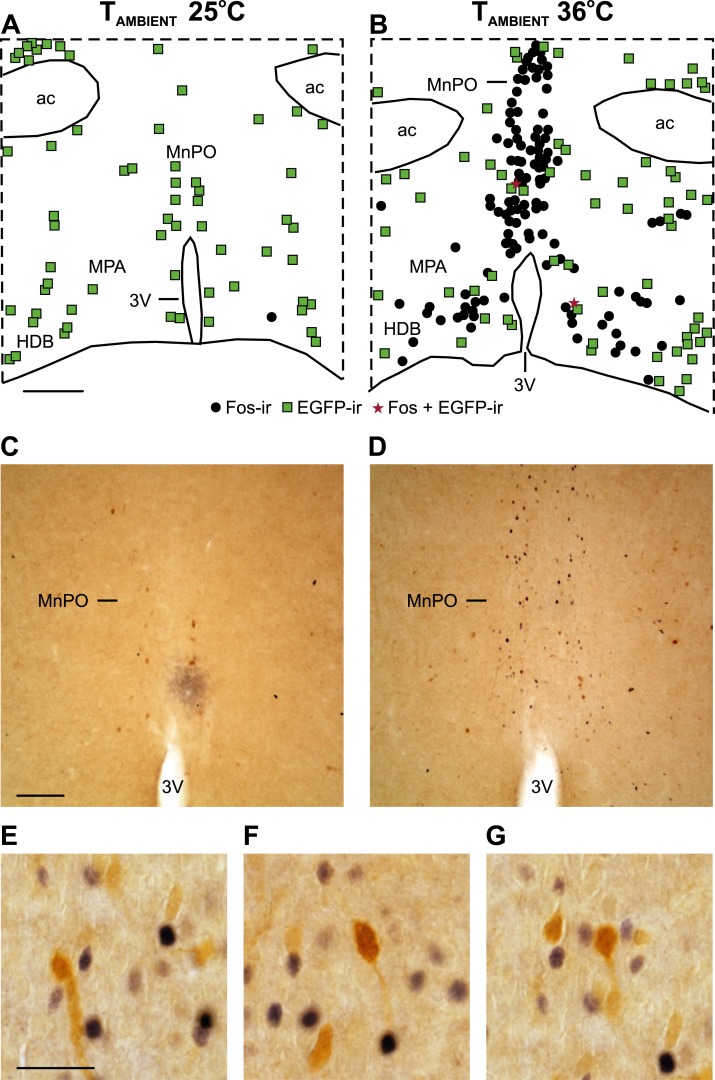
The Fos expression in mice maintained at 25°C was very low (Fig. 7; Table 2), consistent with the long acclimation period in the environmental chamber (Fig. 5E). Mice exposed to the TAMBIENT of 36°C, exhibited a substantial increase in the number of Fos-ir cells in the MnPO and MPA (Fig. 7; Table 2). In contrast, the number of EGFP-ir cells was similar between groups. Only rare EGFP-ir neurons coexpressed Fos in the MnPO or MPA. The higher TAMBIENT did not induce Fos expression in EGFP-ir neurons (Fig. 7; Table 2).
Figure 7.
An increase in the TAMBIENT and TCORE increased Fos in the MnPO and MPA but not in preoptic NK3R neurons. (A, B) Maps of NK3R (EGFP-ir neurons, green squares), Fos-ir neurons (black circles), and dual-labeled neurons (red stars) in mice exposed to TAMBIENT of (A) 25°C and (B) 36°C. Photomicrographs of the MnPO from mice at TAMBIENT of (C) 25°C and (D) 36°C showing the increase in Fos-ir neurons (black, nuclear stain) in mice with the elevated TCORE. EGFP-ir neurons not expressing Fos can also be seen (brown; cytoplasmic stain). (E–G) Photomicrographs of the MnPO from mice exposed to 36°C showing no Fos (black nuclear staining) expression in EGFP (brown cytoplasmic staining) neurons. (A) Scale bar, 250 µm for (A) and (B). (C) Scale bar, 100 µm for (C) and (D). (E) Scale bar, 25 µm for (E)–(G). 3V, third ventricle; ac, anterior commissure; HDB, horizontal limb of the diagonal band.
Table 2.
Effect of Increased TCORE on Average ± SEM Number of Fos, EGFP, and Dual-Labeled Fos/EGFP-ir Cells in Diestrous Tacr3-EGFP Mice
| Variable | TAMBIENT |
|
|---|---|---|
| 25°C (n = 8) | 36°C (n = 12) | |
| MnPO | ||
| Fos | 0.1 ± 0.1 | 39.0 ± 4.3a |
| EGFP | 16.3 ± 2.0 | 16.2 ± 3.8 |
| Fos/EGFP | 0 | 0.4 ± 0.2 |
| MPA | ||
| Fos | 0.3 ± 0.2 | 42.3 ± 7.5a |
| EGFP | 62.3 ± 11.4 | 70.9 ± 9.8 |
| Fos/EGFP | 0 | 0.4 ± 0.1 |
Statistically significant difference between TAMBIENT of 25°C vs 36°C (P < 0.001).
