Abstract
Children and adults prenatally exposed to alcohol show higher rates of mental health problems than unexposed individuals, with depression and anxiety being among the more commonly encountered disorders. Previous studies in rats showed that prenatal alcohol exposure (PAE) can indeed increase depressive- and anxiety-like behavior in adulthood; however, depression and anxiety are often observed in the context of stress and/or a dysregulated stress response system (the hypothalamic-pituitary-adrenal [HPA] axis). PAE can dysregulate the HPA axis, resulting in hyperresponsivity to stress. In turn, this may predispose individuals prenatally exposed to alcohol to the adverse effects of stress compared to unexposed individuals. We have shown previously that PAE animals may be more sensitive to the effects of chronic stress on behavior, showing increased anxiety- and depressive-like behavior following chronic unpredictable stress (CUS) exposure. Here, we investigated the independent and interactive effects of PAE and adult CUS on anxiety-like behavior and receptor systems (corticotropin-releasing hormone receptor type 1 [CRHR1], mineralocorticoid receptor [MR], and glucocorticoid receptor [GR]) underlying stress and emotional regulation, and whether exposure to CUS differentially results in immediate or delayed effects. Adult male and female offspring from PAE, pair-fed (PF), and ad libitum-fed control (C) dams were exposed to either 10 days of CUS or left undisturbed. Behavioral testing began 1 or 14 days post-CUS, and brains were collected following testing. Anxiety-like behaviors were evaluated using the open field, elevated plus maze and dark-light emergence tests. CRHR1, MR, and GR mRNA expression were assessed in the medial prefrontal cortex (mPFC), amygdala, and hippocampal formation, brain areas key to both stress and emotional regulation. We found that PAE differentially increased anxiety-like behavior and altered GR mRNA in males and females compared to their control counterparts. Furthermore, depending on the timing of testing, CUS unmasked alterations in GR and CRHR1 mRNA expression in the mPFC and amygdala in PAE males, and MR mRNA in the hippocampal formation in PAE females compared to their C counterparts. Overall, the changes observed in these receptor systems may underlie the increase in anxiety-like behavior following PAE and CUS exposure in adulthood. That CUS differentially affected brain and behavioral outcome of PAE and C animals, and did so in a sexually-dimorphic manner, has important implications for understanding the etiology of psychopathology in individuals prenatally exposed to alcohol.
Keywords: Prenatal alcohol exposure, Chronic unpredictable stress, Anxiety-like behavior, MR, GR, CRHR1
1. Introduction
Children and adults prenatally exposed to alcohol show higher rates of mental health problems than unexposed individuals, with depression and anxiety being among the more commonly encountered disorders (Famy et al., 1998; O’Connor and Paley, 2009; Pei et al., 2011). Previous studies in rats showed that prenatal alcohol exposure (PAE) can indeed increase depressive- and anxiety-like behavior in adulthood (Brocardo et al., 2012; Cullen et al., 2013; Hofmann et al., 2005; Varlinskaya and Mooney, 2014; Wilcoxon et al., 2005). However, depression and anxiety are often observed in the context of stress and/or a dysregulated stress response system (the hypothalamic-pituitary-adrenal [HPA] axis) (Jacobson, 2014; Nestler et al., 2002). PAE has been shown to result in HPA axis dysregulation. Data from several clinical studies demonstrate higher basal and stress cortisol levels, and studies using animal models extend and support the clinical findings, reporting increased HPA activation and/or a delayed return to basal levels, as well as altered central HPA regulation in PAE compared to control offspring (reviewed in Hellemans et al., 2010). Therefore, HPA dysregulation induced by PAE may predispose these individuals to an increased vulnerability to stress-related disorders such as anxiety or depression following stress over the life course. This would have important clinical relevance because individuals prenatally exposed to alcohol are more likely to encounter stressful environments/experiences during their lifetimes (reviewed in Hellemans et al., 2010). In support of this, we previously found that chronic unpredictable stress (CUS) in adulthood increased anxiety- and depressive-like behavior in PAE rats compared to controls, with differential effects in male and female offspring (Hellemans et al., 2010).
