Abstract
Background
Chronic obstructive pulmonary disease (COPD) is a heterogeneous disease characterised by airflow obstruction and other morbidities such as respiratory symptoms, reduced physical activity and frequent bronchodilator use. Recent advances in personal digital monitoring devices can permit continuous collection of these data in COPD patients, but the relationships among them are not well understood.
Methods
184 individuals from a single centre of the COPDGene cohort agreed to participate in this 3-week observational study. Each participant used a smartphone to complete a daily symptom diary (EXAcerbations of Chronic pulmonary disease Tool, EXACT), wore a wrist-worn accelerometer to record continuously physical activity and completed the Clinical Visit PROactive Physical Activity in COPD questionnaire. 58 users of metered dose inhalers for rescue (albuterol) were provided with an inhaler sensor, which time stamped each inhaler actuation.
Results
Rescue inhaler use was strongly correlated with E-RS:COPD score, while step counts were correlated with neither rescue use nor E-RS:COPD score. Frequent, unpatterned inhaler use pattern was associated with worse respiratory symptoms and less physical activity compared with frequent inhaler use with a regular daily pattern. There was a strong week-by-week correlation among measurements, suggesting that 1 week of monitoring is sufficient to characterise stable patients with COPD.
Discussion
The study highlights the interaction and relevance of personal real-time monitoring of respiratory symptoms, physical activity and rescue medication in patients with COPD. Additionally, visual displays of longitudinal data may be helpful for disease management to help drive conversations between patients and caregivers and for risk-based monitoring in clinical trials.
Keywords: digital health, activity monitors, medication sensors, patient-reported outcomes, COPD
Key messages.
Digitally collected outcomes over the first week of observation were similar to behaviour over the full three weeks of observation suggesting that one week of phenotyping is sufficient for stable COPD.
We identified several distinct individual patterns of rescue medication use, such as infrequent and frequent users and within frequent users, some use was the result of patterned behaviour. Unpattern use was associated with worse COPD outcomes.
Advances in personal digital monitoring devices have allowed recent studies to capture these disease aspects in the real world; however, there are limited data demonstrating how the information can be integrated to support real-time evaluation. This study demonstrates how sensor phenotyping can be integrated in real-time in COPD.
Introduction
Chronic obstructive pulmonary disease (COPD) has multiple subtypes, including emphysema, bronchitis and the frequent exacerbator phenotype.1–3 However, disease progression and mortality are variable and difficult to predict.4–6 Although clinical variables such as age, smoking history, dyspnoea, exacerbation history and body mass index are somewhat useful to model these subtypes, assess disease severity and predict disease progression,7–10 a large amount of unexplained variance remains. For example, airflow obstruction as measured by forced expiratory volume in 1 s (FEV1) is classically used to assess disease severity and predict prognosis,11 but it does not distinguish subtypes and is a poor predictor of disease progression.12
Once diagnosed, management of the patient with COPD is almost entirely based on clinical judgement, with healthcare provider assessments limited to highly subjective patient reports which are of questionable validity.13 Commonly relied on markers for disease state, such as patient reported rescue inhaler use, are highly inaccurate, with studies showing as few as 6% of patients demonstrating appropriate technique and adherence with prescribed inhalers.14 More objective markers, such as a patient’s degree of functional status, are known to be strongly predictive of both morbidity and mortality in COPD. However, the clinical utility of this functional status is greatly limited due to the difficulty in assessing actual home activity at an office visit and infrequent or limited monitoring which occurs in a clinical visit or pulmonary rehabilitation programme.15 For instance, in a population study such as KORA, participants with higher lung function had more activity as measured by Actigraph hip accelerometers, but there were few COPD participants in this study.16 There are also efforts to integrate sensors in pulmonary rehabilitation research, such as a recent pilot study used an Actigraph CT3X+accelerometer to assess changes in steps after randomisation to pulmonary rehabilitation or a clinician-facilitated physical activity intervention.17
Recent technological advances are also now making it possible to conduct objective, real-time, home assessment of symptoms, activity and medication use in patients with COPD.18–20 For instance, 35 patients with COPD who were followed for 12 weeks using electronic inhaler sensors had a 14% increase in inhaler usage during moderate-to-severe exacerbations.21 Using and understanding these real-time, objective, highly granular measures of health in patients with COPD are required steps in achieving the Global Initiative for Chronic Obstructive Lung Disease (GOLD) 2017 mandate that we develop a ‘comprehensive, personalised, patient-tailored approach’ to the management of COPD.22 To date, these types of precision medicine techniques have only been evaluated individually in small pilot studies. Little is known about the utility of performing these types of home digital assessments concurrently, especially in COPD research participants. This study examines the feasibility and value of conducting unassisted, digital assessments of physical activity, daily respiratory symptoms and rescue inhaler use, in the homes of participants from the well-characterised COPDGene cohort.
Methods
Study population
This study and the parent study were approved by the institutional review board at National Jewish Health (Denver, Colorado, USA). Written informed consent was obtained from all participants. Details of the COPDGene study, including recruitment, data collection and longitudinal follow-up are described in online supplementary file 1 and a previous publication.23 COPDGene (NCT02445183) enrolled 10 300 participants ages 45–80 in 19 clinical centres from December 2007 to April 2011. A total of 5835 returned for a single 5-year follow-up visit from 2013 to 2017. Of these, 746 were seen at a single centre (National Jewish Health). Total 255 of these participants were seen from March-October, 2016 and asked to participate in this 3 week observational substudy. Total 184 participants agreed and were consented to participate.
bmjresp-2018-000350supp001.pdf (269.5KB, pdf)
Procedures
Participants were provided with and instructed on the use of the following three devices:1 a Samsung smartphone that administered the EXAcerbations of Chronic pulmonary disease Tool (EXACT);24 they were instructed to complete EXACT daily; if they did not complete it, a research coordinator was informed and they were instructed to complete within 24 hours; at the end of the second week, the participant completed the clinic visit version of the PROactive questionnaire;2 18 participants were fitted with a GT9X or GT3X-BT physical activity monitor (Actigraph, Pensacola, Florida, USA), to be worn continuously for the 3-week study period;3 participants using albuterol rescue metered dose inhalers (MDIs) were offered a Smartinhaler sensor (Adherium, Auckland, New Zealand) to attach to their medication.
