Summary
Keywords: Childhood, obesity, sleep apnoea, sleep-disordered breathing
Background:
Obesity has been recognized as a risk factor for childhood sleep-disordered breathing (SDB), yet it remains unclear how obesity and weight change predict the course of childhood SDB.
Objective:
The objective of the study is to investigate the role of body weight, upper airway abnormalities and developmental trajectories on the persistence and remission of childhood SDB in the transition to adolescence.
Methods:
The Penn State Child Cohort is a representative population sample of 700 children (5–12 years), of whom 421 were followed up as adolescents (12–23 years). Participants underwent a clinical history, physical examination and polysomnography at both time points.
Results:
Obesity and enlarged tonsils were cross-sectionally associated with childhood SDB. Longitudinally, baseline obesity predicted the persistence of childhood SDB (OR = 3.75, 95% CI = 2.00–7.05), while weight loss predicted its remission (OR = 1.67, 95% CI = 1.11–2.50). Children with enlarged tonsils who remitted from SDB had not experienced significant weight loss and only 4.4% had undergone adeno/tonsillectomy. Body fat distribution/composition at follow-up was similar in those who had remitted from childhood SDB as compared with those who had never experienced SDB, while those who persisted with childhood SDB showed significant android distribution and visceral adiposity at follow-up.
Conclusions:
Our data support a causal role for obesity and weight loss in the chronicity and remission, respectively, of childhood SDB in the transition to adolescence and suggest that remission of SDB is related to developmental trajectories of the upper airway in a significant proportion of children. Thus, targeting childhood obesity and weight gain should be a priority in the prevention and treatment of SDB during this critical developmental period.
Introduction
Sleep-disordered breathing (SDB) comprises a range of sleep-related breathing disorders ranging from primary snoring (PS) to obstructive sleep apnoea (OSA). In young children, the prevalence of PS and OSA has been documented in several studies and is estimated to range from 7 to 26% and from 1 to 4%, respectively, depending on the definitions used (1–5). However, data on the natural history of SDB in the transition from childhood to adolescence are lacking, particularly in terms of the clinical factors that predict its persistence and remission.
Childhood SDB is primarily viewed as a disorder caused by anatomical abnormalities of the upper airway (4) such as enlarged tonsils (2,6); therefore, its current first-line treatment is adeno/tonsillectomy (7). However, several recent studies have reported that childhood SDB is likely to remit over time. Specifically, remission rates of 46% for childhood OSA, defined as an apnoea/hypopnoea index (AHI) ≥2 events per hour of sleep, under a 7-month watchful waiting period (7) and up to 70% in observational epidemiological studies with long follow-ups, have been reported (5,8,9). There is a paucity of literature on the factors leading to such high remission rates, which may include clinical as well as developmental changes.
Increased body weight has been recognized as an important risk factor for sleep difficulties in general (10,11) as well as childhood SDB specifically, including both PS and OSA (2,5,6,12). Snoring in childhood has been as associated with increased global adiposity (13), while visceral fat has been shown to be higher in children with obesity and OSA (12). However, these associations have been mostly cross-sectional, and data on whether childhood obesity or weight gain predict the persistence of SDB into adolescence are lacking. The importance of longitudinally examining such factors in adolescence was reflected in our previous study which showed that weight gain, measured as change in childhood body mass index percentile (BMI%), was the best predictor of the incidence of adolescent SDB, defined as an AHI >5 events per hour of sleep (9). A 4-year follow-up study by Sanchez-Armengol et al. demonstrated that SDB in 148 adolescents at both baseline (14.3-years old) and follow-up (18.5-years old) was associated with higher BMI and central fat distribution (14), although the authors were not able to examine trajectories of SDB across different developmental stages (i.e. from childhood to adolescence) due to the age of the sample. While some evidence suggests that SDB in children with obesity is more likely to persist after adeno/tonsillectomy (7,15,16), data indicating a causal relationship are very limited, particularly for subclinical levels, such as when children are overweight.
Thus, the primary aim of this study was to investigate the role of body weight, upper airway abnormalities and developmental trajectories in the persistence and remission of childhood SDB, either PS or OSA, in the transition to adolescence in a large, randomly selected, population-based sample.
Methods
Population
The Penn State Child Cohort (PSCC) has been described in detail in previous studies (2,9). The PSCC is a representative population sample of 700 children (5–12 years old), of whom 421 were followed up approximately 8 years later as adolescents (12–23 years old). There were no differences in demographic or clinical characteristics between the 421 children and the 279 lost to follow-up (9). All children underwent a full clinical history, physical examination and PSG at both baseline and follow-up that allowed an examination of risk factors for the natural history of SDB. The PSCC was established to determine the prevalence and risk factors associated with SDB in a general population sample of children. Thereafter, it was designed to examine the natural history of SDB in a longitudinal design. The study protocol was approved by Penn State University College of Medicine Institutional Review Board. Written informed consent was obtained from participants, and their parents or legal guardians if younger than 18 years.
