Abstract
PURPOSE
Treatment of medulloblastoma has evolved from surgery and radiotherapy to contemporary multimodal regimens. However, the impact on long-term health outcomes remains unknown.
METHODS
Cumulative incidence of late mortality (5 or more years from diagnosis), subsequent neoplasms (SNs), and chronic health conditions were evaluated in the Childhood Cancer Survivor Study among 5-year survivors of medulloblastoma diagnosed between 1970 and 1999. Outcomes were evaluated by treatment exposure, including historical therapy (craniospinal irradiation [CSI] ≥ 30 Gy, no chemotherapy), high risk (CSI ≥ 30 Gy + chemotherapy), standard risk (CSI < 30 Gy + chemotherapy), and by treatment decade (1970s, 1980s, 1990s). Rate ratios (RRs) and 95% CIs estimated long-term outcomes using multivariable piecewise exponential models.
RESULTS
Among 1,311 eligible survivors (median age, 29 years [range, 6 to 60 years]; median time from diagnosis, 21 years [range, 5 to 44 years]), the 15-year cumulative incidence rate of all-cause late mortality was 23.2% (diagnosed 1970s) versus 12.8% (1990s; P = .002), with a recurrence-related mortality rate of 17.7% versus 9.6% (P = .008). Lower late mortality rates as a result of other health-related causes were not observed. Among 997 survivors who completed a baseline survey, the 15-year cumulative incidence of SNs was higher among survivors with multimodal therapy (standard risk, 9.5%; historical, 2.8%; P = .03). Survivors treated in the 1990s had a higher cumulative incidence of severe, disabling, life-threatening, and fatal chronic health conditions (56.5% in 1990s v 39.9% in 1970s; P < .001) and were more likely to develop multiple conditions (RR, 2.89; 95% CI, 1.31 to 6.38). However, survivors of standard-risk therapy were less likely to use special education services than high-risk therapy survivors (RR, 0.84; 95% CI, 0.75 to 0.93).
CONCLUSION
Historical changes in medulloblastoma therapy that improved 5-year survival have increased the risk for SNs and debilitating health conditions for survivors yet reduced the need for special education services.
INTRODUCTION
Medulloblastoma is one of the most common malignant brain tumors in children, accounting for 15% to 20% of pediatric CNS neoplasms.1 Therapy for medulloblastoma has evolved to well-accepted multimodal regimens that include maximal surgical resection, craniospinal irradiation (CSI), and chemotherapy, making medulloblastoma curable in up to 70% to 80% of patients.2-4 Historically, although surgery alone did not achieve cure, the introduction of 30- to 40-Gy CSI with focal boost to the tumor region led to the first reports of survivors of medulloblastoma.5,6 During the 1980s, chemotherapy was incorporated as adjuvant therapy for medulloblastoma and led to improved disease control.7-9 Of note, by the 1990s, patients with medulloblastoma were more routinely risk stratified on the basis of age, extent of surgical resection, and metastatic status at diagnosis, which allowed standard-risk patients to receive reduced-dose CSI (23.4 Gy).2,3 This risk-adapted therapy improved 5-year survival for patients by better identifying those with high-risk disease who required chemotherapy added to a higher dose of CSI while allowing for those with lower-risk disease to receive lower-dose CSI with adjuvant chemotherapy.2,3 The effect of this treatment evolution on long-term outcomes (5 or more years) remains unknown. The recent expansion of the Childhood Cancer Survivor Study (CCSS) cohort to include survivors diagnosed across three decades (1970 to 1999) offers a unique opportunity to explore the effect of temporal changes in medulloblastoma therapy on long-term outcomes when pivotal changes in treatment were made.
METHODS
CCSS Cohort
The CCSS is a retrospective cohort study with longitudinal follow-up of 5-year survivors of childhood cancer treated at 27 participating institutions in the United States and Canada. Eligibility criteria were diagnosis of leukemia, lymphoma, CNS malignancy, Wilms tumor, neuroblastoma, soft tissue sarcoma, or bone cancer before 21 years of age; treatment between 1970 and 1999; and 5-year survival after diagnosis. The study methods and design have been described previously.10,11 Among 35,649 survivors eligible for participation, 1,422 had medulloblastoma, and 1,311 were evaluable for late mortality (vital status). Among these, 997 completed the baseline survey and were evaluable for late outcomes (Data Supplement). Mortality data for survivors who were eligible but did not participate in the study were not available for 73 Canadian survivors and 38 US survivors from a single institution. The study was approved by the institutional review boards of participating institutions; informed consent was obtained from participants or guardians.
Late Mortality, Subsequent Neoplasms, and Chronic Health Condition Outcomes
To ascertain date and cause of death, the National Death Index was reviewed through December 31, 2013, for underlying causes of death according to the International Classification of Diseases, Ninth and Tenth Revisions.12 For deaths that predated the National Death Index (ie, 1975 to 1979), death certificates were requested. Death of 5-year survivors of medulloblastoma was a result of recurrence or progression of medulloblastoma, other health-related causes not a result of medulloblastoma, or external causes (eg, accident, injury, suicide).