Definitions
COPD was defined by postbronchodilator FEV1 to forced vital capacity (FVC) ratio of <0.70. There severity class of airflow limitation1–4 was assessed using GOLD criteria.25 Smoker controls were current or former smokers without evidence of airflow obstruction (FEV1/FVC ≥0.70) and FEV1% predicted ≥80%. Participants with FEV1/FVC ≥0.70) and FEV1%<80% of predicted were categorised as Preserved Ratio Impaired Spirometry (PRISm). Emphysema was quantified by the per cent of voxels with Hounsfield Units (HU)<−950 (%LAA) on CT. Moderate exacerbations were defined as those treated with steroids and/or antibiotics; severe exacerbations were defined as those resulting in hospitalisation. The E-RS: Evaluating Respiratory Symptoms in COPD (E-RS:COPD) measure was calculated from an 11-question subset of the full 14-question daily EXACT questionnaire. Rescue inhaler use where more than one actuation was recorded by the sensor within a 2 min interval was defined as a single rescue occasion. Rescue inhaler use patterns over the 3 weeks were qualitatively assessed independently by three investigators (RPB, NL, MA) who were blinded to participants’ clinical characteristics. Participants were retrospectively categorised into four patterns: infrequent use (<2 rescue events per day), occasional use with rare bad days of ≥2 rescue events per day, frequent use (≥2 rescue events per day) with no specific use pattern and frequent regular use (same time of day) either once daily or two or more times daily (with or without additional use). Group assignment was by consensus. Emphysema progression was assessed using changes in adjusted lung density (g/L) in the previous 5 years. COPD progression was expressed as the change in FEV1 (mL) per year over the previous 5 years. Comorbidities were assessed via a medical history questionnaire assessed at the COPDGene Study Year 5 clinic visit.
Statistical analysis
SAS (V.9.3), S-PLUS (V.7.0.6) and R (V.3.3.1) were used for analysis. Figure graphics were made in R V.3.4. Demographic characteristics of study participants and participant groups were analysed using t-tests and χ² tests. A minimum of 8 hours of wear time for ActiGraph was required for a day to be included in analysis. Summaries of ActiGraph variables were calculated using medians due to the potentially high variance of activity. Summaries of E-RS score and rescue inhaler use were calculated using means. Correlations were calculated using Spearman’s rank correlation. Weekly median summaries for the activity monitoring variables were required to have a minimum of 3 days of valid wear time, identical to the requirements for the PROactive questionnaire. Overall, 3 week summaries of activity monitoring variables were required to have a minimum of 7 days of valid wear time. Weekly mean summaries of E-RS:COPD scores were required to have a minimum of 4 days to be calculated, similar to the requirements for calculating EXACT score at baseline. Similarly, weekly mean summaries of rescue inhaler use needed a minimum of 4 days to be calculated. Beeswarm plots were used to show distributions of variables among subgroups. Boxplots show the IQR, median and whiskers, which represent the minimum of the upper range and the value at the 75th percentile + IQR * 1.5 and the maximum of the lower range and the value at the 25th percentile – IQR * 1.5. Physical Activity Measures During the Scatter plots were used to display relationships between different variables with shaded area representing the 95% CI of the regression line.
Results
Demographics
Of the 184 participants who consented to participate, three withdrew early (on Day 1, 2 and 3, respectively) and one who was non-compliant with study assessments withdrew on Day 7. The analysis population for this study consists of the 180 participants completing at least 1 week. 179 participants completed the full 3-week study. All 180 participants used the electronic diary, 179 used the activity monitor, 58 participants in the cohort used MDI rescue inhalers and 143 participants completed the PROactive questionnaire at Day 14.
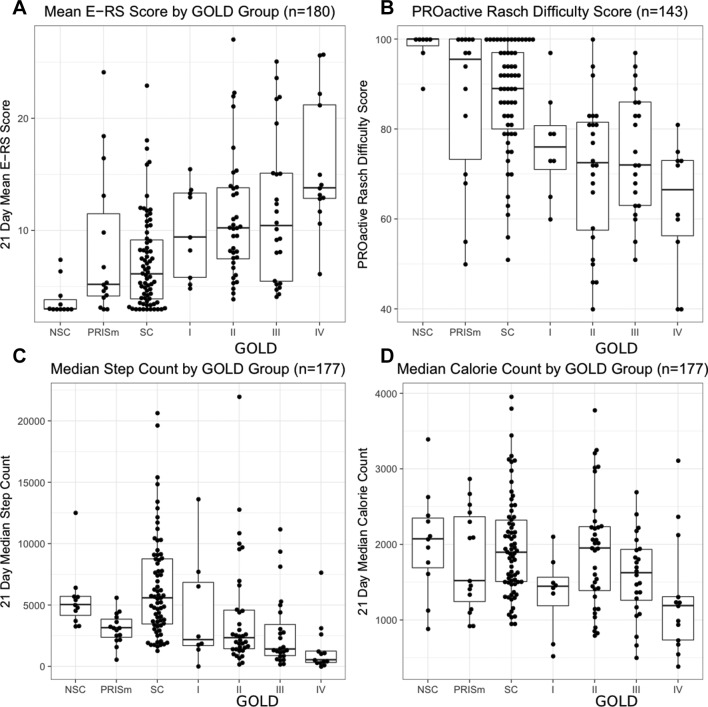
The baseline characteristics for the study population are shown in table 1. Respiratory symptoms and rescue medication use were higher and physical activity was lower for participants with more severe COPD (figure 1). In order to determine an optimal time to phenotype stable participants, we compared each weekly summary of E-RS:COPD total scores, median daily step counts and mean daily inhaler use (online supplementary figure 1-3). The correlation among the 3 weeks ranged from 0.95 to 0.96 for (p<0.001) for steps, 0.91–0.94 for (p<0.001) for E-RS score and 0.88–0.88 for (p<0.001) for rescue inhaler use, suggesting that 1 week of daily symptom diary and actigraphy is sufficient to phenotype a stable COPD participant.
Table 1.