Sleep laboratory
Participants’ sleep was continuously monitored for 9 h with seven-channel polysomnography (PSG), including electroencephalography, electrooculography and electromyography. Respiration was monitored with nasal pressure, thermocouple and thoracic and abdominal strain gauges. Snoring sounds were monitored by a microphone attached to the lateral neck area and were scored blindly by trained sleep technicians. In order to be identified as a snorer, the participant had to have at least two periods of mild (<50% of the night) or moderate-to-severe (>50% of the night) snoring that occurred intermittently or continuously. One single period of snoring at any time during the night did not classify a participant as a snorer. Haemoglobin oxygen saturation (SpO2) was obtained from the finger. The sleep records were scored according to standardized criteria by a registered polysomnography technologist who was blinded to participant characteristics (17). Apnoea and hypopnoea were defined following standardized criteria (18), while taking into account the age of the participants. An apnoea was defined as a cessation of airflow with a minimum duration of 5 s for those aged < 16 years and 10 s for those aged ≥16 years with an associated out-of-phase strain gauge movement. A hypopnoea was defined as a reduction of airflow of approximately 50% with an associated decrease in SpO2 of ≥3% or an associated arousal (18). Apnoea/hypopnoea index was calculated as the number of apnoeas and hypopnoea summed per hour of sleep. Objectively monitored snoring was used in this study, and the presence of childhood PS was determined if AHI was <2 events/hour. The presence of childhood OSA was determined if AHI was ≥2 events/hour. This cut-off was based on the recent CHAT study (7) and commensurate with our previous studies (9,19). The reference group was comprised of subjects without childhood SDB (i.e. AHI < 2 without snoring) who were also absent of adolescent-onset SDB at follow-up (i.e. AHI ≥ 5 based on adult criteria).
Physical examination
A physical examination included measurements of height; weight; hip, waist and neck circumference; and, at baseline, a visual evaluation of the nose and throat by an ENT specialist. The presence of enlarged tonsils was determined based on stages: out of fossa touching, the uvula or out of fossa not touching the uvula (abnormal) and within fossa or post-tonsillectomy (normal). Height was measured in centimetres using a stadiometer (SECA Corp., Hanover, MD, USA), and weight was assessed in kilograms (Cardinal Scale Manufacturing, Webb City, MO, USA). The age-adjusted and sex-adjusted BMI percentile (BMI%) for each participant was calculated based on growth charts (20). Normal weight was defined as a BMI% <85. The waist was measured in centimetres at the top of the iliac crest and the neck at the cricothyroid membrane. We calculated for each body weight parameter the difference between the values from baseline to follow-up, including the change (Δ) in height, weight and BMI%, as well as neck, waist and hip circumferences. Positive ABMI% values indicate an increase in body weight (i.e. weight gain), while negative values indicate a decrease in body weight (i.e. weight loss). Whole-body dual-energy X-ray absorptiometry (DXA) scan was performed to measure the adipose tissue distribution at follow-up (Hologic Discovery W scanner, Hologic Inc., Waltham, MA). Android and gynoid regions and visceral and subcutaneous adipose tissues were selected as regions of interest (ROI), identified by Hologic APEX 4.0 software (Hologic Inc., Bedford, MA), and visually verified by a technician who was blinded to participant characteristics (21,22).
Statistical analyses
Univariate comparisons were assessed using ANOVA or chi-squared analysis for continuous and categorical demographic and clinical variables, respectively. Multivariable general linear models assessed differences between groups in continuous variables of change from childhood to adolescence (e.g. ABMI%), and post-doc comparisons were conducted using Fisher’s LSD. To examine the predictive value and relative independent contribution of childhood weight and change in weight with the natural history of SDB, we further analysed the data using multivariable, multinomial logistic regression. In this longitudinal regression analysis, age, sex and race were forced into the model, while baseline and Δ values for weight, height, BMI%, hip, waist and neck circumference were entered using stepwise, backward elimination to account for multicollinearity. Based on the output of this model, we ran a more clinically meaningful regression model using BMI% as a predictor of the natural course of SDB. Data from these regression models are reported as odds ratios (OR) and 95% confidence interval (95% CI). All analyses were performed using SPSS Statistics v23 (IBM, Armonk, NY, USA).
Results
Our primary aim focused on the persistence and remission of childhood SDB. First, we examined whether the predictors of interest were significantly associated with childhood PS or OSA. As shown in Supplemental Table S1, both childhood PS and OSA were cross-sectionally associated with baseline obesity, while PS was associated with adeno/tonsillectomy and OSA was associated with enlarged tonsils. Second, we examined whether there were any demographic or clinical differences between childhood PS and childhood OSA, and no significant differences were observed in any baseline demographic or clinical characteristic between children with PS or OSA, except the latter being significantly more overweight and having lower Sp02 at baseline. Childhood PS and OSA were only significantly different at follow-up in terms of age and, consequently, Tanner stage. Third, we examined the trajectories of childhood PS and OSA and observed that 28 and 17 children with PS and OSA, respectively, fully remitted in the transition to adolescence (i.e. AHI < 2 without snoring), while 61 children with PS persisted or worsened into OSA (i.e. PS or AHI ≥ 2) and 34 children with OSA persisted or became PS at follow-up (i.e. AHI ≥ 2 or PS). Thus, our primary analyses focused on the natural course of these two levels of childhood SDB combined together, and participants were divided into ‘persistent SDB’ (n = 95) and ‘remitted SDB’ (n = 45). The baseline and follow-up characteristics of the resulting persistent and remitted SDB groups are presented in Table 1. Detailed trajectories adjusted for sex, age and race are presented in Fig. 1 in relation to baseline BMI% ≥85 (i.e. overweight or obese) and ΔBMI%.