The corticotropin-releasing hormone receptor type 1 (CRHR1), mineralocorticoid receptor (MR), and glucocorticoid receptor (GR) are key in regulating HPA activity. CRHR1 is one of the receptors that mediates the neuroregulatory effects of CRH, whereas MR and GR mediate the effects of glucocorticoids. CRHR1, activated by CRH, can both facilitate and depress neurotransmission (Gallagher et al., 2008). This receptor is widely expressed in the brain, including limbic regions such as the medial prefrontal cortex (mPFC), amygdala, and hippocampal formation (Henckens et al., 2016), and has different roles in modulating HPA activity and behavior depending on where it is expressed. MRs are involved in regulating basal HPA tone and HPA activity following stress (ter Heegde et al., 2015), whereas GRs appear to be involved primarily in mediating feedforward/feedback regulation of the stress response (Herman et al., 2016); localization of the MR and GR proteins (i.e. nuclear vs. cytosolic) also plays a role in function (Allan et al., 2014; Caldwell et al., 2014). Importantly, overexpression of CRH, mediated through CRHR1, as well as dysregulation of MR and GR have been suggested to underlie psychopathology such as depression and anxiety (Binder and Nemeroff, 2010; Holsboer and Ising, 2010, 2008; Inda et al., 2017; Joëls and de Kloet, 2017; Veenit et al., 2014).
We and others have shown previously that PAE has widespread effects on these stress-related receptors in the brain. Specifically, PAE increases CRH mRNA expression in the central nucleus of the amygdala in adult males and females (Lan et al., 2015), and differentially decreases CRHR1 mRNA expression in the mPFC, amygdala, hippocampus, and pituitary of adult males and females (Glavas et al., 2007; Raineki et al., 2018, 2016). CRHR1 mRNA and protein expression are also decreased in the hippocampus of adolescent PAE males (Caldwell et al., 2015; Raineki et al., 2018). Additionally, PAE decreases MR mRNA expression in the adult female hippocampus (Sliwowska et al., 2008; Uban et al., 2013), and alters hippocampal MR mRNA expression in adolescent male and GR mRNA expression in adolescent female rats (Raineki et al., 2018). PAE-induced dysregulation of these key receptor systems may predispose individuals to the adverse effects of stress over the life course and increase vulnerability to stress-related disorders such as depression and anxiety. In support of this, we have found that chronic stress during adolescence may further alter mRNA expression of these stress-related receptors and anxiety-like behavior in PAE compared to C animals, and may do so in a sex-dependent manner (Raineki et al., 2018, 2016). As well, we recently showed that PAE and chronic stress in adulthood interact to result in sexually-dimorphic and time-dependent dysregulation of the neurocircuitry underlying behavioral, emotional and stress regulation, as well as alterations in depressive-like (forced swim test) behavior (Lam et al., 2018). However, less is known about whether PAE may interact with chronic stress in adulthood to further impact MR, GR, and CRHR1 expression, and the implications of this interaction for anxiety-like behavior.
The present study aimed to determine the independent and interactive effects of PAE and adult CUS on anxiety-like behavior and receptor systems (CRHR1, MR, and GR) underlying stress and emotional regulation. We also examined whether exposure to CUS results in immediate or delayed effects, as animal studies have shown differential changes in behavior and brain depending on whether or not there is a recovery period between chronic stress or corticosterone exposure and testing (Gourley and Taylor, 2009; Matuszewich et al., 2007). Anxiety-like behaviors of male and female rats were evaluated using the open field (OF), elevated plus maze (EPM), and dark-light emergence (DL) tests. CRHR1, MR, and GR mRNA expression were assessed in the mPFC, amygdala, and hippocampal formation, brain areas key to both stress and emotional regulation.
