Abstract
Lanthanum carbonate is a phosphate binder that is used to reduce serum phosphate levels in patients with end-stage renal disease (ESRD). Lanthanum forms insoluble lanthanum phosphate complexes that are supposed to pass through the gastrointestinal (GI) tract unabsorbed. Phosphate binders have been reported to deposit in the GI tract and can cause mucosal injury. There are few case reports of GI bleeding associated with phosphate binder deposits. This case report presents a patient with iron deficiency anaemia secondary to biopsy-proven lanthanum deposits in the upper GI tract. There were no overt signs of active GI bleeding. Patient’s anaemia improved with discontinuation of the phosphate binder. Lanthanum could be a hidden cause of resistant anaemia among patients with ESRD through asymptomatic GI blood loss.
Keywords: gastrointestinal system, Gi bleeding, drugs: gastrointestinal system, ulcer, oesophagus
Background
Lanthanum carbonate is a phosphate binder that is used to reduce serum phosphate levels in patients with end-stage renal disease (ESRD).1–3 It is described as a white to off-white chewable tablet, and it works by binding dietary phosphorus to form insoluble lanthanum phosphate complexes that are supposed to pass through the gastrointestinal (GI) tract unabsorbed.1 It is generally safe to use and well-tolerated among patients; however, GI events such as nausea, vomiting and abdominal pain were reported as the most common adverse reactions during drug safety trials.1 3 Finding whitish deposits of this medication in the GI tract, therefore, is not a surprise. One retrospective study of seven patients found to have lanthanum deposits in the stomach had endoscopic findings described as ‘annular whitish mucosa’, ‘diffuse whitish mucosa’ or ‘whitish spots’,3 consistent with the description of a non-absorbable white lanthanum tablet. They noted progression of those lesions in three of the patients on repeat examinations with continued use of their phosphate binder.3
The timing from start of drug therapy to GI deposition is also relatively short, considering the typical chronicity of the medication’s use. In a Japanese case report, two ESRD patients (out of the seven total patients in the study) undergoing oesophagogastroduodenoscopy (OGD) who had been on phosphate binders for just over 2 years were already found to have white deposits in the gastric mucosa that were confirmed on histopathological review to be lanthanum. Another patient was found to have deposits after 4 months of taking lanthanum.3
Additionally, publications have associated lanthanum deposits in the GI tract with gastric mucosal alterations such as intestinal metaplasia, regenerative changes and foveolar hyperplasia,4 and one recently associated with upper GI bleeding.5
There are few case reports on the lanthanum-induced GI mucosal injuries.
We present a case of iron deficiency anaemia due to lanthanum deposition in the gastric mucosa with associated gastritis.
Case presentation
A 70-year-old man with ESRD on haemodialysis, diabetes mellitus and chronic mixed iron deficiency anaemia and anaemia of chronic disease presented for his scheduled dialysis session. Over the prior few months, the patient was requiring intravenous iron infusions more frequently than usual, with a new blood transfusion requirement on a few occasions. He required iron transfusions once every 2 months as baseline. During a dialysis session, he complained of worsening baseline shortness of breath and muscle cramps prompting labs that revealed a haemoglobin of 59 g/L, down from his baseline of 9–10 g/dL. Due to his acute drop in haemoglobin, he was admitted to the hospital. He denied any signs or symptoms of bleeding, GI-tract or otherwise. The patient was not on any anticoagulants, but was taking lanthanum for hyperphosphataemia related to chronic kidney disease (CKD) for many years. His darbepoetin dose remained the same. Physical examination was benign. The patient received two units of packed red blood cells, with initial appropriate haemoglobin response and subsequent rapid recurrent drop.