Demographics and clinical characteristics of study participants
| Characteristics | Non-smoking controls (n=11) | PRISm (n=15) | Smoking controls (n=72) | GOLD 1 (n=9) | GOLD 2 (n=36) | GOLD 3 (n=24) | GOLD 4 (n=13) | P value |
| Age (years) | 58 (9) | 68 (6) | 63 (7) | 64 (7) | 67 (8) | 69 (7) | 66 (7) | <0.001 |
| Male, n (%) | 5 (45%) | 6 (40%) | 33 (46%) | 4 (44%) | 19 (53%) | 12 (50%) | 7 (54%) | 0.980 |
| Body mass index (kg/m2) | 25 (4) | 30 (6) | 28 (6) | 24 (4) | 30 (6) | 30 (8) | 27 (5) | 0.020 |
| Current smoker, n (%) | 0 (0%) | 3 (20%) | 25 (35%) | 3 (33%) | 10 (28%) | 6 (25%) | 1 (8%) | 0.427 |
| Smoking pack-year history | n/a | 43 (25) | 39 (23) | 38 (20) | 53 (30) | 55 (25) | 43 (17) | 0.020 |
| Chronic cough, n (%) | 0 (0%) | 1 (7%) | 14 (19%) | 1 (11%) | 20 (56%) | 11 (46%) | 5 (38%) | <0.001 |
| Chronic phlegm, n (%) | 0 (0%) | 3 (20%) | 15 (21%) | 5 (56%) | 16 (44%) | 10 (42%) | 5 (38%) | 0.011 |
| History of wheeze, n (%) | 1 (9%) | 8 (53%) | 25 (35%) | 5 (56%) | 19 (53%) | 18 (75%) | 11 (85%) | <0.001 |
| FEV1, (% predicted) | 105 (11) | 72 (6) | 99 (12) | 91 (8) | 63 (9) | 41 (6) | 24 (5) | <0.001 |
| 6 min walking distance (m) | 581 (69) | 413 (132) | 483 (94) | 459 (134) | 380 (110) | 322 (148) | 278 (120) | <0.001 |
| BODE index | 0.3 (0.5) | 0.8 (1.3) | 0.4 (0.7) | 0.7 (0.9) | 1.7 (1.5) | 3.0 (1.3) | 4.8 (1.1) | <0.001 |
| Emphysema LAA% (−950HU) | 1.3 (1.3) | 1.0 (1.0) | 1.6 (1.7) | 13.8 (9.8) | 8.6 (7.9) | 15.8 (13.2) | 30 (17.1) | <0.001 |
| Lung density (g/L) | 80 (11) | 92 (16) | 83 (15) | 54 (18) | 68 (19) | 55 (21) | 35 (19) | <0.001 |
| Pi10 (mm) | 3.5 (0.1) | 3.6 (0.1) | 3.6 (0.1) | 3.6 (0.1) | 3.6 (0.1) | 3.7 (0.1) | 3.7 (0.1) | <0.001 |
| MMRC dyspnoea score ≥2, n (%) | 0 (0%) | 4 (27%) | 13 (18%) | 4 (44%) | 22 (61%) | 16 (67%) | 13 (100%) | <0.001 |
| SGRQ total score | 2 (4) | 21 (20) | 13 (14) | 24 (16) | 35 (19) | 40 (20) | 51 (18) | <0.001 |
| Prior year exacerbation history | n/a | 0.2 (0.6) | 0.1 (0.3) | 0.6 (1.1) | 0.8 (1.3) | 0.5 (0.7) | 2.0 (1.8) | <0.001 |
| Supplemental oxygen use, n (%) | 0 (0%) | 3 (20%) | 4 (6%) | 1 (11%) | 18 (50%) | 17 (71%) | 13 (100%) | <0.001 |
| Cardiovascular disease, n (%) | 2 (18%) | 3 (20%) | 8 (11%) | 2 (22%) | 9 (25%) | 6 (25%) | 2 (15%) | 0.602 |
| High blood pressure, n (%) | 2 (18%) | 6 (40%) | 29 (40%) | 3 (33%) | 16 (44%) | 12 (50%) | 3 (23%) | 0.518 |
| Gastro-oesophageal reflux, n (%) | 2 (18%) | 4 (27%) | 15 (21%) | 3 (33%) | 16 (44%) | 9 (38%) | 5 (38%) | 0.210 |
| Diabetes, n (%) | 0 (0%) | 0 (0%) | 8 (11%) | 0 (0%) | 4 (11%) | 3 (13%) | 2 (15%) | 0.570 |
FEV1, forced expiratory volume in 1 s; GOLD, Global Initiative for Chronic Obstructive Lung Disease.
Figure 1.
More severe COPD is associated with worse eDiary symptoms and lower measures of activity. Shown are individual participants mean (A) eDiary score and (B) PROactive Rasch Difficulty score; median (C) daily steps and (D) calorie count during the 3-week study period by group. E-RS:COPD, evaluating respiratory symptoms in chronic obstructive pulmonary disease.
Relationship between digital measures
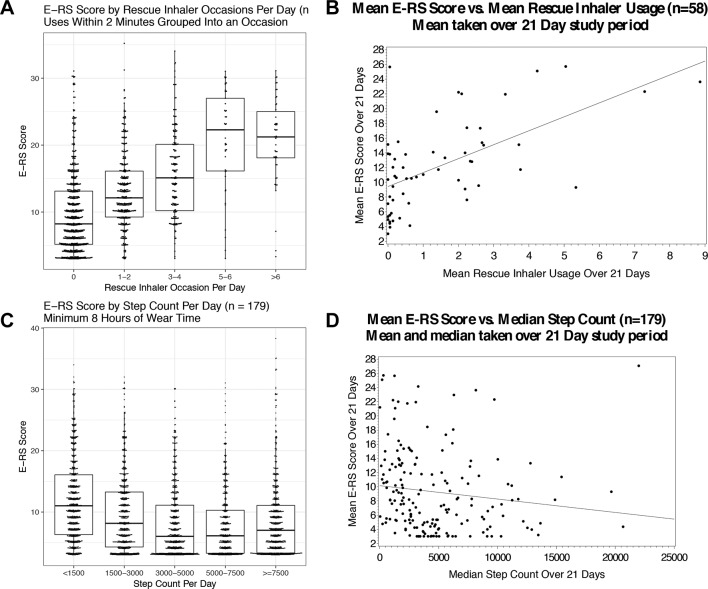
Daily respiratory symptoms (E-RS:COPD total score) were positively associated with more frequent daily rescue inhaler usage (p<0.001) and steps per day (p<0.001) among all participants (figure 2) when assessed day-by-day (over 24 hours). For the entire duration of the study (3 weeks), mean respiratory symptoms was across all patients with COPD (GOLD I-IV, n=82), median step counts measured over 3 weeks were moderately correlated with 6 min walk distance (rho=0.60, p<0.001) and mean respiratory symptoms (E-RS:COPD) over 3 weeks were moderately correlated with CAT (rho=0.72, p<0.001), SGRQ total score (rho=0.70, p<0.001), mMRC (rho=0.60, p<0.001), rescue medication use (rho=0.52, p<0.001) and inversely correlated with SF-36 Physical Function score (rho=−0.48, p<0.001) (online supplementary table 1). Median step counts were not correlated with mean respiratory symptoms (E-RS:COPD score) over 3 weeks (rho=−0.05, p=0.646). Also, more difficulty with performing physical activity (higher PROactive Rasch Difficulty Score) was associated with more rescue inhaler use and E-RS:COPD score (rho=−0.82; p<0.001) (online supplementary figure 4).