All CCSS participants completed a baseline survey, including self- or proxy-reported health conditions. Most conditions were captured from specific questions and a text field for each organ system. A team of nine clinicians/researchers adjudicated the inclusion or exclusion of each condition and graded severity using the National Cancer Institute Common Terminology Criteria for Adverse Events (version 4.03) as mild (grade 1), moderate (grade 2), severe or disabling (grade 3), life threatening (grade 4), or fatal (grade 5).13 Analyses were restricted to grade 3 to 5 events.
Subsequent neoplasms (SNs), defined as new neoplasms (malignant or benign) not including the recurrence of the primary malignancy, were ascertained through self- or proxy-report and confirmed by pathology report or, when not available, confirmed by death certificate or other medical records. Only SNs that occurred 5 or more years after the childhood cancer diagnosis were evaluated.14,15 Among 90 SNs confirmed, 78 were confirmed by pathology report, eight by medical records, and four on the basis of specificity of self-report.
Special education placement, insurance status, and employment status were considered as indicators of psychosocial functioning. For employment status, survivors who were younger than 25 years were excluded. Cancer diagnosis and therapeutic exposure data (including radiotherapy [RT] and chemotherapy) were abstracted from medical records of 1,128 participants as previously described.12,16
Temporal Trends in Treatment Exposure
To account for the historical changes in medulloblastoma therapy across three decades, survivors were stratified according to era of diagnosis (1970s, 1980s, 1990s) and treatment exposure group: historical therapy (surgery + CSI ≥ 30 Gy, no chemotherapy), high-risk multimodal therapy (surgery + CSI ≥ 30 Gy + chemotherapy), and standard-risk multimodal therapy (surgery + CSI < 30 Gy + chemotherapy). Survivors included as standard or high risk had at least one alkylating drug (cyclophosphamide or lomustine) with a platinum compound (cisplatin or carboplatin) on the basis of their common inclusion in most medulloblastoma treatment regimens.
Statistical Analysis
We estimated cumulative incidence of cause-specific death, SNs, and chronic health conditions stratified by treatment era and treatment exposure group. Events that occurred before the cohort entry at 5 years from cancer diagnosis were counted as prevalent at time of cohort entry. For cause-specific deaths, follow-up time ended at the earlier of the date of death or December 31, 2013. For SNs, the follow-up time ended at the earliest of an SN after 5 years since diagnosis, death, or the last questionnaire. For chronic health conditions, follow-up time ended at the earliest of a chronic health condition, SN, late recurrence, death, or last questionnaire.
Piecewise exponential models were used to evaluate associations of treatment era and treatment exposure group with late mortality, SNs, and chronic health conditions, adjusting for age at diagnosis, attained age, sex, and race (not for late mortality). Log-binomial regression was used to assess prevalence of having developed a chronic health condition before cohort entry after medulloblastoma diagnosis; adjusted for age at diagnosis and socioeconomic outcomes (special education, employment status, and insurance status); and adjusted for age at diagnosis, age at baseline survey, sex, and race.
For late mortality, we used multiple imputation for missing treatment information in the cohort.12,17 After generating 10 data sets, statistical analysis was applied to each, yielding results that were summarized by the standard multiple imputation procedure.
RESULTS
Among 1,311 eligible survivors (Data Supplement), median age at diagnosis was 7 years (range, 0 to 21 years), median age at last follow-up was 29 years (range, 6 to 60 years), and median time since diagnosis was 21 years (range, 5 to 44 years). Among 997 participants who completed a baseline survey, 148 (15%) were diagnosed in the 1970s, 349 (35%) in the 1980s, and 500 (50%) in the 1990s (Table 1). Ninety-four percent of survivors received CSI, with 40% from the 1990s receiving CSI less than 30 Gy compared with only 22% to 24% in earlier eras. Survivors from the 1990s were more likely to have received chemotherapy, including platinum and alkylating agents, compared with survivors from the 1970s.
TABLE 1.