Investigations
Due to concerns for obscure GI bleeding, the patient underwent an EGD and colonoscopy. There was no identified source of active bleeding. No occult blood was found in the stool. EGD demonstrated normal duodenal mucosa, but gastric mucosal abnormalities in the peripyloric gastric antrum that were characterised by patchy, whitish, plaque-like lesions (figures 1 and 2) with associated erythema, erosion, friability, granularity and texture change (figure 3). There was also a gastric antral nodule, and gastric antral radial erythematous striping (figures 3 and 4). The gastric cardia and fundus, as well as the oesophagus, were normal. Histopathological review of biopsies taken of the duodenum, antral lesions, antral polyp and erythematous mucosa all separately revealed macrophages in the lamina propria containing refractile material, as well as focal refractile material in the overlying epithelium (figures 5 and 6). Electron microscopy with energy dispersive X-ray analysis exposed particles containing lanthanum, phosphorus and oxygen with focally smaller amounts of calcium, and sulfur, consistent with lanthanum medication deposition.
Figure 1.
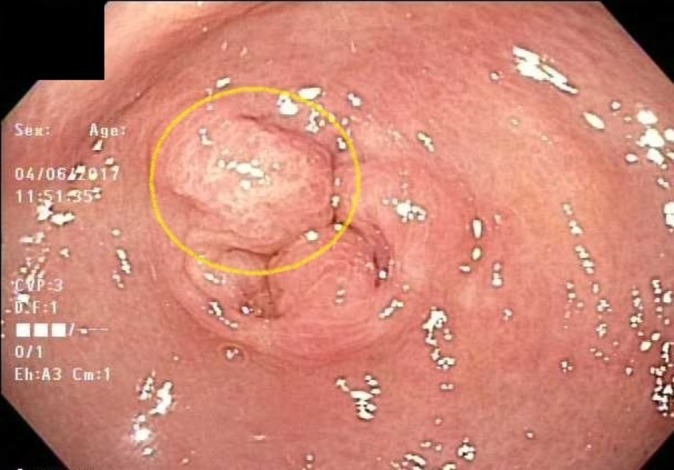
Gastric peripyloric antrum with whitish plaque-like mucosal disposition.
Figure 2.
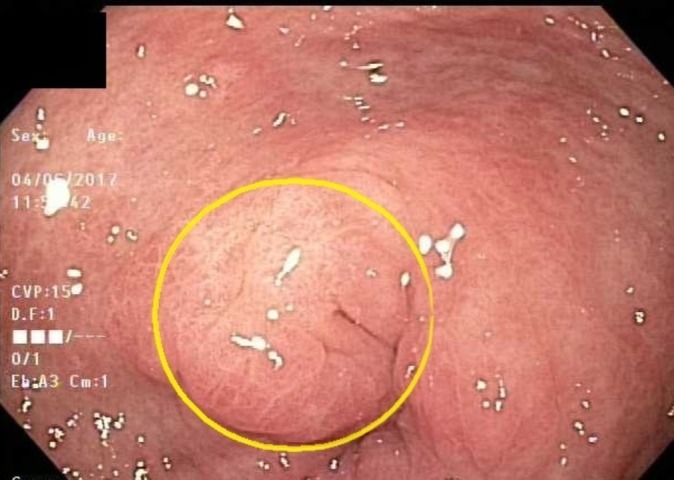
Closer view of figure 1.
Figure 3.
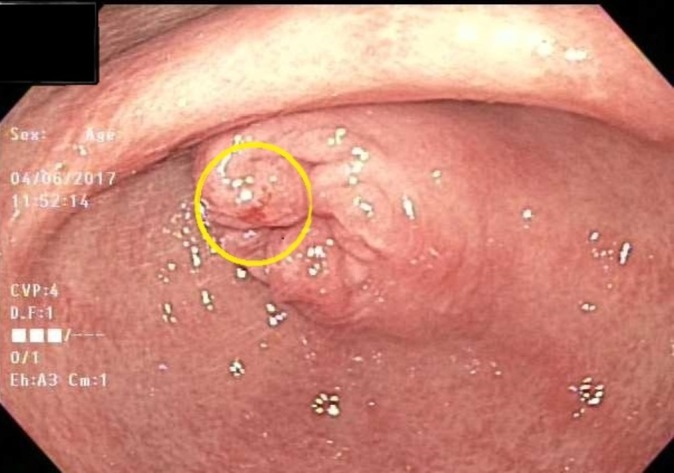
Gastric antrum with diffuse, heterogeneous white versus erythematous mucosa with erosions.
Figure 4.
