Abstract
PURPOSE
Merkel cell carcinoma (MCC) is an aggressive skin cancer often caused by the Merkel cell polyomavirus. Clinical trials of programmed cell death-1 pathway inhibitors for advanced MCC (aMCC) demonstrate increased progression-free survival (PFS) compared with historical chemotherapy data. However, response durability and overall survival (OS) data are limited.
PATIENTS AND METHODS
In this multicenter phase II trial (Cancer Immunotherapy Trials Network-09/Keynote-017), 50 adults naïve to systemic therapy for aMCC received pembrolizumab (2 mg/kg every 3 weeks) for up to 2 years. Radiographic responses were assessed centrally per Response Evaluation Criteria in Solid Tumors (RECIST) v1.1.
RESULTS
Among 50 patients, the median age was 70.5 years, and 64% had Merkel cell polyomavirus–positive tumors. The objective response rate (ORR) to pembrolizumab was 56% (complete response [24%] plus partial response [32%]; 95% CI, 41.3% to 70.0%), with ORRs of 59% in virus-positive and 53% in virus-negative tumors. Median follow-up time was 14.9 months (range, 0.4 to 36.4+ months). Among 28 responders, median response duration was not reached (range, 5.9 to 34.5+ months). The 24-month PFS rate was 48.3%, and median PFS time was 16.8 months (95% CI, 4.6 months to not estimable). The 24-month OS rate was 68.7%, and median OS time was not reached. Although tumor viral status did not correlate with ORR, PFS, or OS, there was a trend toward improved PFS and OS in patients with programmed death ligand-1–positive tumors. Grade 3 or greater treatment-related adverse events occurred in 14 (28%) of 50 patients and led to treatment discontinuation in seven (14%) of 50 patients, including one treatment-related death.
CONCLUSION
Here, we present the longest observation to date of patients with aMCC receiving first-line anti–programmed cell death-1 therapy. Pembrolizumab demonstrated durable tumor control, a generally manageable safety profile, and favorable OS compared with historical data from patients treated with first-line chemotherapy.
INTRODUCTION
Merkel cell carcinoma (MCC) is a rare and aggressive skin cancer with an associated 5-year overall survival (OS) rate of 14% to 27% for advanced or unresectable disease.1 The annual incidence of new patients with MCC in the United States increased by 95% between 2000 and 2013.2 This increase was driven mostly by an increase in the number of persons older than age 60 years, the age at which MCC risk begins to increase.2 Integration of the Merkel cell polyomavirus (MCPyV) occurs in approximately 80% of MCC tumors, and persistent expression of MCPyV T-antigen oncoproteins is required for virus-positive (VP) tumor cells to proliferate.3,4 The remaining 20% of MCCs are thought to be caused by ultraviolet light–mediated DNA damage, on the basis of finding predominant C>T transitions. This virus-negative (VN) MCC subset has a tumor mutational burden that is, on average, 100-fold greater than the low mutational burden associated with VP-MCC.5-7
Multiple lines of evidence support the notion that MCC is an immunogenic cancer, including the fact that MCC incidence is greater than 10-fold higher in chronically immunosuppressed persons.8,9 It is also now clear that non–self-antigens are present in both VP-MCC (in the form of MCPyV oncoproteins) and VN-MCC (as ultraviolet-induced neoantigens).4,5,7 Until recently, cytotoxic chemotherapy was the only systemic treatment option for advanced MCC (aMCC), and it offered limited benefit, with a median progression-free survival (PFS) time of approximately 90 days.10,11 Recent clinical trials of programmed cell death-1 (PD-1) pathway inhibitors in patients with aMCC, as first-line or later therapy, have demonstrated increased PFS and OS compared with historical data from patients receiving chemotherapy.12-14 In 2017, avelumab (anti–programmed death ligand-1 [PD-L1]) became the first drug of any kind to receive US Food and Drug Administration (FDA) approval for treating metastatic MCC in adult and pediatric patients, on the basis of a trial in chemotherapy-refractory patients showing a 33% objective response rate (ORR) with sustained partial and complete responses, indicating durable tumor regression.12 More recently, avelumab was explored in the first-line treatment setting for metastatic MCC, with a 62% objective response rate; responses seemed to be durable over a median follow-up period of 5.1 months.14 In a phase II trial including 25 treatment-naïve or treatment-experienced patients with aMCC, nivolumab (anti–PD-1) demonstrated an ORR of 64%, and median PFS and OS were not reached during a 51-week observation period.15 Finally, we have described the durable efficacy of pembrolizumab (anti–PD-1) as first-line therapy for aMCC in a preliminary report of 26 patients who experienced an ORR of 56%.13 Outcomes from these clinical trials have led to rapid changes in National Comprehensive Cancer Network guidelines for aMCC, in advance of FDA approvals. In 2016, National Comprehensive Cancer Network guidelines listed chemotherapy as the sole treatment option for aMCC; in 2017, pembrolizumab was recommended after chemotherapy; and in 2018, avelumab, nivolumab, and pembrolizumab were all recommended as preferred first-line therapies, ahead of chemotherapy.16
To further explore long-term outcomes from first-line anti–PD-1 therapy in aMCC, we now provide follow-up on the expanded phase II Cancer Immunotherapy Trials Network (CITN)-09/Keynote-017 trial of pembrolizumab (ClinicalTrials.gov identifier: NCT02267603). These data represent the longest follow-up for any anti–PD-1/PD-L1 drug administered in the first-line treatment setting for aMCC.
PATIENTS AND METHODS
Patients
Patients with distant metastatic or recurrent locoregional MCC not amenable to definitive surgery or radiation therapy who had measurable disease per Response Evaluation Criteria in Solid Tumors (RECIST) v1.1 were enrolled.17 Eligibility and exclusion criteria were previously published.13 Patients who had received prior systemic therapy for aMCC were excluded; adjuvant chemotherapy received greater than 6 months before initiating study treatment was allowed. An initial cohort of 26 patients was enrolled between January and December of 2015, and interim results for this group of patients were reported.13 The protocol was then amended to enroll 24 additional patients (March 2016 to May 2017). Results from the full 50-patient cohort are reported here. All patients in this study were treated at sites in the United States.
Study oversight was previously reported.13 Briefly, the study was conducted in accordance with the Declaration of Helsinki and the International Conference on Harmonization Guidelines for Good Clinical Practice. All patients provided written informed consent before study entry. The protocol was approved by the institutional review board at each participating center.
Study Design
This was a phase II, open-label, nonrandomized multicenter study in which at least one response among the first nine treated patients was required (Simon two-stage design)18 to proceed to enroll the initial target of 25 patients. On the basis of early encouraging results,13 a decision was made to expand the cohort to 50 patients. Patients received pembrolizumab 2 mg/kg intravenously every 3 weeks. Treatment continued for up to 2 years or until the development of unacceptable adverse event(s), progressive disease, a complete response with at least 24 weeks of therapy and at least two treatments beyond the date of confirmed complete response, withdrawal of consent, or physician discretion.
Disease Assessment
Computed tomography scans were performed at screening, 12 weeks after starting therapy, and at 9-week intervals thereafter, as previously described.13 After 1 year of treatment, the scan frequency was decreased to 12-week intervals. Patients who had disease progression per RECIST v1.1 were allowed to continue therapy if asymptomatic, if the Eastern Cooperative Oncology Group performance status was less than or equal to 1, and if there was no evidence of rapid progression, with re-evaluation 4 weeks later to assess for delayed response or additional disease progression. RECIST-based evaluations of scans were initially conducted at the institutional level, followed by central radiologic review (reported herein).
Objectives
The primary objective of this study was to determine the efficacy of pembrolizumab as first-line therapy for patients with aMCC. The primary end point was ORR measured by RECIST v1.1. Secondary end points included duration of response (DOR), PFS, and OS. Adverse events were assessed according to National Cancer Institute Common Terminology Criteria for Adverse Events version 4.0.19 Exploratory objectives included correlation of clinical outcomes with analyses of the pretreatment tumor microenvironment, including tumor MCPyV status and PD-L1 expression.
Specimen Acquisition
Pretreatment (immediate or archival) tumor biopsy samples were obtained on all patients, and formalin-fixed paraffin-embedded tumor biopsies or sectioned slides were collected and stored for later use. Blood samples were drawn for correlative laboratory analyses at baseline and at the time of subsequent imaging studies. Serum was collected and processed at local sites and cryopreserved.