Demographic and Treatment Characteristics of Five-Year Survivors of Medulloblastoma Overall and by Treatment Era
All-Cause and Cause-Specific Mortality
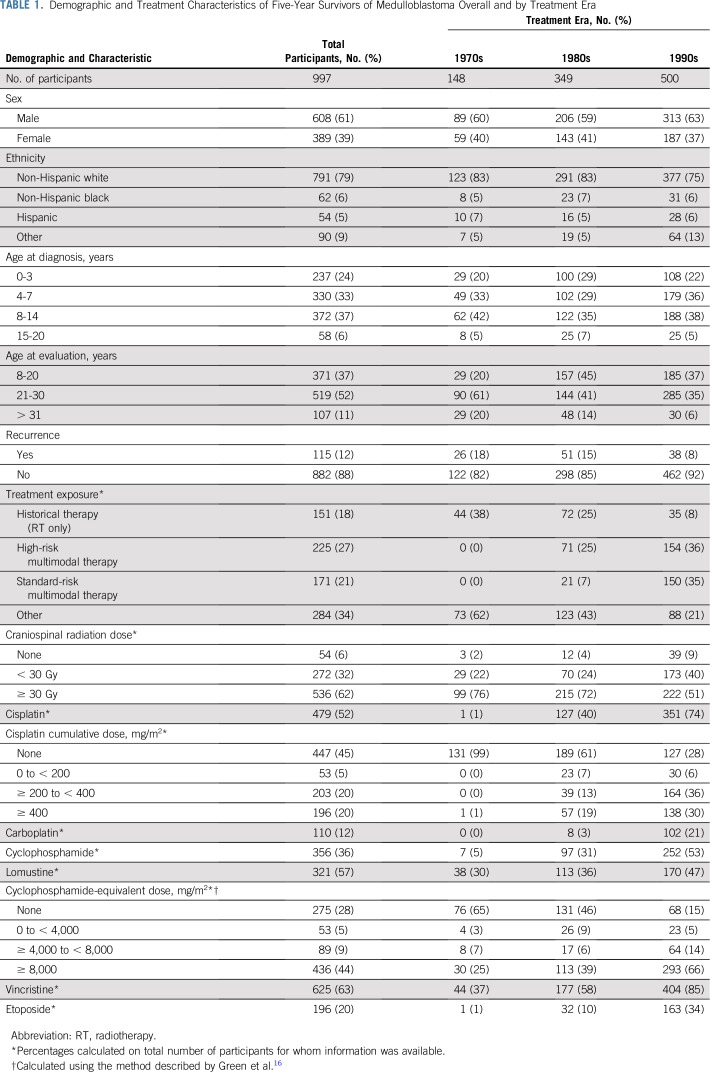
Among the 1,311 survivors evaluable for late mortality, 282 deaths resulted from recurrence or progression of medulloblastoma (n = 177), other health-related causes (n = 102, including 57 SNs), and external causes (n = 3). Across three decades, the 15-year cumulative incidence rate of all-cause late mortality was significantly higher among survivors diagnosed in the 1970s (23.2%; 95% CI, 17.1% to 29.3%) relative to the 1980s (14.4%; 95% CI, 11.2% to 17.6%) and 1990s (12.8%; 95% CI, 10.2% to 15.3%; P = .002; Fig 1A). This finding was driven by a reduction in late mortality as a result of medulloblastoma recurrence or progression from 17.7% (95% CI, 12.1% to 23.2%) for survivors diagnosed in the 1970s to 10.5% (95% CI, 7.7% to 13.4%) and 9.6% (95% CI, 7.4% to 11.9%) from the 1980s and 1990s, respectively (P = .008; Fig 1B). However, no significant reduction was seen in late mortality as a result of other health-related causes overall (Fig 1C). Death as a result of an SN was significantly higher for survivors diagnosed in the 1970s (4.3%, 95% CI, 1.3% to 7.3%) relative to the 1980s (3.0%; 95% CI, 1.4% to 4.6%) and 1990s (1.3%; 95% CI, 0.4% to 2.1%; P = .03; Data Supplement). Treatment exposure group (historical therapy, high-risk multimodal therapy, or standard-risk multimodal therapy) had little effect on cumulative incidence of late mortality as a result of all causes, disease recurrence, and treatment-related causes (Figs 1A to 1C; Data Supplement).
FIG 1.
Fifteen-year cumulative incidence of (A) all-cause, (B) recurrence-related, and (C) health-related cause-specific mortality among 5-year survivors of childhood medulloblastoma by treatment era and treatment exposure. HR, high risk; RT, radiotherapy; SR, standard risk.
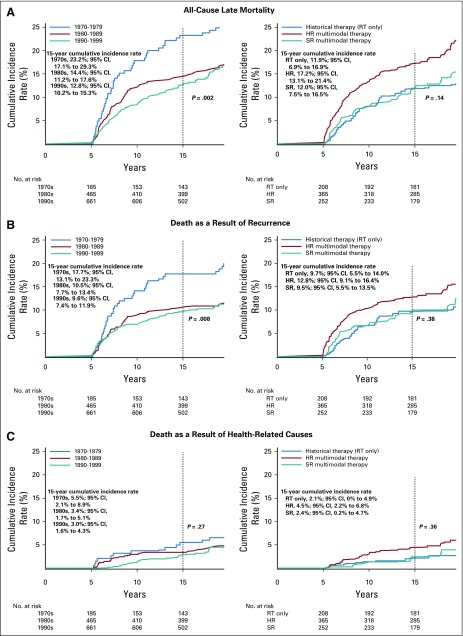
After adjusting for age at diagnosis, attained age, and sex (Table 2), survivors diagnosed in more recent eras demonstrated a reduced rate of death as a result of all causes (1980s v 1970s rate ratio [RR], 0.70 [95% CI, 0.53 to 0.94]; 1990s v 1970s RR, 0.62 [95% CI, 0.46 to 0.84] and recurrence (1980s v 1970s RR, 0.62 [95% CI, 0.42 to 0.91]; 1990s v 1970s RR, 0.58 [95% CI, 0.40 to 0.85]) but not as a result of other health-related causes compared with the 1970s. Treatment exposure group status was not associated with a significant change in rate of death (Table 2).