2. Materials and methods
2.1. Animals and breeding
All animal use and care procedures were in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals, the Canadian Council on Animal Care, and approved by the University of British Columbia Animal Care Committee. Adult virgin male (275–300 g) and female (265–300 g) Sprague-Dawley rats were obtained from Charles River Laboratories (St. Constant, PQ, Canada). Rats, pair-housed and given ad libitum access to standard laboratory chow and water, habituated to the facility for a 7–10-day period, which was maintained on a 12:12 h light/dark cycle (lights on at 07:00 h) and temperature set at 21 ± 1 °C. For breeding, males were paired with a female and the presence of sperm in vaginal lavage samples taken every morning at 08:00 h indicated day 1 of gestation (GD 1).
2.2. Diets and feeding
On GD 1, females were singly housed and randomly assigned to one of three treatment groups: 1) alcohol-fed (PAE; n = 13), receiving a liquid ethanol diet with 36% ethanol-derived calories, 6.7% v/v; 2); 2) pair-fed (PF; n = 11), receiving a liquid control diet with maltose-dextrin isocalorically substituted for ethanol, in an amount consumed by a PAE partner (g/kg body weight/GD); and 3) ad libitum-fed control (C; n = 10), receiving a pelleted control diet. All diet formulations provide optimal nutrition during pregnancy (Weinberg/Keiver High Protein Ethanol [#710324] and Control [#710109] liquid diets, and Weinberg/Keiver High Protein Pelleted Control Diet [#102698] were prepared by Dyets, Inc., Bethlehem, PA, USA) (Lan et al., 2006). Fresh diets were presented daily at 1 h prior to lights off to minimize shifts in the maternal corticosterone circadian rhythm (Gallo and Weinberg, 1981). At the same time, volume of liquid diet consumed since the previous night was recorded. All groups also received ad libitum access to water. To facilitate the transition into a full liquid ethanol diet beginning on GD 3 until GD 21, liquid ethanol diets were introduced gradually: on GD 1, rats were given a 1:2 ratio of liquid ethanol to liquid control diet; on GD 2, they were given a 2:1 ratio. Beginning on GD 22 and throughout lactation, dams received a 19% protein laboratory chow (Teklad Global #2019) and water.
On GD17, blood samples from the tail vein were collected 3 h after lights off from a subset of C, PF, and PAE dams (n = 3 each) and blood alcohol levels were determined using an assay from Pointe Scientific Inc. (Canton, MI, USA); PAE dams had BALs of 134.1 ± 23.5 mg/dl.
On the day of birth (postnatal day 1, PN 1), pups were weighed, and litters randomly culled to 12 (6 males, 6 females when possible). If necessary to maintain litter size, pups from the same prenatal treatment born on the same day were fostered into a litter. Dams and offspring were housed undisturbed except for weekly weighing and cage change. After weaning (PN 22), pups were group-housed with same-sex litter-mates on non-ventilated racks and fed an 18% protein chow (Teklad Global #2018). Pups of the same sex and prenatal treatment, but different litters born ± 2 days, were pair-housed beginning PN 42 ± 2 day.
2.3. Chronic unpredictable stress (CUS) paradigm
Animals (PN 88–99) from each experimental group were randomly assigned to either stress (CUS) or no-stress (non-CUS) conditions. CUS involved 10 days of twice daily exposure to stressors at random times, with a minimum of 2 h between stressors, and in random order; all CUS rats experienced each stressor for the same number of times over the 10-day period. Except for social isolation and wet bedding (see below), all the stressors occurred during the light phase of the circadian cycle.