Figure 2.
Worse eDiary scores are associated the more rescue inhaler usage and reduced step counts over both an individual day and 3-week summary period. Association of daily respiratory symptoms (E-RS:COPD total score) with (A) daily rescue occasions and (C) daily step counts; association of 3-week mean respiratory symptoms with (B) mean rescue inhaler occasions and (D) median steps per day. E-RS:COPD, evaluating respiratory symptoms in chronic obstructive pulmonary disease.
For those patients with COPD requiring supplemental oxygen (n=49), step counts were moderately correlated with CT measures of emphysema (rho=−0.47, p<0.001) and lung density (rho=0.46, p<0.001), along with hours/day of oxygen use (rho=−0.56, p<0.001), while physical function (as measured by SF-36; rho=−0.56, p<0.001) was moderately correlated with respiratory symptoms. For patients with COPD who do not use supplemental oxygen (n=33), age also had a moderate (negative) correlation with step counts (rho=−0.58, p<0.001) (online supplementary table 1).
Higher E-RS:COPD scores were associated with more emphysema progression over the previous 5 years (−0.31±0.11 g/L per one-unit higher E-RS:COPD score; p=0.0058; figure 3), but not with progression in airflow limitation (−0.82±0.67 mL/year per one-unit higher E-RS:COPD score; p=0.22). Median steps per day were associated with neither progression of emphysema (0.04±0.14 g/L per 1000 steps per day; p=0.80) nor progression of airflow obstruction (0.69±0.95 mL/year per 1000 steps per day; p=0.47).
Figure 3.
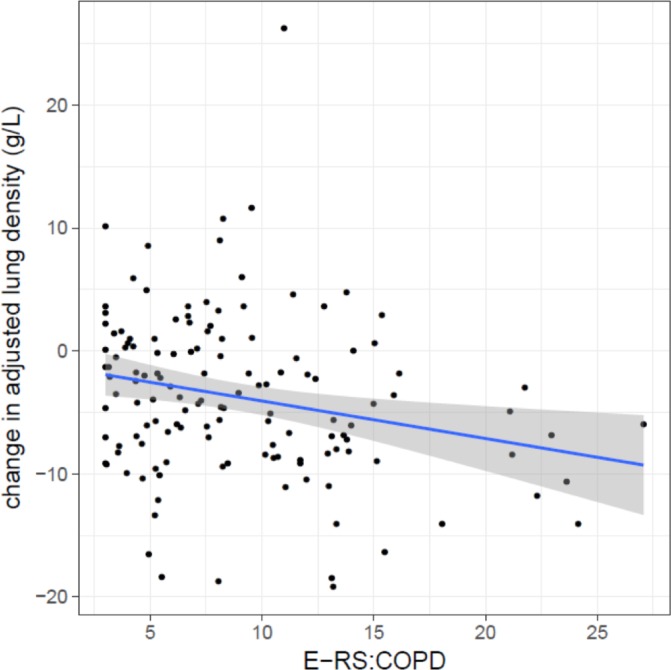
A higher E-RS:COPD score is associated with more emphysema progression. Emphysema progression was measured by change in adjusted lung density over the 5 years prior to the eDairy assessments. E-RS:COPD, evaluating respiratory symptoms in chronic obstructive pulmonary disease.
Patterns of rescue inhaler usage
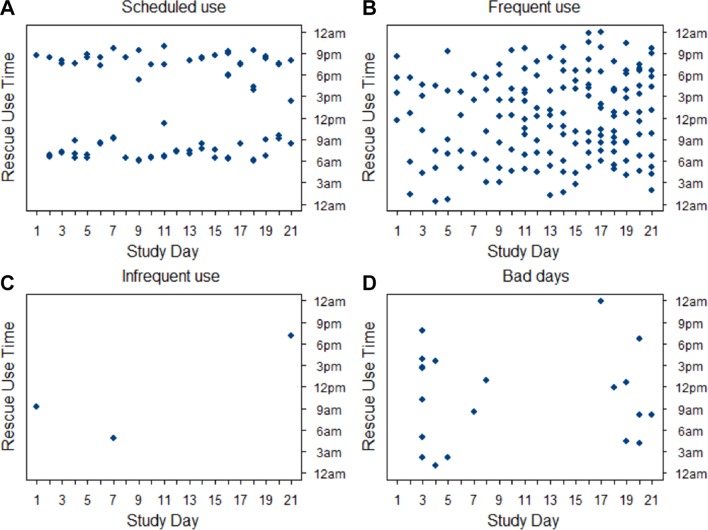
Historically, collection of rescue medication use in clinical trials has been through patient recall using daily diaries. The use of passive sensors on the rescue medications removes recall bias and adds temporal granularity beyond daily summary. We used the inhaler usage data to create temporal plots for each participant with study day on the x-axis, and time of day (00:00 hours to 23:59 hours) on the y-axis and found that the participants broadly fell into four use profiles: participants primarily who use rescue on a regular schedule (a two times per day user example is shown in figure 4); frequent users with no specific pattern (individual example in figure 4); infrequent users (individual example in figure 4) and participants with a few ‘bad days’ (individual example in figure 4). Among the participants with scheduled use, some had one time per day patterns, others two times per day and one that had a three times per day ‘meal time’ pattern. Participant with these use patterns still had occasional rescue use outside of those consistent use times. Participants with frequent albuterol usage were more likely to report wheezing or whistling in their chest (p=0.03) and had higher SGRQ scores (p=0.03) (online supplementary table 2).
Figure 4.
Individual participants demonstrate several different patterns of rescue inhaler use. Typical rescue inhaler use patterns. (A) Scheduled use (at regular times); (B) frequent use (without clear patterned use); (C) infrequent use; (D) bad days (a few days with frequent use).
EXACT events
While this study was not designed to identify healthcare resource utilisation (HCRU) exacerbations, we did review the data from the EXACT diary to identify participants who had a worsening of their COPD symptoms during the study. There were nine participants (5%) who met the criteria of an EXACT-defined event over the 3-week observation period. There was no clear pattern of EXACT-defined events with step counts and rescue medication occasions in these nine participants (four examples of the profiles are found in figure 5 and the remaining five profiles in online supplementary figure 5). Of note, at least one participant in each group, except for non-smoker controls, experienced an EXACT-defined event during the study.
