Abstract
Fibroblast activation protein (FAP) is overexpressed in cancer-associated fibroblasts of several tumor entities. The recent development of quinoline-based PET tracers that act as FAP inhibitors (FAPIs) demonstrated promising results preclinically and already in a few clinical cases. Consequently, these tracers are now applied in our hospital to amend the diagnostics of cancer patients facing the limitations of standard examinations. Here, we analyze the tissue biodistribution and preliminary dosimetry of 2 members of this new class of PET radiopharmaceutical. Methods: A preliminary dosimetry estimate for 68Ga-FAPI-2 and 68Ga-FAPI-4 was based on 2 patients examined at 0.2, 1, and 3 h after tracer injection using the QDOSE dosimetry software suit. Further PET/CT scans of tumor patients were acquired 1 h after injection of either 68Ga-FAPI-2 (n = 25) or 68Ga-FAPI-4 (n = 25); for 6 patients an intraindividual related 18F-FDG scan (also acquired 1 h after injection) was available. For the normal tissue of 16 organs, a 2-cm spheric volume of interest was placed in the parenchyma; for tumor lesions, a threshold-segmented volume of interest was used to quantify SUVmean and SUVmax. Results: Similar to literature values for 18F-FDG, 68Ga-DOTATATE, and 68Ga-PSMA-11, an examination with 200 MBq of 68Ga-FAPI-2 or 68Ga-FAPI-4 corresponds to an equivalent dose of approximately 3–4 mSv. After a fast clearance via the kidneys, the normal organs showed a low tracer uptake with only minimal changes between 10 min and 3 h after injection. In 68Ga-FAPI-2, the tumor uptake from 1 to 3 h after injection decreased by 75%, whereas the tumor retention was prolonged with 68Ga-FAPI-4 (25% washout). Regarding tumor-to-background ratios, at 1 h after injection both 68Ga-FAPI tracers performed equally. In comparison to 18F-FDG, the tumor uptake was almost equal (average SUVmax, 7.41 for 18F-FDG and 7.37 for 68Ga-FAPI-2; not statistically significant); the background uptake in brain (11.01 vs. 0.32), liver (2.77 vs. 1.69), and oral/pharyngeal mucosa (4.88 vs. 2.57) was significantly lower with 68Ga-FAPI. Other organs did not relevantly differ between 18F-FDG and 68Ga-FAPI. Conclusion: FAPI PET/CT is a new diagnostic method in imaging cancer patients. In contrast to 18F-FDG, no diet or fasting in preparation for the examination is necessary, and image acquisition can potentially be started a few minutes after tracer application. Tumor-to-background contrast ratios were equal to or even better than those of 18F-FDG.
Keywords: radiotracer tissue kinetics, cancer-associated fibroblasts, PET/CT, biodistribution, dosimetry, fibroblast activation protein
Fibroblast activation protein (FAP) is highly expressed in the stroma of several tumor entities. Especially breast, colon, and pancreatic carcinomas are characterized by a strong desmoplastic reaction, which means that 90% of the gross tumor mass can consist of stromal but not tumor cells.
Fibroblasts are present ubiquitously in the whole body and show dipeptidyl peptidase 4 expression but no or only a very low FAP expression. In contrast, cancer-associated fibroblasts are specifically characterized by the expression of FAP, which, unlike the closely related dipeptidyl peptidase 4, has not only exopeptidase activity but also endopeptidase activity; that is, proteins can be cleaved not only at their terminal end but at any postproline bond in the amino acid sequence (1). Thus, cancer-associated fibroblasts differ from normal fibroblasts by providing FAP as a target with a relatively high tumor-specific expression, and FAP inhibitors (FAPIs) have already been developed as cancer drugs (2,3).
Based on a quinoline-based FAP-specific inhibitor (2), a new class of radiopharmaceuticals was designed and found preclinically highly promising as molecular targeting imaging probes, and it is hoped that they also will be therapeutically useful (4,5). A few first-in-human cases demonstrated high-contrast tumor imaging and possible appropriateness as a pan-tumor agent (4,5).
Consequently, we are now increasingly using this tracer to amend the diagnostics of cancer patients who are facing the limitations of standard examinations.
Here, we approximated the radiation exposure of serial PET/CT using the ligands 68Ga-FAPI-2 and 68Ga-FAPI-4 and analyzed the normal-tissue biodistribution and tumor uptake of these ligands in comparison to the current standard, 18F-FDG.
MATERIALS AND METHODS
Patients
All patients gave written informed consent to undergo 68Ga-FAPI PET/CT following the regulations of the German Pharmaceuticals Act §13(2b). All patients were referred for the experimental diagnostics by their oncologists, who were facing an unmet diagnostic challenge that could not be solved sufficiently with standard diagnostic means. Examples of such challenges are insufficient tumor delineation for target-volume segmentation before external-beam radiotherapy, suspicion that lesions are false-negative on 18F-FDG, and the need to select target-positive patients for experimental last-line therapy with therapeutic FAPI conjugates. The data were analyzed retrospectively with the approval of the local ethics committee (approval S016/2018). Detailed patient characteristics are provided in Table 1.
TABLE 1.
