Abstract
An ex-30-week gestation, preterm male baby was admitted to a tertiary neonatal unit and noted to have increased ventilator requirements and diagnosed with sepsis. The baby also developed an abscess over the left elbow and over the xiphisternum along with a decrease in movement of the left hand and the right leg. Panton-Valentine leukocidin (PVL)-producing Staphylococcus aureus (SA) was isolated from the blood culture. A whole body MRI showed disseminated abscess with multiple foci in the lung, left elbow and over the xiphisternum. Disseminated sepsis with multiple septic foci has not been previously reported in neonates. We would like to highlight the fact that sepsis due to PVL toxin-producing SA can cause significant morbidity and mortality in neonates. Proper screening should be done to rule out septic foci in neonates. MRI is a good non-invasive investigation to document septic foci in a neonate and rule out multiorgan involvement.
Keywords: nosocomial infections, pneumonia (infectious disease), neonatal intensive care, radiology
Background
Neonatal infections are an important cause of morbidity and mortality. This is more commonly seen in premature infants. In a recent multicentric study from the UK, the incidence of late-onset sepsis was noted to be 3/1000 live births. About 54% were due to coagulase-negative staphylococci, 21% due to Enterobacteriaceae and 18% were due to Staphylococcus aureus (SA). About 71% of infections were noted in infants ≤32 weeks gestation.1 Our index case also was noted to have late-onset staphylococcal sepsis with multiple septic foci in the lungs, left elbow and xiphisternum along with duskiness of the toe and diminished movement of the right leg. We intend to highlight the fact that in episodes of neonatal sepsis, we should be aware of multiple septic foci. MRI is a useful non-invasive tool to rule out disseminated sepsis. Rationalisation of antibiotics and the duration of treatment can be planned accordingly.
Case presentation
An ex-30-week gestation, preterm male baby with a birth weight of 1800 g was born by emergency caesarean section due to fetal bradycardia. Maternal vaginal swabs did not grow any organism. There was no history or evidence suggestive of the mother having sepsis. The baby was born in good condition and commenced on continuous positive airway pressure because of grunting. However, he was intubated soon after because of repeated episodes of apnoea. He was also noted to have bradycardia, hypotension and poor peripheral perfusion from 12 hours of age for which he was transferred to our tertiary neonatal unit for further management. Sepsis screen was unremarkable and blood culture did not grow any organism at base hospital. The patient was screened again for sepsis at our hospital after referral. Blood culture and viral PCR were negative on admission. Chest X-ray was normal on admission. He remained on minimal settings on the ventilator for his haemodynamic instability. He was initially managed with dobutamine and epinephrine and gradually his unexplained hypotension and bradycardia resolved. Baby initially had umbilical lines (umbilical arterial and venous catheter). Percutaneous central venous line was done on day 4 as the patient remained on dobutamine and epinephrine infusions. Trophic feeds with mother’s own milk were started from day 6 and gradually increased as total parenteral nutrition was weaned off. The baby was extubated on day 7 of life when his perfusion and heart rate had improved and he was haemodynamically stable. On day 10, he was found to be tachypnoeic with increasing oxygen requirement for which he was initially commenced on humidified high-flow nasal cannula oxygen and then needed invasive ventilation. Within 24 hours, he was noted to have a dusky fourth toe on his right foot and a developing abscess overlying the anteromedial aspect of his left elbow and xiphisternum. He was also noted to have reduced mobility of the right leg. On examination, he had his right leg flexed at his hip and knee. The right knee was found to be warm and tender.
