Abstract
Owing to increasing international travel, physicians will encounter more infectious diseases acquired overseas, which may be bacterial, fungal or parasitic in nature. 1 Knowledge of the geographic distribution of specific diseases permits the formulation of a differential diagnosis in the context of clinical presentation. Parasitic infestations of the maxillofacial tissues can be caused by a host of different ectoparasites, for example, myiasis, a frequently misdiagnosed disease of tourists returning from exotic locations. For those natives and travellers who are subject to these ‘infestations’, the experience can be both alarming and very distressing.
Keywords: infections, emergency medicine
Background
The human ectoparasitoses myiasis and tungiasis, have now re-emerged as frequently misdiagnosed diseases of tourists returning from exotic destinations. Myiasis, a cutaneous infestation of larvae, caused by the human botfly is rarely encountered in the UK, Europe and the Northern hemisphere. Dermatobia hominis, otherwise known as the human botfly, is native to Central and South America. Infestation is only seen in travellers to these areas. It causes a localised, itchy, erythematous raised skin lesion. The larva may occasionally be visualised protruding through a central punctum. A serous, bloody and rarely purulent discharge can occur. The authors present the case of an 18-year-old Caucasian male with myiasis of the scalp following his return from his travels in Belize. This paper will discuss the epidemiology, life cycle of the human botfly, its clinical presentation, differential diagnosis and methods of larval extraction.
Presentation
An 18-year-old Caucasian young man presented to his local emergency department with a ‘maggot in his scalp’. He had recently returned from a gap year in Belize and reported sustaining several mosquito bites to his head and extremities. Prior to his departure he already had a similar ‘maggot’ removed from his scalp in Belize. His medical history was unremarkable. He was a non-smoker and he did not consume alcohol. Following assessment by the emergency staff, he was reviewed by a member of the oral and maxillofacial team.
Clinical examination revealed a 2 cm pruritic, cutaneous lesion with a central punctum draining a small amount of clear, serosanguinous fluid on his scalp. He reported feeling movement in the lesion and intermittent sharp, stabbing episodes of pain.
Differential diagnosis
A ‘maggot’ was observed projecting and intruding through the punctum. No systemic signs were reported but mild constitutional symptoms such as nausea and lethargy were experienced.
Treatment
Removal of this organism involved a surgical approach. Two per cent of lidocaine with 1:80 000 epinephrine was infiltrated around the lesion and a small elliptical incision made in the scalp (figures 1). The organism was fully exposed and excised. Using pulsatile lavage through a small suction tip, the surgical site was irrigated with 0.9% normal saline solution (figures 2). Primary closure with 5/0 ethilon was achieved. Healing took place uneventfully and no antibiotic therapy was required.
Figure 1.
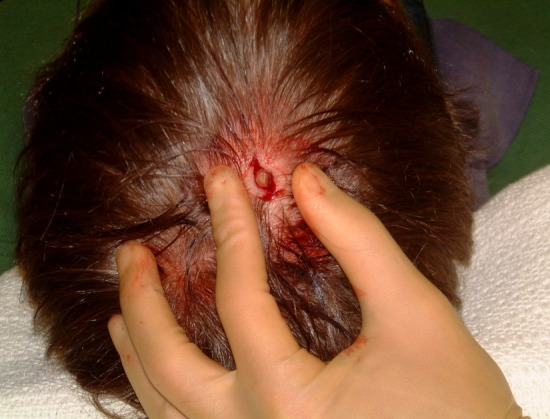
An elliptical incision was performed under local anaesthesia (LA and the larva was identified.
Figure 2.
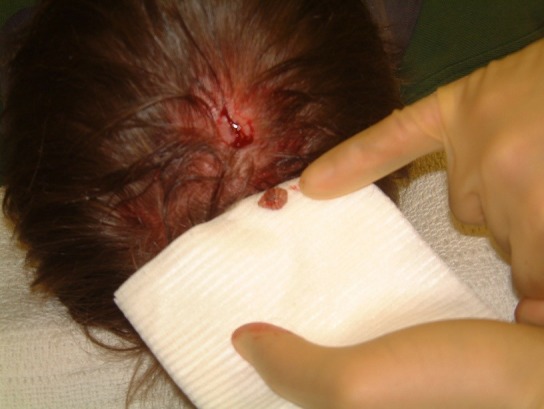
The larva was removed and sent to the entomologist.