Experiment 4: NK3R neurons coexpressed VGLUT2 mRNA in the MnPO and VGAT mRNA in the MPA
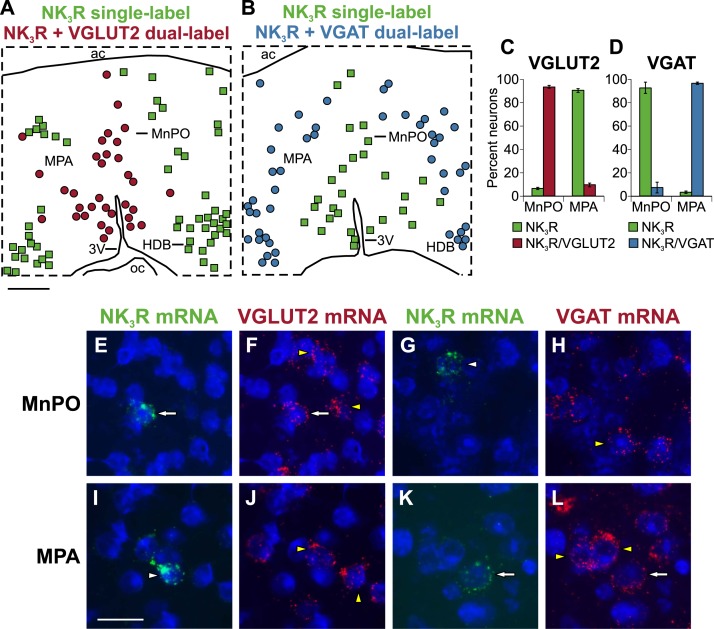
Using in situ hybridization, the NK3R (Tacr3) probe labeled scattered small neurons in the MnPO and MPA. Large neurons in the septal area and diagonal band were also labeled. The distribution of neurons labeled with the Tacr3 probe was consistent with the EGFP immunohistochemistry findings in Tacr3-EGFP mice and in situ hybridization studies posted in the Allen Brain Atlas (available at: www.brain-map.org). The VGLUT2 (Slc17A6) probe highlighted an extensive population of neurons in the MnPO and MPA. In contrast, VGAT (Slc32a1) labeling was much more prominent in the MPA than in the MnPO. The distribution of labeling with the VGLUT2 and VGAT probes was consistent with the in situ hybridization findings posted in the Allen Brain Atlas (available at: www.brain-map.org).
Nearly all (94%) of the NK3R neurons in the MnPO coexpressed VGLUT2 mRNA, with only a small number (7.2%) coexpressing VGAT mRNA (Fig. 8). The labeling pattern was reversed in the MPA, in which 97% of the NK3R neurons expressed VGAT mRNA, with only 9.6% of NK3R neurons expressing VGLUT2 mRNA (Fig. 8). Thus, NK3R neurons in the MnPO were predominantly glutamatergic. In contrast, in the MPA, they were predominantly GABAergic. Although not quantified, many more neurons were found in the MnPO that expressed VGLUT2 mRNA than those coexpressing both NK3R and VGLUT2 mRNA. Similarly, in the MPA, the number of single-labeled GABAergic neurons far outnumbered the number of neurons that expressed both NK3R and VGAT mRNA.
Figure 8.
Dual-label fluorescent in situ hybridization reveals predominantly glutamatergic NK3R neurons in the MnPO and GABAergic NK3R neurons in the MPA. (A, B) Maps of single-labeled NK3R neurons (green squares), dual-labeled NK3R/VGLUT2 neurons (red circles), and dual-labeled NK3R/VGAT neurons (blue circles). (C, D) Graphs showing the percentage of neurons expressing NK3R mRNA that were single-labeled (green bars), dual-labeled with VGLUT2 mRNA (red bars), or dual-labeled with VGAT mRNA (blue bars). Photomicrographs of dual-label fluorescent in situ hybridization in the (Top) MnPO and (Bottom) MPA using probes complementary to (E, F, I, J) NK3R and VGLUT2 mRNA or (G, H, K, L) NK3R and VGAT mRNA. The white arrows in (E) and (F) show a cell that is dual-labeled for NK3R and VGLUT2 mRNA in the MnPO. The white arrows in (K) and (L) show a cell that is dual-labeled for NK3R and VGAT mRNA in the MPA. Single-labeled NK3R neurons are marked with white arrowheads, single-labeled VGLUT2 or VGAT neurons are marked with yellow arrowheads. (A) Scale bar, 250 µm for (A) and (B). (I) Scale bar, 25 µm for (E)–(L).
