Abstract
Aims
The aim of this study was to determine whether patients with metal-on-metal (MoM) arthroplasties of the hip have an increased risk of cardiac failure compared with those with alternative types of arthroplasties (non-MoM).
Patients and Methods
A linkage study between the National Joint Registry, Hospital Episodes Statistics and records of the Office for National Statistics on deaths was undertaken. Patients who underwent elective total hip arthroplasty between January 2003 and December 2014 with no past history of cardiac failure were included and stratified as having either a MoM (n = 53 529) or a non-MoM (n = 482 247) arthroplasty. The primary outcome measure was the time to an admission to hospital for cardiac failure or death. Analysis was carried out using data from all patients and from those matched by propensity score.
Results
The risk of cardiac failure was lower in the MoM cohort compared with the non-MoM cohort (adjusted hazard ratio (aHR) 0.901; 95% confidence interval (CI) 0.853 to 0.953). The risk of cardiac failure was similar following matching (aHR 0.909; 95% CI 0.838 to 0.987) and the findings were consistent in subgroup analysis.
Conclusion
The risk of cardiac failure following total hip arthroplasty was not increased in those in whom MoM implants were used, compared with those in whom other types of prostheses were used, in the first seven years after surgery.
Cite this article: Bone Joint J 2018;100-B:20–7.
Keywords: Hip arthroplasty, Heart failure, Metal-on-metal, Prosthesis
Metal-on-metal (MoM) prostheses of the hip have been widely used,1 but are now known to have unacceptably high rates of failure.2-4 This has triggered regulatory alerts, device recalls and mandatory surveillance programs. The failure of these devices has led to many patients subsequently undergoing early revision.5-8 Corrosion and wear of MoM implants, which are composed of cobalt-chromium alloy, can result in the release of particulate debris and metal ions into the circulation. Alongside localized effects, MoM prostheses have been associated with systemic complications such as cardiotoxicity and mortality.9-14 Concerns have been recently raised about cobalt cardiomyopathy, particularly following the report of a three-fold increased risk in hospital admissions due to cardiac failure in a subgroup of men with MoM prostheses.15,16 The findings have, however, been inconsistent.13,17,18 The symptoms and signs of cardiac failure are common in patients undergoing total hip arthroplasty (THA) and any association between the type of prosthesis and cardiac failure presenting at a later date, and usually to a different clinician, could easily be overlooked.19
Record-linkage studies have been used to investigate the relationship between death due to cancer in patients who have undergone MoM THA, concluding that there is no increased risk.20-22 In this study, we tested the hypothesis that MoM THA was associated with increased risk of cardiac failure compared with other types of prostheses.
Patients and Methods
This was a retrospective cohort study using linked national data sets. The United Kingdom National Joint Registry (NJR), which is the largest arthroplasty registry in the world,23 was the primary source of data for this study. The submission of data to the registry has been mandatory for National Health Service (NHS) hospitals in England, Wales and Northern Ireland since 1 April 2011, with > 95% of primary procedures captured in 2015 and now with information from more than two million arthroplasties.23
Each record describes the demographics of the patient including age, gender, American Society of Anesthesiologists (ASA) grade,24 body mass index (BMI), the type of prosthesis including the bearing surface, size of the components and brand and surgical details. Corresponding data are generated routinely for NHS Hospital Episodes Statistics (HES),25 which specify the reasons for admission, procedures performed and length of stay. The Office for National Statistics (ONS)26 records death certification data on all deaths registered in England and Wales including the date and cause(s) of death.
Individual patient-level record linkage was carried out by NHS Digital (NHS, Leeds, United Kingdom), using the NHS number, date of birth, gender and postcode. Only patients consenting to inclusion in the NJR registry and to the use of their data for research purposes were included. Pseudo-anonymous data from the NJR were provided to the investigators for analysis. The study was undertaken in accordance with the Strengthening the Reporting of Observational studies in Epidemiology (STROBE) checklist.27
The inclusion criteria involved those who underwent elective, primary THA between 1 January 2003 and 31 December 2014 who had linked HES records. This provided a minimum period of six preoperative years in which to establish a record of cardiac failure at the time of THA and a minimum postoperative follow-up of six months. Patients with cardiac failure prior to or in the six months following THA, which would most likely have been attributable to pre-existing disease, were excluded from the overall analysis but included in sensitivity analyses. A step-wise process was used to remove records of insufficient quality and cases with duplicate or conflicting data. The exposure of interest was defined as the type of arthroplasty. The MoM cohort included patients with metal acetabular and femoral components which articulated directly; both resurfacing and stemmed arthroplasties were included. Exposure dated from the first primary arthroplasty. Patients with both a MoM and a non-MoM arthroplasty were excluded.
