Abstract
Theory of mind (ToM) refers to the ability to attribute mental states to others. Behavioral variant frontotemporal dementia (bvFTD) is a neurodegenerative disorder characterized by profound deficits in social cognition, including ToM. We investigate whether bvFTD affects intention attribution tendency while viewing abstract animations and whether this might represent a primary deficit. A sample of 15 bvFTD patients and 19 matched controls were assessed on cognition and performed an implicit ToM task. They were instructed to describe what they observed in movement patterns displayed by geometrical shapes (triangles). These movement patterns either represented animacy, goal-directed actions or manipulation of mental state (ToM). The responses were scored for both accuracy and intentionality attribution. Using Voxel-Based Morphometry, we investigated the structural neuroanatomy associated with intention attribution tendency. The behavioral results revealed deficits in the bvFTD group on intentionality attribution that were specific for the ToM condition after controlling for global cognitive functioning (MMSE-score), visual attention (TMT B-score), fluid intelligence (RCPMT-score) and confrontation naming (BNT-score). In the bvFTD sample, the intention attribution tendency on the ToM-condition was associated with grey matter volume of a cluster in the cerebellum, spanning the right Crus I, Crus II, VIIIb, IX, left VIIb, IX and vermal IX and X. The results reveal a specific, primary, implicit domain-general ToM deficit in bvFTD that cannot be explained by cognitive dysfunction. Furthermore, the findings point to a contribution of the cerebellum in the social-cognitive phenotype of bvFTD.
Keywords: Theory of mind, Frontotemporal dementia, Neuroanatomy, Mentalizing
Highlights
-
•
We show a reduction in intention attribution tendency to abstract shapes in bvFTD.
-
•
Cognitive or subordinate processes did not explain the reduction.
-
•
The reduction was associated with structural integrity of a cerebellar cluster.
1. Introduction
Behavioral variant frontotemporal dementia (bvFTD) is a neurodegenerative disorder characterized by profound deficits in social cognition, including Theory of mind (ToM). ToM is a central notion in social cognition and refers to the ability to attribute mental states to others (Premack and Woodruff, 1978). ToM deficits in bvFTD have been documented using false belief tasks, ToM cartoons and stories, faux pas comprehension and mental state attribution based on eye expression and movement patterns (Baez et al., 2017; Bora et al., 2015; Cerami et al., 2014; Couto et al., 2013; Dermody et al., 2016; Dodich et al., 2016; Henry et al., 2014; Lough et al., 2006; Ramanan et al., 2017; Shany-Ur et al., 2012; Synn et al., 2018; Torralva et al., 2007; Torralva et al., 2009). The ToM impairment in bvFTD is a frequent first clinical manifestation (Desmarais et al., 2018; Pardini et al., 2013) and appears to be more pronounced for more complex functions, such as faux pas comprehension, which may reflect inadequate use of social norms consistent with the clinical phenotype (Kumfor et al., 2017a).
In addition to deficits in ToM, bvFTD is also associated with impaired executive functioning. Considering the relatively substantial cognitive demand of some of the more complex ToM-tasks, it has been suggested that ToM deficits may be secondary to cognitive or executive deficits typically seen in bvFTD. The majority of ToM-studies in bvFTD did not control for cognitive abilities and there is evidence that ToM correlates with executive functioning in bvFTD (Baez et al., 2014; Gregory et al., 2002; Torralva et al., 2015). On the other hand, there is indirect evidence that ToM deficits can not be explained by global cognitive or executive dysfunction (Bertoux et al., 2016; Flanagan et al., 2018). A possible approach to reducing the impact of other cognitive processes on TOM function is the use of an implicit task. Surprisingly, implicit TOM tasks have seldom been performed in bvFTD studies, despite its relevance for this dysexecutive population. In the present study, we make use of an orthogonal task to investigate TOM in bvFTD.
Furthermore, ToM can be conceived as an umbrella term for multiple processes, comprising intentionality attribution (e.g. to movement patterns) or inferring mental state (e.g. from the eyes). It has been proposed that these processes rely on more basic subordinate functions like mimicry, understanding causality and tracking of intentions and goals (Prochazkova et al., 2018; Schaafsma et al., 2015). There is lots to be learned about the specificity of ToM deficits in bvFTD in relation to these subordinate processes. For instance, there is evidence that intentionality attribution is not restricted to animate objects, but also occurs towards movement patterns displayed by abstract shapes, such as triangles. This is in line with the notion that intentionality attribution constitutes a subordinate TOM function that is not conditional on the social category of a stimulus. In the present study, we investigate subordinate TOM functions in bvFTD to reveal whether the TOM deficit is restricted to higher order processes in which subordinate functions are integrated, or whether the TOM deficit in bvFTD is also present at the more basic level of subordinate functions.
At the neural level, ToM deficits in bvFTD have been associated with medial prefrontal, fronto-insular and anterior temporal regions (Baez et al., 2017; Couto et al., 2013; Dermody et al., 2016; Kumfor et al., 2017b; Synn et al., 2018). These results align with findings from functional brain imaging studies in normal subjects, which have consistently implicated these areas in ToM (Mar, 2011). An interesting exception however relates to the involvement of the cerebellum in bvFTD. There is evidence that the cerebellum is affected in bvFTD and associated with bvFTD symptoms (Cash et al., 2018; Chen et al., 2018; Seeley et al., 2008), including ToM deficits (Synn et al., 2018).
However, little is known about the specificity of brain-ToM associations in bvFTD, as no study has included any nuisance variable that controls for cognitive functioning or subordinate processes like understanding goal-directed actions (Van den Stock, 2018). Finally, mostly explicit measures of ToM have been used, either obtained from caregivers or from performance on tasks that use verbal stories or cartoon/picture depictions and explicit judgements of ToM functions like intention attribution.
In the present study, we tackled these issues and investigated the performance of a sample of bvFTD and controls on an implicit ToM task. We investigate whether bvFTD affects intentionality attribution tendency to abstract animations. We evaluate whether the propensity to ascribe thoughts and feelings to movement patterns is impaired in bvFTD and whether this is specific to movement patterns displaying mental state manipulation. Should this be the case, it would support the notion of a domain-general social cognitive dysfunction and shed new light on the social-cognitive profile of bvFTD. Furthermore, we investigate whether any deficits in intentionality attribution can be explained by deficits in cognitive functioning.
We also investigate the regional association of ToM deficits in bvFTD with structural neural integrity. Importantly, we focus on the association that is ToM-specific.
2. Material and method
The study was approved by the Ethical Committee of University Hospitals Leuven and all participants gave written informed consent.
2.1. Participants
A total of 15 patients fulfilling the revised diagnostic criteria for probable bvFTD (Rascovsky et al., 2011) were recruited. In all patients, disturbances in behaviour were the most prominent clinical feature. Patients were recruited via the Memory Clinic of University Hospitals Leuven (N = 3), the Geriatric Psychiatry Department of University Psychiatric Center KU Leuven (N = 7) and the Neurology Department of Onze-Lieve-Vrouw Hospital Aalst-Asse-Ninove, Belgium (N = 5). Diagnosis of bvFTD was based on clinical assessment, neuropsychological testing and patterns of atrophy on structural MRI scans. FDG-PET imaging was performed in ten patients. Visual rating of the structural and functional brain imaging revealed the expected variability in bvFTD anatomical subtypes (right temporal = 6; frontotemporal = 5; frontal = 3; temporal = 1), with a predominance of temporal atrophy (Cerami et al., 2016; Whitwell et al., 2009).
