Abstract
PURPOSE
Predictive biomarkers to identify patients with human epidermal growth factor receptor 2 (HER2)–positive breast cancer who may benefit from targeted therapy alone are required. We hypothesized that early measurements of tumor maximum standardized uptake values corrected for lean body mass (SULmax) on [18F]fluorodeoxyglucose positron emission tomography/computed tomography would predict pathologic complete response (pCR) to neoadjuvant pertuzumab and trastuzumab (PT).
PATIENTS AND METHODS
Patients with stage II/III, estrogen receptor–negative, HER2-positive breast cancer received four cycles of neoadjuvant PT. [18F]Fluorodeoxyglucose positron emission tomography/computed tomography was performed at baseline and 15 days after PT initiation (C1D15). Eighty evaluable patients were required to test the null hypothesis that the area under the curve of percentage of change in SULmax by C1D15 predicting pCR is less than or equal to 0.65, with a one-sided type I error rate of 10%.
RESULTS
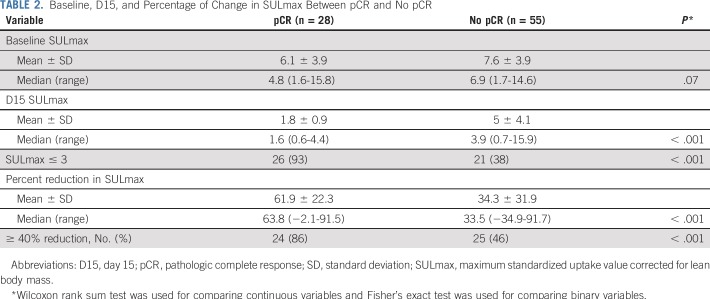
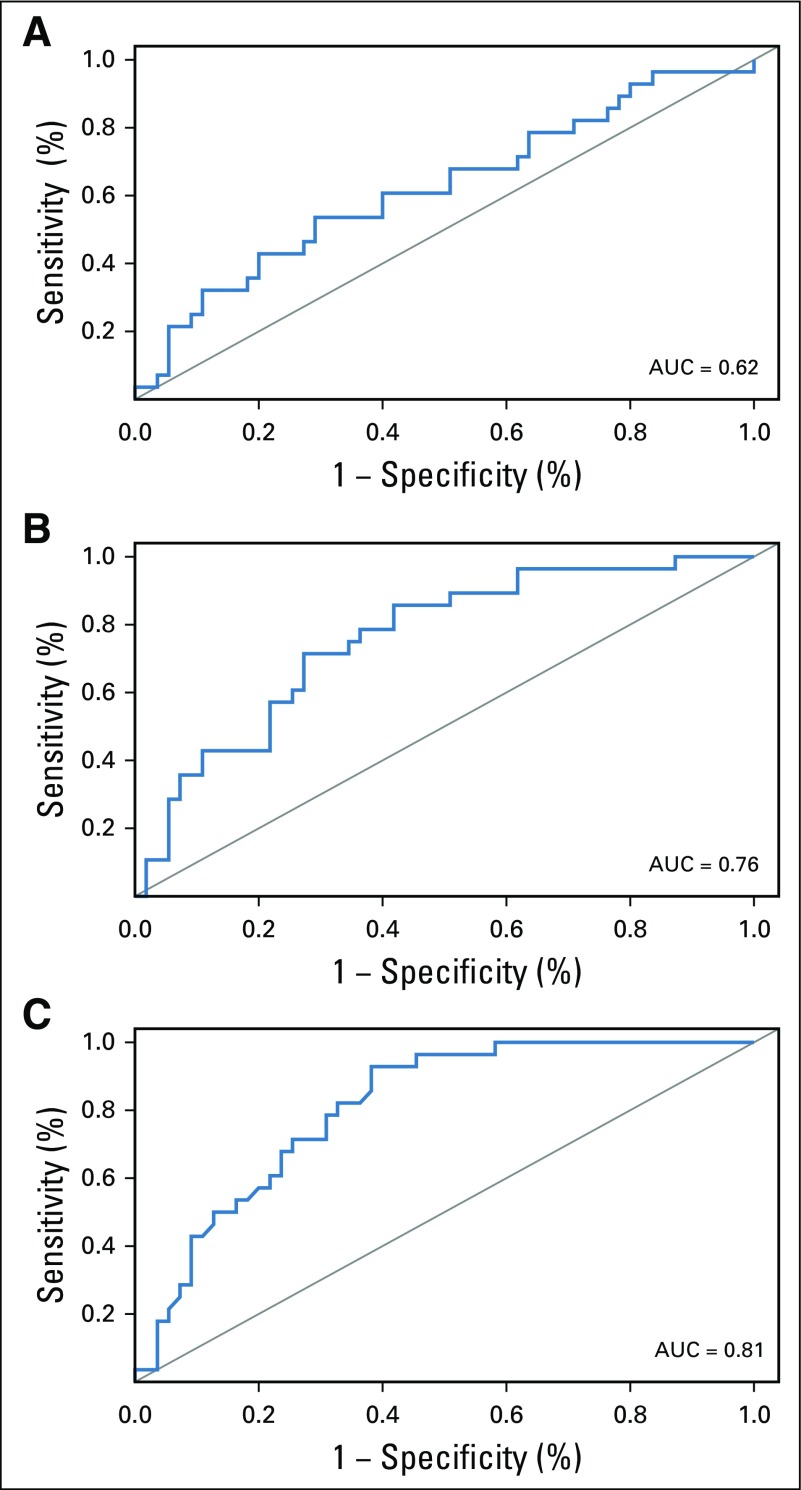
Eighty-eight women were enrolled (83 evaluable), and 85% (75 of 88) completed all four cycles of PT. pCR after PT alone was 34%. Receiver operating characteristic analysis yielded an area under the curve of 0.76 (90% CI, 0.67 to 0.85), which rejected the null hypothesis. Between patients who obtained pCR versus not, a significant difference in median percent reduction in SULmax by C1D15 was observed (63.8% v 33.5%; P < .001), an SULmax reduction greater than or equal to 40% was more prevalent (86% v 46%; P < .001; negative predictive value, 88%; positive predictive value, 49%), and a significant difference in median C1D15 SULmax (1.6 v 3.9; P < .001) and higher proportion of C1D15 SULmax less than or equal to 3 (93% v 38%; P < .001; negative predictive value, 94%; positive predictive value, 55%) were observed.
CONCLUSION
Early changes in SULmax predict response to four cycles of PT in estrogen receptor–negative, HER2-positive breast cancer. Once optimized, this quantitative imaging strategy may facilitate a more tailored approach to therapy in this setting.
