Abstract
Context:
Partner notification for gonorrhea is intended to interrupt transmission and to bring people exposed to infection to care. Partner notification may be initiated through public health professionals (disease intervention specialist: DIS referral) or patients (patient referral). In some cases, patients may carry medications or prescriptions for partners (patient-delivered partner therapy: PDPT).
Objective:
To examine how patterns of notifying and treating partners of persons with gonorrhea differ by partner notification approach.
Design:
From published literature (2005–2012), we extracted 10 estimates of patient referral data from 7 studies (3853 patients, 7490 partners) and 5 estimates of PDPT data from 5 studies (1781 patients, 3125 partners). For DIS referral estimates, we obtained 2010–2012 data from 14 program settings (4581 patients interviewed, 8301 partners). For each approach, we calculated treatment cascades based on the proportion of partners who were notified and treated. We also calculated cascades based on partners notified and treated per patient diagnosed.
Results:
Proportions of partners notified and treated were, for patient referral, 56% and 34%; for PDPT, 57% and 46%; for DIS referral, 25% and 22%. Notification and treatment estimates for patient referral and PDPT were significantly higher than for DIS referral, but DIS referral was more efficacious than the other methods in assuring treatment among those notified (all Ps < .001). The notification and treatment ratios per patient seen were, for patient referral, 0.96 and 0.61; for PDPT, 0.90 and 0.73; for DIS referral, 0.45 and 0.40.
Conclusion:
Patient-based methods had higher proportions of partners treated overall, but provider referral had the highest proportion treated among those notified. These data may assist programs to align the most efficacious strategies with the most epidemiologically or clinically important cases while assuring the best scalable standard of care for others.
Keywords: contact tracing, disease notification, gonorrhea
In the United States, an estimated 350 062 new cases of Neisseria gonorrhoeae infection were reported to the Centers for Disease Control and Prevention in 2014, making gonorrhea the second most commonly reported disease in the nation (with a rate of 110.7 cases per 100 000 population).1 Gonorrhea is a major cause of pelvic inflammatory disease, which can lead to ectopic pregnancy, tubal infertility, and chronic pelvic pain in women. With gonococcal infections, there is also an increased risk of HIV transmission and acquisition.2
Gonorrhea is somewhat geographically concentrated, with a case report rate of 131.4 cases per 100 000 population in the southern region of the United States compared with 84.7 to 106.6 cases per 100 000 in the remaining 3 regions.1 Substantial disparities also exist in cases by age and race. Men and women aged 20 to 24 years have the highest rate of reported cases of gonorrhea (485.6 cases per 100 000 and 533.7 cases per 100 000, respectively). By racial and ethnic groups, the highest rate of reported cases of gonorrhea is among blacks (405.4 cases per 100 000 population). This rate was 10.6 times the rate among whites (38.3 cases per 100 000). Finally, the distribution of gonorrhea is more complex than these summary statistics indicate: for example, the highest current rates of increase are in the West, the highest overall rates are in the South, and the highest rates among the most affected race are mostly in the Midwest.1
One of the areas in which sexually transmitted disease (STD) control programs manage gonorrhea is through partner notification (PN).3 Traditionally, public health programs have interviewed persons with a diagnosis of infection to locate and notify those partners of their exposure and assist them in seeking evaluation and care. This process is most commonly conducted by disease intervention specialists (DIS), who are public health investigators with special training in PN techniques. DIS are typically not focused solely on PN for gonorrhea. Programs must weigh competing priorities, such as the disease incidence in their area, the degree to which gonorrhea is diagnosed outside public health clinical settings, and more pressing public health concerns, largely syphilis incidence and HIV incidence, and disease management. A previous survey estimated that only 17% of gonorrhea cases seen in public clinics were interviewed for PN, with some programs conducting no DIS referral for gonorrhea at all.4
Many programs ask patients to notify their partners, as is the case with most gonorrhea cases diagnosed outside public sector clinics. This approach, patient referral, is often a brief instruction or request and is practiced widely.5,6 Patient referral is typically more efficacious in terms of getting partners notified and treated if the approach is based on point-of-care interactive counseling.7,8 In some areas, patient referral may be accompanied by medications or prescriptions for patients to give to their partners: patient-delivered partner therapy (PDPT).