Patient Characteristics
| Patient no. | Sex | Age | MBq | Diagnosis | Tracer |
| 1 | F | 89 | 312 | Breast cancer | 68Ga-FAPI-2 |
| 2 | M | 55 | 298 | Colorectal cancer | 68Ga-FAPI-2, 18F-FDG |
| 3 | M | 56 | 256 | Cancer of unknown primary | 68Ga-FAPI-2 |
| 4 | M | 64 | 336 | Head and neck cancer | 68Ga-FAPI-2 |
| 5 | M | 66 | 196 | Head and neck cancer | 68Ga-FAPI-2 |
| 6 | F | 64 | 202 | Head and neck cancer | 68Ga-FAPI-2 |
| 7 | F | 65 | 178 | Head and neck cancer | 68Ga-FAPI-2 |
| 8 | M | 59 | 325 | Head and neck cancer | 68Ga-FAPI-2 |
| 9 | M | 68 | 255 | Head and neck cancer | 68Ga-FAPI-2, 18F-FDG |
| 10 | M | 70 | 212 | Hepatocellular carcinoma | 68Ga-FAPI-2 |
| 11 | M | 66 | 308 | Liposarcoma | 68Ga-FAPI-2 |
| 12 | M | 78 | 222 | Non–small cell lung cancer | 68Ga-FAPI-2, 18F-FDG |
| 13 | F | 66 | 268 | Non–small cell lung cancer | 68Ga-FAPI-2 |
| 14 | F | 58 | 126 | Esophagus cancer | 68Ga-FAPI-2, 18F-FDG |
| 15 | M | 70 | 134 | Esophagus cancer | 68Ga-FAPI-2 |
| 16 | M | 31 | 307 | Pancreatic cancer | 68Ga-FAPI-2, 18F-FDG |
| 17 | M | 52 | 167 | Pancreatic cancer | 68Ga-FAPI-2 |
| 18 | M | 56 | 222 | Pancreatic cancer | 68Ga-FAPI-2 |
| 19 | F | 73 | 142 | Pancreatic cancer | 68Ga-FAPI-2 |
| 20 | M | 74 | 122 | Prostate cancer | 68Ga-FAPI-2 |
| 21 | M | 77 | 318 | Prostate cancer | 68Ga-FAPI-2 |
| 22 | M | 60 | 285 | Renal cell carcinoma | 68Ga-FAPI-2 |
| 23 | M | 77 | 225 | Thyroid cancer | 68Ga-FAPI-2, 18F-FDG |
| 24 | M | 55 | 270 | Thyroid cancer | 68Ga-FAPI-2 |
| 25 | F | 60 | 238 | Uterus cancer | 68Ga-FAPI-2 |
| 26 | F | 57 | 263 | Breast cancer | 68Ga-FAPI-4 |
| 27 | F | 44 | 220 | Colorectal cancer | 68Ga-FAPI-4 |
| 28 | M | 66 | 286 | Colorectal cancer | 68Ga-FAPI-4 |
| 29 | M | 55 | 244 | Colorectal cancer | 68Ga-FAPI-4 |
| 30 | M | 46 | 247 | Cancer of unknown primary | 68Ga-FAPI-4 |
| 31 | F | 82 | 236 | Head and neck cancer | 68Ga-FAPI-4 |
| 32 | M | 51 | 263 | Head and neck cancer | 68Ga-FAPI-4 |
| 33 | M | 84 | 246 | Hepatocellular carcinoma | 68Ga-FAPI-4 |
| 34 | M | 77 | 299 | Non–small cell lung cancer | 68Ga-FAPI-4 |
| 35 | F | 58 | 217 | Non–small cell lung cancer | 68Ga-FAPI-4 |
| 36 | M | 64 | 255 | Non–small cell lung cancer | 68Ga-FAPI-4 |
| 37 | F | 56 | 250 | Ovarian cancer | 68Ga-FAPI-4 |
| 38 | F | 67 | 260 | Pancreatic cancer | 68Ga-FAPI-4 |
| 39 | F | 76 | 243 | Pancreatic cancer | 68Ga-FAPI-4 |
| 40 | F | 55 | 293 | Pancreatic cancer | 68Ga-FAPI-4 |
| 41 | M | 52 | 239 | Pancreatic cancer | 68Ga-FAPI-4 |
| 42 | M | 61 | 198 | Pancreatic cancer | 68Ga-FAPI-4 |
| 43 | M | 73 | 277 | Pancreatic cancer | 68Ga-FAPI-4 |
| 44 | M | 57 | 275 | Pancreatic cancer | 68Ga-FAPI-4 |
| 45 | M | 60 | 237 | Pancreatic cancer | 68Ga-FAPI-4 |
| 46 | M | 31 | 233 | Pancreatic cancer | 68Ga-FAPI-4 |
| 47 | M | 71 | 249 | Prostate cancer | 68Ga-FAPI-4 |
| 48 | M | 64 | 227 | Prostate cancer | 68Ga-FAPI-4 |
| 49 | M | 72 | 276 | Thyroid cancer | 68Ga-FAPI-4 |
| 50 | F | 27 | 204 | Thyroid cancer | 68Ga-FAPI-4 |
Radiopharmaceuticals
Synthesis and labeling of 68Ga-FAPI-2 (4) and 68Ga-FAPI-4 (5) have already been described previously. 18F-FDG was obtained commercially (Life Radiopharma f-con GmbH). The chemical structures of 68Ga-FAPI-2 and 68Ga-FAPI-4 are provided in Figure 1.
FIGURE 1.