INTRODUCTION
Dual human epidermal growth factor receptor 2 (HER2)–directed therapy with pertuzumab and trastuzumab (PT) in combination with chemotherapy is more effective than trastuzumab and chemotherapy for patients with HER2-positive breast cancer.1 The TRYPHAENA trials reported high pathologic complete response (pCR) rates with the dual approach, which led to the approval of neoadjuvant PT-based regimens in high-risk HER2-positive breast cancer.2,3 Indeed, pCR is an accepted primary end point in neoadjuvant clinical trials and can result in drug approval contingent on confirmatory results in the adjuvant setting.4 Data from several studies have suggested that a proportion of patients derive benefit from HER2-directed therapy alone and may not require chemotherapy. The NeoSphere and Adjuvant Dynamic Marker-Adjusted Personalized Therapy (ADAPT) trial investigators reported pCR rates of approximately 30% for patients with estrogen receptor (ER)–negative and HER2-positive breast cancer who received neoadjuvant PT without chemotherapy.2,5 Thus, the development of predictive biomarkers that can identify a subgroup of patients with HER2-positive breast cancer who may be treated with HER2-directed therapy alone and potentially spared chemotherapy is of clinical and scientific interest.
Changes in [18F]fluorodeoxyglucose (FDG) uptake on positron emission tomography (PET)/computed tomography (CT) as early as a few weeks after commencement of neoadjuvant therapy has promise as a predictive biomarker in early breast cancer.6,7 In the prospective Neoadjuvant Lapatinib and/or Trastuzumab Treatment Optimization (NeoALTTO) study, changes in standardized uptake value (SUV) on FDG-PET/CT were assessed as a predictor of response to neoadjuvant HER2-directed therapy.8 Patients were randomly assigned to a 6-week biologic window of HER2-directed therapy alone (lapatinib alone, trastuzumab alone, or lapatinib with trastuzumab) followed by the addition of chemotherapy before surgery.9 Metabolic changes in breast tumors were detected as early as 2 weeks after commencing HER2-directed therapy, and pCR rates were found to be twice as high in patients designated as responders versus nonresponders by FDG-PET/CT findings.10 Because chemotherapy was added after HER2-directed therapy in this trial, concluding that early changes in SUV would predict pCR to HER2-directed therapy alone is not possible.
We hypothesized that early changes in maximum SUV corrected for lean body mass (SULmax) on FDG-PET/CT would correlate with pCR in patients with ER-negative, HER2-positive breast cancer who receive neoadjuvant PT without chemotherapy. To test this hypothesis, we performed a multicenter phase II study in which women with stage II/III, ER-negative, and HER2-positive breast cancer received 12 weeks of neoadjuvant PT and incorporated serial FDG-PET/CT imaging and blood and tumor biopsy collection.
PATIENTS AND METHODS
Eligibility
Eligible women were 18 years of age or older with untreated histologically proven infiltrating carcinoma of the breast and clinical stage T2-4(a-c), any N, and M0 disease (American Joint Committee on Cancer 7th Edition staging). Tumors must have been ER less than or equal to 10% and HER2 positive11 by local pathology review. Eastern Cooperative Oncology Group performance status 0 to 1, left ventricular ejection fraction greater than or equal to 50%, and adequate organ function were required. Patients agreed to baseline and follow-up FDG-PET/CT and study-specific procedures and signed a written informed consent approved by the institutional review boards of the participating institutions.
Study Design
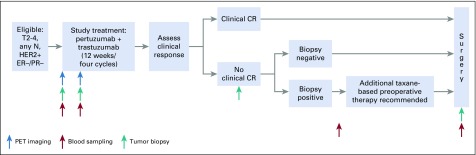
Participants received 12 weeks of neoadjuvant PT (Appendix Fig A1, online only). Pertuzumab (supplied by Genentech, South San Francisco, CA) was administered every 3 weeks (840 mg loading dose, then 420 mg) and trastuzumab every 3 weeks (8 mg/kg loading dose, then 6 mg/kg), both intravenously. Dose modification was not permitted.
FDG-PET/CT was performed before research tumor biopsies, at baseline, and at day 15 after commencement of therapy (C1D15). Tumor tissue was obtained from the surgical specimen on the day of surgery for research purposes. Plasma samples were obtained at baseline and C1D15, post-treatment/presurgery, and postsurgery (Appendix Fig A1). Common Terminology Criteria for Adverse Events (version 4.0) was used to grade toxicity.
Neoadjuvant HER2-directed therapy and chemotherapy given after study treatment with PT and before definitive surgery was allowed per physician discretion for incomplete response or disease progression (Appendix Fig A1). Taxane-based chemotherapy with PT was recommended. Tumor biopsy to confirm presence of residual disease histologically (ie, no pCR) was required before additional chemotherapy to ensure data available for primary end point. Axillary evaluation and type of breast surgery performed were per surgeon discretion. Postoperative systemic therapy and radiation per standard of care were recommended.
FDG-PET/CT
Participants underwent FDG-PET/CT at baseline and C1D15 (3-day window). Approval of FDG-PET/CT facilities included provision of study manual to sites, review of representative clinical scans, and phantom images. After a 60-minute uptake phase that followed intravenous FDG injection, a combined FDG-PET/CT scan was obtained from midskull to midfemur in general conformance with the Uniform Protocols for Imaging in Clinical Trials FDG-PET/CT and Radiological Society of North America Quantitative Imaging Biomarkers Alliance profiles.12 Scans were transmitted digitally, and central review and quantitation were performed by readers blinded to clinical data.6 Measurements were acquired by placing an all-encompassing spherical volume of interest over the target primary breast cancer tissue and recording SULmax, with care taken not to include adjacent normal FDG-avid tissue. SULmax was collected because it is more consistent than SUV in normal tissues from patient to patient, being less weight dependent.13 SULmax of primary breast cancer is reported in this analysis (primary end point).
Statistical Considerations
The primary objective was to correlate baseline and early percentage of change (by C1D15) in SULmax on FDG-PET/CT of the primary breast cancer with pCR after four cycles of neoadjuvant PT. pCR was defined as no viable invasive cancer in breast and axilla by local pathology review. All other patients were classified as non-pCR, including those with histologically confirmed residual disease after 12 weeks of PT or clinical progression on PT. An early stopping rule was in place to suspend or terminate the study if the proportion of clinical progression exceeded 10%.
To be evaluable for the primary analysis, both baseline and C1D15 FDG-PET/CT scans were performed, SULmax data collected, and pCR status after PT evaluated. The study was powered to test the null hypothesis that the area under the receiver operating characteristic (ROC) curve of percentage of change in SULmax at C1D15, a measure used to quantify the overall predictive power of the biomarker, is less than or equal to 0.65. Eighty evaluable patients with a projected pCR rate of 25% would have an 81% or greater power to detect a true area under the curve (AUC) of 0.80 against the null hypothesis (an AUC less than or equal to 0.65 at a one-sided type I error rate of 0.10). The AUC measures how well the biomarker discriminates between responders (those obtaining pCR) and nonresponders (those not obtaining pCR), with a greater AUC indicating higher diagnostic accuracy. The CIs for AUC were obtained by means of U-statistics theory.14 A sample size of up to 88 patients was planned to account for nonevaluable patients. Safety analysis included patients who received at least one dose of any study drug and was based on the frequency of adverse events (AEs).