Discussion
Menopause is characterized by degeneration of ovarian follicles, estrogen withdrawal, and hypertrophy of KNDy neurons in the human hypothalamus (4, 6, 7, 51). Estrogen withdrawal leads to hot flushes, a sudden activation of heat dissipation effectors that frequently results in a drop in the TCORE (3). We hypothesized that KNDy neurons play a role in the generation of hot flushes via NK3R signaling in the MnPO (5). In support of this hypothesis, NKB infusion induces hot flushes in women (27) and NK3R antagonists reduce their number and frequency (28–31). The present experiments were designed to reveal how NK3R neurons in the MnPO relate to the central thermoregulatory pathways for heat defense in the female mouse.
In our first experiment, we monitored the thermoregulatory effects of ablating NK3R neurons in the MnPO of Tacr3-EGFP mice using injections of NK3-SAP. EGFP immunohistochemistry revealed a loss of 80% of NK3R neurons in the MnPO, with a focal loss of neurons in the adjacent MPA. The ablation of NK3R neurons resulted in a consistent elevation in TCORE during the light phase, which occurred regardless of E2 treatment. The amplitude of the TCORE circadian rhythms was blunted by NK3-SAP, but the TSKIN and activity circadian rhythms were unchanged. The TCORE elevation was not high enough to be considered a fever and remained lower or equal to the average TCORE of the dark phase. Thus, ablation of NK3R neurons in the MnPO did not induce a severe thermoregulatory deficit, such as the pronounced hyperthermia reported after the MnPO has been destroyed by electrolytic lesions (52). Despite the shift to the higher TCORE during the light phase, the TCORE frequency distributions were nearly identical, indicating similar thermal stability (49). Thus, MnPO NK3R ablation produced a regulated shift in the balance point of TCORE during the light phase.
One of the main functions of the thermoregulatory system is to defend TCORE against changes in TAMBIENT (53). Failure of thermal homeostasis is manifested by a poikilothermic state, in which a change in TAMBIENT produces parallel effects on TCORE (54). Therefore, to further evaluate thermoregulatory function, we exposed NK3-SAP–injected and control mice to a wide range of TAMBIENT. The mice were exposed to TAMBIENT that were greater than, less than, and within the thermoneutral zone of ~29.0° to 32.0°C in mice (49). Despite the changes in TAMBIENT, the TCORE of NK3R-ablated mice was maintained at a higher level. These data support the concept that the higher TCORE in NK3R ablated mice is a regulated shift in the balance point and not a global failure of thermoregulation.
We have previously described differences between E2 modulation of body temperature in rats and mice (23). E2 treatment of OVX mice lowers the TCORE during the light phase (23) but only lowers the TCORE in rats when exposed to a high TAMBIENT (22, 55). E2 has no effect on TSKIN in OVX mice (23) but lowers TSKIN in OVX rats (22, 56, 57). The effects of NK3R MnPO neuron ablation are also different between rats and mice. In the present study, ablation of MnPO NK3R neurons in mice resulted in a higher TCORE during the light phase, without changing the TSKIN. In rats, ablation of MnPO NK3R neurons lowered the TSKIN without altering the TCORE (21). However, in both mice and rats, ablation of NK3R neurons in the MnPO did not prevent the effects of E2 on thermoregulation (21). Thus, NK3R neurons in the MnPO are not essential for the E2 modulation of thermoregulation in either rats or mice.
The fundamental neural circuit for thermoregulation consists of neurons that are sensitive to thermal challenges (internal or external) and activate behavioral and autonomic thermoeffectors to maintain the TCORE (58). Although little is known in humans, a central thermosensory pathway regulating heat defense has been described in the rat (58). This pathway starts with warm-sensitive cutaneous thermosensors that relay signals to dorsal horn neurons and the dorsal lateral parabrachial nucleus and then to glutamatergic neurons in the MnPO (58). Glutamatergic MnPO neurons synapse on warm-sensitive, GABAergic neurons in the MPA that ultimately influence autonomic thermoeffectors such as cutaneous vasodilation and face washing behavior (58, 59). Recent studies have identified several markers for warm-sensitive neurons in the mouse preoptic area, including the TRPM2 channel, which could act as a warm sensor (60–63). In addition to the core thermosensory pathway, numerous synaptic integration sites allow the body temperature to be regulated by a variety of factors, such as serum osmolality, energy stores, circadian rhythms, hormones, and immune mediators (59, 64). The MnPO, in particular, integrates signals related to body fluid homeostasis, temperature, sleep, cardiovascular function, and the immune system (65, 66).
Induction of Fos has been extensively used to evaluate temperature-sensitive central nervous system circuits (58, 67–71). To evaluate whether preoptic NK3R neurons were sensitive to increased environmental or core temperatures, we exposed Tacr3-EGFP mice to high TAMBIENT and performed dual-label EGFP/Fos immunohistochemistry. Two paradigms were used: a rapid increase in TAMBIENT to stimulate skin thermosensors (experiment 2) and a rapid and then prolonged elevation of the TAMBIENT designed to elevate the TCORE (experiment 3). Stimulation of skin thermosensors induced Fos in the MnPO. The increased TCORE induced Fos in both the MnPO and the MPA. However, no Fos expression was induced in EGFP-ir neurons in either the MnPO or MPA. Thus, we found no evidence that NK3R neurons in the MnPO or MPA are warm sensitive or activated by warm cutaneous or visceral thermosensors.