TABLE 2.
Multivariable RRs of All-Cause and Cause-Specific Late Mortality in Five-Year Survivors of Childhood Medulloblastoma
Temporal Changes in Morbidity
SNs.
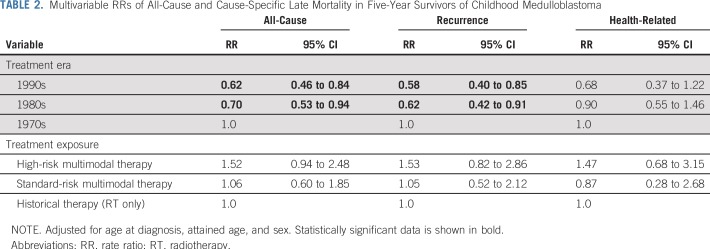
Overall, there were 90 SNs, including eight malignant gliomas and 24 benign meningiomas (Data Supplement). Although no difference in the cumulative incidence of SNs at 15 years since diagnosis was observed by treatment era (Fig 2A; Data Supplement), survivors who received standard-risk multimodal therapy had an increased incidence of SNs (9.5%, 95% CI, 5.1% to 15.4%) compared with survivors of historical therapy (2.8%, 95% CI, 0.9% to 6.5%; P = .02; Fig 2B; Data Supplement).
FIG 2.
Fifteen-year cumulative incidence of subsequent neoplasms among 5-year survivors of childhood medulloblastoma by (A) treatment era and (B) treatment exposure. HR, high risk; RT, radiotherapy; SR, standard risk.
Chronic health conditions.
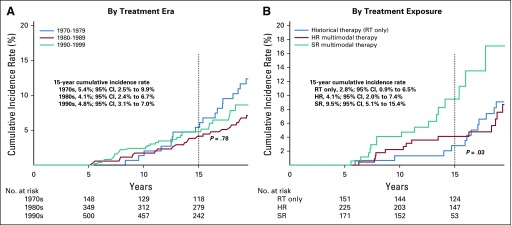
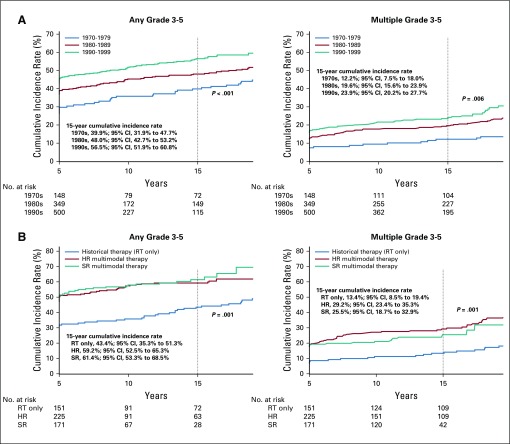
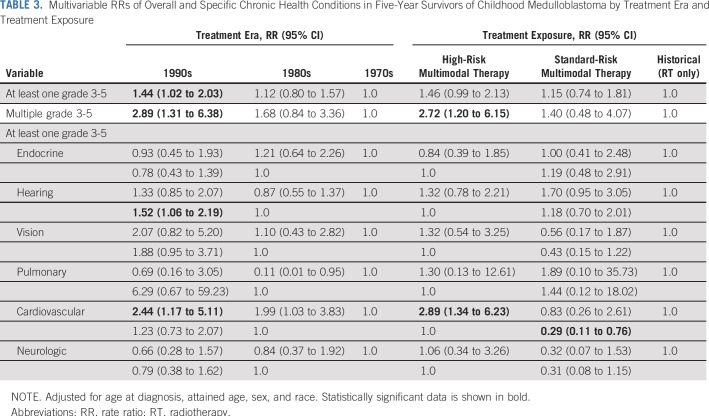
The 15-year cumulative incidence rate of a first grade 3 to 5 condition was 39.9% (95% CI, 31.9% to 47.7%) for survivors diagnosed in the 1970s, 48.0% (95% CI, 42.7% to 53.2%) for those diagnosed in the 1980s, and 56.5% (95% CI, 51.9% to 60.8%) for those diagnosed in the 1990s (P < .001; Fig 3A; Data Supplement). A similar pattern was observed for the incidence of multiple (two or more) grade 3 to 5 conditions from 12.2% (95% CI, 7.5% to 18.0%) in the 1970s to 19.6% (95% CI, 15.6% to 23.9%) in the 1980s and 23.9% (95% CI, 20.2% to 27.7%) in the 1990s (P = .006). Multivariable analysis confirmed a higher rate of grade 3 to 5 conditions (RR, 1.44; 95% CI, 1.02 to 2.03; Table 3) as well as multiple conditions (RR, 2.89; 95% CI, 1.31 to 6.38; Table 3) among survivors diagnosed in the 1990s compared with survivors from the 1970s. In addition, the incidence of at least one and multiple chronic grade 3 to 5 conditions was higher among survivors who received multimodal therapy compared with those who received historical therapy with RTonly (Fig 3B; Data Supplement). Survivors of multimodal high-risk therapy had a higher risk of experiencing multiple grade 3 to 5 conditions compared with survivors who received historical therapy (RR, 2.72; 95% CI, 1.20 to 6.15; Table 3).