PDPT is a version of expedited partner therapy (EPT), a broad term describing various methods where the partners of the index patient receive treatment prior to evaluation.9 Combining patient referral with the provision of medication, the goal of EPT is to speed prophylactic treatment in the exposed partner. Because partners are receiving treatment prior to examination, expedited approaches should also be accompanied by efforts to encourage partners to seek clinical evaluation and to educate them about the need for treatment.10 Studies that have compared patient referral to PDPT suggest that PDPT increases notification and results in increased partner treatment.11–13
Previous studies have assessed the efficacy and effectiveness of PN, using methods and randomizing trials to compare different patient referral approaches. Choosing approaches within the framework of an STD program, however, requires that programs consider the efficacy and availability of different partner services approaches and choose a mix of approaches that maximizes impact.14 Achieving sufficient levels of availability does not necessarily imply 100% coverage for some approaches. For example, an analysis of New York State DIS referral data from the 1990s suggested that interview rates rising to approximately 50% over 12 years were associated with decreasing gonorrhea rates during that time period.15
We used existing data to examine how patterns of notifying and treating partners of persons with gonorrhea differed by PN approaches. We used a simple cascade model in which we began with the number of partners eligible for treatment and then moved to the proportion contacted and the proportion receiving treatment. The objective is to observe what mix of PN interventions could best serve the goals of treating sufficient proportions of partners to interrupt transmission.
Methods
Data sources
We conducted a search of PubMed using the following search terms: “gonorrhea” and “partner notification.” We focused on studies published between 2005 and the present; 2005 was selected as the start time because that is when the Centers for Disease Control and Prevention began to formally support PDPT. To be eligible, articles had to use US data because the mix of PN approaches in the United States is quite distinct from the mix of PN approaches in other countries in that field investigation of gonorrhea is extremely rare outside the United States. For patient referral, we accepted articles with estimates around chlamydial infection as well as gonorrhea because the referral mechanisms for both infections are the same and because PDPT for gonorrhea includes co-treatment for chlamydial infection (ie, 1 g of azithromycin; this also constitutes part of the recommended dual therapy for gonorrhea in the United States).16 For DIS referral, we used STD program data on gonorrhea PN collected between 2010 and 2012. We excluded studies from other countries and also those without notification and treatment data.
For patient referral data, we found 10 estimates in 7 studies published between 2005 and 2011.7,12,13,17–20 For PDPT, we used 5 estimates from 5 studies published between 2005 and 2011, using the same literature search (by keeping the patient referral and PDPT search time frames to the same period, we minimized time confounds).12,13,20–22 There were 1781 patients and 3125 partners. For DIS referral, we used program data on gonorrhea PN collected from a convenience sample of STD programs between 2010 and 2012. These data comprised 14 estimates from 12 STD programs (cities or counties) varying by size and geographic location. Geographic locations included urban and suburban components in each jurisdiction, with 2 areas also including rural components. Most data in programs originate with interviews in a clinic, with partner interviews (via phone or in the field) contributing thereafter. We did not have social or demographic information on the patients or their partners.
Analyses
Our basic goal is to estimate the treatment cascade from the initial patient encounter through to treatment of partners. The raw data required for this purpose are the (1) number of index patients, (2) the number of partners the index patients reported, (3) the number of partners notified of exposure, and (4) the number treated. The data sources for literature estimates are primarily self-reported data gathered from randomized control trials and program evaluations. STD program data used for DIS referral estimates are more likely to include notification and treatment data verified independently of patient report. This is reflected in standard disposition codes, labeled in the Table. The differences between codes are typically germane to program operations; here, we have concatenated codes that indicate notification of a partner and then codes that indicate either treatment or lack of infection. We used binominal tests to compare proportions of notified and treated across patient referral, PDPT, and DIS referral.
TABLE.