The primary outcome measure was the time to an episode of cardiac failure following arthroplasty. The secondary outcome measure was all causes of mortality. The time at risk for the outcome was measured from the day of the arthroplasty until the first hospital admission with cardiac failure, death or the end of the study period (March 2015). Cardiac failure was defined by the appropriate diagnostic codes following discharge from hospital or on a death certificate (see supplementary material). The demographics of the patients and details of the operation were extracted from NJR and comorbidities were extracted from HES data.
The study protocol was approved by the Research Subcommittee of the NJR and the Data Access Advisory Group of NHS Digital.
Statistical analysis
Cox proportional hazards models were used to estimate the hazard ratio (HR) for primary and secondary outcomes by group and were adjusted for potential confounding factors including age at the time of the operation, gender, a history of type 2 diabetes, hypertension, coronary heart disease and other indicators of comorbidity and frailty (Charlson comorbidity index,28 ASA grade and the number and length of hospital admissions in the five years prior to arthroplasty).
In order to control for the indications available in the data set, propensity-matched analyses (adjusted and unadjusted) were performed using one-to-one matching of patients undergoing MoM arthroplasty with those undergoing a non-MoM arthroplasty. The cohort was stratified by gender before propensity matching and matched by age, history of diabetes, heart disease and hypertension, Charlson index and ASA class. A caliper of 0.2 was used for the propensity score.
Further comparisons were performed for subgroups, including those at the highest risk of cardiac failure and those at the highest risk of implant failure,29 including those with an Articular Surface Replacement (ASR) XL Acetabular System (DePuy Orthopaedics, Warsaw, Indiana). BMI was available for approximately half of the patients (n = 278 757) and was included as a further covariate for this subset. Data processing was performed using Microsoft SQL Server 2012 (Redmond, Washington), and statistical analyses with SPSS Statistics version 22.0 (IBM, Armonk, New York). The proportional hazards assumption was tested using the correlation between survival and partial residuals.
Results
A total of 581 954 patients with a THA recorded in the NJR could be linked to HES and ONS data. Following data cleaning and the exclusion of emergency admissions, 550 589 (94.6%) THAs remained. Of these, 535 776 (97.3%) had no history of cardiac failure either before their arthroplasty or within the subsequent six months. This cohort was used in the main analysis (Fig. 1). A total of 53 529 patients (10.0%) had a MoM hip arthroplasty and were matched in the propensity analysis. Notably, the patients were younger (mean age: 58.6 years, sd 11.1) versus 69.2 years, sd 11.0). More were male (61.1% versus 38.1%) with fewer comorbidities, as measured by the prevalence of diabetes, coronary heart disease and hypertension, Charlson index and ASA grade (Table I). By design, propensity matching reduced these differences (Table I).
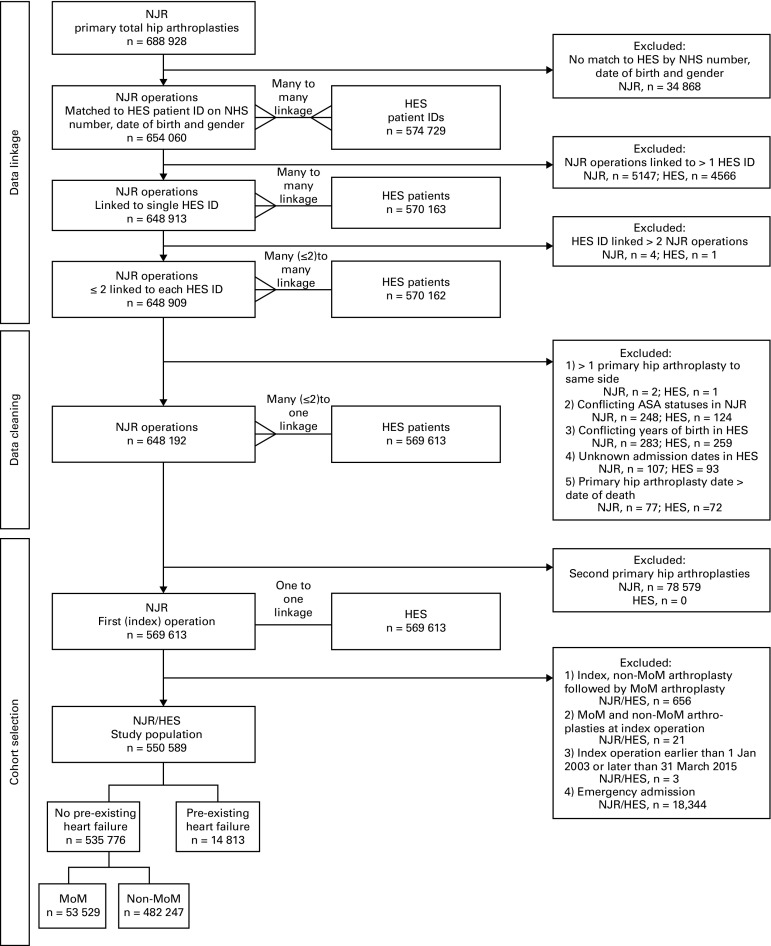
Fig. 1.