In addition, 19 healthy control subjects were recruited from our own database of volunteers. Exclusion criteria consisted of present or past neurological or psychiatric disorders (including substance abuse), major medical comorbidities and the use of medication that influences the central nervous system.
All subjects were right-handed, as assessed by the Edinburgh Handedness Inventory. Table 1 displays the demographic and clinical data of the participants. Group comparisons on the clinical measures displayed in Table 1 were not corrected for multiple comparisons to reduce the risk of Type 2 errors.
Table 1.
Demographic and clinical data.
| bvFTD (n = 15) | Controls (n = 19) | T (χ2) | p | ||
|---|---|---|---|---|---|
| Age (SD) | 67.3 (6.65) | 66.6 (6.45) | −0.286 | 0.784 | |
| Sex (M/F) | 9/6 | 10/9 | (0.185) | 0.667 | |
| Symptom onset (SD) | 2.1 (1.04) | ||||
| MMSE (/30) | 26.4 (1.60) | 29.3 (0.59) | 6.75a | <0.001* | |
| RAVLT# | A1-A5 (/75) | 30.6 (9.22) | 50.8 (7.73) | 6.63 | <0.001* |
| % recall | 60.8 (33.65) | 80.1 (18.11) | 1.89a | 0.078 | |
| Reco (/15) | 12.3 (3.38) | 14.1 (1.35) | 1.77a | 0.098 | |
| BNT (/60) | 39.3 (11.72) | 54.7 (2.49) | 4.99a | <0.001* | |
| AVFμ | 15.4 (6.01) | 22.6 (5.91) | 3.25 | 0.003* | |
| TMT | A (s)μ | 69.7 (52.26) | 33.1 (9.74) | −2.40a | 0.034* |
| B (s)£ | 186.8 (149.55) | 84.9 (36.72) | −2.22a | 0.049* | |
| RCPMT (/24)§ | 16.9 (4.30) | 20.7 (2.85) | 3.02 | 0.005* | |
| AAT (/120) | Compreh | 96.0 (10.90) | 109.3 (5.47) | 4.40 | <0.001* |
SD = standard deviation, M/F = ratio males/females; MMSE = Mini Mental State Examination; RAVLT = Rey's Auditory Verbal Learning test; Reco = recognition; BNT = Boston Naming Test; AVF = Animal Verbal Fluency; TMT = Trail Making Test; RCPMT = Raven's Colored Progressive Matrices Test; AAT = Aachen Aphasia Test; Compreh = comprehension; a = Equal variances not assumed; # = N(bvFTD) = 13; μ = N(bvFTD) = 12; £ = N(bvFTD) = 11; § = N(bvFTD) = 14); * = significant group difference (p < .05).
2.2. Procedures
2.2.1. Cognitive evaluation
All subjects underwent cognitive assessment including Mini-Mental State Examination (MMSE), Rey's Auditory Verbal Learning Test (AVLT), Boston Naming Test (BNT), Animal Verbal Fluency (AVF), Trail Making Test (TMT), Raven's Colored Progressive Matrices Test (RCPMT; series A and B) and Aachen Aphasia Battery (AAT; comprehension subtest).
2.2.2. Happé-Frith animation task
Abell and colleagues (Abell et al., 2000) designed a task for assessing spontaneous mentalizing based solely on the kinematic properties of abstract shapes (further termed here Happé-Frith Animation task; HFA). Movement patterns of two triangles were manipulated as to display either random purposeless movements (RD) (e.g., ‘floating in space’), goal directed movements (GD) where one triangle responded to the behavior of the other one (e.g., ‘following’) or ToM movements, in which one triangle manipulated the other one's mental state or behavior (e.g., ‘seducing’). These conditions are presumed to respectively trigger animacy perception, agency perception and spontaneous mentalizing. Subjects are merely instructed to verbally describe what they see, so the instructions contain no reference to mentalizing. Yet, when the responses are coded according to mental state attributions, these conditions reliably differ (Abell et al., 2000). As subjects are instructed to ‘describe what they see’, the HFA taps more into a tendency rather than an ability. A lower score on intentionality attribution does not imply that a subject is less able to attribute intentions, but reflects a reduced tendency to spontaneously attribute intentions. Indeed, the HFA evaluates proneness, rather than capacity. This implicit task is more sensitive to detect social-cognitive deficits compared to explicit tasks in clinical groups with mentalizing problems like autism spectrum disorders (ASD). ASD patients can make use of compensatory mechanisms like explicit knowledge of social norms or other strategies to perform well within the normal limits on explicit mentalizing tasks. Yet, they are impaired on implicit variants like the HFA (Frith, 2004; Schneider et al., 2013; Senju et al., 2009).
The materials and procedure have been described in detail elsewhere (Abell et al., 2000; Castelli et al., 2002; Castelli et al., 2000). In summary, each of twelve animations show two shapes (a big red triangle and a small blue triangle) moving around against a white background. Each animation lasts between 34 and 45 s and belongs to one of the following three movement patterns: random (RD), shapes bouncing off the walls like billiard balls, or merely drifting about; goal directed (GD), showing shapes either dancing, chasing, imitating, or one leading the other; and finally theory of mind (ToM), showing movements of two interacting shapes, in which the one triangle anticipates or manipulates the mental state of the other: either persuading, bluffing, mocking or surprising. The type of movement is different between conditions, but the basic visual characteristics in terms of shape, overall speed and orientation changes are similar. The 12 stimuli were presented in a fixed (across participants) randomized (across movement patterns) order on an LCD computer screen. After watching each animation, the participant was instructed to describe what he/she observed. This description was recorded with a digital voice recorder. Three independent raters who were blinded regarding group status of the participants subsequently scored the recorded audio clips on intentionality (0–5) and accuracy (0–2). The intentionality score represents the degree to which the participant attributes intentions to the triangles. A score of 0 was assigned when the participant describes an action without intentions and a score of 5 is assigned when the person describes a deliberate action with as explicit goal influencing the other's mental state, such as surprising or persuading. The accuracy score describes the extent to which the description fits the displayed action. A score of 0 reflects that the description did not match with the displayed action and a score of 2 means that the action was described adequately. For each stimulus, the raters received criteria for accurate descriptions as reported elsewhere (Bhatara et al., 2009). The animations have face validity and the scoring system can adequately distinguish the different description types. The task is suitable for measuring subtle differences (Abell et al., 2000).
2.2.3. Analysis
We first evaluated interrater agreement by computing intraclass correlation coefficients (ICC) for the three raters for the accuracy and intentionality scores separately. This was done once over the total score of the 12 items and three times over the total score of the 4 items of each of the movement patterns (RD, GD, ToM). All ICCs ranged between 0.82 and 0.92, indicating excellent interrater agreement (Cicchetti, 1994).