Tumor MCPyV Status Determination
Patients were determined to have VP-MCC if they were found to have small T-antigen–specific antibodies in their serum via a customized Luminex immunoassay (ThermoFisher Scientific, Waltham, MA)20 or large T-antigen expression in tumor biopsies via immunohistochemistry (IHC).21,22
PD-L1 IHC
PD-L1 staining (anti–PD-L1 clone 22C3; Merck Research Laboratories, Kenilworth, NJ) was performed at QualTek Molecular Laboratories (Newtown, PA) on formalin-fixed paraffin-embedded sections from pretreatment MCC biopsies, as previously described.13 Specimens were scored as PD-L1 positive for tumor cell or immune cell expression if at least 1% of the cell type of interest expressed cell surface PD-L1.
Statistical Analysis
Objective response (primary end point), PFS, OS, and safety were assessed in all 50 participants. The DOR was evaluated in responders, defined as those who had a confirmed complete or partial response. Objective response and disease control were evaluated with point estimates and 95% CIs on the basis of the exact binomial method. DOR, PFS, and OS were estimated using the Kaplan-Meier method for censored data. Participant flow through the trial, baseline characteristics, and adverse events were summarized by descriptive statistics. Objective response, PFS, and OS were analyzed on the basis of the PD-L1 and MCPyV status of each tumor. Fisher’s exact test was used to assess associations between objective response and PD-L1 expression or viral status. Data were analyzed using SAS version 9.4 (SAS Institute, Cary, NC). The database cutoff date was February 6, 2018. The study is ongoing for follow-up but was completely enrolled at the time of the database cutoff.
RESULTS
Patient Characteristics and Treatments Received
Fifty patients with aMCC and Eastern Cooperative Oncology Group performance status of 0 to 1 were enrolled from January 2015 to May 2017 and received at least one dose of pembrolizumab. Patients received a median of 10.5 doses of pembrolizumab (standard deviation, 10.92 doses; range, 1.0 to 35.0 doses), and the median duration of treatment was 6.6 months (standard deviation, 7.73 months; range, 1 day to 23.6 months). Data were analyzed as of February 6, 2018. Median follow-up time at the time of database cutoff was 14.9 months (range, 0.4 to 36.4+ months).
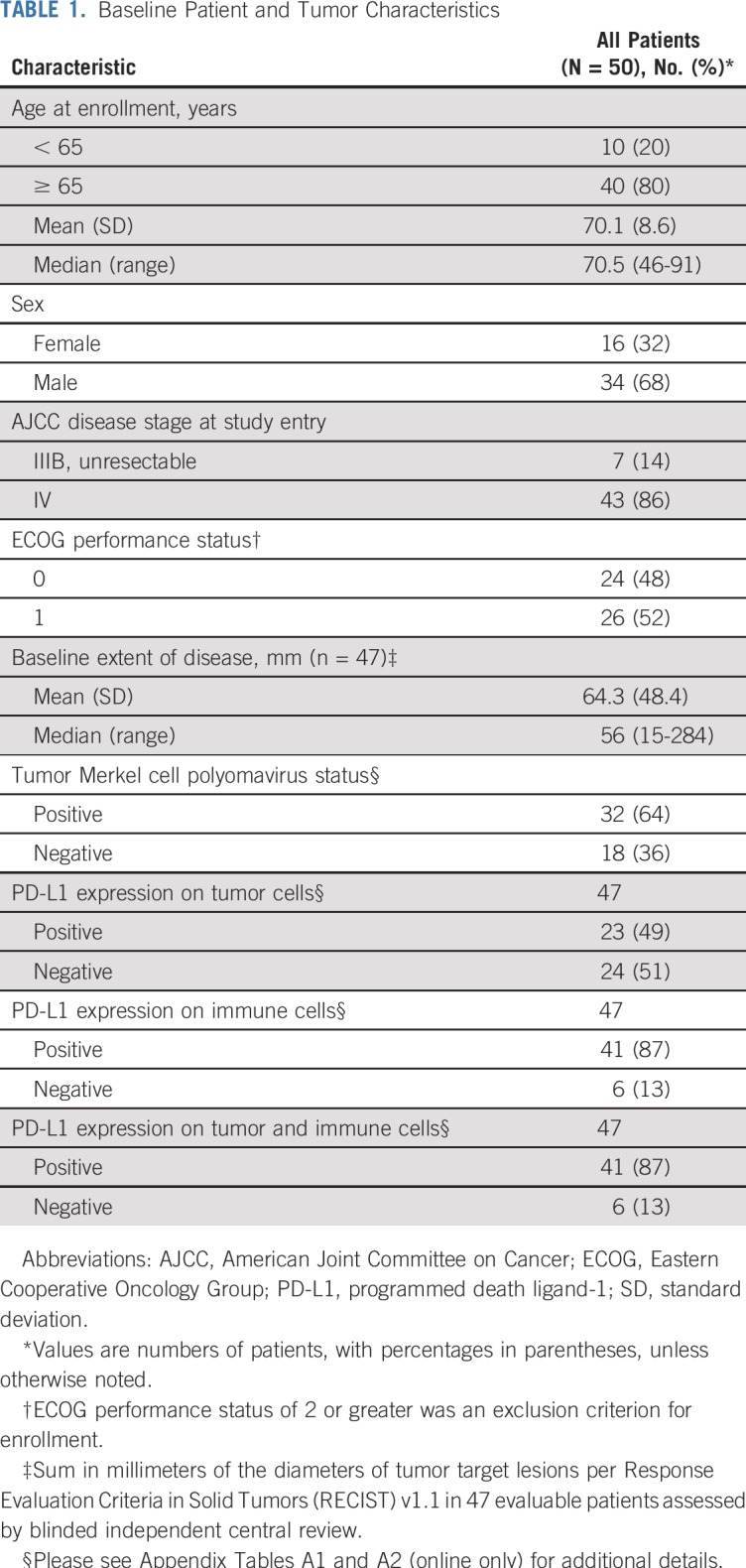
Baseline patient and tumor characteristics are listed in Table 1. As is typical for MCC, the median age was 70.5 years (range, 46 to 91 years), and 80% of patients were older than age 65 years. At the time of study enrollment, 43 patients (86%) had stage IV disease and seven (14%) had unresectable stage IIIB disease. No patient had previously received systemic therapy for aMCC; however, three patients had received adjuvant chemotherapy greater than 6 months before beginning study treatment. Forty-two patients (84%) had previous surgery for MCC, and 35 patients (70%) had previous radiation treatment of MCC. Thirty-two patients (64%) had MCPyV-positive tumors.
TABLE 1.