Stressors included: Platform: Animals individually placed on 20 W × 20 L × 90H cm transparent Plexiglas platforms for 20 min. Restraint: Animals individually restrained in PVC tubes (15 cm × 6 cm for females and 19 cm × 7 cm for males) with ventilation holes for 30 min. Soiled Cage: Pairs of cage mates placed in cages with soiled bedding from other animals for 1 h. Wet Cage: Pairs of cage mates placed in empty cages containing 1 cm of room-temperature water at the bottom for 1 h, without food and water. Social isolation: 12 h of isolation beginning at lights off without food and water, followed by 1 h of water deprivation in the home cage in the morning; Wet bedding: Bedding of home cages were wet with 400 ml of room-temperature water just before lights off. Animals were housed on wet bedding for 13 h and given a clean cage at the end.
2.4. Blood sampling
Basal blood samples were collected via tail nick from both non-CUS and CUS animals on Day 1 of the CUS procedure and on the day following termination of CUS. Samples were collected within 2 min of touching the cage to obtain a true basal measure, centrifuged within 60 min of collection at 2190 g for 10 min at 4 °C, and the serum stored at −20 °C until assayed.
2.5. Radioimmunoassays (RIA)
Total corticosterone (CORT) levels (bound plus free) were measured using a modification of the ImmuChem™ Corticosterone I125 RIA Kit (MP Biomedicals, Orangeburg, NY): all reagents and samples were halved, and the assay was performed according to vendor instructions. The minimum detectable level was 7.7 ng/ml, and the inter- and intra-assay coefficients of variation were < 7.2% and < 10.3%, respectively.
2.6. Behavioral testing
Behavioral testing began 1 day or 14 days after the end of CUS (CUS-1 and CUS-14, respectively). Animal behaviors were assessed on consecutive days, with a one-day break between tests, in the open field (OF), elevated plus maze (EPM), dark-light emergence (DL), and forced swim tests. This paper focuses on the behaviors from the OF, EPM, and DL tests. 24 h prior to each behavioral test, all animals were habituated to the testing room for 20 min. Tests were performed as follows:
The OF apparatus was an 80 × 80 cm × 40 cm square arena enclosed by transparent Plexiglas walls. Animals were tested for 5 min per day over 3 consecutive days. Total distance traveled (cm) in the field, as well as distance travelled, time spent, and frequency and latency of entries into the center zone on Day 1 of the test were analyzed to assess their unconditioned anxiety-like behaviors in response to a novel environment.
The EPM consisted of two open arms and two arms enclosed by 40 cm opaque walls (each arm is 50 × 10 cm, and the center area is 10 × 10 cm) elevated 40 cm above ground. Animals were tested once for 5 min. Time on the open arms as a percent of open and closed arms time, and frequency of closed arm entries were assessed.
The DL apparatus was a white Plexiglas arena (80 × 40 × 40 cm) with an enclosed black box (25 × 40 × 40 cm) placed at one end with an opening (10 × 10 cm) facing out into the lit area. Each rat was tested once for 10 min. Latency to enter and time spent in the light compartment were assessed.
Testing in the FST was performed over 2 days as previously described (Lam et al., 2018). Briefly, animals were placed in a transparent Plexiglas cylinder (20 cm diameter, 60.5 cm height) filled with 25 ± 1 °C water to a 44.5 cm depth (so that the tail would not touch the bottom) for 15 min on Day 1 and 5 min on Day 2 of testing (Detke et al., 1995). Results of the FST were reported previously (Lam et al., 2018).
All behavioral tests were done during the light phase of the circadian cycle, but occurred under dim lighting, except for the dark-light emergence test where 60 lx of lighting was used to illuminate the light compartment. Specifically, the OF test occurred at 12:00 – 16:00 h, the EPM occurred at 12:00 – 15:00 h, the DL occurred at 08:00 – 11:00 h, and the FST occurred at 08:00 – 11:00 h. White noise (30 dB) was used in the background to dampen random noise in the testing room. Behavior in the OF was recorded and scored using Ethovision v3.1 software (Noldus, Wageningen, The Netherlands). All other behaviors were recorded and scored using The Observer 5.0 software (Noldus, Wageningen, The Netherlands). All behaviors were analyzed by an observer blind to the prenatal treatment and CUS condition.