Parametric testing depended on the results of a Shapiro-Wilk test on the residuals of the respective analyses. To investigate group differences on single dependent variables, the residuals were computed following regression of group to this variable. When a normal distribution could be assumed, independent samples t-tests were used in which Levene's test for equality of variances was used and the degrees of freedom were adjusted accordingly if equality could not be assumed. When normality could not be assumed, Mann-Whitney U tests were performed.
Subsequently, we performed linear mixed-model analyses (LMM) with random intercept and group, movement pattern and group x movement pattern as fixed terms. To investigate the effect of cognitive deficits, we performed 4 additional similar LMMs, but each with one supplemental fixed term. Score on MMSE, TMT B, RCMPT and BNT were included as a fixed term to account respectively for global cognitive capacity, visual attention, fluid intelligence and confrontation naming capacity.
All these LMM were performed once on the accuracy and once on the intentionality score. For each LMM, Bonferroni-corrected post-hoc tests were performed on the estimated marginal means of all pairwise comparisons. Post-hoc tests on group x movement pattern interaction effects were performed one-tailed based on previously documented ToM deficits in bvFTD (Kumfor et al., 2017a).
To further investigate any association between intentionality attribution and cognitive functions, we performed a secondary analysis in the bvFTD group and calculated Pearson and Spearman correlation coefficients between the intentionality score on the ToM-condition and the cognitive control measures (MMSE, TMT B, RCPMT and BNT). To minimize the chance of Type II-errors, we did not apply any correction for multiple comparisons in the correlation analyses as this may conceal any association between ToM and cognition.
2.2.4. Brain imaging
All subjects were scanned on the same 3 T Philips Achieva system equipped with a 32-channel head coil. An MPRAGE sequence (TR = 9.6 ms; TE = 4.6 ms; matrix size = 256 × 256; 182 slices) was used to obtain a high-resolution T1-weighted anatomical image (voxel size = 0.98 × 0.98 × 1.22 mm3). Brain imaging data were analyzed using SPM 12 (Wellcome Trust Centre for Neuroimaging, UCL, London, United Kingdom) running under MATLAB R2016B.
T1-weighted structural images were reoriented to the ACPC-plane and centred on the anterior commissure. The CAT 12 toolbox (http://www.neuro.uni-jena.de/cat/) was used for pre-processing. The T1 images were normalized to MNI space and segmented into grey matter (GM), white matter (WM) and cerebrospinal fluid (CSF) using default settings. Following a quality check (sample homogeneity), GM images were smoothed using a kernel with 12 mm FWHM. The atrophic topography was investigated by comparing both groups by means of a 2-sample t-test.
Associations between intentionality attribution on the ToM condition and GM volume in bvFTD were investigated by means of multiple regression analyses with age, sex and total intracranial volume (TIV) as confound predictors. Importantly for the aim of the study to increase the specificity of the results, we also included performance on the RD and GD conditions as confound predictors, as well as MMSE-score. MMSE-score was selected as a confound over RCPMT, TMT B and BNT because it is a domain-general cognitive measure and it was available for all patients. The primary analysis thus included the covariates: intentionality score on the TOM, GD and RD conditions, age, sex, TIV and MMSE.
We also investigated whether the result of the primary analysis was robust with regard to collinearity and the relatively high number of regressors, Therefore, we performed a post-hoc multiple regression analysis, minimizing the control variables and only including intentionality score on the ToM and GD condition (as the most relevant behavioral control condition), MMSE and TIV as predictors.
We ran secondary analyses similar to the primary analysis, but with RCPMT, TMT B and BNT as confounding factors in the areas that showed a significant association between intentionality attribution and GM volume in the primary analysis. As behavioral performance may be dependent on the structural integrity of (presumably compensatory) areas outside the atrophic regions, we performed a whole-brain analysis, hence including the non-atrophic regions. In addition, we investigated whether the clusters resulting from the primary analysis were atrophic in the bvFTD group by inclusively masking them with the group difference in GM volume.
Finally, we investigated whether the significant clusters showing an association in the primary analysis in the bvFTD group could also be found for the total sample.
For the imaging analyses, the statistical threshold was set at voxel-level Pheight < 0.001 combined with cluster-level FWE-correction at Pheight < 0.05, k = 20 voxels for the primary analysis and at voxel-level Pheight < 0.001 for the post-hoc and secondary analyses.
3. Results
3.1. Patient characteristics
The clinical data suggest that the patient sample was in an early disease stage, with an average disease duration of about 2 years. Furthermore, there was no evidence for manifest deficits in global cognitive function, as evidenced by an average MMSE-score of 26.4. Nevertheless, the sample showed specific deficits for confrontation naming (BNT), verbal fluency (AVF), divided attention (TMT), abstract reasoning (RCPMT) and comprehension (AAT). This profile is consistent with the neuropsychological profile of the current diagnostic criteria, specifying deficits in executive tasks and relative sparing of episodic memory (Rascovsky et al., 2011).
3.2. Happé-Frith animation task
Shapiro-Wilk tests indicated normal distribution of the residuals of all intentionality and accuracy scores (all p's > 0.294).
3.2.1. Accuracy score
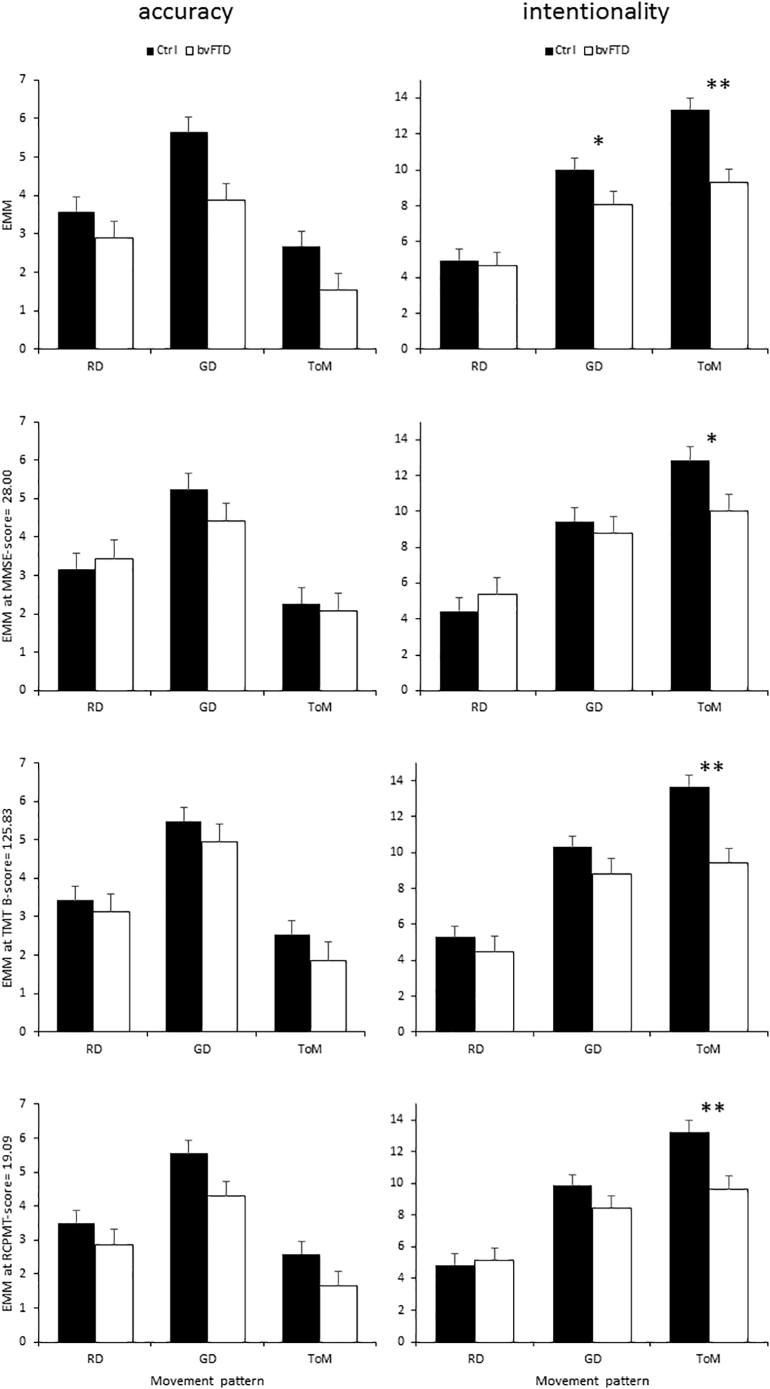
LMM on the accuracy score without any confound variable as a fixed term revealed a main effect of movement pattern (F(2, 64) = 22.632; p < .001) and group (F(1, 32) = 12.042, p = .002). Bonferroni-corrected post-hoc tests revealed significant differences between each pair of movement patterns (all p's < 0.018), with the highest ratings for goal directed movements, followed by random movement and finally ToM.