Construction of Study Variablesa
| Variables | Literature-Based Data for Patient Referral and PDPT |
Program-Based Data for DIS Referral |
|---|---|---|
| Number of index patients | Number enrolled at baseline in study or evaluation | Number of patients interviewed in STD clinic |
| Number of partners | Total number of partners claimedb | Total number of partners claimedb |
| Number of partners notified | Self-report from enrollee (occasional partner corroboration) | Disposition codesc
A: Preventive treatment B: Refused preventative treatment C: Infected, brought to treatment D: Infected, not treated E: Previously treated F: Not infected J: Located, refused testing or treatment |
| Number of partners treated (or other action indicating partner health is assured) | Self-report from enrollee (occasional partner corroboration) | Disposition codes A: Preventive treatment C: Infected, brought to treatment E: Previously treated F: Not infected |
| Notification statistics | ||
| (1) Proportion | (1) Number notified/number of partners | |
| (2) Index | (2) Number notified/number of index patients | |
| Treatment index | ||
| (1) Proportion | (1) Numbertreated/number of partners | |
| (2) Index | (2) Numbertreated/number of index patients | |
Abbreviations: DIS, disease intervention specialist; PDPT, patient-delivered partner therapy; STD, sexually transmitted disease.
The number of gonorrhea-infected patients interviewed is not necessarily the total number of gonorrhea-infected cases because not all clinics interview all cases.
Total numbers of partners are not necessarily exclusive because multiple index patients or study enrollees could claim the same partner. The extent to which there is overlap is rarely known.
Each of these codes used by STD programs to indicate the outcomes of partner services indicates that the partner was notified by someone even if no other disease intervention took place. With disposition E, the route to notification or treatment may not have been through DIS (other than through assuring treatment took place).
From these data, we calculated the notification statistics for the proportion of partners notified and the proportion of index patients notified (Table). These proportions were calculated on the basis of the total partners and index patients, respectively. We calculated the treatment index for partners treated. The treatment proportion was based on the total number of partners, whereas the treatment index used the number of index patients. The notification statistics and the treatment index are important to assess notification and treatment using the index patient as the denominator. The notification statistics and the treatment index inform treatment cascades that were constructed to show per index case, the number of patients notified and treated. Because the denominator was the same, we could describe notification and treatment in terms of index case and not just the proportion of partners notified and the proportion treated.
We used program data to derive all estimates for every program. Two studies (3 estimates) with data for patient referral did not report the number of partners treated (they had index patient reinfection outcomes). These studies were excluded from the calculation of proportions treated and the treatment index. Yu et al20 included 2 patient referral methods and PDPT for a total of 3 estimates and reported analyses by partners, so we could not attribute index patients (N = 743) cleanly by referral method. This study was included for estimates of proportions of partners notified and treated but not in estimations of numbers per index patient.
Results
From the 7 studies extracted from the literature that examined patient referral, there were 3853 patients and 7490 partners in total (see Table, Supplemental Digital Content 1, available at: http://links.lww.com/JPHMP/A234, which shows the data sources and PN efficacy estimates from peer-reviewed literature and STD programs, 2005–2014). Of the partners, 6826 were exclusive to the Yu et al20 study. Excluding the Yu et al study, there were 1.77 partners per index patient. There were 5 studies evaluating PDPT. From those studies, there were 1781 patients reporting 3125 partners. Of these partners, 2941 of them were exclusive to the Yu et al study. Excluding the Yu et al study, there were 1.65 partners per index patient. Combining these patient-mediated referral approaches, our sample included 6377 index patients and 10 615 partners reported, with 1.66 partners per index patient.
Of the 12 programs (14 estimates) from which we obtained data, 2 did not interview any gonorrhea cases at all and 4 others interviewed less than 5.1% of cases. The remaining 6 programs provided a total of 8 estimates because 2 programs provided data for more than 1 year. These programs interviewed 32.9% to 100% of cases. Overall, there were 32 605 gonorrhea-infected patients in the 14 estimates, of whom, 4581 (14.05%) were interviewed. These 4581 patients reported 8301 partners, with 1.81 partners per index patient. Excluding data from programs that interviewed none or a small proportion of gonorrhea cases, the final program data included 7433 cases, 4090 (55.02%) interviewed, and 7377 partners (1.80 partners per index case). In summary, our sample from the literature included 6377 index patients and 10 615 partners reported, with 1.66 partners per index patient; the sample from the program data included 7433 cases, 4090 (55.02%) interviewed, and 7377 partners (1.80 partners per index case).