Flowchart showing data linkage, data cleaning and selection of the cohorts. NJR, National Joint Registry; NHS, National Health Service; HES, hospital episode statistics, MoM, metal-on-metal
Table I.
Baseline characteristics of patients with metal-on-metal and non-metal-on-metal arthroplasties: all patients and those matched by propensity score
| All patients | Propensity-matched patients | |||||
|---|---|---|---|---|---|---|
| MoM | Non-MoM | p-value | MoM | Non-MoM | p-value | |
| Number of patients | 53 529 | 482 247 | N/A | 53 529 | 53 529 | |
| Mean follow-up, yrs (sd) | 7.2 (2.3) | 4.6 (3.0) | < 0.001* | 7.2 (2.3) | 4.6 (3.1) | < 0.001* |
| Mean age, yrs (sd) | 58.6 (11.1) | 69.2 (11.0) | < 0.001* | 58.6 (11.1) | 58.6 (11.8) | 0.004* |
| Gender, n (%) | < 0.001† | 1† | ||||
| Male | 32 700 (61.1) | 183 867 (38.1) | 32 700 (61.1) | 32 700 (61.1) | ||
| Female | 20 829 (38.9) | 298 380 (61.9) | 20 829 (38.9) | 20 829 (38.9) | ||
| Mean body mass index, kg/m2 (sd) | 28.4 (5.2) | 28.5 (5.3) | 0.170* | 28.4 (5.2) | 28.7 (5.2) | < 0.001* |
| Prior type 2 diabetes, n (%) | 2034 (3.8) | 38 575 (8) | < 0.001† | 2034 (3.8) | 1922 (3.6) | 0.070† |
| Prior hypertension, n (%) | 11 171 (20.9) | 197 830 (41.0) | < 0.001† | 11 171 (20.9) | 10 436 (19.5) | < 0.001† |
| Prior coronary heart disease, n (%) | 2966 (5.5) | 53 239 (11) | < 0.001† | 2966 (5.5) | 2710 (5.1) | 0.001† |
| Mean Charlson Index (sd) | 0.2 (0.7) | 0.5 (1) | < 0.001* | 0.2 (0.7) | 0.2 (0.6) | < 0.001* |
| Prior hospitalisations | ||||||
| Mean total admissions (sd) | 1.4 (3.2) | 2.1 (7.6) | < 0.001* | 1.4 (3.2) | 1.4 (2.6) | < 0.001* |
| Mean hospitalized days (sd) | 4.4 (17.6) | 6.5 (20.3) | < 0.001* | 4.4 (17.6) | 4.3 (18.0) | < 0.001* |
| ASA, n (%) | < 0.001† | < 0.001† | ||||
| P1 - Fit and healthy | 20 892 (39) | 79 107 (16.4) | 20 892 (39.0) | 19 998 (37.4) | ||
| P2 - Mild disease not incapacitating | 29 158 (54.5) | 335 415 (69.6) | 29 158 (54.5) | 30 868 (57.7) | ||
| P3 - Incapacitating systemic disease | 3362 (6.3) | 65 896 (13.7) | 3362 (6.3) | 2621 (4.9) | ||
| P4 - Life threatening disease | 100 (0.2) | 1742 (0.4) | 100 (0.2) | 38 (0.1) | ||
| P5 - Expected to die within 24 hrs | 17 (0) | 87 (0) | 17 (0) | 4 (0) | ||
| Ethnicity, n (%) | < 0.001† | < 0.001† | ||||
| Asian | 213 (0.4) | 1531 (0.3) | 213 (0.4) | 330 (0.6) | ||
| Black | 399 (0.7) | 2229 (0.5) | 399 (0.7) | 461 (0.9) | ||
| Chinese | 10 (0) | 158 (0) | 10 (0) | 20 (0) | ||
| Mixed | 98 (0.2) | 638 (0.1) | 98 (0.2) | 137 (0.3) | ||
| Not recorded | 8686 (16.2) | 63 900 (13.3) | 8686 (16.2) | 7617 (14.2) | ||
| Other | 201 (0.4) | 1597 (0.3) | 201 (0.4) | 258 (0.5) | ||
| White | 40 391 (75.5) | 398 401 (82.6) | 40 391 (75.5) | 42 278 (79) | ||
*Student’s t-test
†chi-squared test
MoM, metal-on-metal; sd, standard deviation; ASA, American Society of Anesthesiologists
There were 1431 incidents of cardiac failure in the MoM cohort and 21 245 in the non-MoM cohort. The crude event rates were 3.8 and 9.7 per 1 000 person years’ exposure, respectively, with a crude relative rate of 0.389 (95% confidence interval (CI) 0.368 to 0.410, Table II). Following adjustment, the adjusted hazard ratio (aHR) for the MoM cohort relative to the non-MoM cohort was 0.901 (95% CI 0.853 to 0.953, Table III). In unadjusted analysis, the relative rate of all-cause mortality was 0.389 (95% CI 0.376 to 0.402). Following adjustment, the HR for all-cause mortality was 0.892 (95% CI 0.862 to 0.924).