Investigations
ECG and serial echocardiography during his stay on the unit did not reveal any heart block, arrhythmia or septic focus. Sepsis screen was sent on day 10 as the baby became tachypnoeic with an increase in oxygen requirement. This revealed a C-reactive protein (CRP) of 126 mg/L along with thrombocytopenia with platelets of 86×109/L. White blood cells (WBCs) were 16×109/L. Blood culture grew SA and was positive for Panton-Valentine leukocidin (PVL) toxin. There was no history of recent isolation of SA/PVL from the neonatal/obstetric unit. Neither was there any family history of cutaneous abscess, furunculosis or severe necrotising pneumonia. Repeat echocardiography did not reveal any evidence of infective endocarditis. Over the next 48 hours, the CRP went up to 249 mg/L with WBCs of 42×109/L. Ophthalmological examination was normal. MRI of the whole body was planned to look for any evidence of abscess in the brain and extent of involvement of the left elbow and the right leg. The baby was noted to have cavitating lesions in the right upper lobe, right lung base and also in the left lung, which was not picked up previously on the chest X-ray (figure 1). He had a multiloculated collection in the left antecubital fossa measuring 33×17 mm (figure 2). There was also a lower presternal collection measuring 13×4 mm (figure 1, figure 3). There was no evidence suggestive of pulmonary infection in the initial neonatal period. As a unit, we do not routinely send catheter tip for culture. However, a repeat blood culture sent from a peripheral vein 48 hours after the previous culture also grew SA.
Figure 1.
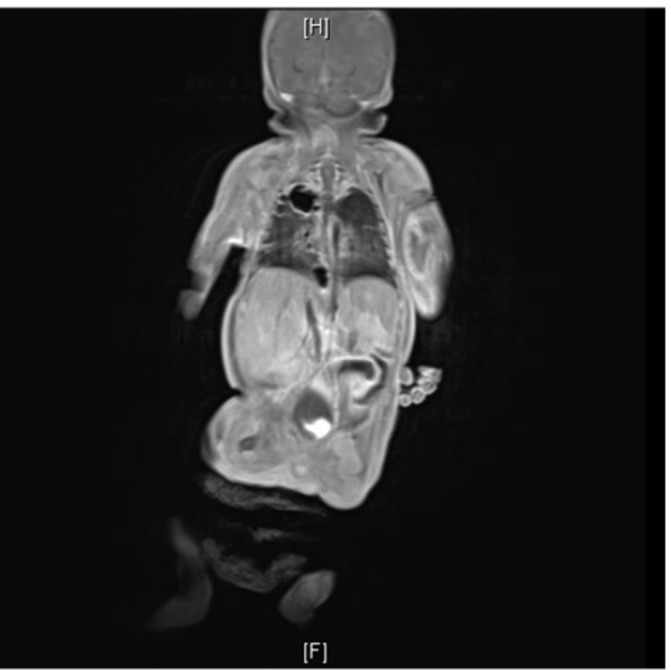
Baby had cavitating lesions in the right upper lobe, right lung base and also in the left lung, which was not picked up previously on the chest X-ray. There was also a lower presternal collection measuring 13×4 mm.
Figure 2.
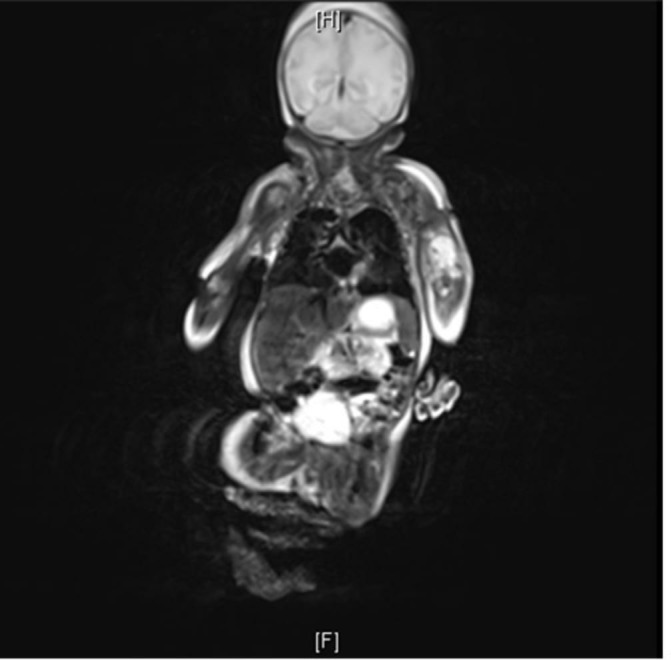
MRI showed multiloculated collection in the left antecubital fossa measuring 33×17 mm.
Figure 3.