The four LMM's with respectively MMSE, TMT B, RCPMT and BNT as confound fixed term all revealed a main effect of movement pattern (all p's < 0.001) and the confound fixed term (all p's < 0.025), except for BNT (p = .264). All Bonferroni-corrected post-hoc tests revealed a similar pattern with significant differences between each pair of movement patterns (all p's < 0.033), where goal-directed movements showed the highest ratings, followed by random movement and ToM.
3.2.2. Intentionality score
LMM on the intentionality score without any confound variable as a fixed term revealed a significant effect of movement pattern (F(2,64) = 96.004, p < .001), group (F(1,32) = 6.537, p = .016) and group x movement pattern interaction (F(2,64) = 7.978, p = .001). Post-hoc tests revealed significant differences between each pair of movement patterns (all p's < 0.001), with the highest ratings for ToM, followed by GD and the lowest ratings for RD. The interaction effect reflected significant group differences on the GD (p = .028) and ToM-condition (p < .001) but not on RD (p = .390).
The three LMM's with a confound fixed term (MMSE, TMT B, RCPMT, BNT) all revealed a significant effect of movement pattern (all p's < 0.001) and group x movement pattern interaction (all p's < 0.002). The former always consisted of significant differences between all movement pattern pairs, where ToM had the highest ratings, followed by GD and RD. The interaction effect always reflected a group difference on ToM (all p's < 0.025), but not on RD (all p's > 0.233) nor GD (all p's > 0.086).
Furthermore, the LMM with TMT B as confound fixed term revealed a main effect of group (F(1,27) = 5.989, p = .021).
The results are displayed in Fig. 1.
Fig. 1.
Behavioral results. Accuracy (left) and intentionality (right) scores displayed as a function of movement pattern and confound terms: (top to bottom) no confound, MMSE, TMT B, RCPMT and BNT. Significant interaction effects are marked as * = p < .05; **p < .005. Ctrl: controls; EMM: Estimated Marginal Means. Error bars represent 1 SEM.
The correlation analyses revealed no significant parametric nor non-parametric association between the ToM intentionality score and any of the cognitive measures (all p's > 0.120).
3.3. Brain imaging
3.3.1. Group difference
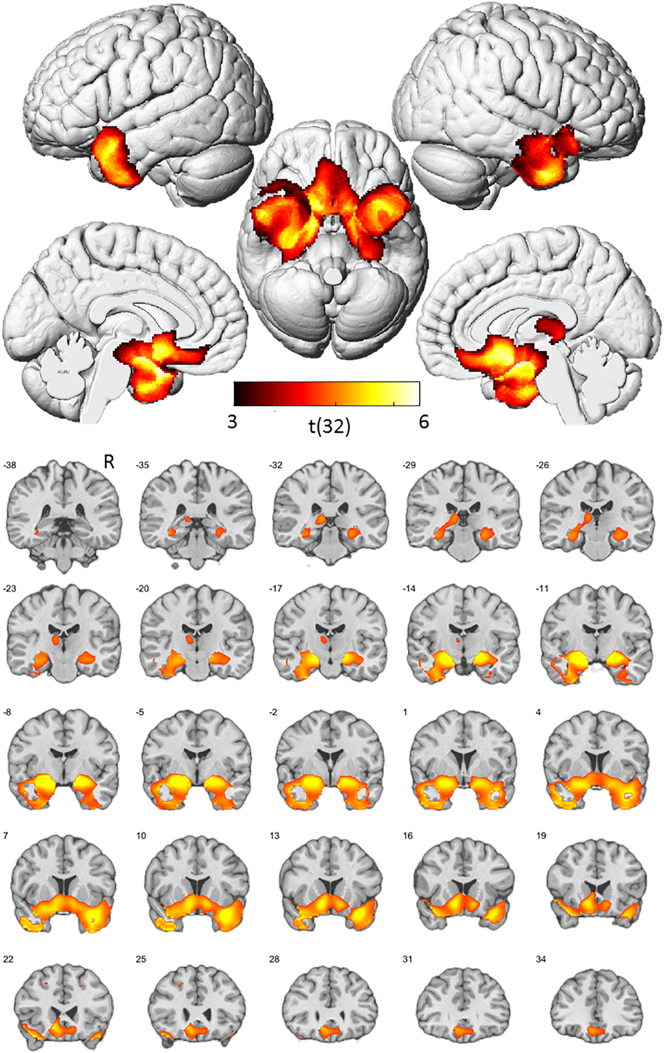
A two sample t-test on the GM volume maps revealed reduced GM volume in the patient group in anterior medio-temporal, insular and ventromedial prefrontal regions, consistent with the typical atrophic topography in bvFTD (Seeley et al., 2008; Whitwell et al., 2009) (see Fig. 2). While the pattern of brain atrophy and the clinical characteristics, such as deficits in confrontation naming (as revealed by decreased score on the BNT), may suggest that some patients fit the profile of semantic variant primary progressive aphasia (svPPA) (Gorno-Tempini et al., 2011), it should be noted that the most prominent clinical feature in all patients was behavioral disturbances, which is an exclusion criterion for svPPA.
Fig. 2.