Continuous variables were listed by means, standard deviations, and medians and ranges and were compared between patients who obtained pCR versus those who did not using Wilcoxon rank sum tests. Binary outcomes were listed by proportions and binomial exact CIs and compared using Fisher’s exact tests. ROCs with SULmax parameters as the predictor were generated with the goal of identifying the SULmax cut point that maximized the sum of sensitivity and specificity. We correlated baseline, C1D15, and percent reduction in SULmax with pCR using logistic regressions and evaluated positive predictive value (PPV) and negative predictive value (NPV) for predicting pCR with selected cutoffs.
All other statistical tests were two-sided and considered statistically significant at P < .05. The analyses were carried out using R version 3.4.2 software packages (http://www.R-project.org; R Foundation for Statistical Computing, Vienna, Austria). The research protocol and article were written by the authors and reviewed by the pharmaceutical funders; the funders had no access to the study database and were not involved in the study analysis or interpretation of results.
RESULTS
Patient Characteristics
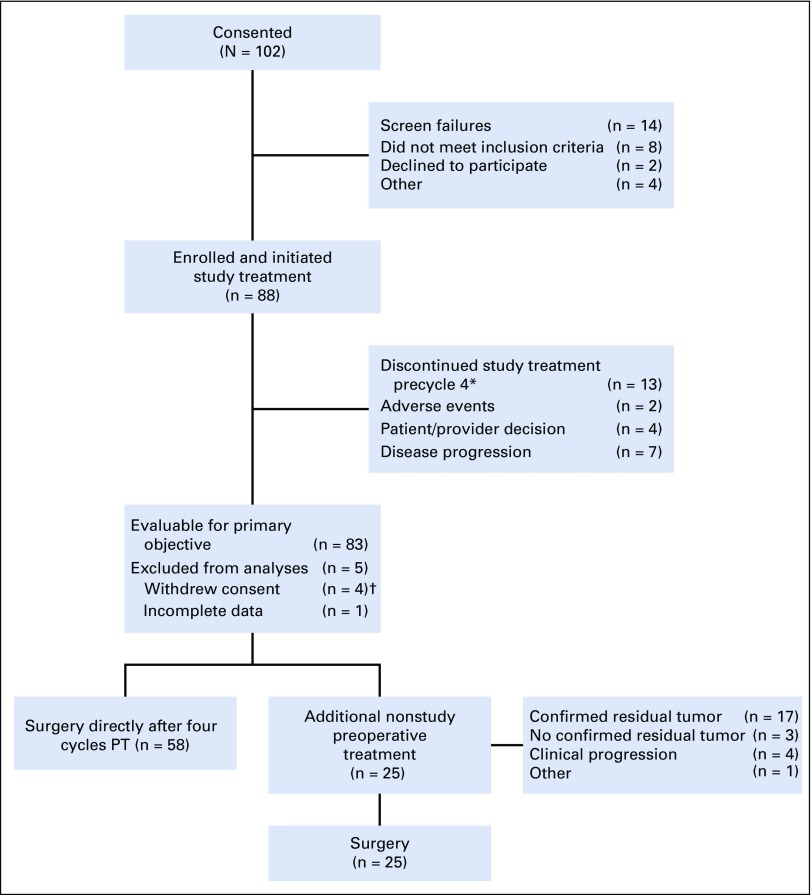
From January 2014 to August 2017, 88 women were enrolled in the study across nine sites. Median age was 58 years (range, 29 to 82 years), and median tumor size was 3.7 cm (range, 2 to 15 cm) by standard imaging (Table 1). Of these women, 83 were evaluable for the primary analysis (Fig 1). All four cycles of PT were completed in 85% of patients (75 of 88), and all 83 evaluable patients underwent the primary surgery. Twenty-five patients (28%) received neoadjuvant nonstudy therapy (Fig 1) and were classified as not obtaining pCR in the intention-to-treat (ITT) analysis. Of note, 22 of these 25 patients met the definition of histologically confirmed residual disease after 12 weeks of PT and/or clinical progression on study therapy. Additional nonstudy neoadjuvant therapy included taxane and carboplatin–based (n = 17) and taxane-based (n = 7) therapy with PT, and one patient received a fifth cycle of PT. Two patients also received anthracycline-based neoadjuvant chemotherapy.
TABLE 1.
Patient Characteristics
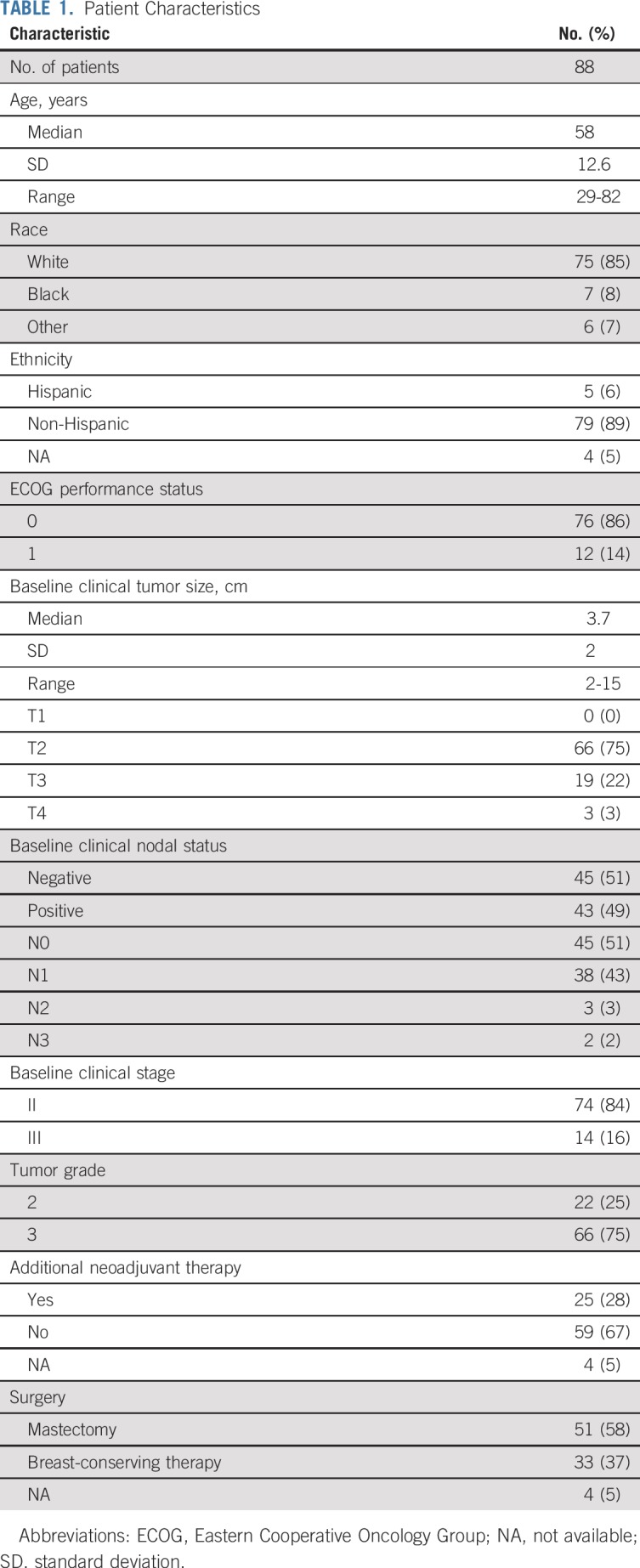
FIG 1.