Partner services cascades
Figure 1 contains the cascades for each PN condition per 100 partners named, calculated from the aggregated data (see Table, Supplemental Digital Content 1 available at: http://links.lww.com/JPHMP/A234, which shows the data sources and PN efficacy estimates from peer-reviewed literature and STD programs, 2005–2014). For patient referral, the aggregate across 10 estimates was 56.4% of partners notified (95% confidence interval [CI], 55.2–57.5) and the aggregate across 7 estimates was 34.0% treated (95% CI, 32.8–35.3). If we constrain the estimate for partners notified to the 7 estimates for which there were also treatment data, the notification percentage drops from 56.4% to 50.2%. Of those notified, 67.8% were treated. For PDPT, the aggregate across 5 estimates was 57.2% of partners notified (95% CI, 55.4–58.9) and 46.5% treated (95% CI, 44.7–48.2). Of those notified, 81.25% were treated. Binomial tests of proportions indicated that notification rates did not differ between patient referral and PDPT (P = .43) but treatment rates did (P < .001).
FIGURE 1.
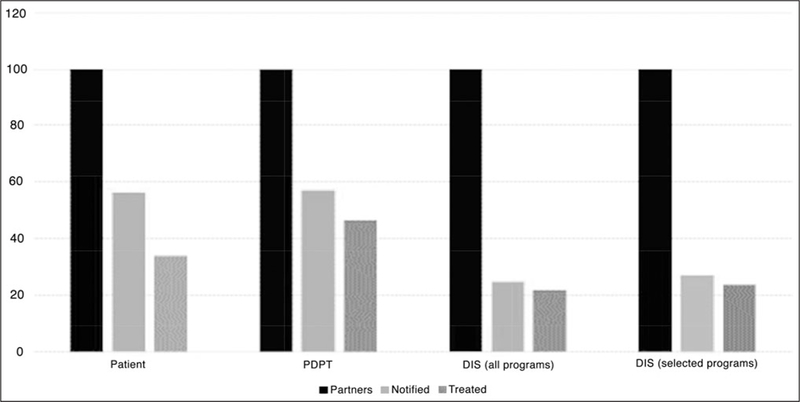
Partner Notification and Treatment Cascades per 100 Partners ClaimedaAbbreviations: DIS, disease intervention specialist; PDPT, patient-delivered partner therapy. aBlack = all partners; medium gray = treated partners; light gray = notified partners.
For DIS referral, we calculated cascades for all programs (n = 14) and for programs that interviewed more than a small fraction of cases (n = 8). These are the 2 right-hand clusters in Figure 1. For all 14 programs, the aggregate proportion of partners notified was 24.8% (95% CI, 23.9–25.7) and the proportion treated was 21.9% (95% CI, 21.0–22.8). For the 8 selected programs, the figures were 27.0% notified (95% CI, 26.0–27.0) and 23.8% treated (95% CI, 22.8–24.8). Of those notified, 88.1% were treated regardless of whether the figures were from all 14 programs or the 8 selected programs. Compared with patient referral (and therefore to PDPT), fewer partners were notified or treated via DIS referral (P < .001 in both cases). This was the case whether comparing with DIS referral in all 14 programs or in the 8 selected programs. However, among those notified,DIS referral (88.1%; 95% CI, 86.6–89.4) was superior in assuring treatment to both patient referral (67.8%; 95% CI, 66.0–69.5) and PDPT (81.3%; 95% CI, 79.4–83.0) (P < .001 in both cases). Results are the same whether the DIS referral estimate is made from 14 programs or 8 selected programs.
Figure 2 contains the results of the analyses using index cases as the denominator. With the index case as the denominators, we will report the notification index and treatment index in terms of whole patients. For patient referral, the notification index was 0.96 and the treatment index was 0.61. That is, almost 1 partner was notified per index case identified and approximately 3 partners were treated per 5 index cases. The corresponding figures for PDPT were 0.90 and 0.73 (3 partners treated per 4 index cases). For all 14 programs, the notification index was 0.45 and the treatment index was 0.40; if the analysis was restricted to 8 programs with higher proportions of cases interviewed, the figures were 0.49 and 0.43. Program data estimates, however, drop if all cases are considered (not just those interviewed). In programs with substantial proportions interviewed, the notification index drops to 0.27 and the treatment index drops to 0.24 (~1 partner treated per 4 index cases); across all 14 estimates, the figures are lower yet: 0.06 and 0.06. These final numbers translate to 1 partner notified or treated for every 18 cases seen in the clinic.
FIGURE 2.

Partner Notification and Treatment Cascades per Index Patienta Abbreviations: DIS, disease intervention specialist; PDPT, patient-delivered partner therapy.aBlack = index; medium gray = notification index; light gray = contact index; lightest gray = treatment index.