Baseline Patient and Tumor Characteristics
Clinical Activity
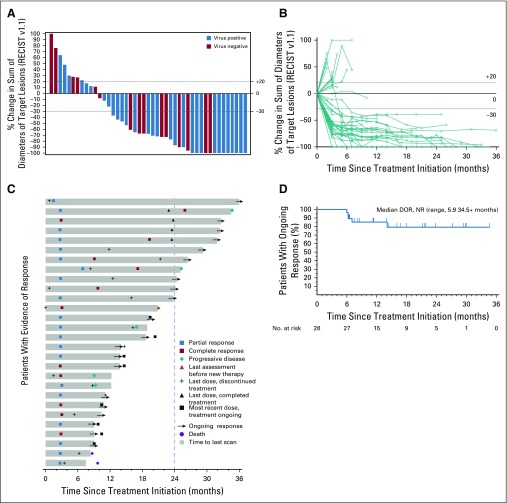
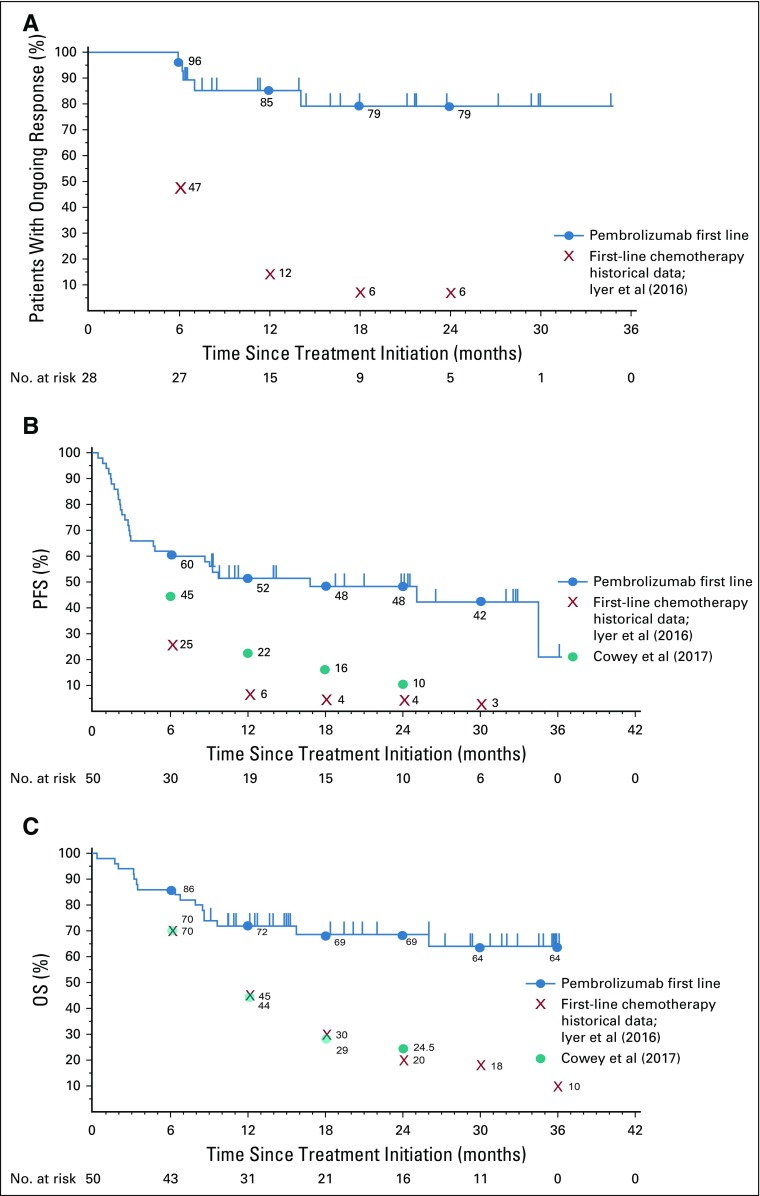
The characteristics of aMCC response to first-line anti–PD-1 therapy are shown in Figures 1A to 1D. Forty-five patients were radiographically evaluable for response including the target lesions according to RECIST v1.1, per central review (Fig 1A). Five patients were unevaluable; three of these patients had no radiographically measurable target lesions at baseline, and two had clinical evidence of disease progression but no on-treatment imaging of target lesions (one patient died before postbaseline assessment, and the other patient had magnetic resonance imaging of the spine showing progression of nontarget lesions per investigator review). Overall target lesion reduction by at least 30% occurred in 31 (69%) of 45 evaluable patients and was not related to tumor viral status. The kinetics of change in tumor burden over time on therapy is shown in Figure 1B. The majority of tumor regressions were rapid and durable. Among responders, the median time to response was 2.8 months (range, 1.5 to 9.7 months), which corresponded to the first planned imaging assessment at 12 weeks after treatment initiation. The treatment courses of 28 patients who achieved an objective response (complete or partial response) are depicted in Figure 1C. At the time of data analysis, 20 of 28 responses were ongoing, and the median durability of response had not been reached. The Kaplan-Meier estimation of response durability at 24 months was 79.1% (Fig 1D). The durability of responses observed with first-line pembrolizumab therapy compares favorably to historical data from patients receiving first-line chemotherapy for aMCC (Appendix Fig A1A, online only).10
FIG 1.
Tumor regression in patients with advanced Merkel cell carcinoma (aMCC) receiving pembrolizumab. (A) Waterfall plot showing the maximum change in tumor burden (sum of target lesion measurements) compared with baseline for 45 radiographically evaluable patients. Patients with Merkel cell polyomavirus–positive tumors are indicated by blue bars. Horizontal dotted lines indicate Response Evaluation Criteria in Solid Tumors (RECIST) v1.1 for partial response (30% or greater decrease in sum of target lesion diameters from baseline, assuming no new lesions) and progressive disease (20% or greater increase in sum of target lesion diameters). Five patients are not depicted; three patients did not have measurable target lesions per RECIST v1.1 at baseline, and two patients with clinical disease progression did not undergo repeat imaging of target lesions. (B) Spider plot showing response kinetics in patients with aMCC receiving pembrolizumab. Percent change in sum of target lesion diameters from baseline is depicted for 45 evaluable patients, based on serial measurements performed approximately every 9 to 12 weeks. (C) Swimmer plot showing durability of response among 28 patients with aMCC who experienced a confirmed partial or complete response to pembrolizumab. In this group of patients, median time to response was 2.8 months (range, 1.5 to 9.7 months). Median duration of response was not reached at the time of data analysis (range, 5.9 to 34.5+ months). Dashed vertical line represents maximum duration of therapy per protocol. (D) Kaplan-Meier curve showing duration of response (DOR) among the 28 patients depicted in panel C. Patients without an event were censored (tick mark) at the last disease assessment date. NR, not reached.
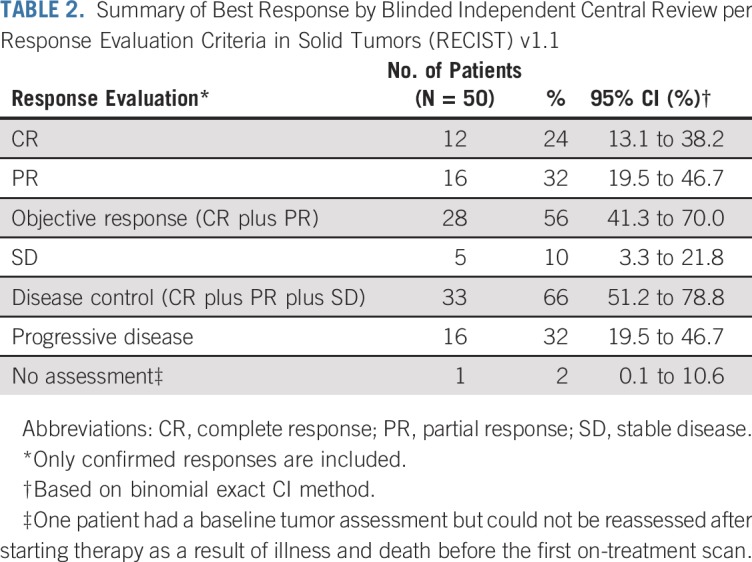
The ORR among all 50 patients, defined by at least one on-treatment tumor assessment and one confirmatory assessment performed at least 4 weeks later, was 56% (28 responses [12 complete responses and 16 partial responses]; 95% CI for ORR, 41.3% to 70.0%; Table 2). Five patients (10%) had stable disease (95% CI, 3.3% to 21.8%), and 16 patients (32%) had progressive disease (95% CI, 19.5% to 46.7%). There was no significant difference between the response rates in patients with VP-MCC (59%; 19 of 32 patients) compared with patients with VN-MCC (53%; nine of 17 patients; P = .765).
TABLE 2.
Summary of Best Response by Blinded Independent Central Review per Response Evaluation Criteria in Solid Tumors (RECIST) v1.1
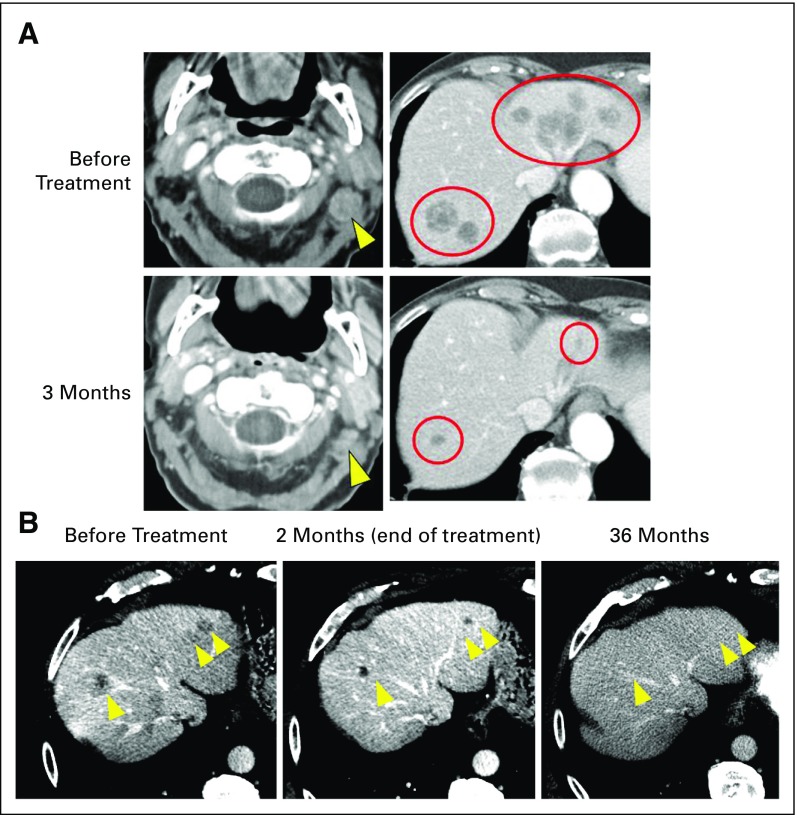
Two representative patients with objective responses are depicted in Figures 2A and 2B. The first patient is a 92-year-old woman with MCC metastatic to multiple organs who experienced substantial tumor regression at the first on-treatment radiologic evaluation. A partial response was ongoing 13 months after treatment initiation, as assessed by the investigator in June 2018 (Fig 2A). The second patient is a 72-year-old man with MCC metastatic to multiple sites, who received two doses of pembrolizumab in February 2015. He experienced a partial response but developed treatment-related hepatitis, requiring discontinuation of pembrolizumab and administration of systemic corticosteroid therapy. Tumors continued to shrink without additional pembrolizumab therapy, with complete resolution of all target lesions as of February 2018, 3 years after treatment initiation (Fig 2B). Consistent with previous reports, administration of corticosteroids did not seem to interfere with this patient’s ongoing antitumor response.23
FIG 2.