Table II.
Incidents, the rate of events and the crude relative risk of heart failure and all-cause mortality for patients with metal-on-metal and non-metal-on-metal arthroplasties: all patients and those matched by propensity score
| MoM | Non-MoM | Crude relative risk (95% CI) | |||||
|---|---|---|---|---|---|---|---|
| Patients | Events | Rate* | Patients | Events | Rate* | ||
| All patients | |||||||
| Cardiac failure | 53 529 | 1431 | 3.8 | 482 247 | 21 245 | 9.7 | 0.389 (0.368 to 0.410) |
| Death | 53 529 | 3728 | 9.7 | 482 247 | 55 875 | 24.9 | 0.389 (0.376 to 0.402) |
| Propensity-matched patients | |||||||
| Cardiac failure | 53 529 | 1431 | 3.8 | 53 529 | 1004 | 4.1 | 0.917 (0.846 to 0.994) |
| Death | 53 529 | 3728 | 9.7 | 53 529 | 2776 | 11.2 | 0.864 (0.823 to 0.908) |
*rate per 1000 person years’ exposure
MoM, metal-on-metal; CI, confidence interval
Table III.
Adjusted hazard ratio of cardiac failure and all-cause mortality for patients with metal-on-metal and non-metal-on-metal arthroplasties: all patients and those matched by propensity score
| Adjusted hazard ratio (95% CI) | p-value* | |
|---|---|---|
| All patients | ||
| Cardiac failure | 0.901 (0.853 to 0.953) | < 0.001 |
| Death | 0.892 (0.862 to 0.924) | < 0.001 |
| Propensity-matched patients | ||
| Cardiac failure | 0.909 (0.838 to 0.987) | 0.023 |
| Death | 0.877 (0.835 to 0.922) | < 0.001 |
*Cox PH model
CI, confidence interval
In subgroup analyses, the aHR was below unity or did not achieve significance for all groups (Fig. 2. In the age-specific subgroup analysis, there was a trend towards an increasing rate of cardiac failure with increasing age, ranging from 0.600 (0.353 to 1.019) in the youngest cohort (≤ 44 years) to 1.15 (0.908 to 1.370) in the oldest (≥ 85 years).
Fig. 2.
Adjusted hazard ratio (aHR) of cardiac failure for patients with metal-on-metal (MoM) and non-MoM arthroplasties: all patients by subgroup. CI, confidence interval; CHD, coronary heart disease; BMI, body mass index; ASA, American Society of Anesthesiologists grade.
In the propensity-matched analysis, there were 1431 incidents of cardiac failure in the MoM cohort and 1004 in the non-MoM cohort. Due to the longer follow-up, the crude event rates were lower in the MoM cohort (3.8 events per 1000 person years versus 4.1 events per 1000 person years), with an unadjusted relative rate of 0.917 (95% CI 0.846 to 0.994, Table II). In adjusted analyses, the aHR was 0.909 (95% CI 0.838 to 0.987, Table III). For all-cause mortality, the aHR was 0.877 (95% CI 0.835 to 0.922, Table III). The subgroup analysis of the propensity-matched cohorts is illustrated in Figure 3. No subgroup had a significantly increased aHR.
Fig. 3.
Adjusted hazard ratio of cardiac failure for patients with metal-on-metal (MoM) and non-MoM arthroplasties: propensity-matched patients by subgroup. CI, confidence interval; aHR, adjusted hazard ratio; CHD, coronary heart disease; BMI, body mass index; ASA, American Society of Anesthesiologists grade.
A total of 557 patients in the MoM cohort and 14 256 in the non-MoM cohort had a previous history of cardiac failure. The respective event rates for the first subsequent admission with cardiac failure were 136.6 and 195.3 per 1000 person years (Table IV). After adjustment, the aHRs for cardiac failure and all-cause mortality were 0.997 (95% CI 0.885 to 1.122; p = 0.952) and 0.983 (95% CI 0.854 to 1.132; p = 0.814), respectively. In the analysis that included BMI as a covariate, the aHRs for cardiac failure and all-cause mortality were 0.920 (95% CI 0.831 to 1.019) and 0.879 (95% CI 0.850 to 0.909), respectively.
Table IV.