Table 1.
Characteristics of the sample stratified by the natural course of childhood sleep-disordered breathing
| 1. No SDB (n = 248) |
2. Persistent SDB (n = 95) |
3. Remitted SDB (n = 45) |
P value | Post-hoc |
|||
|---|---|---|---|---|---|---|---|
| 1 vs. 2 | 1 vs. 3 | 2 vs. 3 | |||||
| Male sex (%) | 52.8 | 57.9 | 35.6 | .044* | .399 | .035* | .015* |
| Ethnic/racial minority (%) | 17.7 | 33.7 | 20.0 | .006* | .002* | .718 | .100 |
| adeno/tonsillectomy (%) | 8.5 | 21.1 | 4.4 | .001* | .002* | .365 | .023* |
| Baseline | |||||||
| Age (years) | 8.54 (1.64) | 8.92 (1.78) | 8.22 (1.69) | .053 | |||
| Height (cm)† | 135.14 (7.27) | 138.50 (7.89) | 135.78 (7.58) | .003* | .001* | .652 | .052 |
| Weight (kg) † | 33.84 (10.14) | 40.90 (11.11) | 36.16(10.73) | <.001* | <.001* | .070 | .017* |
| Neck circumference (cm) † | 28.44 (4.25) | 29.97 (4.39) | 28.96 (4.22) | .016* | .004* | .448 | .200 |
| Hip circumference (cm)† | 71.62 (11.97) | 77.68 (12.28) | 75.58 (11.80) | <.001* | <.001* | .039* | .336 |
| Waist circumference (cm) † | 63.95 (10.39) | 70.05 (10.62) | 66.62 (10.26) | <.001* | <.001* | .111 | .071 |
| BMI% (percentile) | 58.73 (28.35) | 71.08 (28.56) | 65.34 (27.91) | .001* | <.001* | .150 | .264 |
| Normal weight (%) | 77.0 | 54.7 | 62.2 | <.001* | — | — | — |
| Overweight (%) | 9.7 | 11.6 | 22.2 | .189 | .015* | .291 | |
| Obese (%) | 13.3 | 33.7 | 15.6 | <.001* | .425 | .060 | |
| Enlarged tonsils (%) | 34.5 | 43.2 | 50.0 | .099 | |||
| AHI (events/hour of sleep) | 0.46 (0.53) | 1.53 (1.53) | 1.45 (1.38) | <.001* | <.001* | <.001* | .660 |
| Minimum Sp02 | 93.43 (3.52) | 92.62 (3.84) | 90.87 (6.44) | <.001* | .100 | <.001* | .017* |
| Follow-up | |||||||
| Age (years) | 16.70(2.17) | 17.40(2.35) | 16.55 (2.28) | .021* | .009* | .688 | .036* |
| Tanner (stage) | 4.13 (0.76) | 4.28 (0.76) | 4.12 (0.98) | .263 | |||
| Height (cm)‡ | 168.61 (7.78) | 169.44 (7.89) | 166.05 (7.78) | .055 | |||
| Weight (kg) ‡ | 65.65 (16.54) | 76.88 (16.57) | 63.98 (16.37) | <.001* | <.001* | .527 | <.001* |
| Neck circumference (cm) ‡ | 35.05 (4.41) | 36.48 (4.48) | 34.57 (4.43) | .016* | .009* | .501 | .019* |
| Hip circumference (cm) ‡ | 88.42 (13.23) | 96.98 (13.35) | 88.36 (13.15) | <.001* | <.001* | .977 | <.001* |
| Waist circumference (cm) ‡ | 77.82 (12.28) | 86.33 (12.38) | 76.83 (12.14) | <.001* | <.001* | .613 | <.001* |
| BMI% (percentile) | 60.79 (28.34) | 74.76 (25.28) | 61.09 (29.87) | <.001* | <.001* | .947 | .007* |
| Normal weight (%) | 74.2 | 50.5 | 71.1 | <.001* | — | — | — |
| Overweight (%) | 15.7 | 21.1 | 17.8 | .034* | .703 | .284 | |
| Obese (%) | 10.1 | 28.4 | 11.1 | <.001* | .790 | .017* | |
| AHI (events/hour of sleep) | 1.41 (1.08) | 4.04 (5.31) | 0.83 (0.58) | <.001* | <.001* | .196 | <.001* |
| Minimum Sp02 | 91.54 (5.27) | 91.31 (3.86) | 92.58 (5.25) | .347 | |||
| Change | |||||||
| ΔWeight (kg)§ | 32.96 (11.18) | 35.92 (11.50) | 28.08 (11.14) | .001* | .037* | .007* | <.001* |
| ΔNeck circumference (cm) § | 6.21 (4.72) | 7.45 (4.54) | 5.59 (4.47) | .048* | .034* | .412 | .031* |
| ΔHip circumference (cm) § | 15.81 (12.13) | 22.00 (12.24) | 14.13 12.02) | <.001* | <.001* | .392 | <.001* |
| ΔWaist circumference (cm) § | 13.66(9.13) | 17.92 (9.30) | 10.54 (9.10) | <.001* | <.001* | .036* | <.001* |
| ΔBMI% (percentile) § | 1.00 (20.47) | 6.53 (20.76) | −4.39 (20.46) | .011* | .029* | .105 | .004* |
Data are means (SD) except otherwise stated.