Molecular structure of 68Ga-FAPI-2 and 68Ga-FAPI-4.
PET/CT Imaging
All imaging was performed on a Biograph mCT Flow scanner (Siemens). After unenhanced low-dose CT (130 keV, 30 mAs, CARE Dose [Siemens]; reconstructed with a soft-tissue kernel to a slice thickness of 5 mm), PET was acquired in 3-dimensional mode (matrix, 200 × 200) using FlowMotion (Siemens). The emission data were corrected for randoms, scatter, and decay. Reconstruction was performed with ordered-subset expectation maximization using 2 iterations and 21 subsets and Gauss filtration to a transaxial resolution of 5 mm in full width at half maximum. Attenuation correction was performed using the unenhanced low-dose CT data. The injected activity for the FAPI examinations was 122–336 MBq (details provided in Table 1), and the PET scans were started 1 h after injection.
To approximate the dosimetry, imaging was performed 10 min, 1 h, and 3 h after injection of 306 MBq of 68Ga-FAPI-2 in one patient and 258 MBq of 68Ga-FAPI-4 in another patient.
Adverse Events
Standard vital parameters were checked by a medical technician between tracer application and up to 30 min after finishing the examination, and the patients were asked to report any abnormalities.
Radiation Dosimetry Estimate
The dosimetry analysis was performed using the QDOSE dosimetry software suite (ABX-CRO). Kidneys, liver, spleen, urinary bladder content, red marrow, heart content, and remainder of body were included as source organs. Because of blood sampling failure due to poor vein conditions, the red marrow dose was approximated using the activity segmented with volume-of-interest technique in the PET scans. The non–tumor-affected lumbar vertebrae 5 (68Ga-FAPI-2) and 4 (68Ga-FAPI-4) were assumed to contain 2.46% of the total red marrow (6). Using QDOSE, all CT images were coregistered using an automatic deformable coregistration. PET images were coupled to the CT images of the corresponding imaging session. The PET images were transformed according to the transformation matrix of the coupled CT. The volumes of interest of all segmented source organs were drawn in the examination that had the best organ delineation and then were copied onto all other time points to calculate the time–activity curves. Monoexponential curve fitting was then applied to all organ time–activity curves. The cumulative activity à between time 0 and the first measured time point was calculated assuming a linear increase from 0 to the first measured activity. The à between the first measured time point and the last measured time point was integrated numerically using trapezoidal approximation. The à from the last measured time point to infinity was integrated using the fitted function. The total-body à was based on the injected activity and the calculated effective half-life in a total-field-of-view volume of interest, assuming that the limbs behave similar to the torso. This approach was chosen because some parts of the body were not in the field of view of the PET/CT device. The à values of total body and red marrow were added as organs into QDOSE. The à of the remainder of the body was then automatically calculated by subtracting all source-organ à values from the total-body Ã. All source-organ residence times were calculated by dividing the à by the injected activity. Absorbed and effective dose calculations were performed using the International Commission on Radiological Protection (ICRP)–endorsed IDAC-Dose 2.1 and IDAC-Dose 1.0 (7), which are integrated in QDOSE. In addition, the residence times of all included source organs and remainder of body were exported as an OLINDA case file for dose calculation in OLINDA 1.1 (8). Both IDAC-Dose 1.0 and OLINDA 1.1 are based on the Cristy–Eckerman stylized phantom series (9). IDAC-Dose 2.1 is based on the ICRP adult reference computational phantoms (10) and the ICRP-specific absorbed fractions (11). Organ masses were not adapted to individual subject organ masses.
Biodistribution
The tracer biodistribution in patients was quantified by SUVmean and SUVmax at 1 h after injection for 68Ga-FAPI-2, 68Ga-FAPI-4, and 18F-FDG. The interval between 18F-FDG and 68Ga-FAPI examinations was 9 d maximum, and no treatment change took place in between. For calculation of the SUV, circular regions of interest were drawn around the tumor lesions with focally increased uptake in transaxial slices and automatically adapted to a 3-dimensional volume of interest with e.soft software (Siemens) at a 40% isocontour. The normal organs were evaluated with a 1-cm-diameter (for the small organs [thyroid, parotid gland, myocardium, oral mucosa, and spinal cord]) to 2-cm-diameter (brain, muscle, liver, spleen, kidney, fat, aortic lumen content, and lung) sphere placed inside the organ parenchyma.
RESULTS
Adverse Events
All patients tolerated the examination well. No drug-related pharmacologic effects or physiologic responses occurred. All observed parameters (e.g., blood pressure, heart rate, and body temperature) remained normal and unchanged during injection and the 1.5 h of follow-up. No patient reported any symptoms.