Outcome and follow-up
The recovered organism was examined under the microscope by the entomologist. A diagnosis of D. hominis was made with the demonstration and identification of the larva based on its typical morphology.
Discussion
Myiasis, derived from the Greek word ‘myia’ meaning ‘fly’ describes the invasion of tissues and organs of man by larvae of the order Diptera or two-winged flies, producing a furunculoid or boil like lesion. Oral myiasis is a rare condition, a term first introduced by Hope and first described in the literature by Laurence in 1909.2 3 There are three families of ‘two-winged flies’ that cause myiasis, Oestridae (to which D.hominis belongs), Calliphoridae (blow flies) or Sarcophagidae (flesh flies). D. hominis is endemic from Central Mexico, through to Central America and South America. It is commonly found in the forest and jungle areas along rivers. The botfly larvae are obligate parasites, requiring living tissue for development.4 Anatomically, two curved oral hooks are present on the anterior aspect of the larva. At its posterior end, six curved spiracles are present, enabling the larva to breathe. Its life cycle is approximately 3–4 months, the multitude of which is spent in maturation and development. Indeed, the life span of a fully developed adult D. hominis is only 8–9 days. The botfly, unlike others of its species, uses another insect as a mechanical vector for the placement of its eggs. Most commonly, the mosquito is subject to this role. By holding onto the mosquito’s wings using her legs, the female botfly attaches up to 30 eggs to its body. The female botfly can also lay her eggs on plant leaves which may then be deposited on a host as it comes into contact with the leaves. The eggs hatch while the mosquito is feeding on the host, due to the increase in surrounding temperature, and the larvae use the bite wound as the entry point to the skin. The larvae do not migrate from the point of entry and further develop in the subcutaneous tissues for approximately 4–14 weeks. Following maturation of the larvae, they drop out and pupate, usually in the soil. An adult botfly approximately 12–18 mm is finally produced.4 A similar organism found in tropical Africa, the Tumbu Fly (Cordylobia anthropophaga) causes a comparable form of myiasis by contaminating clothing while drying.
There are many cases of oral and cutaneous myiasis and filariasis in the literature, involving species including the house fly and its larval state the screw worm, Cochyliomyia species.
Other flies involved in myiasis include Cordylobia, Chrysomya, Cuterebra (rodent or rabbit botfly), Gasterophilus (horse botfly), Hypoderma (warble fly), Phaenicia (blow fly) and Wohlfahrtia (flesh fly). Cases have been reported involving the scalp, periauricular region and a man’s heel.5–7 To date, only seven cases of cerebral myiasis have been described, with the most serious case involving the green bottle blowfly Phaenicia sericata.8 9
Other non-obligate parasites have been known to infest the tissues of the head and neck by depositing eggs in open wounds, eg, Cochliomyia hominovorax.10
Poor hygiene and a low socioeconomic status are the most important risk factors for acquiring myiasis. The typical furuncular lesion is a papule or nodule with a central punctum that exudes serosanguinous or purulent fluid. In the central pore, by direct visualisation of the posterior part of the larva, intermittent movement of the parasite may be observed. It can also be observed indirectly with bubbling in the discharge. The larvae cause a raised hard painful skin lesion. Pruritus and a sensation of movement of the larvae are the most commonly reported symptoms. Secondary bacterial infection is the most common complication. The diagnosis is rendered clinically in regions where the disease is endemic.
A travel history should be taken including the region where the patient travelled, climate conditions and the species endemic to these regions.
Differential diagnoses have included cutaneous furunculosis, epidermal inclusion cysts, insect pruritus and cutaneous leishmaniasis. As this case report illustrates, cutaneous myiasis should be considered in the differential diagnosis of a subcutaneous mass in the head and neck region, especially in travellers returning from endemic regions. Dermoscopy has been used to identify the posterior parts of a D. hominis maggot within a furuncular lesion and may be helpful if diagnostic doubt is present. A Doppler ultrasound scan can also be used to aid in the diagnosis by visualising larval movements within lesions that were initially thought to be simple furuncles. Laboratory investigations are usually normal except in chronic infestation where an eosinophilia and an elevated immunoglobulin E level may be found.