Estimated marginal means (SD) of childhood variables adjusted for sex, race and age at baseline.
Estimated marginal means (SD) of adolescence variables adjusted for sex, race and age at follow-up.
Estimated marginal means (SD) of change variables adjusted for sex, race, age and their respective baseline value. BMI% = body mass index percentile for age and sex. Normal weight = BMI% < 85. Overweight = BMI% between 85 and 94. Obesity = BMI% ≥ 95.
Statistically significant at P ≤ .05.
Figure 1.
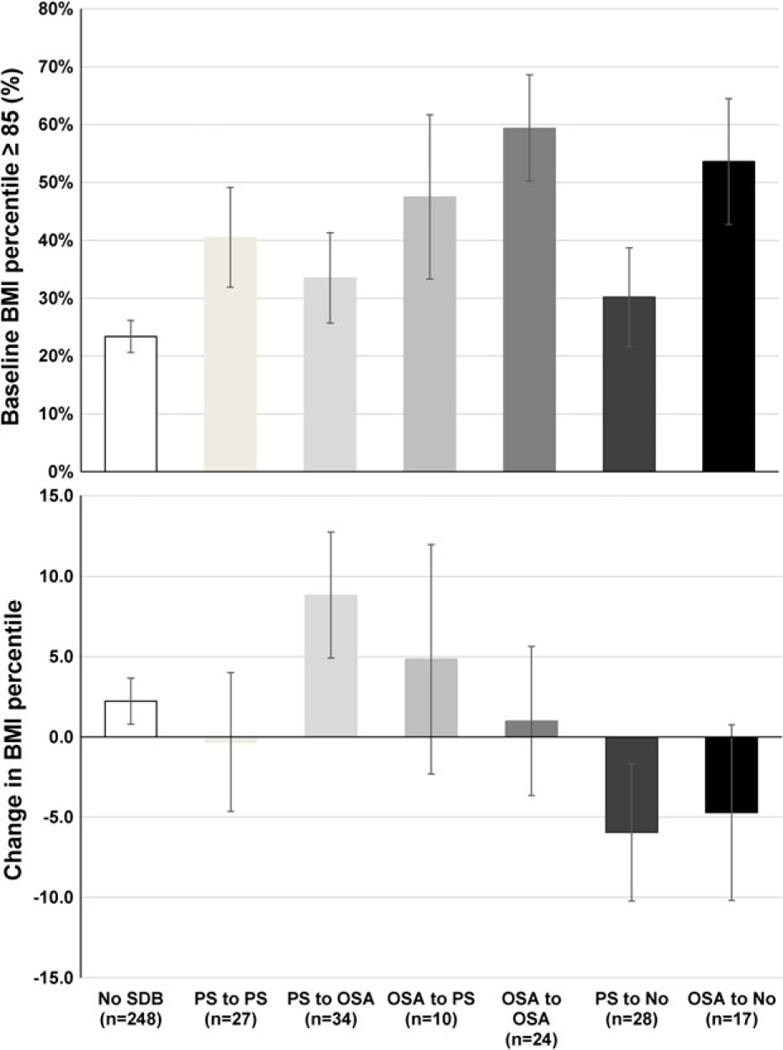
Trajectories of childhood sleep-disordered breathing, baseline and change in body weight in the transition to adolescence. The persistence of childhood PS or childhood OSA was associated with minimal weight change (−0.3 and +1.0 BMI percentiles, respectively) because most of these children were already overweight or obese in childhood (41 and 59%, respectively). Weight gain was associated with childhood PS worsening into adolescent OSA (+8.8 percentiles) as well as with childhood OSA persisting into PS (+4.8 percentiles). In contrast, weight loss was associated with the remission of childhood PS and childhood OSA in the transition to adolescence (−6.0 and −4.7 percentiles, respectively). All data are means and standard errors adjusted for sex, race and age. Change in BMI percentile = BMI percentile at follow-up minus BMI percentile at baseline. OSA, obstructive sleep apnea; PS, primary snoring.