Discussion
Whether estimated via proportions of partners (Figure 1) or per index case (Figure 2), the overall portrait for referral approaches shows that (a) patient referral and PDPT result in more partners notified and (b) that PDPT results in the most partners treated. Mitigating these points, however, is that (c) DIS referral, if used, is the most efficacious of the strategies with respect to assuring treatment among those notified: 88% versus 68% for patient referral and 81% for PDPT.
The populations across the different approaches could differ, and programs may select differentially for DIS referral. These potential confounds mean that direct comparisons have to be treated with caution. Fortunately, no program has to choose a single method of PN. Both PN and patient referral are available almost everywhere.
An important question for STD programs is “What mix of available methods leads to the highest proportion of partners treated?” There are examples of applying this strategy. In Seattle, researchers evaluated the introduction of PDPT on a populationwide level, finding that increased use of PDPT yielded an estimated increase from 39% to 58% in the proportion of partners treated for gonorrhea or chlamydial infection.23 If the effect of DIS referral was added, the proportion rose to 65%. Thus, a mix of PN interventions was associated with a large increase in partners treated at a population level. Another evaluation in the Netherlands subsequent to an assessment of the mix of partner services for men who have sex with men showed that increased attention to PN interventions (which appears to have led to quality improvement) resulted in improved notification rates and even an increase in the proportion of sexually transmitted infection population detected via PN.24 Finally, a recent article used cascades to evaluate syphilis and gonorrhea treatment in 1 US jurisdiction, albeit in greater detail than this review (the authors split our notified step by located and interviewed).25
We found that approximately 1 in 7 patients were interviewed by DIS across programs, although this figure is the result of the bifurcation between programs interviewing no cases and those interviewing around half their cases. More usefully, it appears that DIS resources permit notification of approximately a quarter of partners, of whom, almost 9 in 10 are subsequently treated. The proportion treated as a function of those notified did not differ much according to the size of the program or the proportion of patients interviewed, which suggests efficacy after notification is consistently high across locations.
PDPT is now widely available in terms of legal status, but uptake is, at best, variable.26,27 Moreover, gonorrhea control in the United States must be considered in the context of potential resistance to cephalosporins.28 Because PDPT requires oral medications, programs using this option would not be using the most preferred option of ceftriaxone, which is injected.16 There are few data to indicate how widespread patient referral counseling interventions are even in public clinics.
If the literature reviewed here is a guide, a program that instituted PDPT for all patients whose partners are not notified via DIS should expect to get around 46% of those partners treated. If the program could manage DIS notification for all high-priority cases (88% of partners treated) and get roughly half the remaining partners treated through a brief intervention, that program would manage to treat half or more of all partners. Such a portrait is probably too optimistic, as some high-priority patients will refuse DIS services and gonorrhea morbidity in some programs is largely drawn from men in dense sexual networks and thus difficult to prioritize. For these programs, there are larger issues of the overall value of PN for gonorrhea (this may explain why some programs do not interview cases at all).
Limitations and Future Directions
This assessment was based on existing data, programmatic or in the literature, and this limitation constrained our interpretation. In particular, DIS referral reports typically have verification of outcomes independent of self-report, whereas patient referral and PDPT data were often self-reported with respect to notification and treatment. One study in our sample, however, reported a high concordance between patient and partner reports of treatment (r = 0.88),20 and the estimates from another study with extensive patient follow-up questioning, including for adverse events such as domestic violence, were similar to those from studies with less intensive study.22 Most data were collected in STD clinics or similar facilities; a broader variety of clinical settings would improve estimates for patient referral and PDPT. We also lacked sufficient studies and program estimates to generate distributions of estimates and thus propagate probabilities across the cascades. Because we did not have demographic information on patients in STD program settings, we do not know how well the demographic distribution of patients in these settings matched the primarily heterosexual distribution in patient referral and PDPT studies.
Conclusion
Choosing STD prevention approaches, including PN, to fit populations and the burden of disease remains key. Programs can work to use a mix of PN methods to cover all of the population at some level, evaluate the effects on transmission and care for highly vulnerable people, and then assess how to best distribute interventions and resources for maximum impact. Fortunately, the approaches measured here include low-cost and scalable approaches (patient referral and PDPT) along with higher-efficacy DIS-based approaches. As programs consider how best to reach their priority populations, perhaps they can experiment with a mix of approaches. For this to occur, more program evaluation and additional studies will be needed.