Study flow diagram. Study treatment is pertuzumab and trastuzumab (PT). (*) All patients remained evaluable. (†) Reasons for withdrawal of consent were refusal of further participation after cycle 1, day 1 (n = 1); refusal of further participation after cycle 2, day 1 (n = 1); refusal of further participation after study treatment was completed (n = 1); and withdrawal of consent because of unacceptable toxicity (n = 1).
Treatment Safety and Efficacy
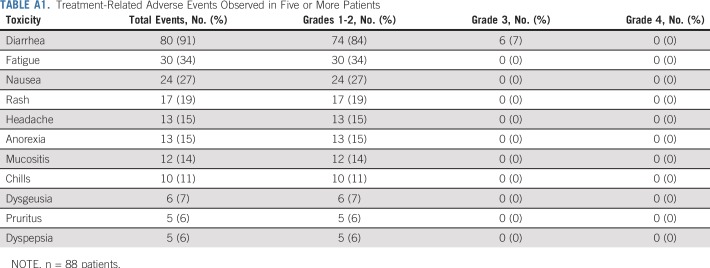
AEs were as expected for this regimen (Appendix Table A1, online only). Grade 3 AEs included diarrhea (8%), anaphylaxis (1%), and left ventricular systolic dysfunction (1%). pCR was observed in 34% patients after four cycles of PT alone (28 of 83 patients; 95% CI, 24% to 45%). The assumption that those who withdrew consent did not obtain pCR led to a conservative estimate of 32% for pCR (28 of 88 patients; 95% CI, 22% to 43%). Of the patients who had histologically confirmed residual disease after 12 weeks of PT or clinical progression and received additional neoadjuvant nonstudy therapy, 54% (12 of 22) had pCR at the time of surgery. Seven (8%) experienced clinical progression on study therapy after two to four cycles (median, three cycles) of PT, and the study did not meet the early stopping rule because the proportion of clinical progression did not exceed 10%.
Baseline and Change in Biomarkers and Correlation With Response to Therapy
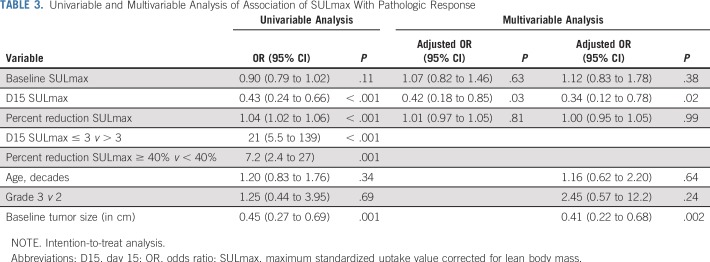
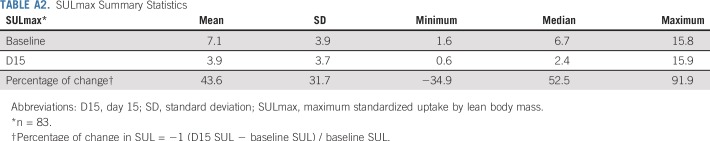
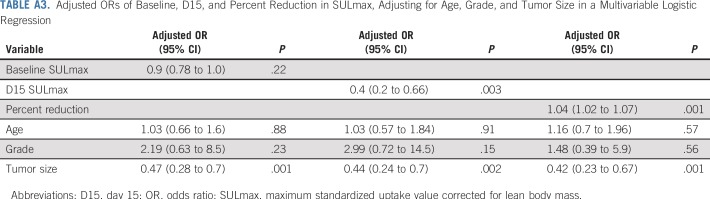
All 88 enrolled patients underwent baseline and C1D15 FDG-PET/CT. The identical scanner was used for baseline and C1D15 scan in 90% of patients, with representative images provided (Appendix Fig A2, online only). Mean injected FDG dose was 10.92 ± 2.3 mCi FDG. Mean uptake time was 64 ± 11.5 minutes. Four patients subsequently withdrew consent, and one patient had incomplete data, which preserved 83 evaluable patients for the ITT analysis. Summary statistics for baseline, C1D15, and percentage of change in SULmax are listed in Appendix Table A2 (online only). Baseline SULmax was not significantly associated with pCR (Table 2). ROC analysis of baseline SULmax yielded an AUC of 0.62 (90% CI, 0.51 to 0.73) for discriminating patients with and without pCR (Fig 2). Univariable logistic regression suggested that baseline SULmax is negatively associated with the odds of obtaining pCR, although the association did not reach statistical significance (Table 3).
TABLE 2.
Baseline, D15, and Percentage of Change in SULmax Between pCR and No pCR
FIG 2.
Receiver operating characteristic curves for (A) baseline and (B) percentage of change in maximum standardized uptake values corrected for lean body mass (SULmax) and (C) 15 days after pertuzumab and trastuzumab initiation (C1D15). AUC, area under the curve.
TABLE 3.
Univariable and Multivariable Analysis of Association of SULmax With Pathologic Response
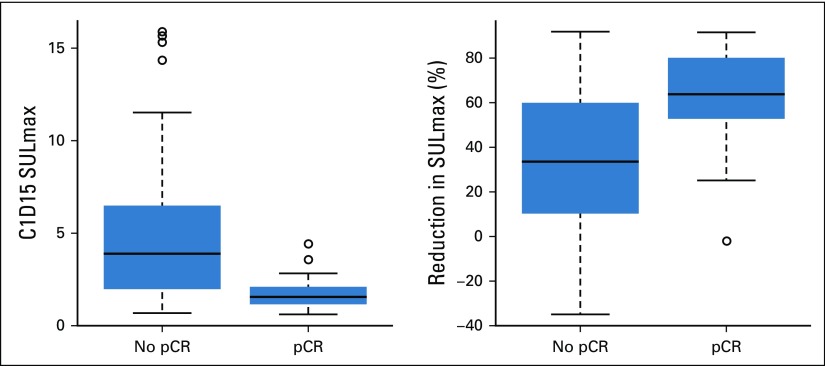
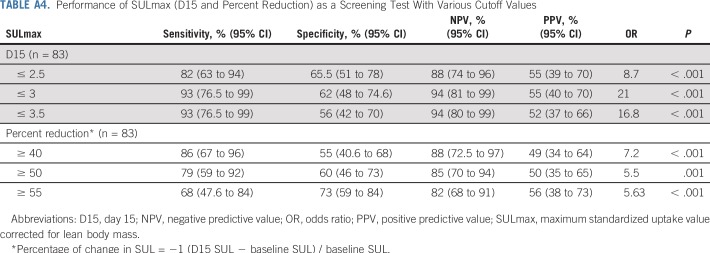
We observed a significant difference in median percent reduction in SULmax by C1D15 between patients who obtained pCR versus those who did not (63.8% v 33.5%; P < .001; Table 2; Fig 3), and the univariable logistic regression estimated an odds ratio (OR) of 1.04 (95% CI, 1.02 to 1.06) associated with each additional 1% reduction in SULmax (Table 3). Similar results were observed in multivariable logistic regression that also adjusted for age, grade, and tumor size (Appendix Table A3, online only). The ROC analysis, with percent reduction in SULmax as the predictor, yielded an AUC of 0.76 (90% CI, 0.67 to 0.85; P = .03) in the evaluable patients, which thus rejected the null hypothesis for the primary end point because the 90% CI excludes AUC less than or equal to 0.65 (Fig 2). The exploratory cutoff of a greater than or equal to 40% versus a less than 40% reduction in SULmax yielded a sensitivity of 86% and specificity of 55% for identifying patients who obtained pCR. Compared with other cut points examined, including the cutoff value of 54.9% determined on the basis of Youden’s index,15 the threshold of 40% is considered clinically optimal for its high NPV (88%) for pCR (ie, ability to predict lack of pCR; Appendix Table A4, online only).