FIG 3.
Fifteen-year cumulative incidence of severe, life-threatening, and fatal health conditions among 5-year survivors of childhood medulloblastoma by (A) treatment era and (B) treatment exposure. HR, high risk; RT, radiotherapy; SR, standard risk.
TABLE 3.
Multivariable RRs of Overall and Specific Chronic Health Conditions in Five-Year Survivors of Childhood Medulloblastoma by Treatment Era and Treatment Exposure
A significantly higher cumulative incidence rate of grade 3 to 4 hearing deficits was found over time (40.6% [95% CI, 36.1% to 44.9%] in the 1990s v 18.2% [95% CI, 12.5% to 24.9%] in the 1970s; P < .001; Data Supplement). Multivariable analyses demonstrated that survivors diagnosed in the 1990s (RR, 1.52; 95% CI, 1.06 to 2.19; Table 3) and survivors who received cisplatin (RR, 2.2; 95% CI, 1.5 to 3.2; Data Supplement) were more likely to experience grade 3 to 4 hearing deficits than those in the 1980s and those who did not receive cisplatin. In addition, there was a higher cumulative incidence rate of grade 3 to 5 cardiovascular conditions, including stroke (75.9%), blood clot (30.1%), and coronary artery disease (7.2%), among survivors treated in more recent eras (RR, 2.44 for 1990s v 1970s; 95% CI, 1.17 to 5.11) and survivors of high-risk therapy (RR, 2.89 for high-risk v historical therapy; 95% CI, 1.34 to 6.23; Table 3; Data Supplement). Although the cumulative incidence rate of grade 3 to 4 visual conditions (reported as cataract [61.7%] and blindness [42.6%]) was higher among survivors diagnosed in the 1990s (10.4%; 95% CI, 7.9% to 13.3%) than among survivors from the 1970s (5.4%; 95% CI, 2.5% to 9.9%; P = .03), multivariable analysis did not show a significant increase in the RR of visual conditions (Table 3; Data Supplement).
Psychosocial function.
Survivors who received standard-risk multimodal therapy were less likely to use special education services than those who received high-risk multimodal therapy (69.9% v 74.0%; RR, 0.84; 95% CI, 0.75 to 0.93; Data Supplement). No statistically significant differences in employment or insurance status were identified across eras of diagnosis and treatment exposure groups.
DISCUSSION
The inclusion of adjuvant chemotherapy in the 1980s and the introduction of risk-stratified therapy in the 1990s represent major milestones in the evolution of treatment of children with medulloblastoma and have substantially improved 5-year survival.2,3,18 Although treatment regimens in the modern era typically have resulted in a decreased risk of late effects in survivors of other childhood cancers,12 we identified a higher risk of morbidity in medulloblastoma survivors of more recent eras. The rise in overall incidence of grade 3 to 5 chronic health conditions from the 1970s to the 1990s seems to be a result of the introduction of adjuvant chemotherapy. One of the key drivers is the higher risk for hearing loss observed in more recent eras associated with the increased use of cisplatin.19 The higher risk for cardiovascular conditions, which almost tripled over three decades among survivors of high-risk therapy compared with those who received RT only, also suggests that chemotherapy may augment the effect of CSI adversely on cardiovascular outcomes. Although this population does not routinely receive anthracycline therapy, this finding is consistent with previous studies that have suggested that non–anthracycline chemotherapeutic agents, including alkylators and cisplatin,20 may be cardiotoxic.
The observed higher 15-year cumulative incidence of SNs among survivors of standard-risk multimodal therapy compared with those exposed to RT only corroborates earlier reports of a higher risk for SNs with adjuvant chemotherapy for medulloblastoma.21,22 Indeed, the long-term follow-up study of Children’s Oncology Group trial A9961 estimated a cumulative 10-year incidence rate of secondary malignancies of 4.2% (95% CI, 1.9% to 6.5%).21 Similarly, the German Society for Pediatric Hematology and Oncology HIT ’91 trial reported 12 (4.3%) secondary neoplasms in 280 patients with medulloblastoma.22 More recently, the 10-year cumulative incidence rate of SNs among medulloblastoma survivors of multimodal therapy at St Jude Children’s Research Hospital was 5.5% (95% CI, 2.8% to 9.6%), which is significantly higher than a historical cohort of patients who received RT only.23 This finding may be explained partly by changes in SN surveillance guidelines and improvement in neuro-imaging techniques to detect indolent neoplasms that coincided with the introduction of multimodal therapy for medulloblastoma. Nevertheless, with approximately 10% of survivors of standard-risk therapy who experienced an SN by only 15 years of follow-up, the question of continued surveillance of the CNS in particular is to be seriously considered.