Response to pembrolizumab in two patients with metastatic Merkel cell carcinoma (MCC). (A) A 92-year-old woman with a history of a virus-negative primary MCC on the scalp in 2016 developed cervical adenopathy and multiple liver lesions. Liver biopsy revealed metastatic MCC. The patient began pembrolizumab in May 2017. She experienced a partial response at 3 months, which was ongoing 13 months after treatment initiation (June 2018). Computed tomography scan images are from baseline and at 3 months (time of response). Yellow arrowheads (left panels) and red circles (right panels) indicate regressing cervical adenopathy and liver metastases, respectively. Treatment-related adverse effects included grade 1 pruritus, anterior uveitis, and creatinine elevation. As of July 2018, the patient’s Eastern Cooperative Oncology Group performance status (ECOG PS) was stable (ECOG PS of 1), and she continued to receive pembrolizumab per protocol. (B) A 72-year-old man developed a virus-negative primary MCC on the scalp in September 2014, which was treated with wide surgical excision. In December 2014, metastatic MCC was identified in the parotid gland, cervical lymph nodes, and numerous sites in the liver (yellow arrowheads). The patient began pembrolizumab therapy in February 2015. After two doses, he experienced a partial response (middle panel), but developed grade 4 immune-mediated hepatitis (peak AST and ALT of 1,223 and 1,281 IU/L, respectively; upper normal limits for AST and ALT are 38 and 48 IU/L, respectively), necessitating discontinuation of pembrolizumab and administration of corticosteroid therapy in March 2015. Hepatic aminotransferases normalized by June 2015. Importantly, the patient’s target lesions continued to shrink with continued observation and no additional cancer therapy and completely resolved by February 2018 (left panel; yellow arrows indicate prior locations of liver metastases). The patient was asymptomatic and without evidence of disease as of August 2018.
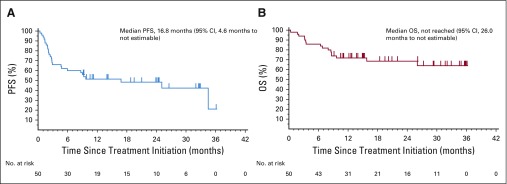
PFS and OS
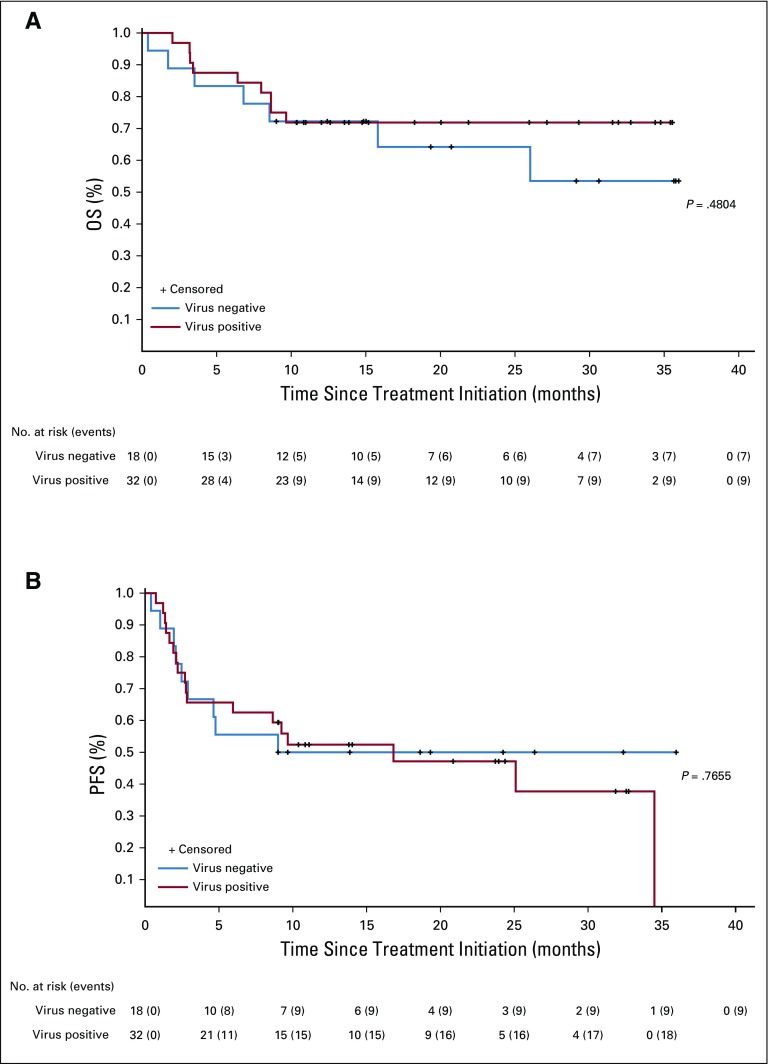
PFS and OS estimates for first-line pembrolizumab therapy in aMCC are shown in Figures 3A and 3B. The median PFS time was 16.8 months (95% CI, 4.6 months to not estimable), and the Kaplan-Meier estimate of PFS rate at 24 months was 48.3% (Fig 3A). Median OS had not been reached at the time of analysis (95% CI, 26.0 months to not estimable; Fig 3B). The Kaplan-Meier estimate for OS rate at 24 months was 68.7%. The PFS and OS data observed here with pembrolizumab therapy compare favorably to historical data obtained from patients who received first-line chemotherapy for aMCC (Appendix Figs A1B and A1C, respectively).10,11 There was no significant difference in OS or PFS when comparing patients with VP-MCC with patients with VN-MCC (P = .48 and P = .77, respectively; Appendix Figs A2A and A2B, online only).
FIG 3.
Progression-free survival (PFS) and overall survival (OS) analyses. (A) Kaplan-Meier curve depicting PFS among 50 patients with advanced Merkel cell carcinoma (aMCC) receiving pembrolizumab. PFS was measured from the time of treatment initiation until either disease progression (Response Evaluation Criteria in Solid Tumors [RECIST] v1.1) or death, whichever occurred first. At 24 months, estimated PFS was 48.3%. Median PFS was 16.8 months (95% CI, 4.6 months to not estimable). (B) Kaplan-Meier curve depicting OS among 50 patients with aMCC receiving pembrolizumab. At 24 months, estimated OS was 68.7%. Median OS was not reached at the time of the analysis (95% CI, 26.0 months to not estimable).