Figure 5.
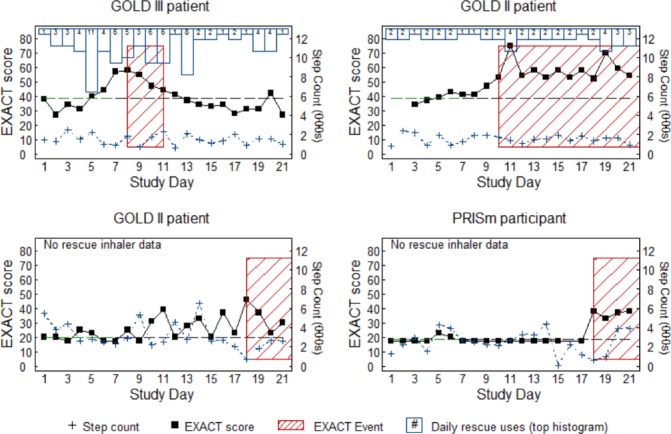
Changes in eDairy scores are only sometimes associated with changes in rescue inhaler use or step counts. Shown are four representative events from nine EXACT defined events (red hashed box). Black squares represent EXACT score with a dashed line representing baseline; blue outlined columns represent frequency of rescue inhaler use for the day; blue crosses represent step counts in thousands of steps per day. EXACT, EXAcerbations of Chronic pulmonary disease Tool.
Discussion
Precision medicine has been defined as ‘an emerging approach for disease treatment and prevention that takes into account individual variability in genes, environment, and lifestyle’.26 While much recent research in COPD has focused on the genes (eg, GWAS and Omics studies) and environment (eg, air pollution), there have been fewer studies investigating day-to-day intraparticipant variability, particularly integrating respiratory symptoms, activity and medication use (ie, lifestyle). This study demonstrates that such an integrated approach is feasible in stable COPD, with the mean daily diary adherence of 91% and mean daily activity monitor adherence (≥8 hours) of 96% over the duration of this 3-week study. Step counts were largely consistent on a day-to-day basis, particularly among patients with COPD. This may partially be due to step counts being quite low overall for these patients relative to other age-matched cohorts such as NHANES27 or possibly due to patients managing their disease by being less active overall. We also found that stable patients have short-term activity, symptom and rescue inhaler patterns which are very reproducible from week-to-week for most participants. This suggests a stable COPD phenotype might be ascertained with as little as 1 week of observation.
In relating the diary and sensor measures to clinical outcomes from the COPDGene study, a link between current symptoms and prior disease progression was suggested by higher E-RS scores, which were associated with more emphysema progression during the initial 5-year observation period of the main COPDGene study, which could be related factors such as inflammation, which have previously been associated with emphysema progression.5 28 For patients using supplemental oxygen, step counts were moderately correlated with CT measurements of emphysema, which may suggest an association between activity and disease severity. Also, step counts were moderately correlated with the clinic visit 6 min walk distance (rho=0.60), which is expected in that they both assess physical activity.
The availability of remote lifestyle data was particularly useful for the daily diary and rescue inhaler usage data. For instance, deviations from the usual pattern in inhaler or diary score could herald the onset of an exacerbation and automated monitoring could lead to early exacerbation identification and treatments. Also, we found that a participant who uses a rescue inhaler 3–4 times per day with regular timed usage is typically less symptomatic and has less severe COPD manifestations than a participant who uses 3–4 times per day with irregular usage. An interesting observation regarding rescue medication use is that the perception of clinical trial outcomes such as ‘mean daily rescue use’ or ‘rescue-free days’ may be insufficient measures due to patterned participant behaviour. This suggests a need to identify habitual users during a run-in period in intervention studies assessing rescue inhaler use as an outcome measure. We speculate that patients who are using their rescue inhalers at approximately the same time each day, potentially with meals or associated with waking up or going to bed (as seen in figure 5) could actually be reclassified as preventative use. This knowledge could provide an opportunity for the healthcare provider to have individual conversations with these patients to better understand their disease management and ensure appropriate use of their rescue inhaler. More research is needed to determine whether these patterns of rescue inhaler use are important when assessing rescue inhaler use as a clinical trial endpoint.
We also found that while the cohort-level digital measures correlated as expected (ie, less activity, more symptoms and more inhaler use in participants with more severe COPD), individual patient behaviour was variable and might obscure patterned behaviour (eg, with inhaler use). With the availability of real-time data, individual patient behaviours could potentially be altered through education or coaching. For example, a patient with COPD who is an extremely frequent user of their rescue medication (figure 5) could be contacted by their healthcare professional to understand whether the patient needs retraining on proper inhaler use or to discuss adherence with their maintenance medication or the need to have their maintenance medication changed or augmented.
Among the diary and sensor measures, rescue medication usage was correlated with E-RS:COPD score at a daily level across all participants. However, the variation in symptom scores was mainly due to differences in severity, rather than an individual’s day-to-day symptom fluctuations. Within the patients with COPD, mean symptoms score and median steps per day were not correlated (online supplementary table 1). The reason for this may be due to the elimination of important day-to-day variability that is inherent in some patients with COPD, along with the relative inactivity of patients with COPD compared with other people their age.
While we have generated visualisations with the large amounts of data in this study, additional work will be needed to develop a platform that will facilitate patient-level summaries for clinical applications. Additionally, machine learning methods could be used to identify more complex data patterns that can be mined for additional insight. As more digital data are gathered in large COPD cohorts, these approaches may inform a provider whether to contact a patient for urgent evaluation or intervention, or conversely, to prompt the patient to contact their healthcare provider.
Our findings also suggest that in a stable group of patients with COPD, 1 week of data collection suffices for short-term profiling using these measures, as there was very high correlation (rho≥0.88) across the individual summaries for each study week (online supplementary figure 1-3). At a cohort level, the outcome measures in this study generally track with disease severity, as we would expect. Specifically, more severe COPD, as defined by FEV1, is associated with fewer steps and calories expended, more rescue medication usage, worse respiratory symptoms (as measured by E-RS:COPD score) and more difficulty with activities (as measured by PROactive difficulty score). Of note, the PRISm group (preserved FEV1/FVC ratio, impaired spirometry (FEV1<80% predicted)) had median step counts and respiratory symptoms more aligned with GOLD I/II than with smoking controls (table 2), which is consistent with a recently published report showing that PRISm participants have more increased dyspnoea, reduced 6 min walk distance and thicker airways on CT scan compared with control smokers with similar smoking history but normal FEV1% (≥80% predicted).29
Table 2.