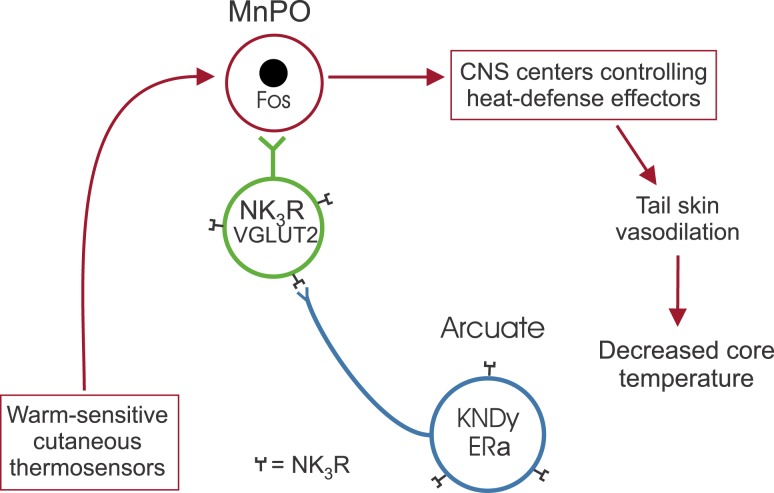
Because preoptic NK3R neurons are not activated by an acute increase in the TAMBIENT or elevated TCORE, they do not appear to be an integral component of the thermosensory heat defense pathway described by Nakamura and Morrison (58) and Morrison (59). Although not part of the thermosensory pathway, a modulatory role on thermoregulation is evident by the higher TCORE in the NK3-SAP–injected mice. These data are consistent with studies showing that NK3R neurons in the MnPO activate heat dissipation effectors to reduce body temperature (20, 21). Combined with previous studies (5, 21, 22, 25, 72), we hypothesize that estrogen-sensitive KNDy neurons do not directly project to the core thermosensory pathway but instead modulate heat dissipation effectors indirectly via a subpopulation of glutamatergic MnPO NK3R-expressing neurons. Interestingly, the number of MnPO neurons expressing fos after infusing an NK3R agonist into the MnPO (20) or due to optogenetic activation of KNDy neurons (25) is similar to that seen in mice exposed to warm stimuli (Fig. 6B and Fig. 7B). These data suggest that glutamatergic MnPO NK3R neurons could activate heat dissipation effectors via local projections within the MnPO to neurons in the heat-defense pathway (Fig. 9). Further studies are needed to determine the validity of this hypothesis.
Figure 9.
Schematic diagram of the relationship between KNDy neurons (blue) and NK3R-expressing neurons in the MnPO (green) and the central thermosensory heat defense pathway (red) in the mouse. We found that ablation of NK3R neurons in the MnPO increases the TCORE without causing thermoregulatory failure. In addition, NK3R neurons in the MnPO are glutamatergic but do not express Fos when mice are exposed to an acute increase in TAMBIENT or higher TCORE. We hypothesize that KNDy neurons activate the central thermosensory pathway for heat defense indirectly via a subpopulation of glutamatergic NK3R-expressing neurons in the MnPO.
To determine whether MnPO NK3R neurons were glutamatergic or GABAergic, we used dual-label fluorescent in situ hybridization to evaluate whether NK3R mRNA is colocalized with VGLUT2 or VGAT mRNA, respectively. Two distinct subpopulations of NK3R neurons were identified, depending on their location in the preoptic area. In the MnPO, the NK3R neurons predominantly expressed VGLUT2 mRNA. In contrast, in the MPA, they expressed VGAT mRNA. In a previous study, optogenetic activation of MnPO glutamatergic neurons in VGLuT2-ires-Cre mice resulted in tail skin vasodilation and hypothermia, underscoring the importance of MnPO glutamatergic neurons in heat defense (73). Identification of glutamatergic NK3R neurons in the MnPO suggests that MnPO NK3R neurons excite the central heat defense pathway, rather than suppressing an inhibitory influence.
Our studies are relevant to recent clinical trials that have effectively reduced hot flushes with oral administration of NK3R antagonists (28–30). Because both NK3R-expressing KNDy neurons and NK3R neurons in the MnPO participate in the activation of heat dissipation effectors (17, 20–22, 25), NK3R antagonists could reduce hot flushes through their actions at either (or both) of these sites. Our data suggest that global thermoregulatory dysfunction (e.g., hyperthermia) would not occur as a side effect of NK3R antagonist administration (21, 22). However, given the elevation of the TCORE after ablation of NK3R preoptic neurons in mice, it would be of interest to monitor baseline temperatures to determine whether more subtle changes occur in thermoregulatory function. Ultimately, information on the chemical and molecular identity of preoptic MnPO NK3R neurons could be useful for designing additional targeted treatments for hot flushes.