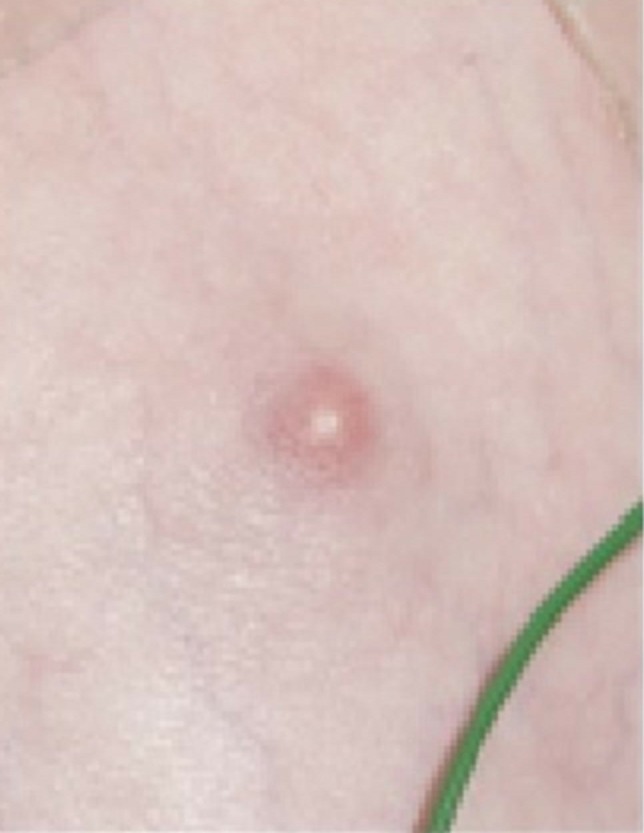
Small abscess overlying xiphisternum.
Differential diagnosis
Disseminated sepsis
Treatment
He was initially started on cefotaxime and vancomycin. Blood culture grew PVL-producing SA, which was sensitive to oxacillin and suggestive of methicillin-sensitive SA (MSSA). Antibiotics were changed to flucloxacillin and linezolid. The baby received flucloxacillin at 25 mg/kg every 8 hours for 2 weeks and then 25 mg/kg every 6 hours for four more weeks. Linezolid was administered as 10 mg/kg at every 8-hour interval. He received regular physiotherapy for his left hand, following which he had gradual improvement in the tone of his hand.
Outcome and follow-up
The patient remained haemodynamically stable and was extubated after 3 days. CRP declined gradually over the course of the next 10 days. However, during this period, he was noted to develop a left-sided wrist drop, which was believed to be a possible complication secondary to the abscess in his left elbow. This was conservatively managed with regular physiotherapy. The baby was treated with linezolid for 10 days and flucloxacillin for 6 weeks. At discharge, the power and tone of his left hand were near normal. The lesions picked up incidentally from his MRI were not followed up with serial MRI scans as the baby was noted to have improved clinically. Follow-up chest X-ray was normal. At the time of discharge, he was breathing in air with normal work of breathing. There was no respiratory compromise noticed in follow-up. Neither was there any other residual complication of SA infection.
Discussion
Infants with a birth weight of less than 2500 g were noted to have 81% of this septic episodes.1 Our patient was a male infant of 30 weeks gestation. He was noted to have bacteraemia due to PVL toxin-producing MSSA. Invasive staphylococcal infections have been documented to cause significant morbidity and mortality in preterm, low birth weight infants as we saw in our patient.2 PVL is a leucotoxin that can penetrate through the cell membrane of humans and cause necrotising inflammation and tissue damage. Leukocidin is a two-component toxin, which targets polymorphonuclear cells, monocytes and macrophages and causes cell death.3 PVL induces a process of necrosis by stimulating granulocyte synthesis of inflammatory mediators. PVL also participates in the extension of the infection by inhibiting phagocyte functions and provoking destruction of these granulocytes.4 Severity is well known in healthy adults and has been also described in preterm infants.5 6
Treatment with flucloxacillin and linezolid was effective in treating the infection. Linezolid has been shown to have better efficacy against PVL toxin-producing SA.7 The baby had disseminated septic foci. MRI incidentally documented the septic foci in the lungs, which had not been noted in the chest X-ray done previously. PVL toxin has been documented to cause necrotising cavitating pneumonia as we saw in our patient.2 He also had collections in the left elbow and a small presternal collection, which resolved gradually.