Persistent and remitted SDB were significantly associated with baseline BMI% ≥85 (45.3 and 37.8%, respectively) when compared with children without SDB (23.0%, P < 0.001 and P = 0.036, respectively). Additionally, persistent SDB was significantly associated with baseline BMI% ≥95 (33.7 vs. 13.3%, P < 0.001 adjusted for sex, race and age) and with a significant mean increase in ΔBMI% (6.53 ± 20.76 vs. 1.00 ± 20.47, P = 0.029 adjusted for sex, race, age and baseline BMI%) when compared with children without SDB (refer to Table 1 for change in all other anthropometric measures). In contrast, remitted SDB was associated with a significant decrease in ΔBMI% when compared with persistent SDB (−4.39 ± 20.46 vs. 6.53 ± 20.76, P = 0.004, adjusted for sex, race, age and baseline BMI%). A significant proportion of children with remitted SDB lost weight (i.e. a ABMI% < −10) (23–25)) as compared with those with persistent SDB (42.2 vs. 13.7%, P < 0.001). This significant weight loss associated with remitted SDB was also reflected in smaller increases in Δweight (35.92 ± 11.50 vs. 28.08 ±11.14, P < 0.001) and Δneck (7.45 ± 4.54 vs. 5.59 ± 4.47, P = 0.031), Δhip (22.00 ±12.24 vs. 14.13 ± 12.02, P < 0.001) and Δwaist (17.92 ± 9.30 vs. 10.54 ± 9.10, P < 0.001) circumferences when compared with persistent SDB (adjusted for sex, race, age and their respective baseline value).
The multivariable, stepwise multinomial regression model adjusted for sex, race and age showed that baseline weight was the best single predictor of persistent SDB (OR = 2.09 95% CI = 1.51–2.89) and Δweight was the best single predictor of remitted SDB (OR = 0.55 95% CI = 0.36–0.86) when compared with children without SDB (Table 2), associations that were similar within males and females (Supplemental Table 2). These data suggested that children who were heavier at baseline were more likely to persist with SDB, while children who lost weight were more likely to remit from SDB. The output of this stepwise model (i.e. weight in kg adjusted for sex and age) was equivalent to BMI% without factoring-in height; thus, we ran a more clinically meaningful multivariable multinomial regression model (Table 2). This model showed that baseline obesity (BMI% ≥95) significantly predicted persistent SDB (OR = 3.75, 95% CI = 2.00–7.05) and baseline overweight (BMI% ≥85 and <95) significantly predicted remitted SDB (OR = 2.57, 95% CI = 1.07–6.19) when compared with children without SDB (Table 2). Baseline obesity (OR = 0.34 95% CI = 0.13–0.93) and an increase in ΔBMI% (OR = 0.60, 95% CI = 0.40–0.90) negatively predicted remitted SDB when compared with persistent SDB (Table 2); in other words, children with normal weight at baseline (OR = 2.94, 95% CI = 1.08–7.69) and those who lost weight (OR = 1.67, 95% CI = 1.11–2.50) were significantly more likely to remit from SDB than to persist with SDB.
Table 2.
Childhood obesity and weight loss predict the persistence and remission of childhood sleep-disordered breathing
| Persistent vs. no SDB | Remitted vs. no SDB | Remitted vs. persistent SDB | |
|---|---|---|---|
| Stepwise mode† | |||
| Baseline weight | 2.09 (1.51–2.89)< .001* | 1.66 (1.07–2.58).023* | 0.80 (0.51–1.25).324 |
| ΔWeight | 1.31 (0.99–1.73).061 | 0.55 (0.36–0.86).009* | 0.42 (0.26–0.68)< .001* |
| BMI model‡ | |||
| Baseline overweight | 2.00 (0.87–4.56).101 | 2.57 (1.07–6.19).035* | 1.29 (0.45–3.68).637 |
| Baseline obesity | 3.75 (2.00–7.05)< .001* | 1.29 (0.50–3.28).599 | 0.34 (0.13–0.93).036* |
| ΔBMI% | 1.31 (0.98–1.74).069 | 0.78 (0.56–1.10).153 | 0.60 (0.40–0.90).014* |
Data are odds ratios (95% confidence interval) and P values from a multivariable, multinomial logistic regression model adjusted for sex, race and age (forced entry) with stepwise (backward elimination) of childhood height, weight, BMI%, hips, waist and neck as well as change in height, change in weight, change in BMI%, change in hips, change in waist and change in neck. Odds ratios for each predicting variable represent an increase in one standard deviation (e.g. change in 13 kg from baseline to follow-up). No SDB (n = 248) served as the common reference group, while persistent SDB (n = 95) served as the reference group for remitted SDB (n = 45).