Safety
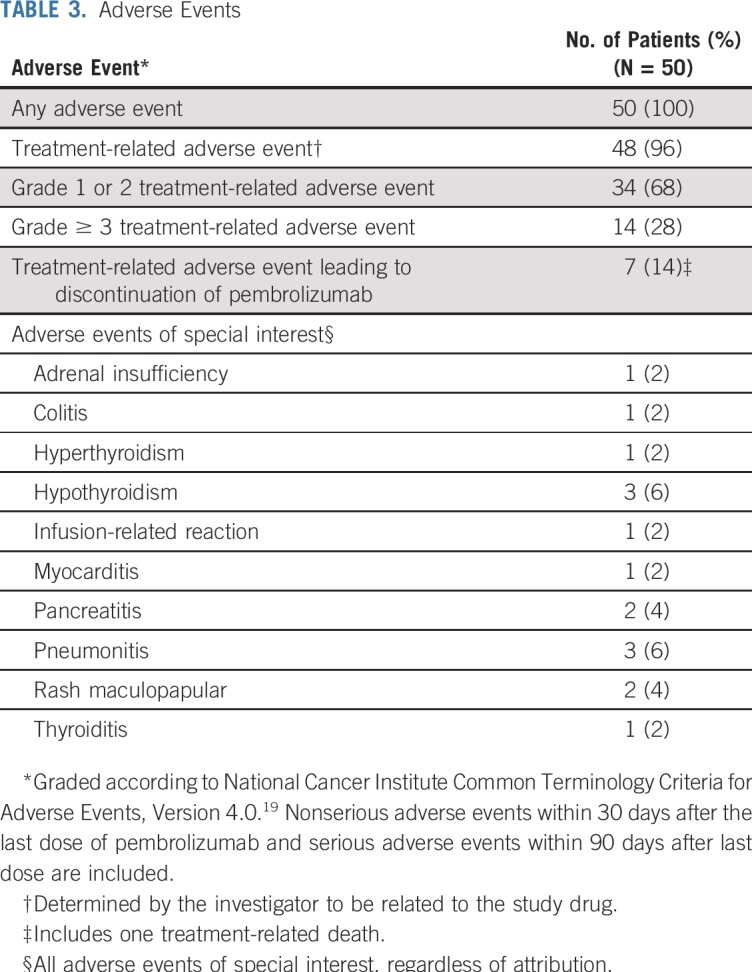
Safety data are listed in Table 3. Treatment-related adverse events (TRAEs) of any grade occurred in 48 (96%) of 50 patients, with grade 3 or greater TRAEs observed in 14 (28%) of 50 patients. Seven patients (14%) discontinued pembrolizumab therapy as a result of TRAEs. There was one death attributed to study therapy; the death occurred in a 73-year-old male patient with widely metastatic MCC and pre-existing atrial fibrillation who developed pericardial and pleural effusions 1 day after a single infusion of pembrolizumab. This patient withdrew from the trial and died 10 days after starting pembrolizumab treatment. Although evaluation was incomplete, progressive MCC and worsening pericardial and pleural effusions likely contributed to this patient’s death.
TABLE 3.
Adverse Events
Tumor PD-L1 Assessments and Correlations With Clinical Outcomes
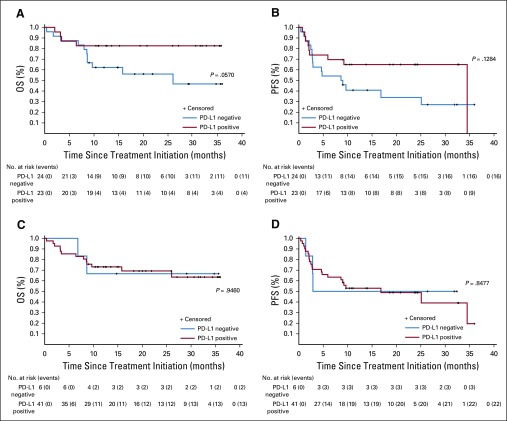
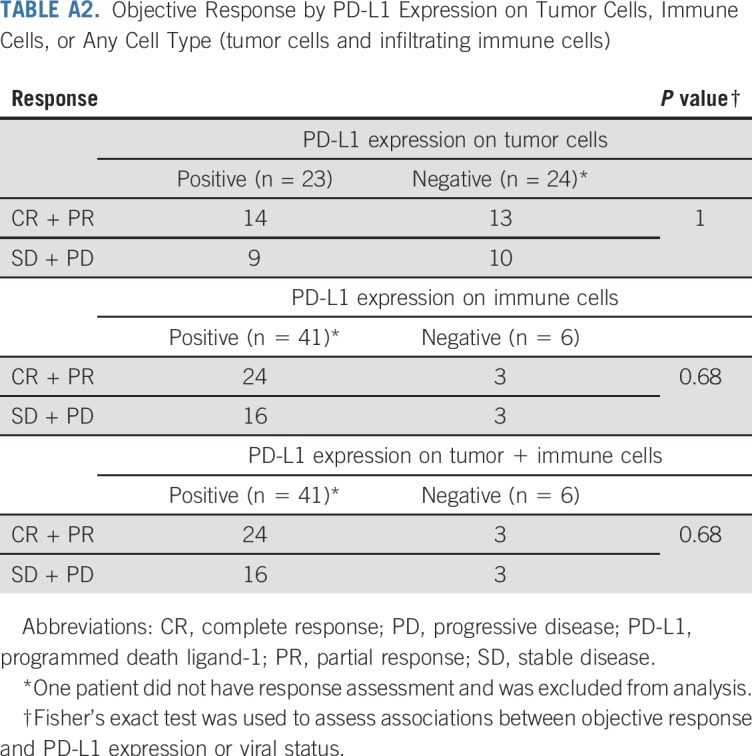
PD-L1 expression was determined via IHC in pretreatment tumor specimens from 47 of the 50 patients. PD-L1 can be expressed on tumor cells and/or tumor-infiltrating immune cells including macrophages, which are prominent in MCC.24-26 We assessed PD-L1 expression on tumor cells, infiltrating immune cells, or any cell type in the tumor specimen (tumor cells and infiltrating immune cells), similar to methods currently used in various commercial analytic PD-L1 IHC assays.27,28 Twenty-three (49%) of 47 specimens showed PD-L1 expression on 1% or more of tumor cells, and 41 (87%) of 47 specimens had 1% or greater PD-L1–positive immune cells. There was a trend for specimens containing PD-L1–positive tumor cells to also be MCPyV positive, as we previously reported in a smaller cohort of specimens from this trial (P = .125; Appendix Table A1, online only).13 Among 46 evaluable patients, PD-L1 expression did not correlate with response (Appendix Table A2, online only). There was a trend toward improved OS and PFS in patients with PD-L1 positivity greater than a 1% threshold on tumor cells, but this did not reach statistical significance (P = .057 for OS; P = .128 for PFS; Appendix Figs A3A and A3B, online only). There was no significant correlation between PD-L1 expression scored on any cell type and OS or PFS (P = .9460 for OS; P = .8477 for PFS; Appendix Figs A3C and A3D).
DISCUSSION
Immunotherapy has revolutionized the treatment landscape for patients with aMCC, which until recently carried a uniformly poor prognosis. This rare malignancy was considered an orphan disease but is receiving increasing attention not only because of its responsiveness to anti–PD-1/PD-L1 therapies, but also because of its intriguing biology. Its two major subtypes, virus induced and carcinogen (ultraviolet light) induced, have dichotomous genetic profiles with exceedingly low versus strikingly high tumor mutational burdens, respectively.5-7 However, as shown here, patients with either MCC subtype who received first-line pembrolizumab therapy experienced similarly high response rates, PFS, and OS. This provides compelling evidence that both the quality and quantity of tumor antigens are important factors driving antitumor immunity and tumor rejection. Clinical outcomes reported here exceed expectations drawn from the chemotherapy literature10,11 and provide the longest follow-up to date in patients with aMCC receiving anti–PD-1/PD-L1 therapy as first-line therapy.
Biomarkers of aMCC response to pembrolizumab, if identified, could further refine treatment strategies. PD-L1 expression assessed by IHC in pretreatment tumors provides a marker favoring the likelihood of anti–PD-1/PD-L1 response in various cancer types, and commercial tests are now FDA approved to guide such therapies for non–small-cell lung cancer and select other malignancies.29 Herein, we report a trend for PD-L1 expression on tumor cells to correlate with an improved PFS and OS in patients with aMCC receiving pembrolizumab. Similarly, Kaufman et al30 reported a trend for higher response rates in chemotherapy-refractory patients with aMCC receiving avelumab, whose pretreatment tumor specimens harbored PD-L1–positive tumor cells. Future research will focus on advanced multiplex and multidimensional tissue markers for their ability to predict anti–PD-1/PD-L1 response in MCC, and early results suggest that the distance between PD-1 and PD-L1 may be a better predictor of response than PD-L1 expression alone in this tumor type.31
Pseudo-progression after initiation of immune checkpoint inhibitor therapy has been described in several other tumor types.32 For MCC, pseudo-progression has not been prominently observed in clinical trial reports to date13,14,30 or within the present cohort. The rapidity of response to immune therapy for MCC (most responses evident by the time of first scan) may mean that it is difficult to detect pseudo-progression, even when it may occur in MCC.
Additional challenges remain to optimize the utility of pembrolizumab in aMCC. Although the majority of 28 objective responders in this report remained in response with a median of 14.9 months of follow-up, eight patients experienced relapse. In-depth studies of pre- and post-treatment tumor biopsies are ongoing in an attempt to unravel factors underlying primary and acquired resistance to pembrolizumab in MCC. Furthermore, although pembrolizumab was generally safe in this elderly patient population (80% of patients were age 65 years or older), 28% of patients experienced a grade 3 or greater TRAE, and there was one death attributed to study treatment. Future studies to optimize treatment regimens, including dose-intensity and treatment duration, may mitigate risk in patients with MCC. Finally, the neoadjuvant and/or adjuvant application of anti–PD-1/PD-L1 therapies in patients with MCC at high risk for postsurgical relapse may be of benefit, and such trials are now in progress (ClinicalTrials.gov identifiers: NCT03271372 and NCT02488759). The rapid response kinetics of MCC to pembrolizumab demonstrated here, with most objective responses documented at the earliest evaluation point at 12 weeks, suggest that a short course of neoadjuvant anti–PD-1 may be efficacious. Indeed, in a recent report, a 4-week course of preoperative anti–PD-1 (nivolumab) was associated with notable response rates by radiographic and pathologic criteria.33 Additional investigations will determine whether the application of anti–PD-1/PD-L1 drugs in stage II to III MCC, in the pre- or postsurgical setting, can extend PFS and OS, providing additional effective treatments for patients who previously had limited options.