We were unable to determine the origin of PVL toxin-producing SA. Central line-associated blood stream infection (CLABSI) was considered as a possible cause for the disseminated disease in our patient. However, confirmation of CLABSI was not possible as we do not routinely send catheter tip for culture. It is also difficult to send blood culture from 2Fr PICC lines. SA could have also come from mother’s own milk, hands of parents and professionals or even from the stool. However, it was unlikely to have come from parents as there was no history of SA infection in the family. Neither was there any SA outbreak on the unit at that point. Mother’s milk and stool were not cultured for isolation of organism.
Preventive strategies, such as adherence to strict hand hygiene, should be followed to prevent the spread of infection in the unit.
Learning points.
Panton-Valentine leukocidin (PVL) toxin-liberating Staphylococcus aureus (SA) can cause severe disseminated sepsis in preterm neonates. It is known to cause necrotising inflammation and tissue damage.
It is essential to use antibiotics, such as linezolid or clindamycin, which has better efficacy against PVL toxin-producing SA.
As transmission of SA is interhuman, we should consider strict hand hygiene to prevent the spread of SA infection in the unit.
Acknowledgments
We acknowledge the parents of the baby who gave us consent for reporting this unusual presentation of sepsis.
Footnotes
Contributors: SGK, ID and DM have been involved in patient care and patient management. SGK, ID and DM have also been involved in data collection. DM has drafted the manuscript with inputs from SGK. ID has overseen the draft of the manuscript. The final manuscript has been ratified by SGK, ID and DM.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
No grant was obtained
Competing interests: None declared.
Provenance and peer review: Not commissioned; externally peer reviewed.
Patient consent for publication: Parental/guardian consent obtained.
References
- 1. Vergnano S, Menson E, Kennea N, et al. Neonatal infections in England: the NeonIN surveillance network. Arch Dis Child Fetal Neonatal Ed 2011;96:F9–F14. 10.1136/adc.2009.178798 [DOI] [PubMed] [Google Scholar]
- 2. Melles DC, van Leeuwen WB, Boelens HA, et al. Panton-valentine leukocidin genes in Staphylococcus aureus. Emerg Infect Dis 2006;12:1174–5. 10.3201/eid1207.050865 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Tomita T, Kamio Y. Molecular biology of the pore-forming cytolysins from Staphylococcus aureus, alpha- and gamma-hemolysins and leukocidin. Biosci Biotechnol Biochem 1997;61:565–72. 10.1271/bbb.61.565 [DOI] [PubMed] [Google Scholar]
- 4. König B, Prévost G, Piémont Y, et al. Effects of Staphylococcus aureus leukocidins on inflammatory mediator release from human granulocytes. J Infect Dis 1995;171:607–13. 10.1093/infdis/171.3.607 [DOI] [PubMed] [Google Scholar]
- 5. Gillet Y, Etienne J, Lina G, et al. Association of necrotizing pneumonia with Panton-Valentine leukocidin-producing Staphylococcus aureus, regardless of methicillin resistance. Clin Infect Dis 2008;47:985–6. 10.1086/591803 [DOI] [PubMed] [Google Scholar]
- 6. Pinto AN, Seth R, Zhou F, et al. Emergence and control of an outbreak of infections due to Panton-Valentine leukocidin positive, ST22 methicillin-resistant Staphylococcus aureus in a neonatal intensive care unit. Clin Microbiol Infect 2013;19:620–7. 10.1111/j.1469-0691.2012.03987.x [DOI] [PubMed] [Google Scholar]
- 7. Diep BA, Equils O, Huang DB, et al. Linezolid effects on bacterial toxin production and host immune response: review of the evidence. Curr Ther Res Clin Exp 2012;73:86–102. 10.1016/j.curtheres.2012.04.002 [DOI] [PMC free article] [PubMed] [Google Scholar]