Despite these higher risks for late outcomes, the current study identified some important improvements in health risk among survivors. First, there was evidence for lower risk for death as a result of medulloblastoma recurrence in more modern eras. This finding is consistent with previous research and suggests that more-recent survivors of medulloblastoma experience more durable remission.12,24 Five-year survivors of medulloblastoma, however, did not experience a similar reduction in risk of death as a result of other health-related conditions, largely late effects, across the three decades.12 This may be attributed to the fact that although a subgroup of standard-risk patients with medulloblastoma was successfully treated with reduced doses of CSI, the addition of adjuvant chemotherapy resulted in an overall intensification of therapy during the studied time period, which differs strikingly from survivors of other childhood cancers treated during these same eras when a clear concurrent reduction in treatment intensity was demonstrated.12 Of note, a lower rate of death as a result of an SN was observed in the current cohort despite a higher incidence of SNs. This observation may be explained by earlier detection and improved treatment of SNs in more recent eras. Second, although risk-adapted therapy did not translate into a significant reduction of late-onset health conditions, the primary goal of CSI dose reduction was to reduce risk for cognitive injury. Thus, that survivors who received standard-risk therapy were less likely to use special education services than those who received high-risk multimodal therapy with greater than 30-Gy CSI is encouraging. However, the absolute difference in rates of special education use was modest and may not be clinically significant. Moreover, access to special education depends on many additional variables that we could not measure.
A number of limitations should be considered when evaluating the current study, including dependence on participants’ self-report of chronic health conditions and psychosocial outcomes; shorter follow-up of survivors from the 1990s, which necessitated the limiting of most comparisons to 15 years of follow-up; and lack of data from survivors of more contemporary medulloblastoma therapies. The inability to measure the effect of advances in screening chronic health conditions over time may have introduced a potential detection bias toward an overestimation of morbidity in more recent eras, but it may be mitigated by the fact that the analysis was restricted to symptomatically severe, life-threatening, and fatal conditions. Also of note, the reported incidence of SNs in this study may be underestimated. Indeed, recent evidence has suggested that a number of late medulloblastoma recurrences diagnosed radiologically may be in fact secondary malignant gliomas histologically.25,26 Finally, the increasing number of 5-year survivors across treatment eras reflects improved 5-year survival from medulloblastoma as a result of improvement in treatment. Although the sample size differs across the decades, comparisons of treatment changes with outcomes across these decades is valid because our cohort represents each era’s 5-year survivor population in an unbiased way. However, a potential exists for survivor bias should the distribution of unmeasured factors, such as genetic predisposition for SNs, change because of changes in therapy.
Taken together, findings from this study highlight the need to focus the efforts of modern medulloblastoma therapy on reducing late effects of treatment of future survivors and to closely monitor contemporary survivors. For hearing deficits, this may be accomplished by early detection and strict stopping rules as well as the potential use of otoprotective agents recently shown to reduce cisplatin-induced hearing loss significantly.27 The use of advanced radiation modalities, including proton beam therapy, also may improve hearing and cardiovascular outcomes.28 For current survivors, it is now imperative, given the high risk for late effects demonstrated in the current study, to implement risk-based screening and guideline-based follow-up. Finally, the improved understanding of medulloblastoma biology may offer the advantage of better risk stratification in modern frontline studies and reduced late effects of therapy in molecularly defined subgroups. This will be tested partially in ongoing trials that further reduce therapy in the WNT subgroup of medulloblastoma known to have very favorable outcomes. Moreover, the introduction of molecularly targeted therapies that better address the biologic diversity of medulloblastoma might have important implications on survival as well as acute and long-term toxicities.
In summary, the CCSS provides evidence that historical changes in medulloblastoma therapy that improved 5-year survival have resulted in an increased risk for severe, life-threatening, and disabling chronic health conditions, including SNs. Now, the challenge for the pediatric neuro-oncology community is to reduce risk while maintaining or improving survival rates.
Footnotes
Presented at the American Society of Clinical Oncology 2017 Annual Meeting, Chicago, IL, June 2-6, 2017. Preprint version available on bioRxiv.
Supported by the National Cancer Institute (CA55727, G.T.A., principal investigator). Support to St. Jude Children’s Research Hospital also provided by a Cancer Center Support (CORE) grant (CA21765, C. Roberts, principal investigator) and the American Lebanese-Syrian Associated Charities.