Atrophic topography of bvFTD sample. Statistical map (p < .001, uncorrected at voxel-level, p < .05, FWE-corrected at cluster level) of group differences (controls>bvFTD) in grey matter volume, represented on rendered views (top) and coronal slices (bottom). Numbers refer to MNI Y-coordinates. Color coding refers to t-values. R = right hemisphere.
3.3.2. Brain-behavior association
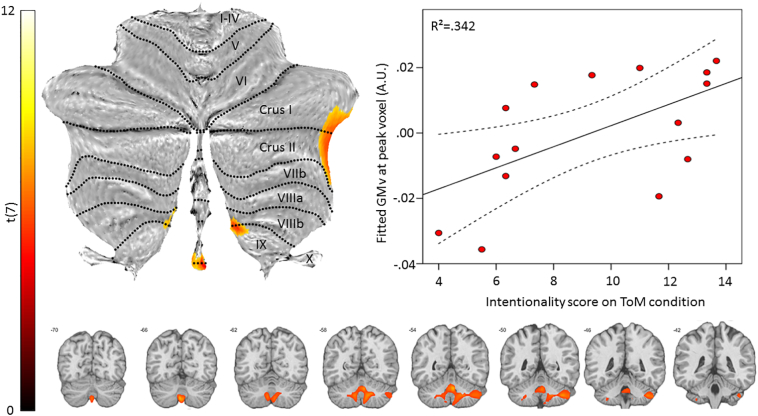
The results of the primary analysis revealed a single significant cluster, located in the cerebellum and covering the vermis and both hemispheres. The association was situated in the right lobules Crus I, Crus II, VIIIb, IX, left VIIb, IX and vermal IX and X (#voxels = 963; Peak MNI-coordinates = 5, −56, −30; Z = 4.47; T = 11.60; see Fig. 3).
Fig. 3.
Brain-behavior association. Statistical map displaying a significant association between intentionality attribution on the ToM condition and grey matter volume superimposed on a flatmap of the cerebellum (top left) and coronal slices (bottom). The top right scatterplot displays the data-points as a function of fitted grey matter volume (GMv) in the peak voxel and intentionality score on the ToM condition. The full line represents the fitted regression line and the dashed lines the 95% mean confidence range. A.U.: arbitrary units.
An orthogonality check revealed no collinearity between any of the variable pairs and the results of the post-hoc multiple regression analysis (with only intentionality score on teh ToM and GD condition, MMSE and TIV as predictors) were primarily localized in the cerebellum, overlapping (97 voxels) with the result of the primary analysis, including the peak result (MNI-coordinates = 6, −54, −30; Z = 3.78; T = 5.88).
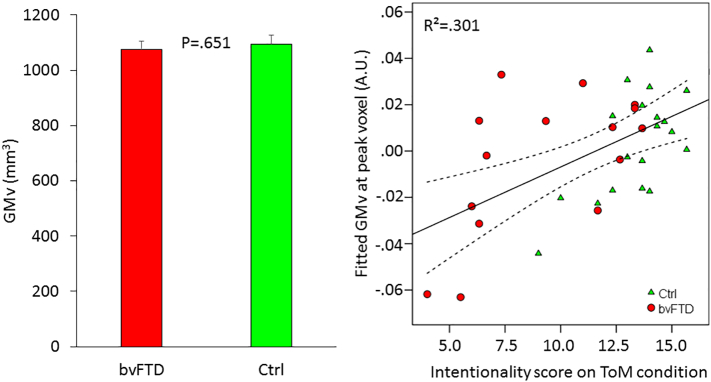
For every subject, we extracted the adjusted grey matter volume in this cluster and compared both groups by means of a 2-sample t-test. This did not reveal a significant result (t(32) = 0.457; p = .651; Fig. 4).
Fig. 4.
ROI analyses. The left bar chart displays the grey matter volume (GMv) in the ROI as a function of group. Error bars represent 1 SEM. The scatterplot displays the data-points from the total sample as a function of group, fitted grey matter volume (GMv) in the peak voxel and intentionality score on the ToM condition. The full line represents the fitted regression line and the dashed lines the 95% mean confidence range. A.U.: arbitrary units.
The secondary analyses with RCPMT, TMT B and BNT as confounds also revealed a significant association in this region, consisting of 491 (51.0%; Peak MNI-coordinates = 6, −56, −32; Z = 3.99; T = 9.81), 38 (3.9%; Peak MNI-coordinates = 5, −51, −33; Z = 3.82; T = 25.39) and 308 (32.0%; Peak MNI-coordinates = 6, −59, −51; Z = 3.45; T = 5.97) voxels respectively.
The analysis on the total sample revealed a significant association in a cluster of 16 voxels (1.7%; Peak MNI-coordinates = −5, −68, −44; Z = 3.05; T = 3.38; Fig. 4).
4. Discussion
The main finding is that the bvFTD sample shows a specific reduction in the tendency to attribute intentionality to movement patterns displaying mental state manipulation. This deficit is specific regarding other subordinate TOM processes (animacy and agency perception) as well as regarding cognitive measures. Furthermore, we did not find evidence for an association between cognitive measures and intentionality attribution in the context of ToM in bvFTD. These findings support the notion that the ToM-deficit in bvFTD is not restricted to high-level TOM function as revealed by previous studies, but deepens towards subordinate processess of intentionality attribution to movement patterns, but not to lower order processess such as animacy and agency perception. In line with this, the results can also not be explained by inaccurate perception of the stimuli, as there was no difference in the accuracy measure, underscoring the specificity of impaired intentionality attribution. The generic nature of the TOM deficit in bvFTD is further reflected in our findings at the implicit level, as the task instructions do not make any reference to ToM whatsoever and the stimuli consist of two basic geometrical shapes. This may explain the discrepancy with previous results reporting an association between ToM and executive functioning (Baez et al., 2014; Torralva et al., 2015). Explicit tasks typically rely on executive functions, at the very least to comply with the task instructions. An implicit task as the one in the present study will trigger more automatic ToM-processes which are less dependent on executive, global cognitive and language functions. Hence, the present result is in line with a primary ToM-deficit in bvFTD.