Events, the rate of events and adjusted hazard ratio of cardiac failure and all-cause mortality for patients with a history of cardiac failure and metal-on-metal and non-metal-on-metal arthroplasties
| MoM | Non-MoM | Adjusted hazard ratio (95% CI) | p-value† | |||||
|---|---|---|---|---|---|---|---|---|
| Patients | Events | Rate* | Patients | Events | Rate* | |||
| Cardiac failure, n | 557 | 293 | 136.6 | 14 256 | 6747 | 195.3 | 0.977 (0.885 to 1.122) | 0.952 |
| Death, n | 557 | 208 | 64.6 | 14 256 | 4933 | 96.0 | 0.983 (0.854 to 1.132) | 0.814 |
*rate per 1000 person years’ exposure
†Cox PH model
MoM, metal-on-metal; CI, confidence interval
Additional analyses were performed on the MoM cohort by the type of device including the type of head, the brand of the acetabular component and the size of the components. For patients receiving a modular head, the aHR approximated unity (aHR = 1.013; 95% CI 0.953 to 1.078). Of these patients, the aHR for those receiving an ASR XL prosthesis was 0.970 (95% CI 0.776 to 1.213). For those having a resurfacing, the aHR was significantly lower (0.656; 95% CI 0.587 to 0.732, Table V). No brand of acetabular component or size of femoral head had an increased aHR for cardiac failure (supplementary fig a).
Table V.
Incidents, the rate of events and adjusted hazard ratio of cardiac failure for patients with either modular or resurfaced metal-on-metal and non-metal-on-metal arthroplasties
| Patients | Events | Event rate* | Adjusted hazard ratio (95% CI) | p-value† | |
|---|---|---|---|---|---|
| Modular | |||||
| Cases | 25 656 | 1084 | 6.1 | 1.013 (0.953 to 1.078) | 0.675 |
| Controls | 482 247 | 21 245 | 9.6 | ||
| ASR XL | |||||
| Cases | 2353 | 78 | 4.5 | 0.970 (0.776 to 1.213) | 0.790 |
| Controls | 482 247 | 21 245 | 9.6 | ||
| Resurfaced | |||||
| Cases | 27 870 | 347 | 1.7 | 0.656 (0.587 to 0.732) | < 0.001 |
| Controls | 482 247 | 21 245 | 9.6 |
*rate per 1000 person years’ exposure
†Cox PH model
CI, confidence interval
Discussion
In this study involving more than half a million patients with arthroplasties of the hip, there was no association between a MoM arthroplasty and an increased incidence of cardiac failure in the first seven years after surgery.
In contrast to the recent report of increased rates of admission with cardiac failure in subgroups of patients with MoM arthroplasties,15 the risk of cardiac failure in these patients appeared to be lower than those with other types of bearing surfaces. These findings persisted after extensive adjustment for confounding factors, after matching by propensity score and even among patients at the highest risk of either cardiac failure or implant failure. Patients with pre-existing cardiac failure had, as expected, significantly higher rates of admission with cardiac failure and death due to cardiac failure. However, this was not influenced by exposure to MoM arthroplasty. In common with other studies,20,21 we found no increase in all-causes of mortality with MoM arthroplasty.
It is however, likely that there are residual confounding factors for which we have failed to account. Our finding that the lower rate for all causes of mortality from MoM arthroplasties was markedly attenuated by multivariate adjustment and matching by propensity score, indicates that this is likely to be due to lower morbidity. These results are in line with previously published studies assessing the risk of cancer and mortality with these patients, in which similarly lower HRs were reported, particularly in patients with a MoM resurfacing.18,21,30 Resurfacing is a bone-conserving option, sought by patients aiming to return to high levels of function. Aspects of better health which are not captured in these data such as exercise and not smoking or socioeconomic status may explain these findings.
The use of modular MoM arthroplasties was more varied, with some patients receiving them because they were deemed to be at high a risk of dislocation. This includes patients with poor musculature, neurological disorders, variations in anatomy and other factors that may be associated with a poorer outcome. These patients therefore have more in common with those undergoing non-MoM arthroplasty than those undergoing a resurfacing This is reflected in our findings, in which the HR for cardiac failure in patients undergoing a modular MoM arthroplasty was close to unity, and thus higher than in those undergoing a resurfacing.
MoM arthroplasties vary both in design and manufacturing process, and have different rates of failure and circulating levels of metal ions.29,31,32 Subgroup analysis by manufacturer found no differences between devices associated with high rates of implant failure such as stemmed implants, those with a large femoral head and brands including the ASR XL and other MoM implants. The ASR XL (DePuy) is a type of large-diameter, stemmed arthroplasty with a high rate of implant failure.33 It was subject to a worldwide device recall and was recently identified as being associated with an increased risk of cardiac failure in elderly men.15
Cobalt cardiomyopathy was first described in 1967 in patients with excess dietary cobalt, ‘Quebec beer-drinkers’ cardiomyopathy’.33 More recently, case reports and small series have reported cardiomyopathy related to failure of MoM implants.7,35-39 However, there were < 30 cases in total and these patients had extreme levels of circulating metal ions. Prentice et al40 performed a cross-sectional observational study of systemic complications and found a 5% lower ejection fraction in 35 patients with MoM resurfacing implants compared with patients with conventional arthroplasties. Subsequent multimodality work41 using both echocardiography and MRI, the benchmark measure of cardiac function, failed to replicate these findings despite including patients with high levels of metal ions in the blood (mean cobalt 30 ppb (range 8 to 118). Using epidemiological approaches, one study found lower standardized mortality ratios for cardiovascular death in a large cohort of patients with a MoM arthroplasty compared with those with a non-MoM arthroplasty.22 Cardiac failure was not specifically studied. A recent report from Australia noted a three-fold increase in the risk of admissions to hospital because of cardiac failure in 63 men with an ASR XL THA with a mean age of 82 years, compared with 1502 men with a metal-on-plastic THA.14 These patients were significantly different from our patients, both in age and risk of cardiac failure. All patients in their study had a high rate of cardiac failure; the incidence of cardiac failure in the control group was 18% at a mean of 7.2 years, and the effect was confined to men. In our study, there was no increased risk of cardiac failure in older patients with an ASR XL THA (supplementary table ii). The numbers were small (n = 786), with wide CIs.