ACKNOWLEDGMENT
We thank the patients and their families and caregivers for participating in this study. We acknowledge the efforts of the Cancer Immunotherapy Trials Network (CITN) across the participating clinical sites in conducting this trial and Judith Kaiser for budgetary and regulatory oversight of CITN; Nichole Pelz, Hannah Thomas, Natalie Miller, and Sumia Dakhil, University of Washington/Fred Hutchinson Cancer Research Center (Seattle, WA), for clinical trial support; Ryan B. O’Malley and Carolyn Wang, University of Washington (Seattle, WA), for assistance with radiology assessments; Trish Brothers, Tianna Dauses, Irwin Freed, Uchechi Nwaba, Katrina Purtell, and Katherine Sheldon, Johns Hopkins Kimmel Cancer Center and Bloomberg–Kimmel Institute for Cancer Immunotherapy (Baltimore, MD), for trial support; and Elliot K. Chartash, Steven M. Townson, Scot Ebbinghaus, Scott Diede, Rachel Lewis, Jenia Booth, Giuseppe Russo, Greg McCormick, and James Anderson, Merck Research Laboratories (Kenilworth, NJ), for collaboration. We also thank Doyel Mitra (ApotheCom, Yardley, PA) for editorial assistance; this assistance was funded by Merck Sharp & Dohme, a subsidiary of Merck, in accordance with Good Publications Practice guidelines.
Appendix
FIG A1.
Kaplan-Meier curves showing response characteristics of pembrolizumab-treated patients in the current trial, compared with published cohorts of patients with advanced Merkel cell carcinoma (aMCC) treated with chemotherapy. Blue circles represent Kaplan-Meier estimates of pembrolizumab data at the indicated time points. Kaplan-Meier estimates of historical first-line chemotherapy cohort data from Iyer et al10 (2016) and from Cowey et al11 (2017) are also indicated. (A) Median duration of response among 28 patients with aMCC who experienced a partial or complete response (RECIST v1.1) to pembrolizumab was not reached (NR) at the time of analysis (range, 5.9 to 34.5+ months, where + indicates an ongoing response). In contrast, Iyer et al10 reported that only 6% of chemotherapy responses persisted at 18 to 24 months. (B) Median progression-free survival (PFS) was 16.8 months for pembrolizumab (95% CI, 4.6 months to not estimable), as compared with historical data from patients receiving first-line chemotherapy, whose median PFS was 3.1 months as reported by Iyer et al10 (red X’s) and 4.6 months (95% CI, 3.0 to 7.0 months) as reported by Cowey et al11 (teal circles). (C) Median overall survival (OS) for pembrolizumab had not been reached at the time of analysis (95% CI, 26.0 months to not estimable). Historical first-line chemotherapy data reported median OS durations of 9.5 months (Iyer et al10) and 10.2 months (95% CI, 7.4 to 15.2 months; Cowey et al11). Tick marks indicate censored events, defined for PFS as the time to the last assessment before the date of data analysis for patients without disease progression or death and defined for OS as the time to the last known alive date before the date of data analysis for patients without a death.
FIG A2.
Kaplan-Meier curves showing (A) overall survival (OS) and (B) progression-free survival (PFS) of pembrolizumab-treated patients, according to tumor Merkel cell polyomavirus (MCPyV) status. There was no statistically significant correlation of OS or PFS with MCPyV status. P values are based on log-rank test.
FIG A3.
Kaplan-Meier curves showing progression-free survival (PFS) and overall survival (OS) of pembrolizumab-treated patients, according to tumor specimen programmed death ligand-1 (PD-L1) expression status. (A) OS and (B) PFS by PD-L1 expression on the surface of tumor cells only. (C) OS and (D) PFS by PD-L1 status on any cell type (tumor cells plus infiltrating immune cells). Patients with PD-L1 expression on tumor cells seemed to have improved OS and PFS, although these trends did not reach statistical significance (P = .057 for OS; P = .128 for PFS). P values are based on log-rank test.
TABLE A1.
Correlation Between MCPyV Status and PD-L1 Expression on Tumor Cells, Immune Cells, or Any Cell Type (tumor cells and infiltrating immune cells)
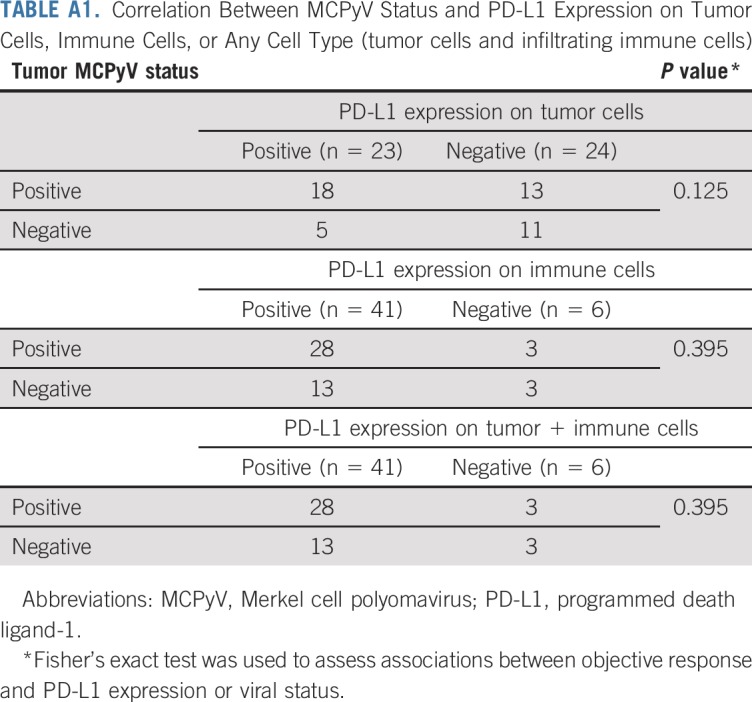
TABLE A2.
Objective Response by PD-L1 Expression on Tumor Cells, Immune Cells, or Any Cell Type (tumor cells and infiltrating immune cells)
Footnotes
Presented in part at the 54th Annual Meeting of the American Society of Clinical Oncology, Chicago, IL, June 1-5, 2018.
Supported by the National Cancer Institute (NCI) Grants No. 1U01CA154967 (M.A.C.), K24CA139052 and R01CA162522 (P.N.), R01CA142779 (S.L.T. and J.M.T.), and T32CA193145 (A.S.); also supported by the National Institutes of Health/NCI Cancer Center Support Grant in Seattle Grant No. P30 CA015704. We also acknowledge support from the Merkel cell carcinoma (MCC) patient gift fund at University of Washington, the Kelsey Dickson MCC Challenge Grant from the Prostate Cancer Foundation, and Merck, which provided pembrolizumab and partial funding for this study.
Processed as a Rapid Communication manuscript.
Clinical trial information: NCT02267603.
Podcast by Dr Drews
AUTHOR CONTRIBUTIONS
Conception and design: Paul Nghiem, Shailender Bhatia, Ragini R. Kudchadkar, Harriet M. Kluger, Kim Margolin, Janis M. Taube, Elad Sharon, Martin A. Cheever, Suzanne L. Topalian
Financial support: Paul Nghiem, Elad Sharon, Martin A. Cheever
Administrative support: Paul Nghiem, Elad Sharon, Martin A. Cheever
Provision of study materials or patients: Paul Nghiem, Shailender Bhatia, Evan J. Lipson, William H. Sharfman, Andrew S. Brohl, Adil Daud, Brian C. Boulmay, Brent A. Hanks, Elad Sharon, Martin A. Cheever
Collection and assembly of data: Paul Nghiem, Shailender Bhatia, Evan J. Lipson, William H. Sharfman, Ragini R. Kudchadkar, Andrew S. Brohl, Phillip A. Friedlander, Adil Daud, Harriet M. Kluger, Sunil A. Reddy, Adam I. Riker, Melissa A. Burgess, Brent A. Hanks, Thomas Olencki, Lisa M. Lundgren, Nirasha Ramchurren, Candice Church, Song Y. Park, Michi M. Shinohara, Janis M. Taube, Nageatte Ibrahim, Steven P. Fling, Blanca Homet Moreno, Elad Sharon, Martin A. Cheever
Data analysis and interpretation: Paul Nghiem, Shailender Bhatia, Evan J. Lipson, William H. Sharfman, Ragini R. Kudchadkar, Phillip A. Friedlander, Adil Daud, Harriet M. Kluger, Sunil A. Reddy, Brian C. Boulmay, Adam I. Riker, Thomas Olencki, Kim Margolin, Abha Soni, Candice Church, Song Y. Park, Michi M. Shinohara, Bob Salim, Janis M. Taube, Steven R. Bird, Nageatte Ibrahim, Steven P. Fling, Blanca Homet Moreno, Elad Sharon, Martin A. Cheever, Suzanne L. Topalian
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS’ DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST AND DATA AVAILABILITY STATEMENT
Durable Tumor Regression and Overall Survival in Patients With Advanced Merkel Cell Carcinoma Receiving Pembrolizumab as First-Line Therapy
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/jco/site/ifc.