Acknowledgments
Financial Support: The present study was supported by the National Institutes of Health, National Institute on Aging (Grant R01 AG047887).
Disclosure Summary: The authors have nothing to disclose.
Glossary
Abbreviations:
- EGFP
enhanced green fluorescent protein
- E2
estradiol-17β
- GABA
γ-aminobutyric acid
- GFP
green fluorescent protein
- HLI
heat loss index
- ir
immunoreactive
- KNDy neurons
arcuate neurons coexpressing kisspeptin, neurokinin B, and dynorphin
- MnPO
median preoptic nucleus
- MPA
medial preoptic area
- NKB
neurokinin B
- NK3R
neurokinin 3 receptor
- OVX
ovariectomized
- SAP
saporin
- TAMBIENT
ambient temperature
- TCORE
core temperature
- TSKIN
skin temperature
- VGAT
vesicular γ-aminobutyric acid transporter
- VGLUT2
vesicular glutamate transporter 2
References
- 1. Santoro N. Symptoms of menopause: hot flushes. Clin Obstet Gynecol. 2008;51(3):539–548. [DOI] [PubMed] [Google Scholar]
- 2. Kronenberg F. Menopausal hot flashes: a review of physiology and biosociocultural perspective on methods of assessment. J Nutr. 2010;140(7):1380S–1385S. [DOI] [PubMed] [Google Scholar]
- 3. Freedman RR. Physiology of hot flashes. Am J Hum Biol. 2001;13(4):453–464. [DOI] [PubMed] [Google Scholar]
- 4. Rance NE, Young WS III. Hypertrophy and increased gene expression of neurons containing neurokinin-B and substance-P messenger ribonucleic acids in the hypothalami of postmenopausal women. Endocrinology. 1991;128(5):2239–2247. [DOI] [PubMed] [Google Scholar]
- 5. Rance NE, Dacks PA, Mittelman-Smith MA, Romanovsky AA, Krajewski-Hall SJ. Modulation of body temperature and LH secretion by hypothalamic KNDy (kisspeptin, neurokinin B and dynorphin) neurons: a novel hypothesis on the mechanism of hot flushes. Front Neuroendocrinol. 2013;34(3):211–227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Rometo AM, Krajewski SJ, Voytko ML, Rance NE. Hypertrophy and increased kisspeptin gene expression in the hypothalamic infundibular nucleus of postmenopausal women and ovariectomized monkeys. J Clin Endocrinol Metab. 2007;92(7):2744–2750. [DOI] [PubMed] [Google Scholar]
- 7. Rance NE, McMullen NT, Smialek JE, Price DL, Young WS III. Postmenopausal hypertrophy of neurons expressing the estrogen receptor gene in the human hypothalamus. J Clin Endocrinol Metab. 1990;71(1):79–85. [DOI] [PubMed] [Google Scholar]
- 8. Abel TW, Voytko ML, Rance NE. The effects of hormone replacement therapy on hypothalamic neuropeptide gene expression in a primate model of menopause. J Clin Endocrinol Metab. 1999;84(6):2111–2118. [DOI] [PubMed] [Google Scholar]
- 9. Sandoval-Guzmán T, Stalcup ST, Krajewski SJ, Voytko ML, Rance NE. Effects of ovariectomy on the neuroendocrine axes regulating reproduction and energy balance in young cynomolgus macaques. J Neuroendocrinol. 2004;16(2):146–153. [DOI] [PubMed] [Google Scholar]
- 10. Rance NE, Krajewski SJ, Smith MA, Cholanian M, Dacks PA. Neurokinin B and the hypothalamic regulation of reproduction. Brain Res. 2010;1364:116–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Lehman MN, Coolen LM, Goodman RL. Minireview: kisspeptin/neurokinin B/dynorphin (KNDy) cells of the arcuate nucleus: a central node in the control of gonadotropin-releasing hormone secretion. Endocrinology. 2010;151(8):3479–3489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Clarkson J, Han SY, Piet R, McLennan T, Kane GM, Ng J, Porteous RW, Kim JS, Colledge WH, Iremonger KJ, Herbison AE. Definition of the hypothalamic GnRH pulse generator in mice. Proc Natl Acad Sci USA. 2017;114(47):E10216–E10223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Herbison AE. The gonadotropin-releasing hormone pulse generator. Endocrinology. 2018;159(11):3723–3736. [DOI] [PubMed] [Google Scholar]
- 14. Casper RF, Yen SSC, Wilkes MM. Menopausal flushes: a neuroendocrine link with pulsatile luteninizing hormone secreation. Science. 1979;205(4408):823–825. [DOI] [PubMed] [Google Scholar]