This study is the largest epidemiological study of systemic effects (cardiac failure) after MoM arthroplasty. Given the nature of the sources of the data, case recruitment should be comprehensive and the findings can therefore be generally followed. The statistical power of the dataset enabled extensive subgroup analysis to address specific concerns such as the differences between gender and brands.15 We accept that before 2005, the data in the NJR include less than 80% of the arthroplasties undertaken in the United Kingdom and some MoM arthroplasties may have been omitted from the analysis.42
Limitations include those of the HES data, where coding is not performed by clinicians and the coding of cardiac failure is imperfect, although this should introduce only analytical noise, rather than bias. Cardiac failure is mainly captured when severe, as in admission to hospital or death, missing cardiac failure diagnosed and managed in a primary care setting. Follow-up was up to seven years only. However, a systematic review suggested that recognized cases occur within a median of 19 months (up to six years) postoperatively.7 We did not include analysis of the levels of metal ions in the blood. Any association of cardiac failure and high levels of metal ions could have been missed, but given the mandated surveillance of these levels and the association of metal ions with local symptoms, most of these patients would have had revision surgery. Retrospective analysis of registry data can rarely be definitive about causality.13,17 There are, however, no prospective observational or sufficiently powered randomized controlled trials, nor are there likely to be in the future. There are only two previous studies, both negative and unpublished,33,42 and no association was reported in a single, small meta-analysis.13
In conclusion, we found a lower incidence of cardiac failure and mortality in patients with MoM arthroplasties compared with other types of arthroplasty of the hip in the first seven years after surgery. While there may be confounding factors by indication, these results should provide reassurance to clinicians and patients alike, regarding the cardiac sequelae associated with these devices. We recommend epidemiological analysis at five-yearly intervals to investigate for any latent effects.
Take home message:
Patients with metal-on-metal hip prostheses (of any type) are not at significantly increased risk of severe heart failure in the first 7 years after elective surgery.
Author contributions:
S. A. Sabah: Study design, Writing paper
J. C. Moon: Study design, Review of manuscript
S. L. Jenkins-Jones: Data preparation, Review of manuscript
C. LI. Morgan: Statistical analysis, Review of manuscript
C. J. Currie: Study design, Statistical analysis, Review of manuscript
J. M. Wilkinson: Study design, Review of manuscript
M. Porter: Review of manuscript
G. Captur: Review of manuscript
J. Henckel: Study design, Review of manuscript
N. Chaturvedi: Study design, Review of manuscript
P. Kay: Review of manuscript
J. A. Skinner: Study design, Writing paper
A. J. Hart: Study design, Writing paper
C. Manisty: Study design, Writing paper
Funding statement:
Funding received from the Medicines and Health care products Regulatory Agency, UK.JCM and CM are directly supported by the National Institute for Health Research (NIHR) University College London Hospitals and Barts Hospitals Biomedical Research Centres.No benefits in any form have been received or will be received from a commercial party related directly or indirectly to the subject of this article.
This is an open-access article distributed under the terms of the Creative Commons Attributions license (CC-BY-NC), which permits unrestricted use, distribution, and reproduction in any medium, but not for commercial gain, provided the original author and source are credited.
We thank the patients and staff of all the hospitals in England, Wales and Northern Ireland who have contributed data to the National Joint Registry (NJR). We are grateful to the Healthcare Quality Improvement Partnership (HQIP), the NJR Research Sub-committee and staff at the NJR Centre for facilitating this work. The authors have conformed to the NJR’s standard protocol for data access and publication. The views expressed represent those of the authors and do not necessarily reflect those of the NJR Steering Committee nor those of the HQIP, who do not vouch for how the information is presented.
This article was primary edited by D. Johnstone and first proof edited by J. Scott.