The second finding relates to the neural substrate of the deficit, which was localized in the cerebellum, covering mainly lobules Crus I, Crus II and IX. The association was also present in the total sample (bvFTD plus controls) in this region. The involvement of these areas in the HFA task has been reported in a previous fMRI-study in normal subjects. Perception of ToM-stimuli triggers more activation in Crus I, Crus II and IX compared to perception of GD-stimuli (Moessnang et al., 2016). There is increasing evidence supporting a role for the cerebellum in social cognitive functions like ToM in normal subjects, where Crus I and IX are proposed to be involved in event mentalizing (mentalizing about events that do not involve human body stimuli) and high-level abstractions in mentalizing (Van Overwalle et al., 2014). The present result adds to these findings and reveals a role for the cerebellum in implicit ToM-deficits in bvFTD. Furthermore, the respective cluster was not atrophic in the present sample. While several studies reported cerebellar atrophy in bvFTD (Chen et al., 2018; Rosen et al., 2002; Seeley et al., 2008), the present sample did not show pronounced reduction in grey matter volume in the cerebellum. This discrepancy may be related to disease stage. While cerebellar atrophy has been reported in non-early samples (i.e. with a mean symptom onset exceeding 3 years), the present sample was in an early stage, with symptom onset averaging at 2.1 years. Presumably, the method used is insufficiently sensitive to detect cerebellar degeneration at an early disease stage, which may be latent in the present sample.Contrary to previous studies, we did not find an association with structural integrity of the medio-frontal cortex, temporal pole and anterior insula. One explanation for the negative result in the temporal pole may relate to the marked atrophy in anterior temporal regions in the present sample. This presumably relates to a compliance selection bias and may result in a floor effect in grey matter volume in the temporal pole, with insufficient variability in the sample to detect any association with performance. There was also no significant association in the anterior insula. It has been proposed that the anterior insula operates as a central and afferent node of the salience network (Seeley et al., 2007) and that external input to the anterior insula is relayed via the semantic appraisal network, including the temporal pole and basolateral amygdala (Van den Stock and Kumfor, 2017; Zhou and Seeley, 2014). These latter areas are heavily affected in the present sample. This may relate to the negative upstream result in the anterior insula. A second explanation for the negative results in the hypothesized areas may relate to the implicity of the task. The vast majority of ToM-studies in bvFTD have used explicit tasks (Kumfor et al., 2017a) and these may rely more on semantic (associated with temporal pole) and interoceptive (associated with anterior insula) functions, compared to implicit ToM-tasks. Another explanation may relate to the stringent control conditions. We used animacy and goal directed action perception as well as cognitive measures as control conditions to isolate ToM-specific processess. Processess that underly the control conditions as well as the condition of interest are filtered out of the results, in line with the aim of the study.
Some limitations are inherent to the present study. The clinical phenotype of bvFTD challenges recruitement as a result of decreased compliance with study protocols. Therefore, the sample size in the present study is relatively small, yet in accordance with typical sample sizes in bvFTD studies. Secondly, we included several cognitive and particularly executive control variables. However, our set of confounds is not exhaustive and the inclusion of other specific tests for abstract reasoning, response inhibition/promotion and problem solving as confounds could result in different effects and provide more insight into the specificity of the TOM deficit. Furthermore, while the MMSE provides a measure of global cognitive functioning, other tests like the Frontal Assessment Battery may also be suitable to control for global frontal functioning. Finally, while the present results increase the knowledge on the TOM deficit in bvFTD, the clinical impact is limited and primarily related to neuropsychological diagnostics (Schroeter et al., 2018).
An interesting question for future studies relates to how intention attribution to abstract shapes in bvFTD differs from intention attribution to social stimuli and how this relates to clinical phenotype and disease progression. For instance, does a deficit at the subordinate level precede a deficit at a higher level, where multiple subordinate processes are integrated (bottom-up progression), or does the deficit in integration of subordinate processes precedes breakdown of specific subordinate processes (top-down progression). Previous studies in bvFTD are in line with a bottom-up progression (Jastorff et al., 2016; Kumfor et al., 2011; Van den Stock et al., 2017). At the neural level, it remains to be investigated whether the clear differences between the neural correlates of ToM deficits for abstract (cerebellum, as revealed by the present study) versus social stimuli (medial prefrontal, fronto-insular and anterior temporal regions (Baez et al., 2017; Couto et al., 2013; Dermody et al., 2016; Kumfor et al., 2017b; Synn et al., 2018)) derive from different neural substrates rather than from the inclusion of specific confound variables. Furthermore, against the background of deficits in emotion processing in bvFTD (Kumfor et al., 2017a), it would be interesting to relate intention attribution to emotion attribution and extend the investigation of structural with functional neural correlates, for instance using a task-based fMRI-paradigm. This may reveal how regional atrophy affects the functional neuro-anatomy of TOM processing in proximal and distant areas (De Winter et al., 2016).
In summary, the behavioral results reflect a specific reduction in implicit intention attribution to abstract ToM stimuli in bvFTD. The imaging results show a bvFTD sample with marked anterior temporal atrophy and a specific brain-behavior association in a non-atrophic cerebellar cluster. The findings indicate that the ToM-deficit in bvFTD is primary and associated with structural integrity of cerebellar lobules crus I, crus II and IX.
Declarations of interest
None.
Funding
This work was supported by the Sequoia-fund for research into ageing and mental health. JV is supported by a KU Leuven starting grant. LV holds an aspirant fellowship granted by Fonds Wetenschappelijk Onderzoek (FWO)- Vlaanderen [11Z8917N]. The funding sources had no role in study design, collection, analysis and interpretation of the data, writing of the report or decision to submit the article for publication.
References
- Abell F., Happé F., Frith U. Do triangles play tricks? Attribution of mental states to animated shapes in normal and abnormal development. Cogn. Dev. 2000;15(1):1–16. [Google Scholar]