Paul Nghiem
Honoraria: EMD Serono, Merck Sharp & Dohme, Sanofi/Regeneron
Consulting or Advisory Role: EMD Serono, Pfizer, Merck Sharp & Dohme
Research Funding: Bristol-Myers Squibb, EMD Serono
Patents, Royalties, Other Intellectual Property: Patent pending for high-affinity T-cell receptors that target the Merkel polyomavirus
Travel, Accommodations, Expenses: Sanofi/Regeneron, Merck Sharp & Dohme
Shailender Bhatia
Honoraria: Genentech, EMD Serono, Bristol-Myers Squibb, Sanofi/Regeneron
Consulting or Advisory Role: Genentech, EMD Serono, Bristol-Myers Squibb, Sanofi/Regeneron
Research Funding: Bristol-Myers Squibb (Inst), Immune Design (Inst), Merck (Inst), EMD Serono (Inst), OncoSec (Inst), NantKwest (Inst)
Travel, Accommodations, Expenses: NantKwest
Evan J. Lipson
Consulting or Advisory Role: Bristol-Myers Squibb, Novartis, EMD Serono, Array BioPharma, Regeneron/Sanofi Genzyme, Macrogenics, Merck, Millennium
Research Funding: Bristol-Myers Squibb (Inst), Merck (Inst), Sysmex (Inst)
Patents, Royalties, Other Intellectual Property: Method of preventing organ transplantation rejections using agonists to the PD-1 checkpoint pathway (Inst)
William H. Sharfman
Honoraria: Bristol-Myers Squibb
Consulting or Advisory Role: Merck, Bristol-Myers Squibb, Novartis, Regeneron
Research Funding: Bristol-Myers Squibb (Inst), Merck, Novartis
Ragini R. Kudchadkar
Honoraria: Bristol-Myers Squibb, Array BioPharma
Consulting or Advisory Role: Bristol-Myers Squibb, Novartis, Array BioPharma
Research Funding: Merck
Andrew S. Brohl
Expert Testimony: GlaxoSmithKline (I)
Phillip A. Friedlander
Stock and Other Ownership Interests: Allergan, Merrimack, Incyte, Clovis Oncology, Bristol-Myers Squibb, Avalanche Biotechnologies
Consulting or Advisory Role: Array BioPharma, Seattle Genetics, Castle Biosciences, Genentech, Regeneron, Aspyrian Therapeutics
Travel, Accommodations, Expenses: Array BioPharma, Seattle Genetics, Castle Biosciences, Regeneron
Adil Daud
Stock and Other Ownership Interests: OncoSec
Honoraria: EMD Serono, Inovio Pharmaceuticals
Consulting or Advisory Role: OncoSec, GlaxoSmithKline, Genoptix, Merck
Research Funding: Merck/Schering Plough (Inst), GlaxoSmithKline (Inst), Pfizer (Inst), Genentech (Inst), OncoSec (Inst)
Patents, Royalties, Other Intellectual Property: Patent relating to test for immunotherapy
Harriet M. Kluger
Consulting or Advisory Role: Alexion Pharmaceuticals, Genentech, Corvus Pharmaceuticals, Nektar, Biodesix, Pfizer, Celldex, Iovance Biotherapeutics, Merck
Research Funding: Merck (Inst), Bristol-Myers Squibb (Inst), Apexigen (Inst)
Adam I. Riker
Honoraria: Genomic Health, Castle Biosciences
Consulting or Advisory Role: Novartis
Speakers' Bureau: Genomic Health, Castle Biosciences
Melissa A. Burgess
Consulting or Advisory Role: EMD Serono
Travel, Accommodations, Expenses: Eli Lilly
Brent A. Hanks
Stock and Other Ownership Interests: Bellicum Pharmaceuticals
Honoraria: Virginia Oncology Associates, CE Concepts, Duke Oncology Network
Consulting or Advisory Role: G1 Therapeutics, Fujifilm, Gerson Lehrman Group, Guidepoint Global
Speakers' Bureau: CE Concepts, Merck
Research Funding: Merck, OncoMed, AstraZeneca, GlaxoSmithKline, Tempest Therapeutics, Leap Therapeutics, D3 A*STAR
Patents, Royalties, Other Intellectual Property: Modulating the metabolism of dendritic cells to enhance antitumor immunity (Inst); Inhibiting paracrine Wnt ligand signaling to augment checkpoint inhibitor immunotherapy (Inst)
Expert Testimony: Kinnard, Clayton, & Beveridge
Travel, Accommodations, Expenses: Cambridge Health Institute, CE Concepts, Virginia Oncology Associates
Kim Margolin
Consulting or Advisory Role: ImaginAb, Iovance Biotherapeutics, Nektar, Guidepoint Global
Research Funding: Bristol-Myers Squibb (Inst), Amgen (Inst), Genentech (Inst), Oncolytics (Inst), Checkmate Pharmaceuticals (Inst)
Lisa M. Lundgren
Stock and Other Ownership Interests: Amgen, Celgene
Candice Church
Research Funding: EMD Serono (Inst)
Patents, Royalties, Other Intellectual Property: Patent: 1. Chapuis A, Nghiem P, McAfee M, Miller N, Paulson K, Koelle D, Schmitt T, Church C. Merkel Cell Polyomavirus T Antigen-Specific TCRs and Uses Thereof. PCT/US2017/061645 (Inst)
Michi M. Shinohara
Research Funding: Soligenix, Elorac, Actelion
Janis M. Taube
Consulting or Advisory Role: Bristol-Myers Squibb, AstraZeneca, Merck, Amgen
Research Funding: Bristol-Myers Squibb
Travel, Accommodations, Expenses: Bristol-Myers Squibb, AstraZeneca, Merck, Amgen
Steven R. Bird
Employment: Merck Sharp & Dohme
Stock and Other Ownership Interests: Merck Sharp & Dohme
Nageatte Ibrahim
Employment: Merck
Stock and Other Ownership Interests: Merck, GlaxoSmithKline
Steven P. Fling
Research Funding: Merck
Travel, Accommodations, Expenses: Cambridge Healthtech Institute
Blanca Homet Moreno
Employment: Merck Sharp & Dohme
Suzanne L. Topalian
Stock and Other Ownership Interests: Aduro Biotech (I), Compugen (I), Potenza Therapeutics (I), Jounce Therapeutics (I), Five Prime Therapeutics, Tizona Therapeutics (I), DNAtrix (I), FLX Bio (I), WindMIL (I), Dragonfly Therapeutics, ERVAXX (I)
Consulting or Advisory Role: Five Prime Therapeutics, Amgen (I), MedImmune (I), Merck, Abbvie, Compugen (I), DNAtrix (I), FLX Bio (I), Tizona Therapeutics (I), WindMIL (I), Dragonfly Therapeutics, Bayer (I), Dynavax (I), ERVAXX (I), Immunomic Therapeutics (I), Janssen Oncology (I)
Research Funding: Bristol-Myers Squibb, Compugen (I), Potenza Therapeutics (I)
Patents, Royalties, Other Intellectual Property: Aduro Biotech (I), Bristol-Myers Squibb (I), Immunonomic Therapeutics (I)
Travel, Accommodations, Expenses: Bristol-Myers Squibb, Five Prime Therapeutics
No other potential conflicts of interest were reported.