FIG 3.
Box plots of 15 days after pertuzumab and trastuzumab initiation (C1D15) and percent reduction in maximum standardized uptake value corrected for lean body mass (SULmax) in patients with pathologic complete response (pCR) v no pCR. The horizontal line inside each box shows the median. The lower and upper hinges of each box represent the 25th and 75th percentiles, respectively. The circles represent actual values of C1D15 and percent reduction in SULmax.
A significant difference was observed in median C1D15 SULmax between patients who obtained pCR versus those who did not (1.6 v 3.9; P < .001; Table 2; Fig 3), and the univariable logistic regression estimated an OR of 0.43 (95% CI, 0.24 to 0.66) for a one-unit increase in C1D15 SULmax (Table 3). The association remained statistically significant after adjusting for age, grade, and tumor size (Appendix Table A3). The exploratory ROC analysis, with C1D15 SULmax as the predictor yielded an AUC of 0.81 (90% CI, 0.73 to 0.88; P < .001; Fig 2). The cutoff of less than or equal to 3 versus greater than 3 in C1D15 SULmax yielded a high NPV for pCR over other cut points and maximized the Youden’s index. A significantly higher proportion of C1D15 SULmax less than or equal to 3 was observed in patients who obtained pCR versus those who did not (93% v 38%; P < .001). NPV and positive predictive value for pCR associated with this retrospectively determined threshold were 94% and 55%, respectively.
Multivariable logistic regression that adjusted for baseline SULmax and percent reduction in SULmax revealed that C1D15 SULmax remained significantly predictive of pCR (adjusted OR, 0.42; 95% CI, 0.18 to 0.85; Table 3). The inclusion of age, grade, and tumor size in the regression model did not change the predictive value of the PET parameters (Appendix Table A3). Tumor stage was not included in the analysis because no patients with stage III disease obtained pCR.
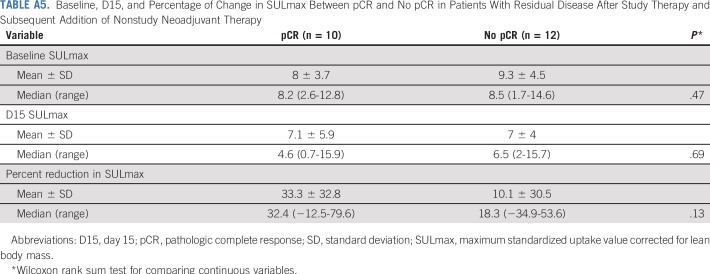
Among the 22 patients who received a subsequent addition of nonstudy neoadjuvant therapy (Fig 1), a predefined exploratory analysis was performed to correlate SULmax with pCR. Baseline, median percent reduction, and C1D15 SULmax were not significantly associated with pCR in this small patient population (Appendix Table A5, online only).
DISCUSSION
In the TBCRC026 trial, we investigated whether early changes in SULmax on FDG-PET/CT can predict pCR after HER2-directed therapy with PT alone (no chemotherapy) in ER-negative, HER2-positive early-stage breast cancer. In an ITT analysis, our study met its primary end point, which demonstrated that a percent reduction in SULmax by C1D15 and C1D15 SULmax were statistically significantly different between patients who obtained pCR after four cycles of PT versus those who did not. ROC analysis revealed a higher proportion of SULmax reduction of greater than or equal to 40% in those who obtained pCR with high sensitivity (86%) and NPV (88%). The exploratory analysis of C1D15 SULmax less than or equal to 3 may have an even greater sensitivity (93%) and NPV (94%) as a predictor of pCR. Because 56% of evaluable patients had a C1D15 SULmax less than or equal to 3, both biomarkers have the potential to be useful early response assessment tools.
Our results suggest that optimized FDG-PET/CT quantitative imaging-based biomarkers may facilitate intensification and de-intensification of neoadjuvant therapy. Because of a high NPV, reduction in SULmax on FDG-PET/CT during the first cycle of PT may predict most optimally those patients who will not achieve pCR with PT alone and should be recommended alternative regimens in future clinical trials. One can consider an analogy to our most important predictive biomarkers in breast cancer where ER- and HER2-negative phenotypes have a very high NPV for lack of benefit from targeted therapies.11,16
We previously reported that early change in SULmax on FDG-PET/CT by C1D15 after initiating neoadjuvant chemotherapy is successful in predicting pCR in patients with HER2-negative breast cancer.6 A limited number of studies have been performed in patients with HER2-positive breast cancer. In patients who receive trastuzumab emtansine for metastatic disease, a combination of results of pretreatment HER2-PET/CT with [89Zr]trastuzumab with those of an early FDG-PET/CT metabolic response assessment (baseline and before the second cycle of trastuzumab emtansine) showed promise in predicting response.17 The NeoALTTO substudy identified that metabolic changes detected on FDG-PET/CT in early-stage breast tumors 2 weeks after commencing HER2-directed therapy predict for pCR rates that are twofold higher in metabolic responders compared with nonresponders by imaging criteria.8 The TBCRC026 trial, however, aimed to identify an optimal SULmax cut point that could help to predict response to PT. This is in contrast to the NeoALTTO substudy that used a predefined cut point for response per European Organisation for Research and Treatment of Cancer imaging criteria at 2 weeks (greater than 15% reduction in SUV), which may not represent the most optimal cut point in the study population to predict response.10
PT in combination with chemotherapy is now a standard of care in the high-risk early breast cancer setting yet can be associated with serious toxicities.18 Investigators thus have attempted to de-escalate therapy. For example, 12 weeks of adjuvant paclitaxel and 1 year of trastuzumab were associated with high survival rates among women with small, node-negative HER2-positive breast cancer.19 Chemotherapy-free approaches also have been investigated using dual HER2-directed therapy concurrent with endocrine therapy for those with ER-positive cancers.5,20,21 Pertuzumab- or lapatinib-based approaches demonstrate similar pCR rates to the 34% rate we observed with neoadjuvant PT alone in this ER-negative, HER2-positive subtype.5,20,21
Studies in the neoadjuvant setting are ideal for investigating promising predictors of sensitivity to HER2-directed therapy. In the PAMELA trial, investigators administered 18 weeks of neoadjuvant lapatinib and trastuzumab (and endocrine therapy if ER positive) in patients with HER2-positive early breast cancer. Stromal tumor-infiltrating lymphocytes and tumor cellularity at C1D15 were independently associated with pCR.22 The HER2-enriched intrinsic subtype (defined by PAM50) also was identified as a potential predictor of pCR.20 In a study that investigated 12 weeks of lapatinib and trastuzumab, TBCRC006 investigators reported that activation of the phosphoinositide 3-kinase pathway is associated with resistance to therapy.23 Approaches that use a combination of imaging-, blood-, and tissue-based biomarkers may provide the most optimal biomarker of response to HER2-directed therapy without chemotherapy.