Dosimetry Estimate
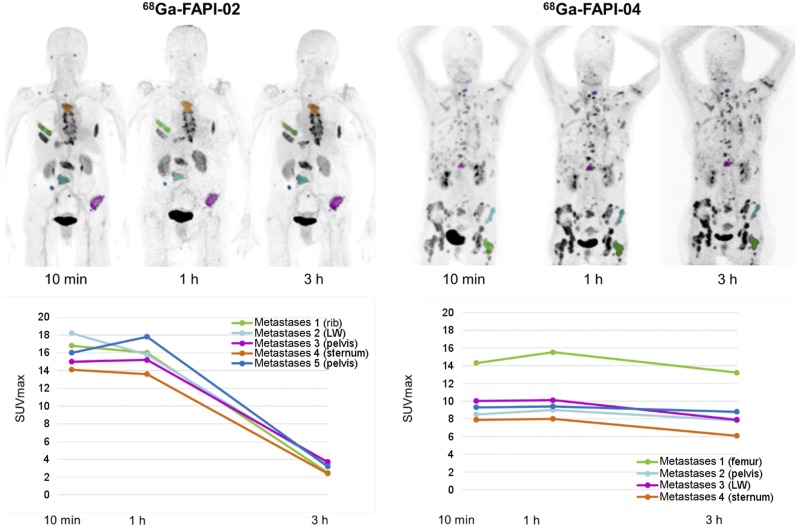
Maximum-intensity projections of the PET scans used for source-organ segmentation are shown in Figure 2. The approximated dosimetry for the 2 patients is presented in Table 2. The effective dose of 68Ga-FAPI-2 was 1.80E−2 mSv/MBq calculated with OLINDA (1.82E−2 with IDAC1/ICRP60 and 1.79E−2 with IDAC2/ICRP103). The effective dose for 68Ga-FAPI-4 PET/CT was 1.64E−2 mSv/MBq calculated with OLINDA (1.66E−2 with IDAC1/ICRP60 and 1.35E−2 with IDAC2/ICRP103). If the delayed scan at 3 h after injection is omitted in clinical practice, the routine activity for a FAPI examination could be reduced to 200 MBq of 68Ga; consequently, the radiation dose of such a 68Ga-FAPI PET/CT scan would be 3–4 mSv.
FIGURE 2.
68Ga-FAPI-2 and 68Ga-FAPI-4 at different imaging time points (10 min, 1 h, and 3 h after injection) in 2 patients with metastasized breast cancer. Rapid tumor targeting and fast blood clearance are followed by long plateau phase without relevant change in image contrast (top). In comparison to 68Ga-FAPI-2, 68Ga-FAPI-4 is characterized by prolonged tumor retention time (bottom). LW = lumbar body.
TABLE 2.
Dosimetry Estimate (OLINDA)
| Site | 68Ga-FAPI-2 | 68Ga-FAPI-4 |
| Adrenals | 1.23E−02 | 1.12E−02 |
| Brain | 9.54E−03 | 9.11E−03 |
| Breasts | 9.58E−03 | 8.88E−03 |
| Gallbladder wall | 1.19E−02 | 1.13E−02 |
| Lower large intestine wall | 1.23E−02 | 1.17E−02 |
| Small intestine | 1.19E−02 | 1.13E−02 |
| Stomach wall | 1.13E−02 | 1.06E−02 |
| Upper large intestine wall | 1.17E−02 | 1.11E−02 |
| Heart wall | 4.73E−02 | 2.02E−02 |
| Kidneys | 4.45E−02 | 4.43E−02 |
| Liver | 1.51E−02 | 1.46E−02 |
| Lungs | 1.09E−02 | 9.89E−03 |
| Muscle | 1.04E−02 | 9.91E−03 |
| Ovaries | 1.24E−02 | 1.19E−02 |
| Pancreas | 1.23E−02 | 1.13E−02 |
| Red marrow | 3.28E−02 | 2.08E−02 |
| Osteogenic cells | 2.94E−02 | 2.16E−02 |
| Skin | 9.01E−03 | 8.63E−03 |
| Spleen | 2.62E−02 | 1.05E−02 |
| Testes | 1.04E−02 | 1.01E−02 |
| Thymus | 1.15E−02 | 1.01E−02 |
| Thyroid | 1.03E−02 | 9.82E−03 |
| Urinary bladder wall | 8.89E−02 | 9.91E−02 |
| Uterus | 1.33E−02 | 1.30E−02 |
| Total body | 1.19E−02 | 1.09E−02 |
| Effective dose (mSv/MBq) | 1.80E−02 | 1.64E−02 |
Biodistribution
The 2 patients examined 10 min to 3 h after injection demonstrated that both FAPI tracers rapidly reached their stable physiologic biodistribution. In normal tissue, changes between 10 min and 3 h after injection were minimal. Tumor uptake declined by a mean of 75% from 1 h to 3 h after injection using 68Ga-FAPI-2; less washout, only 25% (mean), between 1 h and 3 h after injection (i.e., longer tumor retention) was observed with 68Ga-FAPI-4 (Fig. 2, bottom). However, at 1 h after injection (the time point also chosen for comparison to 18F-FDG), both 68Ga-FAPI tracers performed equally with regard to tumor-to-background ratios.