Data are odds ratios (95% confidence interval) and P values from multivariable, multinomial logistic regression models for baseline BMI% groups and change in BMI% adjusted for sex, race and age (all forced entry). Odds ratios for change in BMI% represent an increase in one standard deviation (e.g. increase in 13 kg from baseline to follow-up). Odds ratios for change in BMI% represent an increase in one standard deviation (i.e. increase in 23 percentiles of BMI from baseline to follow-up). No SDB (n = 248) served as the common reference group, while persistent SDB (n = 95) served as the reference group for remitted SDB (n = 45). BMI% = body mass index percentile for age and sex. Childhood overweight = BMI percentile between 85 and 94. Childhood obesity = BMI percentile ≥95.
Statistically significant at P ≤ .05.
Given that 50% of children with remitted SDB had a history of enlarged tonsils at baseline, we examined whether the observed weight loss occurred independently of such history. As shown in Supplemental Fig. S1, remitted SDB was associated with a significant decrease in ΔBMI% among those with normal tonsil size in childhood (−10.06 ± 20.18 percentiles, P = 0.031), but not among children with enlarged tonsils (−0.03 ± 26.77 percentiles). About 43% of children with persistent SDB had a history of enlarged tonsils at baseline; however, those with persistent SDB experienced an increase in ΔBMI% regardless of baseline tonsil size. Of note, only 4.4% of children with remitted SDB had a history of adeno/ tonsillectomy a figure significantly lower than those with persistent SDB (21.1%, P = 0.023). These results remained similar and in the same direction when other oropharyngeal or nasopharyngeal abnormalities (e.g. long palate, turbinate hypertrophy, nasal drainage, cervical adenopathy) were included together with enlarged tonsils.
To examine how the weight gain and weight loss associations observed above were reflected on specific body fat tissue distribution and composition at follow-up, we examined differences in DXA- measured adiposity between groups as shown in Supplemental Fig. S2. While there were no significant differences across groups in gynoid/whole body ratio at follow-up (P = 0.683), significant differences were present in android/whole body ratio (P = 0.008), subcutaneous adipose tissue (P < 0.001) and visceral adipose tissue (P < 0.001), indicating that, while remitted SDB’s body fat distribution and composition was similar to those without SDB (P = 0.850, P = 0.716, P = 0.566 and P = 0.591, respectively), persistent SDB was associated with central adiposity at follow-up compared with those without SDB (P = 0.422, P = 0.003, P < 0.001and P < 0.001, respectively).
Discussion
Previous studies have shown that childhood SDB is associated with obesity (5,8,9). In our study, 43% of children with SDB were overweight in childhood, of whom about 28% were obese. Consistently, childhood obesity predicted the persistence of SDB in the transition to adolescence, while weight loss predicted its remission during the same developmental period. Importantly, the association of childhood obesity and weight gain with persistent SDB was present regardless of childhood tonsil size or history of adeno/tonsillectomy. In fact, more children with persistent SDB had a history of adeno/tonsillectomy compared with the other groups, which suggests that some of these children were indeed identified to suffer from SDB and provided with the current first-line treatment; however, adeno/tonsillectomy did not resolve their SDB. Our novel findings indicate that children with obesity and SDB are very likely to persist or worsen over time and present in adolescence with central fat distribution and increased visceral adiposity. Thus, it is imperative that childhood obesity and weight gain be a primary focus of our preventive and therapeutic efforts during this critical developmental period (23,25).
In our study, the association of weight loss with remission of childhood SDB was observed not only in terms of a decrease in BMI percentile but also in the body fat distribution and composition in adolescence, which was similar to those who had never experienced SDB. Interestingly, weight loss did not predict the remission of childhood SDB among those with enlarged tonsils. Surgical treatments, such as adeno/tonsillectomy can indeed be effective in resolving SDB in some children with upper airway abnormalities (7). Thus, one may conclude that most of the children in our study remitted from SDB because they underwent adeno/tonsillectomy. However, only 4.4% of those who remitted had a history of such surgery. These data are consistent with other recent studies reporting a high rate of natural remission of childhood SDB over long follow-ups (5,8) and even after 7 months of watchful waiting (7). Thus, our data also support that in a sizeable proportion of children, the remission of SDB in the transition to adolescence might be related to normal developmental trajectories such as widening or growth of the upper airway and other anatomical structures (26–28). Together, these data suggest that a multidisciplinary approach to treatment should integrate and focus on weight loss, even in children who are overweight, for a large proportion of paediatric patients.
There are some potential limitations of the present study. There was a lack of an intermediate follow-up in between childhood and adolescence; thus, there is the potential for a waxing-and-waning pattern between PS and OSA that would not have been detected. Additionally, capnography was not included in the PSG recording, which limited the ascertainment of hypoventilation syndrome; however, this type of SDB is more common in clinical than general population samples. An ENT exam was not conducted at follow-up, and thus, we could not assess the presence or absence of enlarged tonsils when participants were adolescents; however, these upper airway anatomical abnormalities are not likely to be associated with SDB at this developmental stage. Dual-energy X-ray absorptiometry scan was only conducted at follow-up; thus, the change in body fat distribution/composition could not be examined from baseline to follow-up. Similarly, variables related to diet and physical activity were not collected at baseline, and therefore, the potential contribution of changes in these lifestyle factors on body weight and on the natural history of SDB could not be examined.