Strengths of our study include its multicenter prospective design and inclusion of a homogenous group of patients with ER-negative, HER2-positive breast cancer. We chose the C1D15 time point for the second FDG-PET/CT scan to define as early as possible in the treatment course those patients who might require an alternative approach. The exploratory single C1D15 time point seems promising as a predictive biomarker that if confirmed, would indicate that baseline imaging may not be required. Limitations of our study include the identification of a baseline primary tumor SULmax value below the liver reference value (mean + 2 × standardized deviation [n = 4 patients]), which may affect accurate measurement of percentage of change in SULmax.13 These patients were included in our analysis because they were eligible per protocol, but they may be excluded in future studies.
This approach to biomarker development that incorporates FDG-PET/CT is feasible across multiple sites, is less invasive than performing a tumor biopsy for biomarker evaluation, and has been shown to be useful in other tumor types.24,25 We identified percentage of change and C1D15 cutoffs for SULmax that best predict resistance to HER2-directed therapy with PT and will design prospective studies in which we will determine the clinical utility of altering therapy on the basis of early changes in SULmax. We also will investigate other imaging parameters, including those described in the PET Response Criteria in Solid Tumors version 1.0.13
In conclusion, the results have the potential to provide a more individualized approach to neoadjuvant therapy in women with stage II/III ER-negative, HER2-positive breast cancer. Such an approach could identify patients who may receive HER2-directed therapy alone and be spared chemotherapy as well as those who require an aggressive approach.
ACKNOWLEDGMENT
We thank Martin Lodge for imaging assistance during study initiation. We also thank Zhe Zhang, Susan Hilsenbeck, Stacie Jeter, and Bridget Walsh for valuable contributions during the study design and conduct.
Appendix
FIG A1.
TBCRC026 study schema. CR, complete response; ER, estrogen receptor; HER2, human epidermal growth factor receptor 2; PET, positron emission tomography; PR, progesterone receptor.
FIG A2.
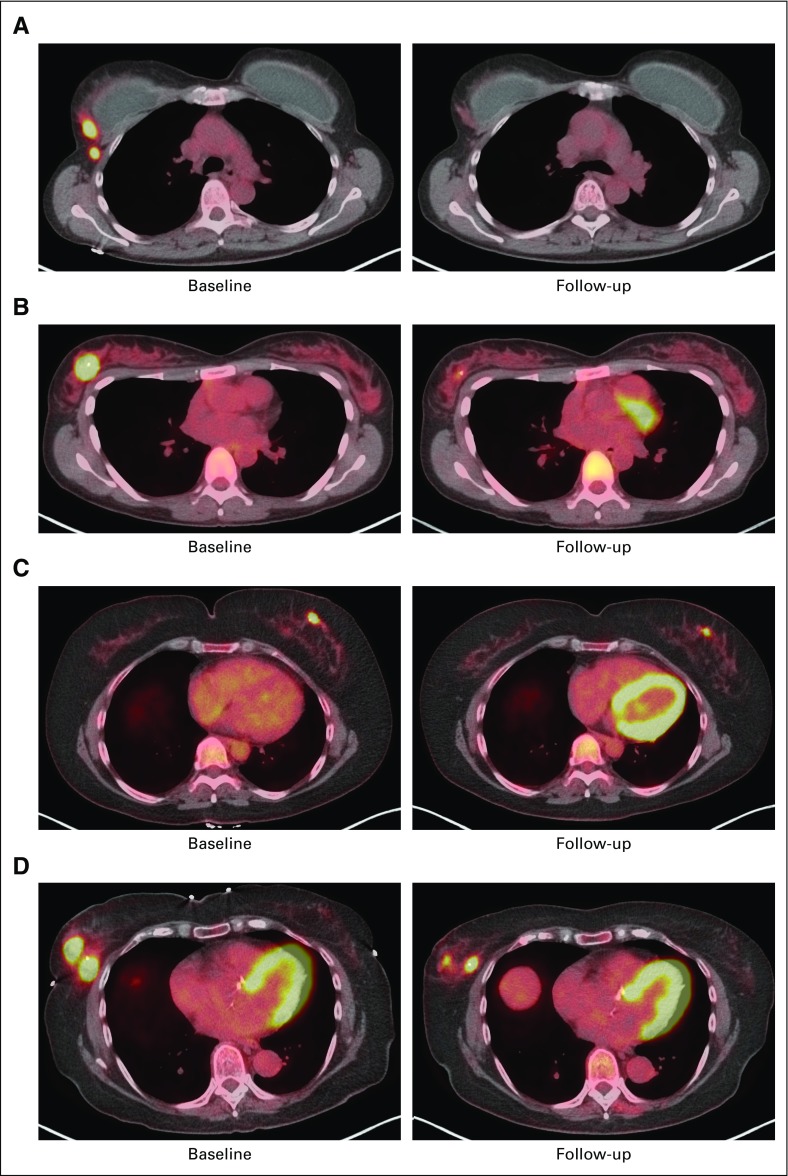
Sample [18F]fluorodeoxyglucose positron emission tomography images (baseline and day 15) in patients (A and B) with and (C and D) without pathologic complete response.
TABLE A1.
Treatment-Related Adverse Events Observed in Five or More Patients
TABLE A2.
SULmax Summary Statistics
TABLE A3.
Adjusted ORs of Baseline, D15, and Percent Reduction in SULmax, Adjusting for Age, Grade, and Tumor Size in a Multivariable Logistic Regression
TABLE A4.
Performance of SULmax (D15 and Percent Reduction) as a Screening Test With Various Cutoff Values
TABLE A5.
Baseline, D15, and Percentage of Change in SULmax Between pCR and No pCR in Patients With Residual Disease After Study Therapy and Subsequent Addition of Nonstudy Neoadjuvant Therapy
Footnotes
Presented at the 2018 American Society of Clinical Oncology Annual Conference, Chicago, IL, June 1-5, 2018.