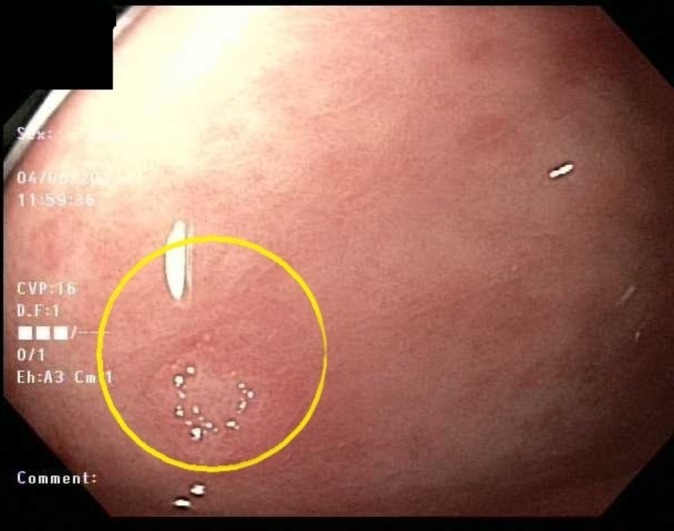
Gastric antral nodule with whitish plaque-like deposition over erythematous base.
Figure 5.
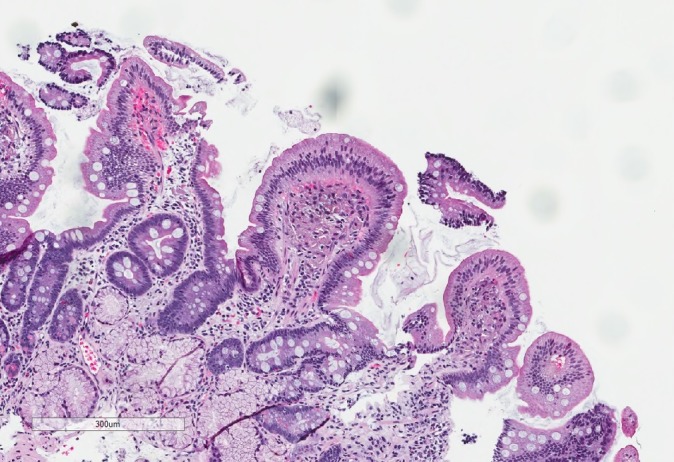
Duodenal histiocytosis. Histiocyte collections at the tips of duodenal villi (H&E, original magnification ×8).
Figure 6.
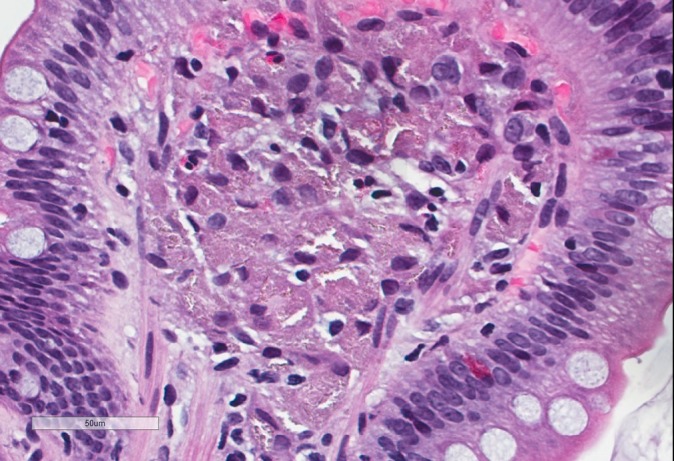
Duodenal histiocytosis. Histiocyte collections filled with amorphous material (H&E, original magnification ×40).
Subsequent capsule endoscopy revealed 2–3 small, non-bleeding angioectasias, and further endoscopy was not pursued.
Differential diagnosis
We believe that our patient’s iron deficiency anaemia was due to the biopsy proved lanthanum deposits and associated mucosal injury. The haemoglobin drop was abrupt, persistent, recurrent, without overt signs or symptoms of bleeding, and there was no other significant potential source for bleeding identified. EGD, colonoscopy and capsule endoscopy were otherwise without significant findings. The patient had no evidence of atrophic autoimmune gastritis or small bowel enteropathy, and was not on any gastric acid-reducing medication that may have otherwise interfered with iron absorption. His intravenous iron requirement has been gradually decreasing since stopping the lanthanum.