AUTHOR CONTRIBUTIONS
Conception and design: Ralph Salloum, Roger Packer, Wendy Leisenring, Kevin C. Oeffinger, Maryam Fouladi, Gregory T. Armstrong
Financial support: Leslie L. Robison, Gregory T. Armstrong
Administrative support: Leslie L. Robison, Gregory T. Armstrong
Provision of study material or patients: Roger Packer, Leslie L. Robison, Kevin C. Oeffinger, Gregory T. Armstrong
Collection and assembly of data: Ralph Salloum, Wendy Leisenring, Rebecca Howell, Leslie L. Robison, Kevin C. Oeffinger, Gregory T. Armstrong
Data analysis and interpretation: Ralph Salloum, Yan Chen, Yutaka Yasui, Roger Packer, Elizabeth Wells, Allison King, Todd M. Gibson, Kevin R. Krull, Kevin C. Oeffinger, Maryam Fouladi, Gregory T. Armstrong
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Late Morbidity and Mortality Among Medulloblastoma Survivors Diagnosed Across Three Decades: A Report From the Childhood Cancer Survivor Study
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/jco/site/ifc.
Allison King
Stock and Other Ownership Interests: Magenta Therapeutics
Consulting or Advisory Role: Amphivena Therapeutics (I), Celgene (I), Cellworks (I), Incyte (I), Karyopharm Therapeutics (I), RiverVest Venture Partners (I), Tioma Therapeutics (I)
Research Funding: Novimmune (I)
Other Relationship: WUGEN (I)
Kevin R. Krull
Patents, Royalties, Other Intellectual Property: Royalties from Wolters Kluwer
Maryam Fouladi
Research Funding: Merck (Inst)
No other potential conflicts of interest were reported.
REFERENCES
- 1.Massimino M, Biassoni V, Gandola L, et al. Childhood medulloblastoma. Crit Rev Oncol Hematol. 2016;105:35–51. doi: 10.1016/j.critrevonc.2016.05.012. [DOI] [PubMed] [Google Scholar]
- 2.Packer RJ, Gajjar A, Vezina G, et al. Phase III study of craniospinal radiation therapy followed by adjuvant chemotherapy for newly diagnosed average-risk medulloblastoma. J Clin Oncol. 2006;24:4202–4208. doi: 10.1200/JCO.2006.06.4980. [DOI] [PubMed] [Google Scholar]
- 3.Gajjar A, Chintagumpala M, Ashley D, et al. Risk-adapted craniospinal radiotherapy followed by high-dose chemotherapy and stem-cell rescue in children with newly diagnosed medulloblastoma (St Jude Medulloblastoma-96): Long-term results from a prospective, multicentre trial. Lancet Oncol. 2006;7:813–820. doi: 10.1016/S1470-2045(06)70867-1. [DOI] [PubMed] [Google Scholar]
- 4.von Bueren AO, Kortmann RD, von Hoff K, et al. Treatment of children and adolescents with metastatic medulloblastoma and prognostic relevance of clinical and biologic parameters. J Clin Oncol. 2016;34:4151–4160. doi: 10.1200/JCO.2016.67.2428. [DOI] [PubMed] [Google Scholar]
- 5.Paterson E, Farr RF. Cerebellar medulloblastoma: Treatment by irradiation of the whole central nervous system. Acta Radiol. 1953;39:323–336. doi: 10.3109/00016925309136718. [DOI] [PubMed] [Google Scholar]
- 6.Stiller CA, Lennox EL. Childhood medulloblastoma in Britain 1971-77: Analysis of treatment and survival. Br J Cancer. 1983;48:835–841. doi: 10.1038/bjc.1983.274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Packer RJ, Sutton LN, Elterman R, et al. Outcome for children with medulloblastoma treated with radiation and cisplatin, CCNU, and vincristine chemotherapy. J Neurosurg. 1994;81:690–698. doi: 10.3171/jns.1994.81.5.0690. [DOI] [PubMed] [Google Scholar]
- 8.Tait DM, Thornton-Jones H, Bloom HJ, et al. Adjuvant chemotherapy for medulloblastoma: The first multi-centre control trial of the International Society of Paediatric Oncology (SIOP I) Eur J Cancer. 1990;26:463–469. [PubMed] [Google Scholar]
- 9.Packer RJ, Goldwein J, Nicholson HS, et al. Treatment of children with medulloblastomas with reduced-dose craniospinal radiation therapy and adjuvant chemotherapy: A Children’s Cancer Group Study. J Clin Oncol. 1999;17:2127–2136. doi: 10.1200/JCO.1999.17.7.2127. [DOI] [PubMed] [Google Scholar]
- 10.Robison LL, Armstrong GT, Boice JD, et al. The Childhood Cancer Survivor Study: A National Cancer Institute-supported resource for outcome and intervention research. J Clin Oncol. 2009;27:2308–2318. doi: 10.1200/JCO.2009.22.3339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Leisenring WM, Mertens AC, Armstrong GT, et al. Pediatric cancer survivorship research: Experience of the Childhood Cancer Survivor Study. J Clin Oncol. 2009;27:2319–2327. doi: 10.1200/JCO.2008.21.1813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Armstrong GT, Chen Y, Yasui Y, et al. Reduction in late mortality among 5-year survivors of childhood cancer. N Engl J Med. 2016;374:833–842. doi: 10.1056/NEJMoa1510795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Oeffinger KC, Mertens AC, Sklar CA, et al. Chronic health conditions in adult survivors of childhood cancer. N Engl J Med. 2006;355:1572–1582. doi: 10.1056/NEJMsa060185. [DOI] [PubMed] [Google Scholar]