Supplementary material. Further information including tables and figures are available alongside the online version of this article at www.bjj.boneandjoint.org.uk
References
- 1.Bozic KJ, Kurtz S, Lau E, et al. The epidemiology of bearing surface usage in total hip arthroplasty in the United States. J Bone Joint Surg [Am] 2009;91-A:1614–1620. [DOI] [PubMed] [Google Scholar]
- 2.Pandit H, Glyn-Jones S, McLardy-Smith P, et al. Pseudo-tumours associated with metal-on-metal hip resurfacings. J Bone Joint Surg [Br] 2008;90-B:847–851. [DOI] [PubMed] [Google Scholar]
- 3.Matharu GS, Judge A, Pandit HG, Murray DW. Which factors influence the rate of failure following metal-on-metal hip arthroplasty revision surgery performed for adverse reactions to metal debris? an analysis from the National Joint Registry for England and Wales. Bone Joint J 2017;99-B:1020–1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Matharu GS, Nandra RS, Berryman F, et al. Risk factors for failure of the 36 mm metal-on-metal Pinnacle total hip arthroplasty system: a retrospective single-centre cohort study. Bone Joint J 2017;99-B:592–600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Medicines and Healthcare products Regulatory Agency. Gov. UK. Metal-on-metal (MoM) hip replacements - updated advice with patient follow ups. June 2012. https://www.gov.uk/drug-device-alerts/medical-device-alert-metal-on-metal-mom-hip-replacements-updated-advice-with-patient-follow-ups (date last accessed 6 October 2017).
- 6.U.S. Food & Drug Administration. Implants and Prosthetics: Metal-on-Metal Hip Implants. 2015. http://www.fda.gov/medicaldevices/productsandmedicalprocedures/implantsandprosthetics/metalonmetalhipimplants/default.htm (date last accessed 6 October 2017).
- 7.Devlin JJ, Pomerleau AC, Brent J, et al. Clinical features, testing, and management of patients with suspected prosthetic hip-associated cobalt toxicity: a systematic review of cases. J Med Toxicol 2013;9:405–415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hart AJ, Sabah SA, Bandi AS, et al. Sensitivity and specificity of blood cobalt and chromium metal ions for predicting failure of metal-on-metal hip replacement. J Bone Joint Surg [Br] 2011;93-B:1308–1313. [DOI] [PubMed] [Google Scholar]
- 9.Cheung AC, Banerjee S, Cherian JJ, et al. Systemic cobalt toxicity from total hip arthroplasties: review of a rare condition Part 1 - history, mechanism, measurements, and pathophysiology. Bone Joint J 2016;98-B:6–13. [DOI] [PubMed] [Google Scholar]
- 10.Bradberry SM, Wilkinson JM, Ferner RE. Systemic toxicity related to metal hip prostheses. Clin Toxicol (Phila) 2014;52:837–847. [DOI] [PubMed] [Google Scholar]
- 11.Abdel-Gadir A, Berber R, Porter JB, et al. Detection of metallic cobalt and chromium liver deposition following failed hip replacement using T2* and R2 magnetic resonance. J Cardiovasc Magn Reson 2016;18:29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Vasukutty NL, Minhas THA. Systemic effects of cobalt toxicity after revision hip replacement can manifest in intermediate to long term follow-up. Hip Int 2016;26:31–34. [DOI] [PubMed] [Google Scholar]
- 13.Pijls BG, Meessen JMTA, Schoones JW, et al. Increased mortality in metal-on-metal versus non-metal-on-metal primary total hip arthroplasty at 10 years and longer follow-up: a systematic review and meta-analysis. PLoS One 2016;11:0156051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Visuri T, Borg H, Pulkkinen P, Paavolainen P, Pukkala E. A retrospective comparative study of mortality and causes of death among patients with metal-on-metal and metal-on-polyethylene total hip prostheses in primary osteoarthritis after a long-term follow-up. BMC Musculoskelet Disord 2010;11:78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gillam MH, Pratt NL, Inacio MC, et al. Heart failure after conventional metal-on-metal hip replacements. Acta Orthop 2017;88:2–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Packer M. Cobalt cardiomyopathy: a critical reappraisal in light of a recent resurgence. Circ Heart Fail 2016;9:003604. [DOI] [PubMed] [Google Scholar]
- 17.Kandala N- B, Connock M, Pulikottil-Jacob R, et al. Response to two recent BMJ papers on mortality after hip replacement: comparative modelling study. BMJ 2014;348:1506. [DOI] [PubMed] [Google Scholar]
- 18.Kendal AR, Prieto-Alhambra D, Arden NK, Carr A, Judge A. Mortality rates at 10 years after metal-on-metal hip resurfacing compared with total hip replacement in England: retrospective cohort analysis of hospital episode statistics. BMJ 2013;347:6549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Townsend N, Williams J, Bhatnagar P, Wickramasinghe K, Rayner MBritish Heart Foundation Cardiovascular Disease Statistics. 61. 2014. https://www.bhf.org.uk/publications/statistics/cardiovascular-disease-statistics-2014 (date last accessed 16th November 2017).