Treatment
The patient’s lanthanum phosphate binder was stopped in order to prevent further medication deposition and mucosal injury. He was then selectively started on ferric citrate liquid iron supplementation for his iron deficiency anaemia, as well as to prevent risk of subsequent iron tablet-induced gastritis in a patient already suffering from mucosal injury from medication deposition. Discontinuation of the phosphate binder is the only currently reported strategy for prevention or treatment of GI injury related to its deposition, and was described as the mainstay of treatment in a case report on sevelamer-induced GI injury.6 There is no other reported treatment for lanthanum-induced GI injury and/or deposits.
Outcome and follow-up
Since stopping the lanthanum, the patient has continued his oral iron regimen, but required less frequent iron infusions, previously one time per month, now every other month. His darbepoetin needs have also incidentally decreased. The patient has never demonstrated overt bleeding.
A repeat EGD and colonoscopy was performed 6 months later for surveillance. It revealed persistent appearance of erythematous gastropathy and antral mucosal deposits. Repeat biopsies of the duodenum, gastric body and pylorus showed persistent reactive changes and macrophages containing refractile material consistent with lanthanum deposits. Biopsies from the lesser and greater curve of the gastric antrum showed gastric mucosa with focal chronic inflammation. Lanthanum medication deposits and inflammation were not present in biopsies from the gastric incisura, cardia or colonic polyps.
Discussion
To the best of our knowledge, this is the first case report demonstrating iron deficiency anaemia secondary to lanthanum deposits. In total, there have been <20 published cases on phosphate binder-induced GI injuries in the medical literature, and most of those are related to sevelamer. Lanthanum-associated GI mucosal injury is a relatively novel entity among lanthanum users in the literature, and thus has not yet been fully characterised. Presentation of a phosphate binder’s deposits, such as lanthanum or sevelamer, can range from asymptomatic deposits to acute obstruction requiring surgical intervention.7 One study demonstrated that CKD patients with a history of diabetes, abdominal surgery or chronic constipation were more likely to have phosphate binder-induced GI complications.7 We propose, based on the drug’s mechanism of action, that this may be secondary to slowed luminal clearance, increased deposition during slow transit, and subsequent complications of the drug’s deposition or luminal collection. This idea would also be consistent with our patient’s demonstration of medication deposition in the motility-dependent areas of the antral stomach and small bowel. As over half of the patients in the USA with CKD have diabetes,8 and a reported 20% of patients starting dialysis in the UK have diabetes,6 this greater impact of phosphate binders on the GI tract of diabetic patients may be substantial. Our patient had CKD with diabetes, and demonstrated GI-related complications of medication deposition.
Endoscopic findings of patients on phosphate binders included reports of erosions, ulcerations and inflammatory and peptic changes. The histopathological presentation included acute inflammation, ulceration, ischaemic injury and necrosis.7 These prior reports are consistent with our patient case, which demonstrated endoscopic erythema, erosion and histopathological acute inflammation.
There are variable data regarding the reversibility of these deposits. In one case report, after stopping the phosphate binder the patient’s symptoms resolved and follow-up EGD demonstrated complete resolution of prior deposits.9 Separately, another case reported the endoscopic findings associated with lanthanum deposits remained unchanged after 8 months of drug cessation.10 Our patient’s deposits did not change on the repeat EGD done 6 months after cessation. Reversibility of lanthanum deposition and related lesions may be time-dependent, patient-dependent, related to comorbid disease or irreversible. Discontinuation of the lanthanum, however, showed clinical improvement of our patient’s anaemia. Further studies are needed.
Lanthanum, or other phosphate binder, could be a hidden cause of resistant anaemia among patients with CKD on these medications. We believe that our patient’s persistent iron deficiency anaemia is likely due to small volume GI bleeding secondary to lanthanum deposits with coinciding mucosal injury. It is important for lanthanum-induced GI lesions to be considered as the aetiology for iron deficiency in a patient with CKD taking lanthanum, or other phosphate binders, particularly if presenting with GI symptoms. Although the overall report rate currently seen in the published literature is small, the true number of patient cases for such a common disease and common treatment regimen is likely much higher.