Support for TBCRC026 and its partners (AVON Foundation, Breast Cancer Research Foundation, and Susan G. Komen for the Cure) by Sidney Kimmel Comprehensive Cancer Center Core grant P30-CA006973; National Cancer Institute Quantitative Imaging Network contract 5U01-CA140204; and Genentech, including supply of pertuzumab and trastuzumab. Grant funding was obtained from the ASCO Conquer Cancer Foundation Career Development Award (2013) and the AVON Center of Excellence.
Clinical trial information: NCT01937117.
AUTHOR CONTRIBUTIONS
Conception and design: Roisin M. Connolly, Mothaffar Rimawi, Ian E. Krop, Eric P. Winer, Antonio C. Wolff, Ben H. Park, Richard L. Wahl, Vered Stearns
Financial support: Roisin M. Connolly, Vered Stearns
Administrative support: Ashley Carpenter
Provision of study material or patients: Roisin M. Connolly, Lisa A. Carey, Minetta C. Liu, Anna Maria Storniolo, Vicente Valero, Christos Vaklavas, Melissa Camp, Antonio C. Wolff, Vered Stearns
Collection and assembly of data: Roisin M. Connolly, Lilja Solnes, Ashley Carpenter, Katy Gaffney, Vandana Abramson, Lisa A. Carey, Minetta C. Liu, Mothaffar Rimawi, Jennifer Specht, Anna Maria Storniolo, Vicente Valero, Christos Vaklavas, Melissa Camp, Robert S. Miller, Ashley Cimino-Mathews, Vered Stearns
Data analysis and interpretation: Roisin M. Connolly, Jeffrey P. Leal, Lilja Solnes, Chiung-Yu Huang, Mothaffar Rimawi, Anna Maria Storniolo, Vicente Valero, Antonio C. Wolff, Ashley Cimino-Mathews, Ben H. Park, Richard L. Wahl, Vered Stearns
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS’ DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST AND DATA AVAILABILITY STATEMENT
TBCRC026: Phase II Trial Correlating Standardized Uptake Value With Pathologic Complete Response to Pertuzumab and Trastuzumab in Breast Cancer
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/jco/site/ifc.
Roisin M. Connolly
Research Funding: Genentech (Inst), Novartis (Inst), Puma Biotechnology (Inst), Merck (Inst), Merrimack Pharmaceuticals (Inst), MacroGenics (Inst)
Travel, Accommodations, Expenses: Syndax Pharmaceuticals, Genentech
Lilja Solnes
Research Funding: Progenics Pharmaceuticals, Endocyte
Chiung-Yu Huang
Employment: University of California at San Francisco, Johns Hopkins University
Vandana Abramson
Consulting or Advisory Role: Novartis, Eisai
Research Funding: Genentech, Roche, Astellas Pharma
Lisa A. Carey
Research Funding: GlaxoSmithKline (Inst), Genentech (Inst), Roche (Inst)
Patents, Royalties, Other Intellectual Property: Royalty-sharing agreement, investorship interest in licensed intellectual property to startup company, Falcon Therapeutics, that is designing neural stem-cell–based therapy for glioblastoma multiforme (I)
Minetta C. Liu
Research Funding: Eisai (Inst), Seattle Genetics (Inst), Novartis (Inst), Roche (Inst), Genentech (Inst), GRAIL (Inst), Merck (Inst), Janssen Pharmaceuticals (Inst), Tesaro (Inst)
Travel, Accommodations, Expenses: GRAIL, Merck, Celgene, Agena Bioscience, Menarini Silicon Biosystems, Cynvenio Biosystems, Pfizer
Mothaffar Rimawi
Consulting or Advisory Role: Genentech, Novartis, MacroGenics, Daiichi Sankyo
Research Funding: Pfizer (Inst)
Jennifer Specht
Consulting or Advisory Role: Nektar, Genomic Health
Research Funding: Pfizer (Inst), Genentech (Inst), Roche (Inst), Celgene (Inst), Nektar (Inst), Seattle Genetics (Inst), Merck (Inst), Celldex (Inst), AbbVie (Inst), Juno Therapeutics (Inst), Novartis (Inst), Myriad Pharmaceuticals (Inst), Cascadian Therapeutics (Inst)
Travel, Accommodations, Expenses: Nektar
Anna Maria Storniolo
Employment: Boston Pharmaceuticals (I)
Stock or Other Ownership: Amgen, AbbVie, Merck, Flexion Therapeutics, Juno Therapeutics, Intellia Therapeutics, Editas Medicine, Eli Lilly (I)
Consulting or Advisory Role: Eli Lilly
Research Funding: Pfizer (Inst), AstraZeneca (Inst), MedImmune (Inst)
Vicente Valero
Honoraria: Roche, Genentech
Consulting or Advisory Role: Roche, Genentech
Travel, Accommodations, Expenses: Roche, Genentech
Christos Vaklavas
Research Funding: Roche (Inst), Genentech (Inst), Incyte (Inst), Pharmacyclics (Inst), Pfizer (Inst), TRACON Pharmaceuticals (Inst), Zymeworks (Inst), Novartis (Inst), H3 Biomedicine (Inst)
Ian E. Krop
Employment: AMAG Pharmaceuticals (I)
Leadership: AMAG Pharmaceuticals (I)
Stock or Other Ownership: AMAG Pharmaceuticals (I)
Honoraria: Roche, Genentech
Consulting or Advisory Role: Roche, Genentech, MacroGenics, Taiho Pharmaceutical, Seattle Genetics, Context Therapeutics
Research Funding: Roche, Genentech
Eric P. Winer
Stock or Other Ownership: Verastem
Honoraria: Genentech, Leap Therapeutics, InfiniteMD, Eli Lilly, Carrick Therapeutics, GlaxoSmithKline
Antonio C. Wolff
Research Funding: Pfizer (Inst), BioMarin (Inst), Celldex (Inst)
Patents, Royalties, Other Intellectual Property: Named as inventor on one or more issued patents or pending patent applications related to methylation in breast cancer and assigned his rights to Johns Hopkins University and participates in a royalty-sharing agreement with Johns Hopkins University
Ashley Cimino-Mathews
Research Funding: Bristol-Myers Squibb
Ben H. Park
Leadership: Loxo Oncology
Stock or Other Ownership: Loxo Oncology
Consulting or Advisory Role: Horizon Discovery, Foundation Medicine, Loxo Oncology
Research Funding: Foundation Medicine
Richard L. Wahl
Stock or Other Ownership: Endocyte, Progenics Pharmaceuticals
Honoraria: Actinium Pharmaceuticals
Consulting or Advisory Role: Nihon Medi-Physics, Clarity Pharmacies
Travel, Accommodations, Expenses: Actinium Pharmaceuticals
Vered Stearns
Consulting or Advisory Role: Iridium Therapeutics
Research Funding: AbbVie (Inst), Merck (Inst), Pfizer (Inst), MedImmune (Inst), Novartis, (Inst), Celgene (Inst), Puma Biotechnology (Inst), Biocept (Inst)
Other Relationship: Immunomedics
No other potential conflicts of interest were reported.