Summary of remote data measurements during the 3-week study period
| Non-smoking controls (n=11) |
PRISm (n=15) |
Smoking controls (n=72) |
GOLD 1 (n=9) |
GOLD 2 (n=36) |
GOLD 3 (n=24) |
GOLD 4 (n=13) |
P value | |
| Physical activity | ||||||||
| Median [IQR] steps per day* | 5038 [3744–5721] | 3156 [2198–4036] | 5585 [3429–8784] | 2182 [1601–7144] | 2338 [1437–4608] | 1420 [870–3753] | 544 [321–1249] | <0.001 |
| Median [IQR] calories per day* | 2074 [1615–2387] | 1521 [1147–2427] | 1896 [1509–2333] | 1447 [1020–1633] | 1952 [1364–2238] | 1625 [1232–1938] | 1190 [735–1308] | 0.006 |
| Mean PROactive Rasch Difficulty Score | 98 (4.1) | 85.9 (17.8) | 87.3 (12.3) | 76.5 (11.7) | 70.9 (16.3) | 74.6 (13.4) | 63 (14.5) | <0.001 |
| Daily symptoms | ||||||||
| Mean E-RS:COPD Total Score | 3.9 (1.6) | 8.4 (6.6) | 7.2 (4.2) | 9.9 (4.1) | 11.1 (5.5) | 11.8 (6.6) | 15.8 (6) | <0.001 |
| EXACT-defined events | 0 (0%) | 1 (7%) | 1 (1%) | 1 (11%) | 3 (8%) | 2 (8%) | 1 (8%) | 0.550 |
| Rescue medication | n/a | (n=2) | (n=9) | (n=2) | (n=22) | (n=15) | (n=8) | |
| Mean rescue inhaler use (occasions per day) | n/a | 0.1 (0.1) | 0.7 (1.0) | 0.2 (0.1) | 1.3 (1.7) | 2.5 (2.5) | 1.6 (1.7) | 0.150 |
Higher PROactive Rasch Difficulty score means less difficulty (0-100 scale).
*Steps and calories based on participants with ≥7 days with 8 or more hours of wear time.
E-RS:COPD, evaluating respiratory symptoms in chronic obstructive pulmonary disease; EXACT, EXAcerbations of Chronic pulmonary disease Tool; GOLD, Global Initiative for Chronic Obstructive Lung Disease; IQR, Inter-quartile range.
Limitations
While there was good representation across the groups from the COPDGene cohort, the study was conducted at an individual site (National Jewish Health, Denver, Colorado, USA) and may not be fully representative of the individual subgroups (controls and patients with COPD). The study was not designed to detect acute exacerbations of COPD, therefore the relationship between EXACT-defined events and HCRU exacerbations could not be fully explored. Also, while we did see a few instances of COPD worsening (through EXACT-defined events and deterioration of E-RS:COPD scores) and adherence with the devices and diaries was high, the short-term nature of the study (3 weeks) limited our ability to look at longitudinal changes in the digital measures and evaluate longer term patient adherence to the technology being used.
Conclusion
Integrated, home based digital monitoring is becoming more popular and likely represents the future of medicine. This study demonstrates that such monitoring is feasible in patients with COPD and can identify useful insights into individual patient behaviour. Since large volumes of data are generated, there is a great need for the development of automated algorithms to process, analyse and report data in a way that can be easily interpretable by healthcare providers. In some cases, the data could be used as part of patient self-management strategies as well. Although larger and longer studies need to be done, one can envision that digital monitoring may lead to more patient engagement and education regarding their disease. Our study suggests integrated digital home monitoring needs to be expanded to determine whether it is useful for the early identification of an exacerbation and whether the early identification could lead to meaningful clinical interventions and improved outcomes. This more targeted approach could lead to more engagement than, for example, a generic model where reimbursement or rewards are given by healthcare payers for individual use of pedometers with no specific feedback from a healthcare specialist. For designing clinical trials, this study provides understanding of how remote measurements of digital patient correlate with each other and with traditional clinic visit assessments and long-term outcomes in the stable patient with COPD.
Acknowledgments
Grant Support: National Heart, Lung and Blood Institute (NHLBI RO1HL 095432, U01 HL089856, U01 HL089897, HHSN26820090020CP30); COPD Foundation.
Footnotes
Collaborators: COPDGene Investigators: Administrative Core: James Crapo, MD (PI), Edwin Silverman, MD, PhD (PI), Barry Make, MD, Elizabeth Regan, MD, PhD, Rochelle Lantz, Lori Stepp, Sandra Melanson Genetic Analysis Core: Terri Beaty, PhD, Barbara Klanderman, PhD, Nan Laird, PhD, Christoph Lange, PhD, Michael Cho, MD, Stephanie Santorico, PhD, John Hokanson, MPH, PhD, Dawn DeMeo, MD, MPH, Nadia Hansel, MD, MPH, Craig Hersh, MD, MPH, Peter Castaldi, MD, MSc, Merry-Lynn McDonald, PhD, Jin Zhou, MD, PhD, Manuel Mattheissen, MD, PhD, Emily Wan, MD, Megan Hardin, MD, Jacqueline Hetmanski, MS, Margaret Parker, MS, Tanda Murray, MS Imaging Core: David Lynch, MB, Joyce Schroeder, MD, John Newell, Jr., MD, John Reilly, MD, Harvey Coxson, PhD, Philip Judy, PhD, Eric Hoffman, PhD, George Washko, MD, Raul San Jose Estepar, PhD, James