- 15. Tataryn IV, Meldrum DR, Lu KH, Frumar AM, Judd HL. LH, FSH and skin temperature during the menopausal hot flash. J Clin Endocrinol Metab. 1979;49(1):152–154. [DOI] [PubMed] [Google Scholar]
- 16. Oakley AE, Steiner RA, Chavkin C, Clifton DK, Ferrara LK, Reed SD. κ Agonists as a novel therapy for menopausal hot flashes. Menopause. 2015;22(12):1328–1334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Burke MC, Letts PA, Krajewski SJ, Rance NE. Coexpression of dynorphin and neurokinin B immunoreactivity in the rat hypothalamus: Morphologic evidence of interrelated function within the arcuate nucleus. J Comp Neurol. 2006;498(5):712–726. [DOI] [PubMed] [Google Scholar]
- 18. Krajewski SJ, Burke MC, Anderson MJ, McMullen NT, Rance NE. Forebrain projections of arcuate neurokinin B neurons demonstrated by anterograde tract-tracing and monosodium glutamate lesions in the rat. Neuroscience. 2010;166(2):680–697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Shughrue PJ, Lane MV, Merchenthaler I. In situ hybridization analysis of the distribution of neurokinin-3 mRNA in the rat central nervous system. J Comp Neurol. 1996;372(3):395–414. [DOI] [PubMed] [Google Scholar]
- 20. Dacks PA, Krajewski SJ, Rance NE. Activation of neurokinin 3 receptors in the median preoptic nucleus decreases core temperature in the rat. Endocrinology. 2011;152(12):4894–4905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Mittelman-Smith MA, Krajewski-Hall SJ, McMullen NT, Rance NE. Neurokinin 3 receptor-expressing neurons in the median preoptic nucleus modulate heat-dissipation effectors in the female rat. Endocrinology. 2015;156(7):2552–2562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Mittelman-Smith MA, Williams H, Krajewski-Hall SJ, McMullen NT, Rance NE. Role for kisspeptin/neurokinin B/dynorphin (KNDy) neurons in cutaneous vasodilatation and the estrogen modulation of body temperature. Proc Natl Acad Sci USA. 2012;109(48):19846–19851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Krajewski-Hall SJ, Blackmore EM, McMinn JR, Rance NE. Estradiol alters body temperature regulation in the female mouse. Temperature (Austin). 2017;5(1):56–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Krull AA, Larsen SA, Clifton DK, Neal-Perry G, Steiner RA. A comprehensive method to quantify adaptations by male and female mice with hot flashes induced by the neurokinin B receptor agonist, senktide. Endocrinology. 2017;158(10):3259–3268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Padilla SL, Johnson CW, Barker FD, Patterson MA, Palmiter RD. A neural circuit underlying the generation of hot flushes. Cell Reports. 2018;24(2):271–277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Crandall CJ, Manson JE, Hohensee C, Horvath S, Wactawski-Wende J, LeBlanc ES, Vitolins MZ, Nassir R, Sinsheimer JS. Association of genetic variation in the tachykinin receptor 3 locus with hot flashes and night sweats in the Women’s Health Initiative Study. Menopause. 2017;24(3):252–261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Jayasena CN, Comninos AN, Stefanopoulou E, Buckley A, Narayanaswamy S, Izzi-Engbeaya C, Abbara A, Ratnasabapathy R, Mogford J, Ng N, Sarang Z, Ghatei MA, Bloom SR, Hunter MS, Dhillo WS. Neurokinin B administration induces hot flushes in women. Sci Rep. 2015;5(1):8466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Prague JK, Roberts RE, Comninos AN, Clarke S, Jayasena CN, Nash Z, Doyle C, Papadopoulou DA, Bloom SR, Mohideen P, Panay N, Hunter MS, Veldhuis JD, Webber LC, Huson L, Dhillo WS. Neurokinin 3 receptor antagonism as a novel treatment for menopausal hot flushes: a phase 2, randomised, double-blind, placebo-controlled trial. Lancet. 2017;389(10081):1809–1820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Fraser GL, Depypere H, Timmerman D, Donders G, Sieprath P, Ramael S, Combalbert J, Hoveyda HR. Clinical evaluation of the NK3 receptor antagonist fezolinetant (a.k.a. ESN364) for the treatment of menopausal hot flashes. Endocrine Soc Abstr.2017:OR16-15. [DOI] [PubMed]