Supplementary Material
Implications for Policy & Practice.
-
■
The primary programmatic question lies in which index patients (or even which partners—an index patient with relatively low-risk factors may have a partner who is much more central to transmission) are most worth pursuing.
-
■
Beside using DIS to reach those who are most likely to transmit disease, we also note that most programs, as well as partner services recommendations, consider patient or partner vulnerability to disease or sequelae (eg, prioritizing pregnant women for PN to prevent congenital syphilis).29
-
■
A more subtle question around prioritizing cases for care is when to stop DIS referral even if resources permit it. That is, if a program had DIS resources left over after interviewing and conducting referral for all “important” cases, how efficient is it to keep interviewing less important cases with the same methods?
-
■
This question is tied to another, which is what is available as an alternative PN approach.
-
■
Here, programs could benefit by “raising the floor,” in which PDPT or patient referral counseling is used routinely, and by choosing one of these cheaper alternatives to DIS referral if patient priority is low enough.
-
■
DIS are not limited to performing PN and so saved resources could be devoted to other STD prevention activities.
Footnotes
The authors appreciate the programs that shared data for this project.
The findings and conclusions in this article are those of the authors and do not necessarily represent the views of the Centers for Disease Control and Prevention.
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal’s Web site (http://www.JPHMP.com).
The authors have no conflicts or conflicts of interest, and no funding was given for the work of the authors.
References
- 1.Centers for Disease Control and Prevention. Sexually Transmitted Disease Surveillance 2014. Atlanta, GA: US Department of Health and Human Services; 2015. [Google Scholar]
- 2.Fleming DT, Wasserheit JN. From epidemiological synergy to public health policy and practice: the contribution of other sexually transmitted diseases to sexual transmission of HIV infection. Sex Transm Infect. 1999;75(1):3–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hogben M Partner notification for sexually transmitted diseases. Clin Infect Dis. 2007;44(suppl 3):S160–S174. [DOI] [PubMed] [Google Scholar]
- 4.Golden MR, Hogben M, Handsfield HH, St Lawrence JS, Potterat JJ, Holmes KK. Partner notification for HIV and STD in the United States: low coverage for gonorrhea, chlamydial infection, and HIV. Sex Transm Dis. 2003;30:490–496. [DOI] [PubMed] [Google Scholar]
- 5.Hogben M, St Lawrence JS, Montaño DE, Kasprzyk D, Leichliter JS, Phillips WR. Physicians’ opinions about partner notification methods: case reporting, patient referral, and provider referral. Sex Transm Infect. 2004;80:30–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hogben M, Burstein GR, Golden MR. Partner notification in the clinician’s office: patient health, public health and interventions. Curr Opin Obstet Gynecol. 2009;21:365–370. [DOI] [PubMed] [Google Scholar]
- 7.Wilson TE, Hogben M, Malka E, et al. Reducing sexually transmitted infection rates by improving patient referral for treatment: a randomized, controlled trial. Am J Pub Health. 2009;99(suppl): S104–S110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ferreira A, Young T, Mathews C, Zunza M, Low N. Strategies for partner notification for sexually transmitted infections, including HIV. Cochrane Database Syst Rev. 2013;(10):CD002843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hogben M, Kidd S, Burstein GR. Expedited partner therapy for sexually transmitted infections. Curr Opin Obstet Gynecol. 2012;24:299–304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Centers for Disease Control and Prevention. Expedited Partner Therapy in the Management of Sexually Transmitted Diseases. Atlanta, GA: US Department of Health and Human Services; 2006. [Google Scholar]
- 11.Schillinger JA, Kissinger P, Calvet H, et al. Patient-delivered partner treatment with azithromycin to prevent repeated Chlamydia trachomatis infection among women: a randomized, controlled trial. Sex Transm Dis. 2003;30:49Y56. [DOI] [PubMed] [Google Scholar]