- Baez S., Manes F., Huepe D., Torralva T., Fiorentino N., Richter F.…Ibanez A. Primary empathy deficits in frontotemporal dementia. Front. Aging Neurosci. 2014;6 doi: 10.3389/fnagi.2014.00262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baez S., Pinasco C., Roca M., Ferrari J., Couto B., Garcia-Cordero I.…Torralva T. Brain structural correlates of executive and social cognition profiles in behavioral variant frontotemporal dementia and elderly bipolar disorder. Neuropsychologia. 2017 doi: 10.1016/j.neuropsychologia.2017.02.012. [DOI] [PubMed] [Google Scholar]
- Bertoux M., O'Callaghan C., Dubois B., Hornberger M. In two minds: executive functioning versus theory of mind in behavioural variant frontotemporal dementia. J. Neurol. Neurosurg. Psychiatry. 2016;87(3):231–234. doi: 10.1136/jnnp-2015-311643. [DOI] [PubMed] [Google Scholar]
- Bhatara A.K., Quintin E.M., Heaton P., Fombonne E., Levitin D.J. The effect of music on social attribution in adolescents with autism spectrum disorders. Child Neuropsychol. 2009;15(4):375–396. doi: 10.1080/09297040802603653. [DOI] [PubMed] [Google Scholar]
- Bora E., Walterfang M., Velakoulis D. Theory of mind in behavioural-variant frontotemporal dementia and Alzheimer's disease: a meta-analysis. J. Neurol. Neurosurg. Psychiatry. 2015;86(7):714–719. doi: 10.1136/jnnp-2014-309445. [DOI] [PubMed] [Google Scholar]
- Cash D.M., Bocchetta M., Thomas D.L., Dick K.M., van Swieten J.C., Borroni B.…Genetic Ftd Initiative G. Patterns of gray matter atrophy in genetic frontotemporal dementia: results from the GENFI study. Neurobiol. Aging. 2018;62:191–196. doi: 10.1016/j.neurobiolaging.2017.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castelli F., Happe F., Frith U., Frith C. Movement and mind: a functional imaging study of perception and interpretation of complex intentional movement patterns. Neuroimage. 2000;12(3):314–325. doi: 10.1006/nimg.2000.0612. [DOI] [PubMed] [Google Scholar]
- Castelli F., Frith C., Happe F., Frith U. Autism, Asperger syndrome and brain mechanisms for the attribution of mental states to animated shapes. Brain. 2002;125(Pt 8):1839–1849. doi: 10.1093/brain/awf189. [DOI] [PubMed] [Google Scholar]
- Cerami C., Dodich A., Canessa N., Crespi C., Marcone A., Cortese F.…Cappa S.F. Neural correlates of empathic impairment in the behavioral variant of frontotemporal dementia. Alzheimers Dement. 2014;10(6):827–834. doi: 10.1016/j.jalz.2014.01.005. [DOI] [PubMed] [Google Scholar]
- Cerami C., Dodich A., Lettieri G., Iannaccone S., Magnani G., Marcone A.…Perani D. Different FDG-PET metabolic patterns at single-subject level in the behavioral variant of fronto-temporal dementia. Cortex. 2016;83:101–112. doi: 10.1016/j.cortex.2016.07.008. [DOI] [PubMed] [Google Scholar]
- Chen Y., Kumfor F., Landin-Romero R., Irish M., Hodges J.R., Piguet O. Cerebellar atrophy and its contribution to cognition in frontotemporal dementias. Ann. Neurol. 2018 doi: 10.1002/ana.25271. [DOI] [PubMed] [Google Scholar]
- Cicchetti D.V. Guidelines, criteria, and rules of thumb for evaluating normed and standardized assessment instruments in psychology. Psychol. Assess. 1994;6(4):284–290. [Google Scholar]
- Couto B., Manes F., Montanes P., Matallana D., Reyes P., Velasquez M.…Ibanez A. Structural neuroimaging of social cognition in progressive non-fluent aphasia and behavioral variant of frontotemporal dementia. Front. Hum. Neurosci. 2013;7 doi: 10.3389/fnhum.2013.00467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Winter F.L., Van den Stock J., de Gelder B., Peeters R., Jastorff J., Sunaert S.…Vandenbulcke M. Amygdala atrophy affects emotion-related activity in face-responsive regions in frontotemporal degeneration. Cortex. 2016;82:179–191. doi: 10.1016/j.cortex.2016.06.001. [DOI] [PubMed] [Google Scholar]
- Dermody N., Wong S., Ahmed R., Piguet O., Hodges J.R., Irish M. Uncovering the neural bases of cognitive and affective empathy deficits in Alzheimer's disease and the Behavioral-variant of Frontotemporal dementia. J. Alzheimers Dis. 2016;53(3):801–816. doi: 10.3233/JAD-160175. [DOI] [PubMed] [Google Scholar]
- Desmarais P., Lanctot K.L., Masellis M., Black S.E., Herrmann N. Social inappropriateness in neurodegenerative disorders. Int. Psychogeriatr. 2018;30(2):197–207. doi: 10.1017/S1041610217001260. [DOI] [PubMed] [Google Scholar]
- Dodich A., Cerami C., Crespi C., Canessa N., Lettieri G., Iannaccone S.…Cacioppo J.T. Differential impairment of cognitive and affective mentalizing abilities in neurodegenerative dementias: evidence from behavioral variant of frontotemporal dementia, Alzheimer's disease, and mild cognitive impairment. J. Alzheimers Dis. 2016;50(4):1011–1022. doi: 10.3233/JAD-150605. [DOI] [PubMed] [Google Scholar]
- Flanagan E.C., Lagarde J., Hahn V., Guichart-Gomez E., Sarazin M., Hornberger M., Bertoux M. Executive and social-cognitive determinants of environmental dependency syndrome in behavioral frontotemporal dementia. Neuropsychology. 2018;32(4):377–384. doi: 10.1037/neu0000433. [DOI] [PubMed] [Google Scholar]
- Frith U. Emanuel Miller lecture: confusions and controversies about Asperger syndrome. J. Child Psychol. Psychiatry. 2004;45(4):672–686. doi: 10.1111/j.1469-7610.2004.00262.x. [DOI] [PubMed] [Google Scholar]
- Gorno-Tempini M.L., Hillis A.E., Weintraub S., Kertesz A., Mendez M., Cappa S.F.…Grossman M. Classification of primary progressive aphasia and its variants. Neurology. 2011;76(11):1006–1014. doi: 10.1212/WNL.0b013e31821103e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gregory C., Lough S., Stone V., Erzinclioglu S., Martin L., Baron-Cohen S., Hodges J.R. Theory of mind in patients with frontal variant frontotemporal dementia and Alzheimer's disease: theoretical and practical implications. Brain. 2002;125:752–764. doi: 10.1093/brain/awf079. Pt 4. [DOI] [PubMed] [Google Scholar]
- Henry J.D., Phillips L.H., von Hippel C. A meta-analytic review of theory of mind difficulties in behavioural-variant frontotemporal dementia. Neuropsychologia. 2014;56:53–62. doi: 10.1016/j.neuropsychologia.2013.12.024. [DOI] [PubMed] [Google Scholar]
- Jastorff J., De Winter F.L., Van den Stock J., Vandenberghe R., Giese M.A., Vandenbulcke M. Functional dissociation between anterior temporal lobe and inferior frontal gyrus in the processing of dynamic body expressions: insights from behavioral variant frontotemporal dementia. Hum. Brain Mapp. 2016;37(12):4472–4486. doi: 10.1002/hbm.23322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumfor F., Miller L., Lah S., Hsieh S., Savage S., Hodges J.R., Piguet O. Are you really angry? The effect of intensity on facial emotion recognition in frontotemporal dementia. Soc. Neurosci. 2011;6(5–6):502–514. doi: 10.1080/17470919.2011.620779. [DOI] [PubMed] [Google Scholar]
- Kumfor F., Hazelton J.L., De Winter F.-L., de Langavant L.C., Van den Stock J. Clinical studies of social neuroscience: a lesion model approach. In: Ibáñez A., Sedeño L., García A.M., editors. Neuroscience and social science: the missing link. Springer International Publishing; Cham: 2017. pp. 255–296. [Google Scholar]