- 30. Prague JK, Roberts RE, Comninos AN, Clarke S, Jayasena CN, Mohideen P, Lin VH, Stern TP, Panay N, Hunter MS, Webber LC, Dhillo WS. Neurokinin 3 receptor antagonism rapidly improves vasomotor symptoms with sustained duration of action. Menopause. 2018;25(8):862–869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Skorupskaite K, George JT, Veldhuis JD, Millar RP, Anderson RA. Neurokinin 3 receptor antagonism reveals roles for neurokinin B in the regulation of gonadotropin secretion and hot flashes in postmenopausal women. Neuroendocrinology. 2018;106(2):148–157. [DOI] [PubMed] [Google Scholar]
- 32. Cully M. Deal watch: neurokinin 3 receptor antagonist revival heats up with Astellas acquisition. Nat Rev Drug Discov. 2017;16(6):377. [DOI] [PubMed] [Google Scholar]
- 33. Faubion SS, Stuenkel CA. Neurokinin 3 receptor antagonists for treatment of vasomotor symptoms: a new panacea or just a flash in the pan? Menopause. 2018;25(8):859–861. [DOI] [PubMed] [Google Scholar]
- 34. Gong S, Zheng C, Doughty ML, Losos K, Didkovsky N, Schambra UB, Nowak NJ, Joyner A, Leblanc G, Hatten ME, Heintz N. A gene expression atlas of the central nervous system based on bacterial artificial chromosomes. Nature. 2003;425(6961):917–925. [DOI] [PubMed] [Google Scholar]
- 35. Mittelman-Smith MA, Williams H, Krajewski-Hall SJ, Lai J, Ciofi P, McMullen NT, Rance NE. Arcuate kisspeptin/neurokinin B/dynorphin (KNDy) neurons mediate the estrogen suppression of gonadotropin secretion and body weight. Endocrinology. 2012;153(6):2800–2812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Sadowski S, Huang R-RC, Fong TM, Marko O, Cascieri MA. Characterization of the binding of [125I-iodo-histidyl, methyl-Phe7] neurokinin B to the neurokinin-3 receptor. Neuropeptides. 1993;24(6):317–319. [DOI] [PubMed] [Google Scholar]
- 37. Corboz MR, Rivelli MA, Eckel SP. Bronchoconstrictor effect of the tachykinin NK3-receptor agonists [MePhe7]-neurokinin B and senktide in the isolated guinea pig lung. Exp Lung Res. 2010;36(9):509–521. [DOI] [PubMed] [Google Scholar]
- 38. Drapeau G, d’Orléans-Juste P, Dion S, Rhaleb N-E, Regoli D. Specific agonists for neurokinin B receptors. Eur J Pharmacol. 1987;136(3):401–403. [DOI] [PubMed] [Google Scholar]
- 39. Leffler A, Ahlstedt I, Engberg S, Svensson A, Billger M, Öberg L, Bjursell MK, Lindström E, von Mentzer B. Characterization of species-related differences in the pharmacology of tachykinin NK receptors 1, 2 and 3. Biochem Pharmacol. 2009;77(9):1522–1530. [DOI] [PubMed] [Google Scholar]
- 40. Watson RE Jr, Wiegand SJ, Clough RW, Hoffman GE. Use of cryoprotectant to maintain long-term peptide immunoreactivity and tissue morphology. Peptides. 1986;7(1):155–159. [DOI] [PubMed] [Google Scholar]
- 41.RRID:AB_2307313, http://scicrunch.org/resolver/AB_2307313.
- 42.RRID:AB_2106755, http://scicrunch.org/resolver/AB_2106755.
- 43. Patronas P, Horowitz M, Simon E, Gerstberger R. Differential stimulation of c-fos expression in hypothalamic nuclei of the rat brain during short-term heat acclimation and mild dehydration. Brain Res. 1998;798(1-2):127–139. [DOI] [PubMed] [Google Scholar]
- 44. Elmquist JK, Ahima RS, Elias CF, Flier JS, Saper CB. Leptin activates distinct projections from the dorsomedial and ventromedial hypothalamic nuclei. Proc Natl Acad Sci USA. 1998;95(2):741–746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.RRID:AB_2336114, http://scicrunch.org/resolver/AB_2336114.
- 46.RRID:AB_2336819, http://scicrunch.org/resolver/AB_2336819.
- 47.RRID:AB_2313606, http://scicrunch.org/resolver/AB_2313606.