A variety of methods of treatment and removal have been documented. Potentially effective treatments include occlusion of the spiracular plate with petroleum jelly, bees wax, nail polish or bacon fat as they interfere with the larva’s respiration and force it to extrude itself.
However, if occlusive therapy is unsuccessful, the larvae may suffer asphyxia necessitating their removal. Wooden spatulas have been used effectively to apply pressure to pop the larva out. Larvae can also be removed with suction. Lidocaine injections underneath the cyst creates pressure that pushes the larva out. Surgical debridement using a scalpel is the treatment of choice. After removal, the maggot should be killed by immersion for 30 s in very hot water which prevents decay and maintains the natural colour. The larvae should then be preserved in a solution of 70%–95% ethanol. Tetanus prophylaxis should be administered. Furthermore, insect repellents play a role in keeping mosquitoes carrying larval eggs away. It is important that travellers wear protective clothing. Identification can be very challenging with specific knowledge of the morphological aspects of the larva necessary for the identification of the maggot’s species, a task usually done by an entomologist.
Admittedly, a high index of suspicion is required in individuals presenting with a new skin lesion following their return from travelling to endemic areas.
Learning points.
Dermatobia hominis is endemic to South America and causes skin furuncular myiasis at the site of egg penetration.
This case highlights the importance of establishing a travel history, especially in individuals returning from travel to endemic regions.
The only effective treatment is complete surgical excision and prompt communication between the surgeon and entomologist to establish the diagnosis.
Footnotes
Contributors: LD: wrote the case report. VS: edited the paper.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: None declared.
Provenance and peer review: Not commissioned; externally peer reviewed.
Patient consent for publication: Obtained.
References
- 1. Diaz JH. The epidemiology, diagnosis, management, and prevention of ectoparasitic diseases in travelers. J Travel Med 2006;13:100–11. 10.1111/j.1708-8305.2006.00021.x [DOI] [PubMed] [Google Scholar]
- 2. Hope FW. On insects and their larvae occasionally found in human body. Royal Entomological Society Transactions 1840;2:236–71. [Google Scholar]
- 3. Laurence SM. Dipterous larvae infection. BMJ 1909;9:88. [Google Scholar]
- 4. Garvin KW, Singh V. Case report: cutaneous myiasis caused by Dermatobia hominis, the human botfly. Travel Med Infect Dis 2007;5:199–201. 10.1016/j.tmaid.2006.09.005 [DOI] [PubMed] [Google Scholar]
- 5. Liebert PS, Madden RC. Human botfly larva in a child’s scalp. J Pediatr Surg 2004;39:629–30. 10.1016/j.jpedsurg.2003.12.035 [DOI] [PubMed] [Google Scholar]
- 6. Boruk M, Rosenfeld RM, Alexis R. Human botfly infestation presenting as peri-auricular mass. Int J Pediatr Otorhinolaryngol 2006;70:335–8. 10.1016/j.ijporl.2005.06.025 [DOI] [PubMed] [Google Scholar]
- 7. Grosshans EM, Kremer M. Maleville J: Du role des demodex folliculorum dans l’histogenese de la rosacee granulomateuse. Bull Soc Dermatol Syphilgr 1972;79:639–46. [Google Scholar]
- 8. Cheshier SH, Bababeygy SR, Higgins D, et al. Cerebral myiasis associated with angiosarcoma of the scalp: case report. Neurosurgery 2007;61:E167 10.1227/01.neu.0000279738.15307.37 [DOI] [PubMed] [Google Scholar]
- 9. Froomin LL. Kaznelson AB: Intradural cyst of parasitic origin [myiasis clinic]. Zh Ushn Nos Gorl Bolezn 1939;16:427–33. [Google Scholar]
- 10. Zeltser R, Lustmann J. Oral myiasis. Int J Oral Maxillofac Surg 1988;17:288–9. 10.1016/S0901-5027(88)80004-3 [DOI] [PubMed] [Google Scholar]