Ross, MSc, Mustafa Al Qaisi, MD, Jordan Zach, Alex Kluiber, Jered Sieren, Tanya Mann, Deanna Richert, Alexander McKenzie, Jaleh Akhavan, Douglas Stinson PFT QA Core, LDS Hospital, Salt Lake City, UT: Robert Jensen, PhD Biological Repository, Johns Hopkins University, Baltimore, MD: Homayoon Farzadegan, PhD, Stacey Meyerer, Shivam Chandan, Samantha Bragan Data Coordinating Center and Biostatistics, National Jewish Health, Denver, CO: Douglas Everett, PhD, Andre Williams, PhD, Carla Wilson, MS, Anna Forssen, MS, Amber Powell, Joe Piccoli Epidemiology Core, University of Colorado School of Public Health, Denver, CO: John Hokanson, MPH, PhD, Marci Sontag, PhD, Jennifer Black-Shinn, MPH, Gregory Kinney, MPH, PhDc, Sharon Lutz, MPH, PhD. COPDGene Investigators:Ann Arbor VA: Jeffrey Curtis, MD, Ella Kazerooni, MD Baylor College of Medicine, Houston, TX: Nicola Hanania, MD, MS, Philip Alapat, MD, Venkata Bandi, MD, Kalpalatha Guntupalli, MD, Elizabeth Guy, MD, Antara Mallampalli, MD, Charles Trinh, MD, Mustafa Atik, MD, Hasan Al-Azzawi, MD, Marc Willis, DO, Susan Pinero, MD, Linda Fahr, MD, Arun Nachiappan, MD, Collin Bray, MD, L. Alexander Frigini, MD, Carlos Farinas, MD, David Katz, MD, Jose Freytes, MD, Anne Marie Marciel, MD Brigham and Women’s Hospital, Boston, MA: Dawn DeMeo, MD, MPH, Craig Hersh, MD, MPH, George Washko, MD, Francine Jacobson, MD, MPH, Hiroto Hatabu, MD, PhD, Peter Clarke, MD, Ritu Gill, MD, Andetta Hunsaker, MD, Beatrice Trotman-Dickenson, MBBS, Rachna Madan, MD Columbia University, New York, NY: R. Graham Barr, MD, DrPH, Byron Thomashow, MD, John Austin, MD, Belinda D’Souza, MD Duke University Medical Center, Durham, NC: Neil MacIntyre, Jr., MD, Lacey Washington, MD, H Page McAdams, MD Fallon Clinic, Worcester, MA: Richard Rosiello, MD, Timothy Bresnahan, MD, Joseph Bradley, MD, Sharon Kuong, MD, Steven Meller, MD, Suzanne Roland, MD Health Partners Research Foundation, Minneapolis, MN: Charlene McEvoy, MD, MPH, Joseph Tashjian, MD Johns Hopkins University, Baltimore, MD: Robert Wise, MD, Nadia Hansel, MD, MPH, Robert Brown, MD, Gregory Diette, MD, Karen Horton, MD Los Angeles Biomedical Research Institute at Harbor UCLA Medical Center, Los Angeles, CA: Richard Casaburi, MD, Janos Porszasz, MD, PhD, Hans Fischer, MD, PhD, Matt Budoff, MD, Mehdi Rambod, MD Michael E. DeBakey VAMC, Houston, TX: Amir Sharafkhaneh, MD, Charles Trinh, MD, Hirani Kamal, MD, Roham Darvishi, MD, Marc Willis, DO, Susan Pinero, MD, Linda Fahr, MD, Arun Nachiappan, MD, Collin Bray, MD, L. Alexander Frigini, MD, Carlos Farinas, MD, David Katz, MD, Jose Freytes, MD, Anne Marie Marciel, MD Minneapolis VA: Dennis Niewoehner, MD, Quentin Anderson, MD, Kathryn Rice, MD, Audrey Caine, MD Morehouse School of Medicine, Atlanta, GA: Marilyn Foreman, MD, MS, Gloria Westney, MD, MS, Eugene Berkowitz, MD, PhD. National Jewish Health, Denver, CO: Russell Bowler, MD, PhD, David Lynch, MB, Joyce Schroeder, MD, Valerie Hale, MD, John Armstrong, II, MD, Debra Dyer, MD, Jonathan Chung, MD, Christian Cox, MD Temple University, Philadelphia, PA: Gerard Criner, MD, Victor Kim, MD, Nathaniel Marchetti, DO, Aditi Satti, MD, A. James Mamary, MD, Robert Steiner, MD, Chandra Dass, MD, Libby Cone, MD University of Alabama, Birmingham, AL: William Bailey, MD, Mark Dransfield, MD, Michael Wells, MD, Surya Bhatt, MD, Hrudaya Nath, MD, Satinder Singh, MD, University of California, San Diego, CA: Joe Ramsdell, MD, Paul Friedman, MD University of Iowa, Iowa City, IA: Alejandro Cornellas, MD, John Newell, Jr., MD, Edwin JR van Beek, MD, PhD University of Michigan, Ann Arbor, MI: Fernando Martinez, MD, MeiLan Han, MD, Ella Kazerooni, MD University of Minnesota, Minneapolis, MN: Christine Wendt, MD, Tadashi Allen, MD University of Pittsburgh, Pittsburgh, PA: Frank Sciurba, MD, Joel Weissfeld, MD, MPH, Carl Fuhrman, MD, Jessica Bon, MD, Danielle Hooper, MD University of Texas Health Science Center at San Antonio, San Antonio, TX: Antonio Anzueto, MD, Sandra Adams, MD, Carlos Orozco, MD, Mario Ruiz, MD, Amy Mumbower, MD, Ariel Kruger, MD, Carlos Restrepo, MD, Michael Lane, MD.
Contributors: RPB and RTS designed the study. MA, SJ, NL and RPB analysed data. NL and RPB interpreted data and wrote the manuscript. BEM and RTS reviewed the manuscript. MA, NL, SJ and RPB had full access to all the data in the study, interpreted the data prepared the manuscript independently and had final responsibility for the decision to submit for publication.
Funding: This study was funded by the COPD Foundation (Miami, FL, USA). GSK provided digital equipment (eDiary, activity monitors, medication sensors) to the participating site (National Jewish Health, Denver, CO).
Competing interests: RB, SJ and AM have no financial or personal relationships with people or organisations that could inappropriately influence this work. NL, BM and RT-S are employees of GSK and hold stock. MA is an employee of GSK.