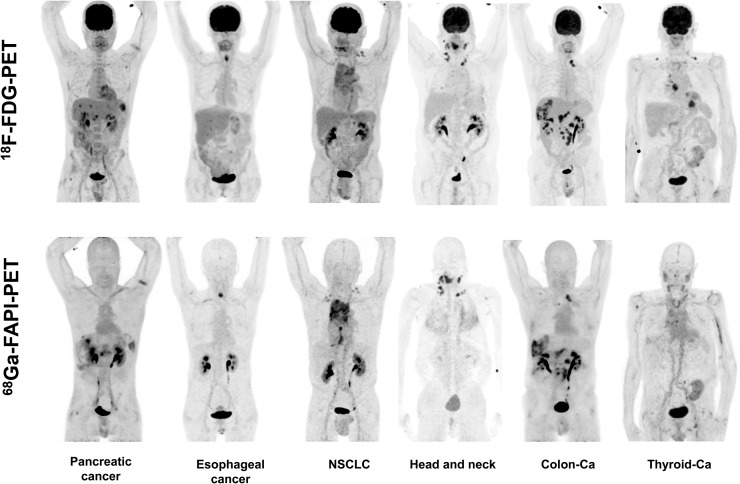
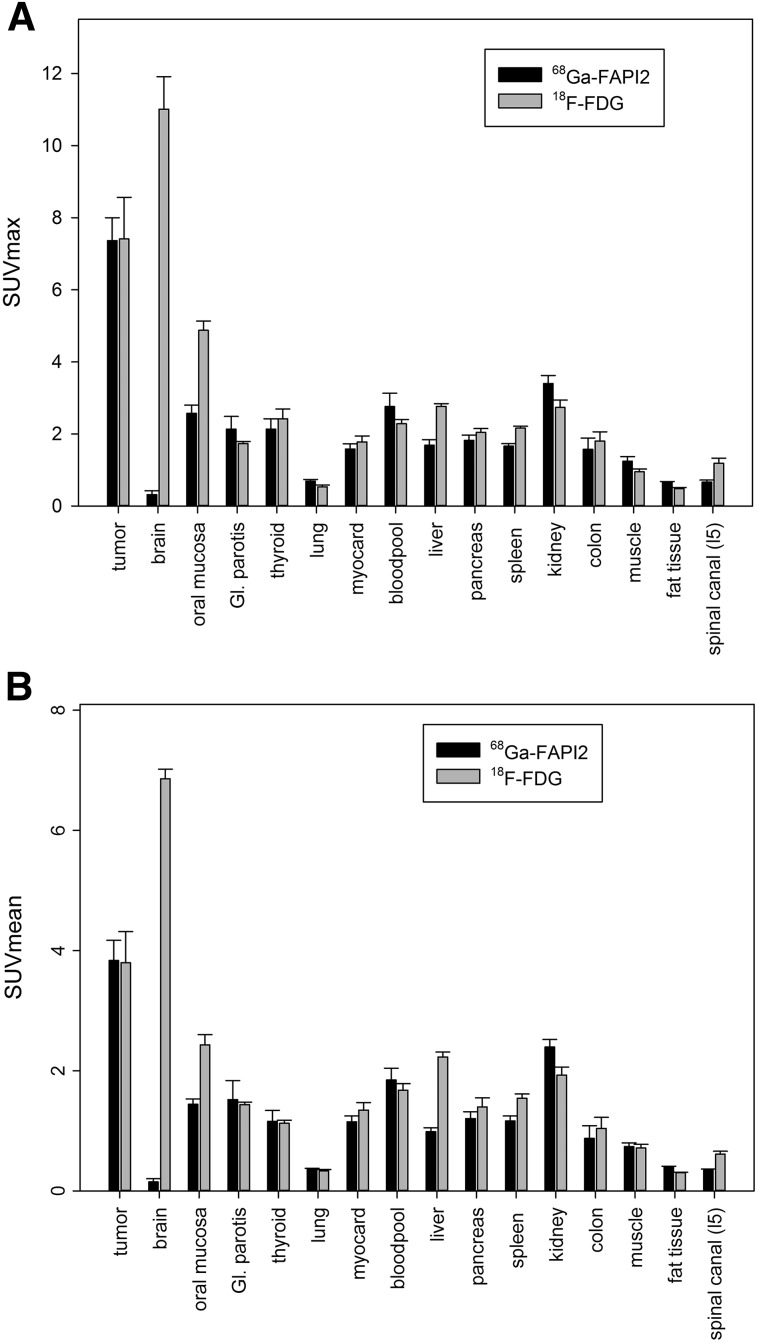
The quantitative tumor uptake of FAPI PET was similar to that of the current oncologic PET standard of reference, 18F-FDG (average SUVmax, 7.41 for 18F-FDG and 7.37 for 68Ga-FAPI-2; not statistically significant). In pancreatic, esophageal, lung, head and neck, and colorectal cancer, the quantitative tumor uptake was noninferior to that of 18F-FDG. In contrast, dedifferentiated thyroid cancer with flip-flop uptake of 18F-FDG was not accumulating 68Ga-FAPI (Fig. 3). Regarding background activity, the average SUVmax of 68Ga-FAPI-2 was significantly lower in brain (0.32 vs. 11.01), liver (1.69 vs. 2.77), and oral/pharyngeal mucosa (2.57 vs. 4.88), thus improving the contrast ratios for liver metastases of pancreatic and colorectal cancer and delineation of the esophageal cancer (Fig. 3). For all other organs, 68Ga-FAPI-2 presented no significant difference from 18F-FDG (Fig. 4A).
FIGURE 3.
Intraindividual comparison of 6 patients with 6 different tumor entities undergoing 18F-FDG PET and 68Ga-FAPI PET imaging within less than 9 d. Five of 6 patients present similar strong tumor uptake with 18F-FDG and 68Ga-FAPI, and 3 of 6 could benefit from lower background in liver or pharyngeal mucosa. In contrast, iodine-negative thyroid cancer patient presented only minor 68Ga-FAPI tracer uptake compared with 18F-FDG. Ca = cancer; NSCLC = non–small cell lung cancer.