- 12.Golden MR, Whittington WL, Handsfield HH, et al. Effect of expedited treatment of sex partners on recurrent or persistent gonorrhea or chlamydial infection. N Engl J Med. 2005;352:676–685. [DOI] [PubMed] [Google Scholar]
- 13.Kissinger P, Mohammed H, Richardson-Alston G, et al. Patientdelivered partner treatment for male urethritis: a randomized, controlled trial. Clin Infect Dis. 2005;41:623–629. [DOI] [PubMed] [Google Scholar]
- 14.St Louis ME, Holmes KK. Conceptual framework for STD/HIV prevention and control In: Holmes KK, Sparking PF, Mardh P-A, et al. eds. Sexually Transmitted Diseases. 3rd ed New York, NY: McGraw-Hill; 1999:1239–1253. [Google Scholar]
- 15.Du P, Coles B, Gerger T, et al. Effects of partner notification on reducing gonorrhea incidence rate. Sex Transm Dis. 2007;34:189–194. [DOI] [PubMed] [Google Scholar]
- 16.Workowski KA, Bolan GA. Sexually transmitted diseases treatment guidelines, 2015. MMWR Recomm Rep. 2015;64(3):1–137. [PMC free article] [PubMed] [Google Scholar]
- 17.Menza TW, St. De Lore J, Fleming M, Golden MR. Partner notification for gonococcal and chlamydial infections in men who have sex with men: success is underestimated by traditional disposition codes. Sex Transm Dis. 2008;35:84–90. [DOI] [PubMed] [Google Scholar]
- 18.Thurman AR, Shain RN, Holden AEC, Champion JD, Perdue ST, Piper JM. Partner notification of sexually transmitted infections: a large cohort of Mexican American and African American women. Sex Transm Dis. 2008;35:136–140. [DOI] [PubMed] [Google Scholar]
- 19.Gursahaney PR, Jeong K, Dixon BW, Weisenfeld HC. Partner notification of sexually transmitted diseases: practices and preferences. Sex Transm Dis. 2011;38:821–827. [DOI] [PubMed] [Google Scholar]
- 20.Yu YY, Frasure-Williams JA, Dunne EF, Bolan G, Markowitz L, Bauer HM. Chlamydia partner services for females in California family planning clinics. Sex Transm Dis. 2011;38:913–918. [DOI] [PubMed] [Google Scholar]
- 21.Kerani RP, Fleming M, DeYoung B, Golden MR. A randomized, controlled trial of inSPOT and patient-delivered partner therapy for gonorrhea and chlamydial infection among men who have sex with men. Sex Transm Dis. 2011;38:941–946. [DOI] [PubMed] [Google Scholar]
- 22.Temkin E, Klassen AC, Mmari K, Gillespie DG. A qualitative study of patients’ use of expedited partner therapy. Sex Transm Dis. 2011; 38(7):651–656. [DOI] [PubMed] [Google Scholar]
- 23.Golden MR, Hughes JP, Brewer DD, et al. Evaluation of a population-based program of expedited partner therapy for gonorrhea and chlamydial infection. Sex Transm Dis. 2007;34:598–603. [DOI] [PubMed] [Google Scholar]
- 24.van Aar F, van Weert Y, Spiker R, et al. Partner notification among men who have sex with men and heterosexuals with STI/HIV: different outcomes and challenges. Int J STD AIDS. 2015;26: 565–573. [DOI] [PubMed] [Google Scholar]
- 25.Murphy RD, Wohl AR, Ma Y, Kobeissi L, Oduyemi O, Perez MJ. Adaptation of the HIV care continuum as a method for evaluating syphilis and gonorrhea disease control activities in Los Angeles County. Sex Transm Dis. 2015;42:686–690. [DOI] [PubMed] [Google Scholar]
- 26.Golden MR, Kerani RP, Stenger A, et al. Uptake and populationlevel impact of expedited partner therapy (EPT) on Chlamydia trachomatis and Neisseria gonorrhoeae: the Washington State community-level randomized trial of EPT. PLoS Med. 2015;12(1): e1001777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cramer R, Leichliter JS, Stenger MR, Loosier PS, Slive L; SSuN Working Group. The legal aspects of expedited partner therapy practice: do state laws and policies really matter? Sex Transm Dis. 2013;40:657–662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kirkcaldy RD, Bolan GA, Wasserheit JN. Cephalosporin-resistant gonorrhea in North America. JAMA. 2013;309:185–187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Centers for Disease Control and Prevention. Recommendations for partner services programs for HIV infection, syphilis, gonorrhea, and chlamydial infection. MMWR Recomm Rep. 2008;57(RR-9): 1–83. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