Patient consent for publication: Not required.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1. Agusti AG. COPD, a multicomponent disease: implications for management. Respir Med 2005;99:670–82. 10.1016/j.rmed.2004.11.006 [DOI] [PubMed] [Google Scholar]
- 2. Vestbo J, Agusti A, Wouters EF, et al. . Should we view chronic obstructive pulmonary disease differently after eclipse? A clinical perspective from the Study Team. Am J Respir Crit Care Med 2014;189:1022–30. 10.1164/rccm.201311-2006PP [DOI] [PubMed] [Google Scholar]
- 3. Hurst JR, Vestbo J, Anzueto A, et al. . Susceptibility to exacerbation in chronic obstructive pulmonary disease. N Engl J Med Overseas Ed 2010;363:1128–38. 10.1056/NEJMoa0909883 [DOI] [PubMed] [Google Scholar]
- 4. Vestbo J, Edwards LD, Scanlon PD, et al. . Changes in forced expiratory volume in 1 second over time in COPD. N Engl J Med 2011;365:1184–92. 10.1056/NEJMoa1105482 [DOI] [PubMed] [Google Scholar]
- 5. Coxson HO, Dirksen A, Edwards LD, et al. . The presence and progression of emphysema in COPD as determined by CT scanning and biomarker expression: a prospective analysis from the eclipse study. Lancet Respir Med 2013;1:129–36. 10.1016/S2213-2600(13)70006-7 [DOI] [PubMed] [Google Scholar]
- 6. Decramer M, Celli B, Kesten S, et al. . Effect of tiotropium on outcomes in patients with moderate chronic obstructive pulmonary disease (UPLIFT): a prespecified subgroup analysis of a randomised controlled trial. The Lancet 2009;374:1171–8. 10.1016/S0140-6736(09)61298-8 [DOI] [PubMed] [Google Scholar]
- 7. Rabe KF, Hurd S, Anzueto A. Global initiative for chronic obstructive lung D. global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: gold executive summary. Am J Respir Crit Care Med 2007;176:532–55. [DOI] [PubMed] [Google Scholar]
- 8. Celli BR, Cote CG, Marin JM, et al. . The body-mass index, airflow obstruction, dyspnea, and exercise capacity index in chronic obstructive pulmonary disease. N Engl J Med 2004;350:1005–12. 10.1056/NEJMoa021322 [DOI] [PubMed] [Google Scholar]
- 9. Puhan MA, Garcia-Aymerich J, Frey M, et al. . Expansion of the prognostic assessment of patients with chronic obstructive pulmonary disease: the updated bode index and the ado index. The Lancet 2009;374:704–11. 10.1016/S0140-6736(09)61301-5 [DOI] [PubMed] [Google Scholar]
- 10. Jones RC, Donaldson GC, Chavannes NH, et al. . Derivation and validation of a composite index of severity in chronic obstructive pulmonary disease: the dose index. Am J Respir Crit Care Med 2009;180:1189–95. 10.1164/rccm.200902-0271OC [DOI] [PubMed] [Google Scholar]
- 11. Fletcher C, Peto R. The natural history of chronic airflow obstruction. Br Med J 1977;1:1645–8. 10.1136/bmj.1.6077.1645 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Nishimura K, Izumi T, Tsukino M, et al. . Dyspnea is a better predictor of 5-year survival than airway obstruction in patients with COPD. Chest 2002;121:1434–40. 10.1378/chest.121.5.1434 [DOI] [PubMed] [Google Scholar]
- 13. Anderson WH, Ha JW, Couper DJ, et al. . Variability in objective and subjective measures affects baseline values in studies of patients with COPD. PLoS One 2017;12:e0184606 10.1371/journal.pone.0184606 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Sulaiman I, Cushen B, Greene G, et al. . Objective assessment of adherence to inhalers by patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2017;195:1333–43. 10.1164/rccm.201604-0733OC [DOI] [PubMed] [Google Scholar]
- 15. Garcia-Aymerich J, Lange P, Benet M, et al. . Regular physical activity reduces hospital admission and mortality in chronic obstructive pulmonary disease: a population based cohort study. Thorax 2006;61:772–8. 10.1136/thx.2006.060145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Luzak A, Karrasch S, Thorand B, et al. . Association of physical activity with lung function in lung-healthy German adults: results from the KorA FF4 study. BMC Pulm Med 2017;17 10.1186/s12890-017-0562-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. O'Neill B, O'Shea O, McDonough S, et al. . Clinician-Facilitated physical activity intervention versus pulmonary rehabilitation for improving physical activity in COPD: a feasibility study. COPD 2018;15:254–64. 10.1080/15412555.2018.1486396 [DOI] [PubMed] [Google Scholar]
- 18. Gimeno-Santos E, Raste Y, Demeyer H, et al. . The proactive instruments to measure physical activity in patients with chronic obstructive pulmonary disease. Eur Respir J 2015;46:988–1000. 10.1183/09031936.00183014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Breda Cushena NM, Hennigan K, Sulaiman I. A pilot study to monitor changes in spirometry and lung volume, following an exacerbation of chronic obstructive pulmonary disease (COPD), as part of a supported discharge program. Science Direct 2016;119:55–62. [DOI] [PubMed] [Google Scholar]
- 20. Himes BE, Weitzman ER. Innovations in health information technologies for chronic pulmonary diseases. Respir Res 2016;17 10.1186/s12931-016-0354-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Sumino K, Locke ER, Magzamen S, et al. . Use of a remote inhaler monitoring device to measure change in inhaler use with chronic obstructive pulmonary disease exacerbations. J Aerosol Med Pulm Drug Deliv 2018;31:191–8. 10.1089/jamp.2017.1383 [DOI] [PubMed] [Google Scholar]
- 22. Roversi S, Corbetta L, Clini E. Gold 2017 recommendations for COPD patients: toward a more personalized approach. COPD Research and Practice 2017;3 10.1186/s40749-017-0024-y [DOI] [Google Scholar]
- 23. Regan EA, Hokanson JE, Murphy JR, et al. . Genetic epidemiology of COPD (COPDGene) study design. COPD: Journal of Chronic Obstructive Pulmonary Disease 2011;7:32–43. 10.3109/15412550903499522 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Leidy NK, Wilcox TK, Jones PW, et al. . Standardizing measurement of chronic obstructive pulmonary disease exacerbations. reliability and validity of a patient-reported diary. Am J Respir Crit Care Med 2011;183:323–9. 10.1164/rccm.201005-0762OC [DOI] [PubMed] [Google Scholar]
- 25. Global Initiative for Chronic Obstructive Lung Disease 2017 , 2017. Available: http://goldcopd.org/ [Accessed 30 Oct 2017].
- 26. Bethesda AS, 2017. U.S. Department of Health and Human Services; October 2017 [updated April 2015] What is precision medicine?] updated April 2015]What is precision medicine?]. Available from Available: https://ghr.nlm.nih.gov/primer/precisionmedicine/definition
- 27. Rejeski WJ, Mihalko SL. Physical activity and quality of life in older adults. J Gerontol A Biol Sci Med Sci 2001;56 23–35. 10.1093/gerona/56.suppl_2.23 [DOI] [PubMed] [Google Scholar]
- 28. Zemans RL, Jacobson S, Keene J, et al. . Multiple biomarkers predict disease severity, progression and mortality in COPD. Respir Res 2017;18 10.1186/s12931-017-0597-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Wan ES, Castaldi PJ, Cho MH, et al. . Epidemiology, genetics, and subtyping of preserved ratio impaired spirometry (PriSM) in COPDGene. Respir Res 2014;15 10.1186/s12931-014-0089-y [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjresp-2018-000350supp001.pdf (269.5KB, pdf)