REFERENCES
- 1.Baselga J, Cortés J, Kim SB, et al. : Pertuzumab plus trastuzumab plus docetaxel for metastatic breast cancer. N Engl J Med 366:109-119, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gianni L, Pienkowski T, Im YH, et al. : Efficacy and safety of neoadjuvant pertuzumab and trastuzumab in women with locally advanced, inflammatory, or early HER2-positive breast cancer (NeoSphere): A randomised multicentre, open-label, phase 2 trial. Lancet Oncol 13:25-32, 2012 [DOI] [PubMed] [Google Scholar]
- 3.Schneeweiss A, Chia S, Hickish T, et al. : Pertuzumab plus trastuzumab in combination with standard neoadjuvant anthracycline-containing and anthracycline-free chemotherapy regimens in patients with HER2-positive early breast cancer: A randomized phase II cardiac safety study (TRYPHAENA). Ann Oncol 24:2278-2284, 2013 [DOI] [PubMed] [Google Scholar]
- 4.Prowell TM, Pazdur R: Pathological complete response and accelerated drug approval in early breast cancer. N Engl J Med 366:2438-2441, 2012 [DOI] [PubMed] [Google Scholar]
- 5.Nitz UA, Gluz O, Christgen M, et al. : De-escalation strategies in HER2-positive early breast cancer (EBC): Final analysis of the WSG-ADAPT HER2+/HR- phase II trial: Efficacy, safety, and predictive markers for 12 weeks of neoadjuvant dual blockade with trastuzumab and pertuzumab ± weekly paclitaxel. Ann Oncol 28:2768-2772, 2017 [DOI] [PubMed] [Google Scholar]
- 6.Connolly RM, Leal JP, Goetz MP, et al. : TBCRC 008: Early change in 18F-FDG uptake on PET predicts response to preoperative systemic therapy in human epidermal growth factor receptor 2-negative primary operable breast cancer. J Nucl Med 56:31-37, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wahl RL, Zasadny K, Helvie M, et al. : Metabolic monitoring of breast cancer chemohormonotherapy using positron emission tomography: Initial evaluation. J Clin Oncol 11:2101-2111, 1993 [DOI] [PubMed] [Google Scholar]
- 8.Gebhart G, Gámez C, Holmes E, et al. : 18F-FDG PET/CT for early prediction of response to neoadjuvant lapatinib, trastuzumab, and their combination in HER2-positive breast cancer: Results from Neo-ALTTO. J Nucl Med 54:1862-1868, 2013 [DOI] [PubMed] [Google Scholar]
- 9. doi: 10.1016/S0140-6736(11)61847-3. Baselga J, Bradbury I, Eidtmann H, et al: Lapatinib with trastuzumab for HER2-positive early breast cancer (NeoALTTO): A randomised, open-label, multicentre, phase 3 trial. Lancet 379:633-640, 2012 [Erratum: Lancet 379:616, 2012] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Therasse P, Arbuck SG, Eisenhauer EA, et al. : New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst 92:205-216, 2000 [DOI] [PubMed] [Google Scholar]
- 11.Wolff AC, Hammond ME, Hicks DG, et al. : Recommendations for human epidermal growth factor receptor 2 testing in breast cancer: American Society of Clinical Oncology/College of American Pathologists clinical practice guideline update. J Clin Oncol 31:3997-4013, 2013 [DOI] [PubMed] [Google Scholar]
- 12.Graham MM, Wahl RL, Hoffman JM, et al. : Summary of the UPICT protocol for 18F-FDG PET/CT imaging in oncology clinical trials. J Nucl Med 56:955-961, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wahl RL, Jacene H, Kasamon Y, et al. : From RECIST to PERCIST: Evolving considerations for PET response criteria in solid tumors. J Nucl Med 50:122S-150S, 2009. (suppl 1) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.DeLong ER, DeLong DM, Clarke-Pearson DL: Comparing the areas under two or more correlated receiver operating characteristic curves: A nonparametric approach. Biometrics 44:837-845, 1988 [PubMed] [Google Scholar]
- 15.Youden WJ: Index for rating diagnostic tests. Cancer 3:32-35, 1950 [DOI] [PubMed] [Google Scholar]
- 16.Hammond ME, Hayes DF, Dowsett M, et al. : American Society of Clinical Oncology/College of American Pathologists guideline recommendations for immunohistochemical testing of estrogen and progesterone receptors in breast cancer. J Clin Oncol 28:2784-2795, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gebhart G, Lamberts LE, Wimana Z, et al. : Molecular imaging as a tool to investigate heterogeneity of advanced HER2-positive breast cancer and to predict patient outcome under trastuzumab emtansine (T-DM1): The ZEPHIR trial. Ann Oncol 27:619-624, 2016 [DOI] [PubMed] [Google Scholar]
- 18.Gao J, Swain SM: Pertuzumab for the treatment of breast cancer: A safety review. Expert Opin Drug Saf 15:853-863, 2016 [DOI] [PubMed] [Google Scholar]
- 19.Tolaney SM, Barry WT, Dang CT, et al. : Adjuvant paclitaxel and trastuzumab for node-negative, HER2-positive breast cancer. N Engl J Med 372:134-141, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Llombart-Cussac A, Cortés J, Paré L, et al. : HER2-enriched subtype as a predictor of pathological complete response following trastuzumab and lapatinib without chemotherapy in early-stage HER2-positive breast cancer (PAMELA): An open-label, single-group, multicentre, phase 2 trial. Lancet Oncol 18:545-554, 2017 [DOI] [PubMed] [Google Scholar]
- 21.Rimawi MF, Mayer IA, Forero A, et al. : Multicenter phase II study of neoadjuvant lapatinib and trastuzumab with hormonal therapy and without chemotherapy in patients with human epidermal growth factor receptor 2-overexpressing breast cancer: TBCRC 006. J Clin Oncol 31:1726-1731, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nuciforo P, Pascual T, Cortés J, et al. : A predictive model of pathologic response based on tumor cellularity and tumor-infiltrating lymphocytes (CelTIL) in HER2-positive breast cancer treated with chemo-free dual HER2 blockade. Ann Oncol 29:170-177, 2018 [DOI] [PubMed] [Google Scholar]
- 23.Rimawi MF, De Angelis C, Contreras A, et al. : Low PTEN levels and PIK3CA mutations predict resistance to neoadjuvant lapatinib and trastuzumab without chemotherapy in patients with HER2 over-expressing breast cancer. Breast Cancer Res Treat 167:731-740, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Engert A, Haverkamp H, Kobe C, et al. : Reduced-intensity chemotherapy and PET-guided radiotherapy in patients with advanced stage Hodgkin’s lymphoma (HD15 trial): A randomised, open-label, phase 3 non-inferiority trial. Lancet 379:1791-1799, 2012 [DOI] [PubMed] [Google Scholar]
- 25.Lordick F, Ott K, Krause BJ, et al. : PET to assess early metabolic response and to guide treatment of adenocarcinoma of the oesophagogastric junction: The MUNICON phase II trial. Lancet Oncol 8:797-805, 2007 [DOI] [PubMed] [Google Scholar]