- 14.Neglia JP, Friedman DL, Yasui Y, et al. Second malignant neoplasms in five-year survivors of childhood cancer: Childhood Cancer Survivor Study. J Natl Cancer Inst. 2001;93:618–629. doi: 10.1093/jnci/93.8.618. [DOI] [PubMed] [Google Scholar]
- 15.Turcotte LM, Liu Q, Yasui Y, et al. Temporal trends in treatment and subsequent neoplasm risk among 5-year survivors of childhood cancer, 1970-2015. JAMA. 2017;317:814–824. doi: 10.1001/jama.2017.0693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Green DM, Nolan VG, Goodman PJ, et al. The cyclophosphamide equivalent dose as an approach for quantifying alkylating agent exposure: A report from the Childhood Cancer Survivor Study. Pediatr Blood Cancer. 2014;61:53–67. doi: 10.1002/pbc.24679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Taylor JMG, Muñoz A, Bass SM, et al. Estimating the distribution of times from HIV seroconversion to AIDS using multiple imputation. Multicentre AIDS Cohort Study. Stat Med. 1990;9:505–514. doi: 10.1002/sim.4780090504. [DOI] [PubMed] [Google Scholar]
- 18.Thomas PR, Deutsch M, Kepner JL, et al. Low-stage medulloblastoma: Final analysis of trial comparing standard-dose with reduced-dose neuraxis irradiation. J Clin Oncol. 2000;18:3004–3011. doi: 10.1200/JCO.2000.18.16.3004. [DOI] [PubMed] [Google Scholar]
- 19.Brock PR, Knight KR, Freyer DR, et al. Platinum-induced ototoxicity in children: A consensus review on mechanisms, predisposition, and protection, including a new International Society of Pediatric Oncology Boston Ototoxicity Scale. J Clin Oncol. 2012;30:2408–2417. doi: 10.1200/JCO.2011.39.1110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Madeddu C, Deidda M, Piras A, et al: Pathophysiology of cardiotoxicity induced by nonanthracycline chemotherapy. J Cardiovasc Med (Hagerstown) 17:e12-e18, 2016 (suppl) [DOI] [PubMed]
- 21.Packer RJ, Zhou T, Holmes E, et al. Survival and secondary tumors in children with medulloblastoma receiving radiotherapy and adjuvant chemotherapy: Results of Children’s Oncology Group Trial A9961. Neuro-oncol. 2013;15:97–103. doi: 10.1093/neuonc/nos267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.von Hoff K, Hinkes B, Gerber NU, et al. Long-term outcome and clinical prognostic factors in children with medulloblastoma treated in the prospective randomised multicentre trial HIT’91. Eur J Cancer. 2009;45:1209–1217. doi: 10.1016/j.ejca.2009.01.015. [DOI] [PubMed] [Google Scholar]
- 23.Tsui K, Gajjar A, Li C, et al. Subsequent neoplasms in survivors of childhood central nervous system tumors: Risk after modern multimodal therapy. Neuro-oncol. 2015;17:448–456. doi: 10.1093/neuonc/nou279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Möller TR, Garwicz S, Perfekt R, et al. Late mortality among five-year survivors of cancer in childhood and adolescence: Differences between the Nordic countries. Acta Oncol. 2004;43:711–718. doi: 10.1080/02841860410002860. [DOI] [PubMed] [Google Scholar]
- 25.Weintraub L, Miller T, Friedman I, et al. Misdiagnosing recurrent medulloblastoma: The danger of examination and imaging without histological confirmation. J Neurosurg Pediatr. 2014;13:33–37. doi: 10.3171/2013.10.PEDS13231. [DOI] [PubMed] [Google Scholar]
- 26.Phi JH, Park AK, Lee S, et al. Genomic analysis reveals secondary glioblastoma after radiotherapy in a subset of recurrent medulloblastomas. Acta Neuropathol. 2018;135:939–953. doi: 10.1007/s00401-018-1845-8. [DOI] [PubMed] [Google Scholar]
- 27.Brock PR, Maibach R, Childs M, et al. Sodium thiosulfate for protection from cisplatin-induced hearing loss. N Engl J Med. 2018;378:2376–2385. doi: 10.1056/NEJMoa1801109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yock TI, Yeap BY, Ebb DH, et al. Long-term toxic effects of proton radiotherapy for paediatric medulloblastoma: A phase 2 single-arm study. Lancet Oncol. 2016;17:287–298. doi: 10.1016/S1470-2045(15)00167-9. [DOI] [PubMed] [Google Scholar]