In conclusion, our data support a causal role for obesity and weight loss in the chronicity and remission, respectively, of SDB in the transition from childhood to adolescence. Obesity predicted the chronicity of SDB regardless of childhood tonsil size or adeno/tonsillectomy while weight loss did not predict the remission of SDB among children with a history of enlarged tonsils. This latter lack of association was not explained by a history of adeno/tonsillectomy suggesting that the remission of SDB in a sizeable proportion of children is also related to normal developmental trajectories of the upper airway. Therefore, watchful waiting as short as 7 months (7) appears to be indicated in normal weight children with SDB. Weight loss should become a priority in preventing childhood SDB from becoming a chronic disorder in the transition to adolescence. Thus, preventative and therapeutic efforts should be directed not only towards children with obesity but also those who are already overweight.
Supplementary Material
Characteristics of the sample stratified by the presence of childhood sleep disordered breathing
Body weight as a predictor of the natural history of sleep-disordered breathing (SDB) in multivariable, stepwise multinomial logistic regression models
Role of childhood history of enlarged tonsils in the association of weight loss with the remission of childhood sleep-disordered breathing. Weight loss was associated with the remission of childhood SDB in the transition to adolescence, but not among those who had a childhood history of enlarged tonsils. All data are means and standard errors adjusted for sex, race, age and childhood body mass index percentile. Change in BMI percentile = BMI percentile at follow-up minus BMI percentile at baseline. SDB = sleep-disordered breathing.
Body fat distribution and composition in adolescence associated with persistent and remitted childhood sleep-disordered breathing. Remitted childhood SDB was associated with normal body fat composition and distribution in adolescence, while persistent SDB since childhood was associated with central adiposity, as measured by dual-energy X-ray absorptiometry android distribution and visceral adiposity. All data are means and standard errors adjusted for sex, race, age and childhood body mass index percentile, enlarged tonsils and adeno/tonsillectomy. SDB = sleep-disordered breathing.
Acknowledgments
Funding source
This research is funded in part by the National Institutes of Health grants R01 HL63772, R01 HL97165, UL1 RR033184 and C06 RR16499.
Abbreviations:
- AHI
Apnoea/hypopnoea index
- BMI
Body mass index
- OSA
Obstructive sleep apnoea
- PS
Primary snoring
- PSG,
Polysomnography
- SDB
Sleep-disordered breathing
- SpO2
Haemoglobin oxygen saturation
Footnotes
Supporting information
Additional supporting information may be found online in the Supporting Information section at the end of the article.
Conflict of interest statement
Drs. Fernandez-Mendoza, Frye, Calhoun, Gaines, Vgontzas, Liao and Bixler report grants from National Institutes of Health, during the conduct of the study.
References
- 1.Anuntaseree W, Kuasirikul S, Suntornlohanakul S. Natural history of snoring and obstructive sleep apnea in Thai school-age children. Pediatr Pulmonol 2005; 39: 415–420. [DOI] [PubMed] [Google Scholar]
- 2.Bixler EO, Vgontzas AN, Lin HM, et al. Sleep disordered breathing in children in a general population sample: prevalence and risk factors. Sleep 2009; 32: 731–736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lumeng JC, Chervin RD. Epidemiology of pediatric obstructive sleep apnea. Proc Am Thorac Soc 2008; 5: 242–252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Marcus CL, Hamer A, Loughlin GM. Natural history of primary snoring in children. Pediatr Pulmonol 1998; 26: 6–11. [DOI] [PubMed] [Google Scholar]
- 5.Goodwin JL, Vasquez MM, Silva GE, Quan SF. Incidence and remission of sleep-disordered breathing and related symptoms in 6- to 17-year old children—the Tucson children’s assessment sleep apnea study. J Pediatr 2010; 157: 57–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dehlink E, Tan HL. Update on paediatric obstructive sleep apnoea. J Thorac Dis 2016; 8: 224–235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Marcus CL, Moore RH, Rosen CL, et al. A randomized trial of adenotonsillectomy for childhood sleep apnea. N Engl J Med 2013; 368: 2366–2377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Spilsbury JC, Storfer-Isser A, Rosen CL, Redline S. Remission and incidence of obstructive sleep apnea from middle childhood to late adolescence. Sleep 2015; 38: 23–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bixler EO, Fernandez-Mendoza J, Liao D, et al. Natural history of sleep disordered breathing in prepubertal children transitioning to adolescence. Eur Respir J 2016; 47: 1402–1409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hart CN, Hawley N, Davey A, et al. Effect of experimental change in children’s sleep duration on television viewing and physical activity. PediatrObes 2017; 12: 462–467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mendelson M, Borowik A, Michallet A, et al. Sleep quality, sleep duration and physical activity in obese adolescents: effects of exercise training. Pediatr Obes 2016; 11: 26–32. [DOI] [PubMed] [Google Scholar]