- 20.Lalmohamed A, MacGregor AJ, de Vries F, Leufkens HGM, van Staa TP. Patterns of risk of cancer in patients with metal-on-metal hip replacements versus other bearing surface types: a record linkage study between a prospective joint registry and general practice electronic health records in England. PloS One 2013;8:65891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Smith AJ, Dieppe P, Porter M, Blom AW. National Joint Registry of England and Wales. Risk of cancer in first seven years after metal-on-metal hip replacement compared with other bearings and general population: linkage study between the National Joint Registry of England and Wales and hospital episode statistics. BMJ 2012;344:2383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mäkelä KT, Visuri T, Pulkkinen P, et al. Cancer incidence and cause-specific mortality in patients with metal-on-metal hip replacements in Finland. Acta Orthop 2014;85:32–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.National Joint Registry for England, Wales and Northern Ireland. NJR 14th Annual Report, 2017. http://www.njrreports.org.uk/Portals/0/PDFdownloads/NJR%2014th%20Annual%20Report%202017.pdf (Date last accessed November 11 2017).
- 24.Saklad M. Grading of patients for surgical procedures. Anesthesiol 1941;2:281–284. [Google Scholar]
- 25.No authors listed. http://content.digital.nhs.uk/hes (date last accessed November 16th 2017).
- 26.No authors listed. https://www.ons.gov.uk/peoplepopulationandcommunity/birthsdeathsandmarriages/deaths. (date last accessed November 16th 2017).
- 27.von Elm E, Altman DG, Egger M, et al. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. J Clin Epidemiol 2008;61:344–349. [DOI] [PubMed] [Google Scholar]
- 28.Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis 1987;40:373–383. [DOI] [PubMed] [Google Scholar]
- 29.Jameson SS, Baker PN, Mason J, et al. Independent predictors of revision following metal-on-metal hip resurfacing: a retrospective cohort study using National Joint Registry data. J Bone Joint Surg [Br] 2012;94-B:746–754. [DOI] [PubMed] [Google Scholar]
- 30.Mäkelä KT, Visuri T, Pulkkinen P, et al. Risk of cancer with metal-on-metal hip replacements: population based study. BMJ 2012;345:4646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.de Steiger RN, Hang JR, Miller LN, Graves SE, Davidson DC. Five-year results of the ASR XL Acetabular System and the ASR Hip Resurfacing System: an analysis from the Australian Orthopaedic Association National Joint Replacement Registry. J Bone Joint Surg [Am] 2011;93-A:2287–2293. [DOI] [PubMed] [Google Scholar]
- 32.Smith AJ, Dieppe P, Vernon K, et al. Failure rates of stemmed metal-on-metal hip replacements: analysis of data from the National Joint Registry of England and Wales. Lancet 2012;379:1199–1204. [DOI] [PubMed] [Google Scholar]
- 33.National Joint Registry for England, Wales and Northern Ireland. NJR 13th Annual Report, 2016. http://www.njrreports.org.uk/Portals/0/PDFdownloads/NJR%2013th%20Annual%20Report%202016.pdf (date last accessed 6 October 2017).
- 34.Morin YL, Foley AR, Martineau G, Roussel J. Quebec beer-drinkers’ cardiomyopathy: forty-eight cases. Can Med Assoc J 1967;97:881–883. [PMC free article] [PubMed] [Google Scholar]
- 35.Pelclova D, Sklensky M, Janicek P, Lach K. Severe cobalt intoxication following hip replacement revision: clinical features and outcome. Clin Toxicol (Phila) 2012;50:262–265. [DOI] [PubMed] [Google Scholar]
- 36.Khan AH, Verma R, Bajpai A, Mackey-Bojack S. Unusual case of congestive heart failure: cardiac magnetic resonance imaging and histopathologic findings in cobalt cardiomyopathy. Circ Cardiovasc Imaging 2015;8:003352. [DOI] [PubMed] [Google Scholar]
- 37.Dahms K, Sharkova Y, Heitland P, Pankuweit S, Schaefer JR. Cobalt intoxication diagnosed with the help of Dr House. Lancet 2014;383:574. [DOI] [PubMed] [Google Scholar]
- 38.Allen LA, Ambardekar AV, Devaraj KM, Maleszewski JJ, Wolfel EE. Clinical problem-solving. Missing elements of the history. N Engl J Med 2014;370:559–566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Machado C, Appelbe A, Wood R. Arthroprosthetic cobaltism and cardiomyopathy. Heart Lung Circ 2012;21:759–760. [DOI] [PubMed] [Google Scholar]
- 40.Prentice JR, Clark MJ, Hoggard N, et al. Metal-on-metal hip prostheses and systemic health: a cross-sectional association study 8 years after implantation. PloS One 2013;8:66186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Berber R, Abdel-Gadir A, Rosmini S, et al. Assessing for cardiotoxicity from metal-on-metal hip implants with advanced multimodality imaging techniques. J Bone Joint Surg [Am] 2017;99-A:1827–1835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.National Joint Registry for England and Wales. NJR 7th Annual Report, 2010. http://www.njrcentre.org.uk/NjrCentre/Portals/0/NJR%207th%20Annual%20Report%202010.pdf (date last accessed 6 October 2017).