FIGURE 4.
PET-based biodistribution analysis of 6 patients intraindividually comparing 18F-FDG PET and 68Ga-FAPI PET, imaged at 1 h after injection. GI = gastrointestinal.
FIGURE 5.
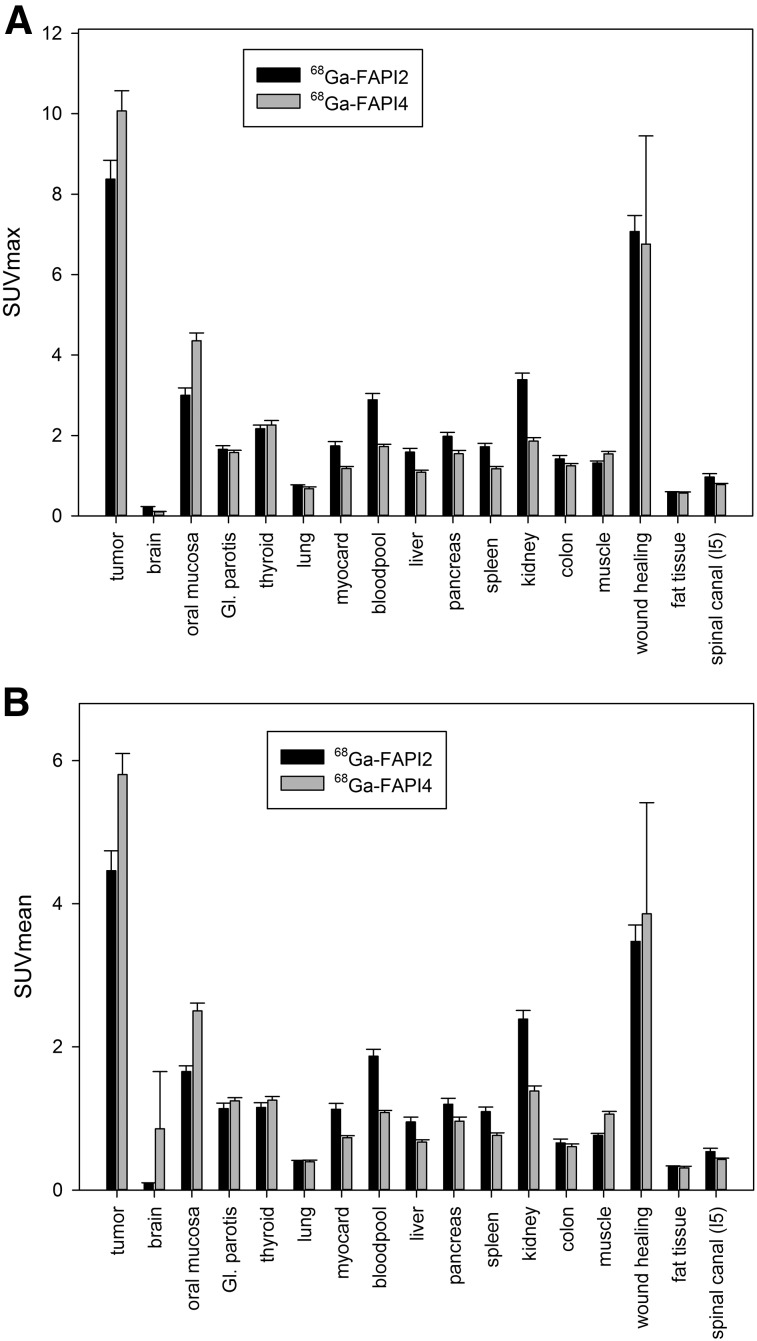
Interindividual comparison of 25 patients examined with 68Ga-FAPI-2 and 25 patients examined with 68Ga-FAPI-4 PET at 1 h after injection. GI = gastrointestinal.
Comparing uptake of 68Ga-FAPI-2 versus 68Ga-FAPI-4 by tumors, the average SUVmax presented no relevant differences (8.37 for 68Ga-FAPI-2 and 10.07 for 68Ga-FAPI-4; not statistically significant). Both tracers had unspecific uptake in locations of wound healing after surgical intervention (7.07 vs. 6.76; not statistically significant). Only minor differences between 68Ga-FAPI-2 and 68Ga-FAPI-4 were observed regarding normal-organ uptake (Fig. 4B).
DISCUSSION
Recently, cancer-associated fibroblasts have been reported as a promising new multitumor target for small-molecule nuclear diagnostics (4,5). In this work, we initially approximated the radiation exposure of 68Ga-FAPI-2 and 68Ga-FAPI-4 PET and found it to be 1.4 and 1.8 mSv/100 MBq, respectively. We further analyzed the normal-tissue biodistribution of these 68Ga-FAPI ligands in comparison to 18F-FDG, the current standard in oncologic PET, and found comparable tumor uptake and, excepting a lower brain, liver, and oral mucosa uptake, comparable background uptake in normal organs.
The approximated dosimetry was limited by evaluating only one patient per tracer. However, the effective organ half-life and hence the radiation exposure is dominated more by the short physical half-life of 68Ga (68 min) than by the biologic half-life of the shuttle molecule, and thus it was no surprise that the effective dose of 68Ga-FAPI-2 and 68Ga-FAPI-4 PET (1.4–1.8 mSv/100 MBq) is similar to that of other 68Ga-based tracers, such as 68Ga-DOTATOC and 68Ga-DOTATATE (2.1 mSv/100 MBq) (12), 68Ga-PSMA-11 (1.6–2.4 mSv/100 MBq) (13,14), 68Ga-PSMA-617 (2.1 mSv/100 MBq), and 18F-FDG (2 mSv/100 MBq) (15), the current standards in oncologic PET.