Further studies are needed to assess the presence of lanthanum in GI lesions in patients at risk, as well as the influence of dose reduction or discontinuation of lanthanum in patients with GI complications. Additional studies to evaluate prevalence of iron deficiency anaemia in patients on lanthanum with deposits in the GI tract are also needed to determine the possible significance for this significant population. Further risk stratification to assess the effects of comorbid diabetes, chronic constipation or prior abdominal surgeries with presence or severity of GI complications may be additionally illuminating.
Learning points.
It is important for lanthanum-induced gastrointestinal (GI) lesions to be considered as the aetiology for iron deficiency in a patient with CKD taking lanthanum, or other phosphate binders, regardless of presence of GI symptoms.
Lanthanum-induced GI symptoms range from being asymptomatic to having an acute abdomen requiring surgical intervention.
Lanthanum could be a hidden cause of resistant anaemia among CKD patients through asymptomatic GI blood losses.
A patient with CKD and comorbid diabetes may be at higher risk for phosphate binder-induced GI complications.
Footnotes
Contributors: CA performed all the background research, studied the patient’s case, endoscopic and pathology findings. KG is the expert on the case. She is the gastroenterologist who follows the patient. She provided CA with all the necessary documents for the case. She reviewed the case report and provided feedback. ES is the pathologist who reviewed the patient’s slides and commented on the findings as stated in the case report.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: None declared.
Provenance and peer review: Not commissioned; externally peer reviewed.
Patient consent for publication: Obtained.
References
- 1. Fosrenol (lanthanum) [prescribing information]. Lexington, MA: Shire US Inc, 2016. [Google Scholar]
- 2. Makino M, Kawaguchi K, Shimojo H, et al. Extensive lanthanum deposition in the gastric mucosa: the first histopathological report. Pathol Int 2015;65:33–7. 10.1111/pin.12227 [DOI] [PubMed] [Google Scholar]
- 3. Murakami N, Yoshioka M, Iwamuro M, et al. Clinical characteristics of seven patients with lanthanum phosphate deposition in the stomach. Intern Med 2017;56:2089–95. 10.2169/internalmedicine.8720-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Ban S, Suzuki S, Kubota K, et al. Gastric mucosal status susceptible to lanthanum deposition in patients treated with dialysis and lanthanum carbonate. Ann Diagn Pathol 2017;26:6–9. 10.1016/j.anndiagpath.2016.10.001 [DOI] [PubMed] [Google Scholar]
- 5. Mancano MA. Gastrointestinal nodules and bleeding with long-term lanthanum use; DRESS and hepatotoxicity due to rivaroxaban; thrombocytopenia induced by pentoxifylline; amlodipine-induced Schamberg’s disease; varenicline-induced acute liver injury. Hospital Pharmacy 2016;51:284–7. 10.1310/hpj5104-284 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Kidneyresearchuk.org. Diabetes symptoms, types & treatments | kidney research UK [online]. https://www.kidneyresearchuk.org/health-information/diabetes (Accessed 30 May 2018).
- 7. Yuste C, Mérida E, Hernández E, et al. Gastrointestinal complications induced by sevelamer crystals. Clin Kidney J 2017;10:539–44. 10.1093/ckj/sfx013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Kidney Disease Statistics for the United States. National institute of diabetes and digestive and kidney diseases: U.S. Department of Health and Human Services, 2016. [Google Scholar]
- 9. Magee J, Robles M, Dunaway P. Sevelamer-induced gastrointestinal injury presenting as gastroenteritis. Case Rep Gastroenterol 2018;12:41–5. 10.1159/000486192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Namie S, Hamabe S, Kawatomi M, et al. Investigation of deposition of lanthanum on gastric mucosa in hemodialysis patients with lanthanum therapy. Nihon Toseki Igakkai Zasshi 2015;48:169–77. 10.4009/jsdt.48.169 [DOI] [Google Scholar]