- 12.Canapari CA, Hoppin AG, Kinane TB, Thomas BJ, Torriani M, Katz ES. Relationship between sleep apnea, fat distribution, and insulin resistance in obese children. J Clin Sleep Med 2011; 7: 268–273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Farr OM, Rifas-Shiman SL, Oken E, Taveras EM, Mantzoros CS. Current child, but not maternal, snoring is bi-directionally related to adiposity and cardiometabolic risk markers: a cross-sectional and a prospective cohort analysis. Metabolism 2017; 76: 70–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sanchez-Armengol A, Ruiz-Garcia A, Carmona-Bernal C, et al. Clinical and polygraphic evolution of sleep-related breathing disorders in adolescents. Eur Respir J 2008; 1: 1016–1022. [DOI] [PubMed] [Google Scholar]
- 15.Bhattacharjee R, Kheirandish-Gozal L, Spruyt K, et al. Adenotonsillectomy outcomes in treatment of obstructive sleep apnea in children: a multicenter retrospective study. Am J Respir Crit Care Med 2010; 182: 676–683. [DOI] [PubMed] [Google Scholar]
- 16.Costa DJ, Mitchell R. Adenotonsillectomy for obstructive sleep apnea in obese children: a meta-analysis. Otolaryngol Head Neck Surg 2009; 140: 455–460. [DOI] [PubMed] [Google Scholar]
- 17.Rechtschaffen A, Kales A. A Manual of Standardized Terminology, Techniques and Scoring System for Sleep Stages of Human Subjects. NIMH Publication; 204: Washington, US, 1968. [DOI] [PubMed] [Google Scholar]
- 18.Iber C, Ancoli-Israel S, Chesson AL, Quan SF. TheAASM Manual for the Scoring of Sleep and Associated Events: Rules, Terminology and Technical Specifications. American Academy of Sleep Medicine: Westchester, IL, 2007. [Google Scholar]
- 19.Frye SS, Fernandez-Mendoza J, Calhoun SL, et al. Neurocognitive and behavioral functioning in adolescents with sleep disordered breathing: a population-based, dual-energy X-ray absorptiometry study. Int J Obes (Lond) (in press). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kuczmarski RJ, Ogden CL, Guo SS, et al. 2000 CDC growth charts for the United States: methods and development. Vital Health Stat 2002; 246: 1–190. [PubMed] [Google Scholar]
- 21.Hologic Inc. Body composition user guide, Document No. MAN-02354 Revision 001. Beford, Hologic Inc., 2010. [Google Scholar]
- 22.Kelly TL, Wilson KE, Ruth CR. Estimating visceral fat by dual-energy X-ray absorptiometry. US patent application number US2010–0234719, Hologic, Inc., 2010.
- 23.Goldschmidt AB, Wilfley DE, Paluch RA, Roemmich JN, Epstein LH. Indicated prevention of adult obesity: how much weight change is necessary for normalization of weight status in children? JAMA Pediatr 2013; 167: 21–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kalarchian MA, Levine MD, Arslanian SA, et al. Family- based treatment of severe pediatric obesity: randomized, controlled trial. Pediatrics 2009; 124: 1060–1068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ford AL, Hunt LP, Cooper A, Shield JP. What reduction in BMI SDS is required in obese adolescents to improve body composition and cardiometabolic health? Arch Dis Child 2010; 95: 256–261. [DOI] [PubMed] [Google Scholar]
- 26.Marcus CL. Sleep-disordered breathing in children. Am J Respir Crit Care 2001; 164: 16–30. [DOI] [PubMed] [Google Scholar]
- 27.Ronen O, Malhotra A, Pillar G. Influence of gender and age on upper-airway length during development. Pediatrics 2007; 120: e1028–e1034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Papaioannou G, Kambas I, Tsaoussoglou M, Panaghiotopoulou-Gartagani P, Chrousos G, Kaditis AG. Age-dependent changes in the size of adenotonsillar tissue in childhood: implications for sleep-disordered breathing. J Pediatr 2013; 162: 269–274. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Characteristics of the sample stratified by the presence of childhood sleep disordered breathing
Body weight as a predictor of the natural history of sleep-disordered breathing (SDB) in multivariable, stepwise multinomial logistic regression models
Role of childhood history of enlarged tonsils in the association of weight loss with the remission of childhood sleep-disordered breathing. Weight loss was associated with the remission of childhood SDB in the transition to adolescence, but not among those who had a childhood history of enlarged tonsils. All data are means and standard errors adjusted for sex, race, age and childhood body mass index percentile. Change in BMI percentile = BMI percentile at follow-up minus BMI percentile at baseline. SDB = sleep-disordered breathing.
Body fat distribution and composition in adolescence associated with persistent and remitted childhood sleep-disordered breathing. Remitted childhood SDB was associated with normal body fat composition and distribution in adolescence, while persistent SDB since childhood was associated with central adiposity, as measured by dual-energy X-ray absorptiometry android distribution and visceral adiposity. All data are means and standard errors adjusted for sex, race, age and childhood body mass index percentile, enlarged tonsils and adeno/tonsillectomy. SDB = sleep-disordered breathing.