It was not scope of this work to evaluate diagnostic accuracy such as the sensitivity and specificity of the new modality for a respective tumor entity. However, in a small cohort of challenging patients harboring various tumor diseases, the quantitative tumor uptake, as well as the background activity in most normal organs, was equal to that of 18F-FDG. A lower uptake in brain, liver, and oral–laryngeal mucosa might be promising for evaluation of brain or liver metastases, liver tumors, or head and neck tumors.
68Ga-FAPI PET/CT should also be considered complementary for tumor entities known to perform poorly with 18F-FDG, such as hepatocellular carcinoma or pancreatic cancer. Both have well-known limitations regarding 18F-FDG that are not yet completely covered by specific PET tracers (16,17).
We imaged several tumor entities with tumor-to-background ratios comparable to 68Ga-FAPI and 18F-FDG. We found high 68Ga-FAPI uptake in pancreatic cancer, esophageal cancer, non–small cell lung cancer, head and neck cancer, and colon cancer. In contrast, dedifferentiated thyroid cancer showed a low uptake or was 68Ga-FAPI–negative (Fig. 3). In this setting, the new imaging probes might benefit from their independence from blood sugar level, needing no dietary preparation. The rapid tumor uptake at 10 min after injection, as demonstrated in Figure 2, also indicates the possibility of early imaging. This could increase patient comfort because of a shorter waiting and scan time, which can be relevant in sick patients, and as a side aspect the radiation burden of the examination might be reduced if the injected activity can be reduced. Thus, the diagnostic performance of early versus late 68Ga-FAPI imaging should be evaluated more systematically in future studies. The possibility of early 68Ga-FAPI imaging would also avoid the 1-h uptake time with the patients resting, which is considered mandatory for 18F-FDG; consequently, 68Ga-FAPI PET could simplify the clinical workflow.
In contrast to 18F-FDG, the 68Ga-FAPI ligands contain DOTA as chelator, which can also be labeled with various therapeutic radionuclides. Taking into account the recent successes of radioligand therapy in neuroendocrine (18) and prostate (19) cancer, targeting FAP also presents a promising new approach in the treatment of these FAP-positive tumors. However, a further increase in tumor retention time, as in part already achieved in the development step from 68Ga-FAPI-2 to 68Ga-FAPI-4 (Fig. 2) (5), would still be required to refine the potential of FAPI-targeting radionuclide therapy, for example, with 153Sm or 90Y.
Similar to 18F-FDG, we observed some uptake in postsurgical wound healing because in this condition fibroblasts are also activated. Thus, we would not consider 68Ga-FAPI a more tumor-specific PET tracer than 18F-FDG. However, 18F-FDG is known to accumulate in acute inflammation, whereas FAP activation is typical of chronic inflammation already causing a fibrotic reaction (20,21). Localized FAP activation has also been reported in other diseases that are followed by tissue remodeling, such as myocardial infarction. Thus, 68Ga-FAPI PET/CT could play a complementary role to 18F-FDG in the field of chronic inflammatory cardiac diseases (22) or other diseases with tissue remodeling.
Although the discussed approaches were pursued, no final conclusion about their validity can be drawn from this first proof-of-concept investigation, which was intended to evaluate the dosimetry of 68Ga-labeled 68Ga-FAPI-2 and 68Ga-FAPI-4 diagnostics, identifying physiologic biodistribution and preliminary target validation in selected tumor entities. Further studies dedicated to evaluating the diagnostic performance in the respective clinically relevant settings are highly warranted.
CONCLUSION
68Ga-FAPI PET/CT is a promising new diagnostic method for imaging various kinds of cancer, in particular pancreatic, head and neck, colon, lung, and breast cancer, with tumor-to-background contrast ratios equal to or even better than those of 18F-FDG. The favorable characteristics of the new ligands include fast tracer kinetics that seem appropriate for imaging patients even less than 1 h after injection; low background uptake in liver, oral mucosa, and brain; and independence from blood sugar. Because the 68Ga-FAPI tracers contain the universal DOTA-chelator, a theranostic approach—after labeling the ligand with an appropriate therapeutic radionuclide—also seems feasible.
DISCLOSURE
Uwe Haberkorn, Anastasia Loktev, Thomas Lindner, and Walter Mier have filed a patent application for quinoline based FAP-targeting agents for imaging and therapy in nuclear medicine. No other potential conflict of interest relevant to this article was reported.