REFERENCES
- 1.Harms KL, Healy MA, Nghiem P, et al. Analysis of prognostic factors from 9387 Merkel cell carcinoma cases forms the basis for the new 8th Edition AJCC Staging System. Ann Surg Oncol. 2016;23:3564–3571. doi: 10.1245/s10434-016-5266-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Paulson KG, Park SY, Vandeven NA, et al. Merkel cell carcinoma: Current US incidence and projected increases based on changing demographics. J Am Acad Dermatol. 2018;78:457–463.e2. doi: 10.1016/j.jaad.2017.10.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Feng H, Shuda M, Chang Y, et al. Clonal integration of a polyomavirus in human Merkel cell carcinoma. Science. 2008;319:1096–1100. doi: 10.1126/science.1152586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Becker JC, Stang A, DeCaprio JA, et al. Merkel cell carcinoma. Nat Rev Dis Primers. 2017;3:17077. doi: 10.1038/nrdp.2017.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wong SQ, Waldeck K, Vergara IA, et al. UV-associated mutations underlie the etiology of MCV-negative Merkel cell carcinomas. Cancer Res. 2015;75:5228–5234. doi: 10.1158/0008-5472.CAN-15-1877. [DOI] [PubMed] [Google Scholar]
- 6.Harms PW, Vats P, Verhaegen ME, et al. The distinctive mutational spectra of polyomavirus-negative Merkel cell carcinoma. Cancer Res. 2015;75:3720–3727. doi: 10.1158/0008-5472.CAN-15-0702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Goh G, Walradt T, Markarov V, et al. Mutational landscape of MCPyV-positive and MCPyV-negative Merkel cell carcinomas with implications for immunotherapy. Oncotarget. 2016;7:3403–3415. doi: 10.18632/oncotarget.6494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Heath M, Jaimes N, Lemos B, et al. Clinical characteristics of Merkel cell carcinoma at diagnosis in 195 patients: The AEIOU features. J Am Acad Dermatol. 2008;58:375–381. doi: 10.1016/j.jaad.2007.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Engels EA, Frisch M, Goedert JJ, et al. Merkel cell carcinoma and HIV infection. Lancet. 2002;359:497–498. doi: 10.1016/S0140-6736(02)07668-7. [DOI] [PubMed] [Google Scholar]
- 10.Iyer JG, Blom A, Doumani R, et al. Response rates and durability of chemotherapy among 62 patients with metastatic Merkel cell carcinoma. Cancer Med. 2016;5:2294–2301. doi: 10.1002/cam4.815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cowey CL, Mahnke L, Espirito J, et al. Real-world treatment outcomes in patients with metastatic Merkel cell carcinoma treated with chemotherapy in the USA. Future Oncol. 2017;13:1699–1710. doi: 10.2217/fon-2017-0187. [DOI] [PubMed] [Google Scholar]
- 12.Kaufman HL, Russell J, Hamid O, et al. Avelumab in patients with chemotherapy-refractory metastatic Merkel cell carcinoma: A multicentre, single-group, open-label, phase 2 trial. Lancet Oncol. 2016;17:1374–1385. doi: 10.1016/S1470-2045(16)30364-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nghiem PT, Bhatia S, Lipson EJ, et al. PD-1 blockade with pembrolizumab in advanced Merkel-cell carcinoma. N Engl J Med. 2016;374:2542–2552. doi: 10.1056/NEJMoa1603702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.D’Angelo SP, Russell J, Lebbé C, et al. Efficacy and safety of first-line avelumab treatment in patients with stage IV metastatic Merkel cell carcinoma: A preplanned interim analysis of a clinical trial. JAMA Oncol. 2018;4:e180077. doi: 10.1001/jamaoncol.2018.0077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Topalian S, Bhatia S, Hollebecque A, et al. Non-comparative, open-label, multiple cohort, phase 1/2 study to evaluate nivolumab (NIVO) in patients with virus-associated tumors (CheckMate 358): Efficacy and safety in Merkel cell carcinoma (MCC) Cancer Res. 2017;77(abstr CT074) [Google Scholar]
- 16.Bichakjian CK, Olencki T, Aasi SZ, et al. Merkel Cell Carcinoma, Version 1.2018, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw. 2018;16:742–774. doi: 10.6004/jnccn.2018.0055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: Revised RECIST guideline (version 1.1) Eur J Cancer. 2009;45:228–247. doi: 10.1016/j.ejca.2008.10.026. [DOI] [PubMed] [Google Scholar]
- 18.Simon R. Optimal two-stage designs for phase II clinical trials. Control Clin Trials. 1989;10:1–10. doi: 10.1016/0197-2456(89)90015-9. [DOI] [PubMed] [Google Scholar]
- 19.National Institutes of Health, National Cancer Institute Common Terminology Criteria for Adverse Events v4.0., May 28, 2009. https://www.eortc.be/services/doc/ctc/CTCAE_4.03_2010-06-14_QuickReference_5x7.pdf
- 20.Paulson KG, Carter JJ, Johnson LG, et al. Antibodies to Merkel cell polyomavirus T antigen oncoproteins reflect tumor burden in Merkel cell carcinoma patients. Cancer Res. 2010;70:8388–8397. doi: 10.1158/0008-5472.CAN-10-2128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shuda M, Arora R, Kwun HJ, et al. Human Merkel cell polyomavirus infection I: MCV T antigen expression in Merkel cell carcinoma, lymphoid tissues and lymphoid tumors. Int J Cancer. 2009;125:1243–1249. doi: 10.1002/ijc.24510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Moshiri AS, Doumani R, Yelistratova L, et al. Polyomavirus-negative Merkel cell carcinoma: A more aggressive subtype based on analysis of 282 cases using multimodal tumor virus detection. J Invest Dermatol. 2017;137:819–827. doi: 10.1016/j.jid.2016.10.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Weber JS, Hodi FS, Wolchok JD, et al. Safety profile of nivolumab monotherapy: A pooled analysis of patients with advanced melanoma. J Clin Oncol. 2017;35:785–792. doi: 10.1200/JCO.2015.66.1389. [DOI] [PubMed] [Google Scholar]
- 24.Lipson EJ, Vincent JG, Loyo M, et al. PD-L1 expression in the Merkel cell carcinoma microenvironment: Association with inflammation, Merkel cell polyomavirus and overall survival. Cancer Immunol Res. 2013;1:54–63. doi: 10.1158/2326-6066.CIR-13-0034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dowlatshahi M, Huang V, Gehad AE, et al. Tumor-specific T cells in human Merkel cell carcinomas: A possible role for Tregs and T-cell exhaustion in reducing T-cell responses. J Invest Dermatol. 2013;133:1879–1889. doi: 10.1038/jid.2013.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Behr DS, Peitsch WK, Hametner C, et al. Prognostic value of immune cell infiltration, tertiary lymphoid structures and PD-L1 expression in Merkel cell carcinomas. Int J Clin Exp Pathol. 2014;7:7610–7621. [PMC free article] [PubMed] [Google Scholar]
- 27.Rimm DL, Han G, Taube JM, et al. A prospective, multi-institutional, pathologist-based assessment of 4 immunohistochemistry assays for PD-L1 expression in non-small cell lung cancer. JAMA Oncol. 2017;3:1051–1058. doi: 10.1001/jamaoncol.2017.0013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Büttner R, Gosney JR, Skov BG, et al. Programmed death-ligand 1 immunohistochemistry testing: A review of analytical assays and clinical implementation in non-small-cell lung cancer. J Clin Oncol. 2017;35:3867–3876. doi: 10.1200/JCO.2017.74.7642. [DOI] [PubMed] [Google Scholar]
- 29.Topalian SL. Targeting immune checkpoints in cancer therapy. JAMA. 2017;318:1647–1648. doi: 10.1001/jama.2017.14155. [DOI] [PubMed] [Google Scholar]
- 30.Kaufman HL, Russell JS, Hamid O, et al. Updated efficacy of avelumab in patients with previously treated metastatic Merkel cell carcinoma after ≥1 year of follow-up: JAVELIN Merkel 200, a phase 2 clinical trial. J Immunother Cancer. 2018;6:7. doi: 10.1186/s40425-017-0310-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Giraldo NA, Nguyen P, Engle EL, et al. Multidimensional, quantitative assessment of PD-1/PD-L1 expression in patients with Merkel cell carcinoma and association with response to pembrolizumab. J Immunother Cancer. 2018;6:99. doi: 10.1186/s40425-018-0404-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wang Q, Gao J, Wu X. Pseudoprogression and hyperprogression after checkpoint blockade. Int Immunopharmacol. 2018;58:125–135. doi: 10.1016/j.intimp.2018.03.018. [DOI] [PubMed] [Google Scholar]
- 33.Topalian SL, Bhatia S, Kudchadkar RR, et al. Nivolumab (Nivo) as neoadjuvant therapy in patients with resectable Merkel cell carcinoma (MCC) in CheckMate 358. J Clin Oncol. 2018;36(suppl; abstr 9505) doi: 10.1200/JCO.20.00201. [DOI] [PMC free article] [PubMed] [Google Scholar]