- Kumfor F., Honan C., McDonald S., Hazelton J.L., Hodges J.R., Piguet O. Assessing the "social brain" in dementia: applying TASIT-S. Cortex. 2017;93:166–177. doi: 10.1016/j.cortex.2017.05.022. [DOI] [PubMed] [Google Scholar]
- Lough S., Kipps C.M., Treise C., Watson P., Blair J.R., Hodges J.R. Social reasoning, emotion and empathy in frontotemporal dementia. Neuropsychologia. 2006;44(6):950–958. doi: 10.1016/j.neuropsychologia.2005.08.009. [DOI] [PubMed] [Google Scholar]
- Mar R.A. The neural bases of social cognition and story comprehension. Annu. Rev. Psychol. 2011;62:103–134. doi: 10.1146/annurev-psych-120709-145406. [DOI] [PubMed] [Google Scholar]
- Moessnang C., Schafer A., Bilek E., Roux P., Otto K., Baumeister S.…Tost H. Specificity, reliability and sensitivity of social brain responses during spontaneous mentalizing. Soc. Cogn. Affect. Neurosci. 2016;11(11):1687–1697. doi: 10.1093/scan/nsw098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pardini M., Emberti Gialloreti L., Mascolo M., Benassi F., Abate L., Guida S.…Cocito L. Isolated theory of mind deficits and risk for frontotemporal dementia: a longitudinal pilot study. J. Neurol. Neurosurg. Psychiatry. 2013;84(7):818–821. doi: 10.1136/jnnp-2012-303684. [DOI] [PubMed] [Google Scholar]
- Premack D.G., Woodruff G. Does the chimpanzee have a theory of mind? Behav. Brain Sci. 1978;1:515–526. [Google Scholar]
- Prochazkova E., Prochazkova L., Giffin M.R., Scholte H.S., De Dreu C.K.W., Kret M.E. Pupil mimicry promotes trust through the theory-of-mind network. Proc. Natl. Acad. Sci. U. S. A. 2018 doi: 10.1073/pnas.1803916115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramanan S., de Souza L.C., Moreau N., Sarazin M., Teixeira A.L., Allen Z.…Bertoux M. Determinants of theory of mind performance in Alzheimer's disease: a data-mining study. Cortex. 2017;88:8–18. doi: 10.1016/j.cortex.2016.11.014. [DOI] [PubMed] [Google Scholar]
- Rascovsky K., Hodges J.R., Knopman D., Mendez M.F., Kramer J.H., Neuhaus J.…Miller B.L. Sensitivity of revised diagnostic criteria for the behavioural variant of frontotemporal dementia. Brain. 2011;134:2456–2477. doi: 10.1093/brain/awr179. Pt 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosen H.J., Gorno-Tempini M.L., Goldman W.P., Perry R.J., Schuff N., Weiner M.…Miller B.L. Patterns of brain atrophy in frontotemporal dementia and semantic dementia. Neurology. 2002;58(2):198–208. doi: 10.1212/wnl.58.2.198. [DOI] [PubMed] [Google Scholar]
- Schaafsma S.M., Pfaff D.W., Spunt R.P., Adolphs R. Deconstructing and reconstructing theory of mind. Trends Cogn. Sci. 2015;19(2):65–72. doi: 10.1016/j.tics.2014.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider D., Slaughter V.P., Bayliss A.P., Dux P.E. A temporally sustained implicit theory of mind deficit in autism spectrum disorders. Cognition. 2013;129(2):410–417. doi: 10.1016/j.cognition.2013.08.004. [DOI] [PubMed] [Google Scholar]
- Schroeter M.L., Pawelke S., Bisenius S., Kynast J., Schuemberg K., Polyakova M.…Diehl-Schmid J. A modified reading the mind in the eyes test predicts behavioral variant frontotemporal dementia better than executive function tests. Front. Aging Neurosci. 2018;10 doi: 10.3389/fnagi.2018.00011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seeley W.W., Menon V., Schatzberg A.F., Keller J., Glover G.H., Kenna H.…Greicius M.D. Dissociable intrinsic connectivity networks for salience processing and executive control. J. Neurosci. 2007;27(9):2349–2356. doi: 10.1523/JNEUROSCI.5587-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seeley W.W., Crawford R., Rascovsky K., Kramer J.H., Weiner M., Miller B.L., Gorno-Tempini M.L. Frontal paralimbic network atrophy in very mild behavioral variant frontotemporal dementia. Arch. Neurol. 2008;65(2):249–255. doi: 10.1001/archneurol.2007.38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Senju A., Southgate V., White S., Frith U. Mindblind eyes: an absence of spontaneous theory of mind in Asperger syndrome. Science. 2009;325(5942):883–885. doi: 10.1126/science.1176170. [DOI] [PubMed] [Google Scholar]
- Shany-Ur T., Poorzand P., Grossman S.N., Growdon M.E., Jang J.Y., Ketelle R.S.…Rankin K.P. Comprehension of insincere communication in neurodegenerative disease: lies, sarcasm, and theory of mind. Cortex. 2012;48(10):1329–1341. doi: 10.1016/j.cortex.2011.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Synn A., Mothakunnel A., Kumfor F., Chen Y., Piguet O., Hodges J.R., Irish M. Mental states in moving shapes: distinct cortical and subcortical contributions to theory of mind impairments in dementia. J. Alzheimers Dis. 2018;61(2):521–535. doi: 10.3233/JAD-170809. [DOI] [PubMed] [Google Scholar]
- Torralva T., Kipps C.M., Hodges J.R., Clark L., Bekinschtein T., Roca M.…Manes F. The relationship between affective decision-making and theory of mind in the frontal variant of fronto-temporal dementia. Neuropsychologia. 2007;45(2):342–349. doi: 10.1016/j.neuropsychologia.2006.05.031. [DOI] [PubMed] [Google Scholar]
- Torralva T., Roca M., Gleichgerrcht E., Bekinschtein T., Manes F. A neuropsychological battery to detect specific executive and social cognitive impairments in early frontotemporal dementia. Brain. 2009;132:1299–1309. doi: 10.1093/brain/awp041. Pt 5. [DOI] [PubMed] [Google Scholar]
- Torralva T., Gleichgerrcht E., Torres Ardila M.J., Roca M., Manes F.F. Differential cognitive and affective theory of mind abilities at mild and moderate stages of Behavioral variant Frontotemporal dementia. Cogn. Behav. Neurol. 2015;28(2):63–70. doi: 10.1097/WNN.0000000000000053. [DOI] [PubMed] [Google Scholar]
- Van den Stock J. Interaction between identity and emotion versus visual basic object recognition deficits: a commentary on Biotti & Cook. Cortex. 2018;101:294–297. doi: 10.1016/j.cortex.2017.03.003. [DOI] [PubMed] [Google Scholar]
- Van den Stock J., Kumfor F. Behavioural-variant frontotemporal dementia: at the interface of interoception, emotion and social cognition? Cortex. 2017 doi: 10.1016/j.cortex.2017.08.013. (in press) [DOI] [PubMed] [Google Scholar]
- Van den Stock J., Stam D., De Winter F.L., Mantini D., Szmrecsanyi B., Van Laere K.…Vandenbulcke M. Moral processing deficit in behavioral variant frontotemporal dementia is associated with facial emotion recognition and brain changes in default mode and salience network areas. Brain Behav. 2017;7(12) doi: 10.1002/brb3.843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Overwalle F., Baetens K., Marien P., Vandekerckhove M. Social cognition and the cerebellum: a meta-analysis of over 350 fMRI studies. Neuroimage. 2014;86:554–572. doi: 10.1016/j.neuroimage.2013.09.033. [DOI] [PubMed] [Google Scholar]
- Whitwell J.L., Przybelski S.A., Weigand S.D., Ivnik R.J., Vemuri P., Gunter J.L.…Josephs K.A. Distinct anatomical subtypes of the behavioural variant of frontotemporal dementia: a cluster analysis study. Brain. 2009;132:2932–2946. doi: 10.1093/brain/awp232. Pt 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou J., Seeley W.W. Network dysfunction in Alzheimer's disease and frontotemporal dementia: implications for psychiatry. Biol. Psychiatry. 2014;75(7):565–573. doi: 10.1016/j.biopsych.2014.01.020. [DOI] [PubMed] [Google Scholar]