REFERENCES
- 1.Hamson EJ, Keane FM, Tholen S, Schilling O, Gorrell MD. Understanding fibroblast activation protein (FAP): substrates, activities, expression and targeting for cancer therapy. Proteomics Clin Appl. 2014;8:454–463. [DOI] [PubMed] [Google Scholar]
- 2.Jansen K, Heirbaut L, Cheng JD, et al. selective inhibitors of fibroblast activation protein (FAP) with a (4-quinolinoyl)-glycyl-2-cyanopyrrolidine scaffold. ACS Med Chem Lett. 2013;4:491–496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Poplawski SE, Lai JH, Li Y, et al. Identification of selective and potent inhibitors of fibroblast activation protein and prolyl oligopeptidase. J Med Chem. 2013;56:3467–3477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Loktev A, Lindner T, Mier W, et al. A tumor-imaging method targeting cancer-associated fibroblasts. J Nucl Med. 2018;59:1423–1429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lindner T, Loktev A, Altmann A, et al. Development of quinoline-based theranostic ligands for the targeting of fibroblast activation protein. J Nucl Med. 2018;59:1415–1422. [DOI] [PubMed] [Google Scholar]
- 6.Hindorf C, Glatting G, Chiesa C, Linden O, Flux G. EANM Dosimetry Committee guidelines for bone marrow and whole-body dosimetry. Eur J Nucl Med Mol Imaging. 2010;37:1238–1250. [DOI] [PubMed] [Google Scholar]
- 7.Andersson M, Johansson L, Eckerman K, Mattsson S. IDAC-Dose 2.1, an internal dosimetry program for diagnostic nuclear medicine based on the ICRP adult reference voxel phantoms. EJNMMI Res. 2017;7:88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Stabin MG, Sparks RB, Crowe E. OLINDA/EXM: the second-generation personal computer software for internal dose assessment in nuclear medicine. J Nucl Med. 2005;46:1023–1027. [PubMed] [Google Scholar]
- 9.Cristy M, Eckerman KF. Specific Absorbed Fractions of Energy at Various Ages from Internal Photon Sources. Oak Ridge, TN: Oak Ridge National Laboratory; 1987. ORNL/TM-8381/V1–V7. [Google Scholar]
- 10.Menzel HG, Clement C, DeLuca P. ICRP publication 110: realistic reference phantoms—an ICRP/ICRU joint effort. A report of adult reference computational phantoms. Ann ICRP. 2009;39:1–164. [DOI] [PubMed] [Google Scholar]
- 11.Bolch WE, Jokisch D, Zankl M, et al. ICRP publication 133: the ICRP computational framework for internal dose assessment for reference adults: —specific absorbed fractions. Ann ICRP. 2016;45:5–73. [DOI] [PubMed] [Google Scholar]
- 12.Sandström M, Velikyan I, Garske-Román U, et al. Comparative biodistribution and radiation dosimetry of 68Ga-DOTATOC and 68Ga-DOTATATE in patients with neuroendocrine tumors. J Nucl Med. 2013;54:1755–1759. [DOI] [PubMed] [Google Scholar]
- 13.Pfob CH, Ziegler S, Graner FP, et al. Biodistribution and radiation dosimetry of 68Ga-PSMA HBED CC: a PSMA specific probe for PET imaging of prostate cancer. Eur J Nucl Med Mol Imaging. 2016;43:1962–1970. [DOI] [PubMed] [Google Scholar]
- 14.Afshar-Oromieh A, Hetzheim H, Kübler W, et al. Radiation dosimetry of 68Ga-PSMA-11 (HBED-CC) and preliminary evaluation of optimal imaging timing. Eur J Nucl Med Mol Imaging. 2016;43:1611–1620. [DOI] [PubMed] [Google Scholar]
- 15.Johansson L, Mattsson S, Nosslin B, Leide-Svegborn S. Effective dose from radiopharmaceuticals. Eur J Nucl Med. 1992;19:933–938. [DOI] [PubMed] [Google Scholar]
- 16.Yun M, Bang SH, Kim JW, Park JY, Kim KS, Lee JD. The importance of acetyl coenzyme A synthetase for 11C-acetate uptake and cell survival in hepatocellular carcinoma. J Nucl Med. 2009;50:1222–1228. [DOI] [PubMed] [Google Scholar]
- 17.Strobel O, Büchler MW. Pancreatic cancer: FDG-PET is not useful in early pancreatic cancer diagnosis. Nat Rev Gastroenterol Hepatol. 2013;10:203–205. [DOI] [PubMed] [Google Scholar]
- 18.Strosberg J, El-Haddad G, Wolin E, et al. Phase 3 trial of 177Lu-Dotatate for midgut neuroendocrine tumors. N Engl J Med. 2017;376:125–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hofman MS, Violet J, Hicks RJ, et al. 177Lu-PSMA-617 radionuclide treatment in patients with metastatic castration-resistant prostate cancer (LuPSMA trial): a single-centre, single-arm, phase 2 study. Lancet Oncol. 2018;19:825–33. [DOI] [PubMed] [Google Scholar]
- 20.Egger C, Cannet C, Gérard C, et al. Effects of the fibroblast activation protein inhibitor, PT100, in a murine model of pulmonary fibrosis. Eur J Pharmacol. 2017;809:64–72. [DOI] [PubMed] [Google Scholar]
- 21.Uitte de Willige S, Malfliet JJ, Janssen HL, Leebeek FW, Rijken DC. Increased N-terminal cleavage of alpha-2-antiplasmin in patients with liver cirrhosis. J Thromb Haemost. 2013;11:2029–2036. [DOI] [PubMed] [Google Scholar]
- 22.Tillmanns J, Hoffmann D, Habbaba Y, et al. Fibroblast activation protein alpha expression identifies activated fibroblasts after myocardial infarction. J Mol Cell Cardiol. 2015;87:194–203. [DOI] [PubMed] [Google Scholar]