- 48. Paxinos G, Franklin KBJ. The Mouse Brain in Stereotaxic Coordinates. 3rd ed.New York: Academic Press;2008. [Google Scholar]
- 49. Gordon CJ. Thermal physiology of laboratory mice: defining thermoneutrality. J Therm Biol. 2012;37(8):654–685. [Google Scholar]
- 50. Romanovsky AA, Ivanov AI, Shimansky YP. Selected contribution: ambient temperature for experiments in rats: a new method for determining the zone of thermal neutrality. J Appl Physiol (1985). 2002;92(6):2667–2679. [DOI] [PubMed] [Google Scholar]
- 51. Rometo AM, Rance NE. Changes in prodynorphin gene expression and neuronal morphology in the hypothalamus of postmenopausal women. J Neuroendocrinol. 2008;20(12):1376–1381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Romanovsky AA, Sugimoto N, Simons CT, Hunter WS. The organum vasculosum laminae terminalis in immune-to-brain febrigenic signaling: a reappraisal of lesion experiments. Am J Physiol Regul Integr Comp Physiol. 2003;285(2):R420–R428. [DOI] [PubMed] [Google Scholar]
- 53. Kanosue K, Crawshaw LI, Nagashima K, Yoda T. Concepts to utilize in describing thermoregulation and neurophysiological evidence for how the system works. Eur J Appl Physiol. 2010;109(1):5–11. [DOI] [PubMed] [Google Scholar]
- 54. Gordon CJ. Thermal biology of the laboratory rat. Physiol Behav. 1990;47(5):963–991. [DOI] [PubMed] [Google Scholar]
- 55. Dacks PA, Rance NE. Effects of estradiol on the thermoneutral zone and core temperature in ovariectomized rats. Endocrinology. 2010;151(3):1187–1193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Bowe J, Li XF, Kinsey-Jones J, Heyerick A, Brain S, Milligan S, O’Byrne K. The hop phytoestrogen, 8-prenylnaringenin, reverses the ovariectomy-induced rise in skin temperature in an animal model of menopausal hot flushes. J Endocrinol. 2006;191(2):399–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Williams H, Dacks PA, Rance NE. An improved method for recording tail skin temperature in the rat reveals changes during the estrous cycle and effects of ovarian steroids. Endocrinology. 2010;151(11):5389–5394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Nakamura K, Morrison SF. A thermosensory pathway mediating heat-defense responses. Proc Natl Acad Sci USA. 2010;107(19):8848–8853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Morrison SF. Central control of body temperature. F1000 Res. 2016;5:5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Tan CL, Cooke EK, Leib DE, Lin Y-C, Daly GE, Zimmerman CA, Knight ZA. Warm-sensitive neurons that control body temperature. Cell. 2016;167(1):47–59.e15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Song K, Wang H, Kamm GB, Pohle J, Reis FC, Heppenstall P, Wende H, Siemens J. The TRPM2 channel is a hypothalamic heat sensor that limits fever and can drive hypothermia. Science. 2016;353(6306):1393–1398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Yu S, Qualls-Creekmore E, Rezai-Zadeh K, Jiang Y, Berthoud H-R, Morrison CD, Derbenev AV, Zsombok A, Münzberg H. Glutamatergic preoptic area neurons that express leptin receptors drive temperature-dependent body weight homeostasis. J Neurosci. 2016;36(18):5034–5046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Tan CL, Knight ZA. Regulation of body temperature by the nervous system. Neuron. 2018;98(1):31–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Morrison SF, Madden CJ, Tupone D. Central control of brown adipose tissue thermogenesis. Front Endocrinol (Lausanne). 2012;3(5):3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Lazarus M, Yoshida K, Coppari R, Bass CE, Mochizuki T, Lowell BB, Saper CB. EP3 prostaglandin receptors in the median preoptic nucleus are critical for fever responses. Nat Neurosci. 2007;10(9):1131–1133. [DOI] [PubMed] [Google Scholar]
- 66. McKinley MJ, Yao ST, Uschakov A, McAllen RM, Rundgren M, Martelli D. The median preoptic nucleus: front and centre for the regulation of body fluid, sodium, temperature, sleep and cardiovascular homeostasis. Acta Physiol (Oxf). 2015;214(1):8–32. [DOI] [PubMed] [Google Scholar]
- 67. Bachtell RK, Tsivkovskaia NO, Ryabinin AE. Identification of temperature-sensitive neural circuits in mice using c-Fos expression mapping. Brain Res. 2003;960(1-2):157–164. [DOI] [PubMed] [Google Scholar]
- 68. Bratincsák A, Palkovits M. Activation of brain areas in rat following warm and cold ambient exposure. Neuroscience. 2004;127(2):385–397. [DOI] [PubMed] [Google Scholar]
- 69. Scammell TE, Price KJ, Sagar SM. Hyperthermia induces c-fos expression in the preoptic area. Brain Res. 1993;618(2):303–307. [DOI] [PubMed] [Google Scholar]
- 70. Yoshida K, Konishi M, Nagashima K, Saper CB, Kanosue K. Fos activation in hypothalamic neurons during cold or warm exposure: projections to periaqueductal gray matter. Neuroscience. 2005;133(4):1039–1046. [DOI] [PubMed] [Google Scholar]
- 71. Yoshida K, Li X, Cano G, Lazarus M, Saper CB. Parallel preoptic pathways for thermoregulation. J Neurosci. 2009;29(38):11954–11964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Yeo S-H, Herbison AE. Projections of arcuate nucleus and rostral periventricular kisspeptin neurons in the adult female mouse brain. Endocrinology. 2011;152(6):2387–2399. [DOI] [PubMed] [Google Scholar]
- 73. Abbott SBG, Saper CB. Median preoptic glutamatergic neurons promote thermoregulatory heat loss and water consumption in mice. J Physiol. 2017;595(20):6569–6583. [DOI] [PMC free article] [PubMed] [Google Scholar]