2.7. Tissue collection
Whole brains were collected via decapitation 30 min after the onset of testing on Day 2 of the forced swim test. Brains were collected and snap frozen on powdered dry ice and stored at −80 °C.
2.8. In situ hybridization
20 μm coronal sections were mounted on slides (Superfrost slides, Fisher Scientific) in a cryostat at −16 °C, and stored at −80 °C. Ribonucleotide probes were used to detect CRHR1 and GR mRNA in the medial prefrontal cortex (mPFC; anterior cingulate [Cg1], and prelimbic [PrL] and infralimbic [IL] cortices), amygdala (central, medial, lateral, and basal nuclei), and the hippocampal formation (dentate gyrus [DG], CA3, CA1, and ventral subiculum). A cRNA ribonucleotide probe was also used to detect MR mRNA in the hippocampal formation. The rat CRHR1 ribonucleotide probe was prepared using a 1.4 kb template (GenBank Accession Number: L24096) that encodes a 415 amino acid protein provided by Dr. Victor Viau (The University of British Columbia, Canada). The rat MR ribonucleotide probe was prepared using a 550 bp template (complementary to the coding region and 3′ untranslated region of rat MR mRNA) from Dr. James Herman (University of Cincinnati, USA). The rat GR ribonucleotide probe was prepared using a 456 bp template (complementary to the coding region and 3′ untranslated region of rat GR mRNA), also provided by Dr. James Herman (Herman et al., 1999). The ribonucleotide probes were labeled with 35S-UTP (Amersham Biosciences, NJ, USA) using Polymerase T7 (CRHR1 and GR) or T3 (MR) and Promega Riboprobe System (Promega Corporation, Madison, WI, USA). All probes were purified using Micro Bio-Spin 30 Columns (Bio-Rad, CA, USA). 1 M of DTT was added to prevent oxidation.
In situ hybridization was performed following previously described procedures (Raineki et al., 2016). Briefly, thawed slides underwent a series of washes, dehydrated through a graded series of ethanol, delipidated in chloroform, and finally air dried. After hybridization buffer mixed with the probe (activity of 1 × 106 cpm/slide) was applied, slides were covered with HybriSlips (Sigma-Aldrich, ON, Canada). Following incubation overnight at 55 °C in humidified chambers (75% formamide), HybriSlips were removed and slides were rinsed through a series of washes, dehydrated through a graded series of ethanol, and air dried overnight.
2.9. Densitometric analysis
Kodak BioMax MR autoradiography films were exposed to hybridized slides. The exposed autoradiography films were developed using Kodak GBX developer and fixer, and scanned and analyzed using Scion Image 4.0.3.2 (National Institutes of Health, USA) according to Paxinos and Watson (2004). Two sections each of the left and right subregions for each brain region per animal were traced freehand to determine mean grey density levels. Mean grey density levels were measured from Bregma 3.00 mm to 2.76 mm for the mPFC; Bregma −2.64 mm to −3.00 mm for the amygdala; and Bregma −4.80 mm to −5.28 mm for the hippocampal formation. The Bregma range chosen for the hippocampal formation corresponds to the ventral/temporal hippocampus which primarily relates to emotion and stress regulation (Fanselow and Dong, 2010; Strange et al., 2014). Background was measured from white matter areas on the same side: forceps minor for the mPFC, internal capsule for the amygdala, and corpus callosum for the hippocampal formation. Corrected mean grey values were obtained by subtracting the background level from each of the four measurements and the four measurements were then averaged together for analysis.
2.10. Statistical analyses
Univariate analyses of variance (ANOVAs) were performed using IBM Statistical Package for the Social Sciences (SPSS) Statistics 20 software (IBM, Armonk, NY, USA). Because main or interactive effects of sex were revealed for the behavioral measures, all ANOVAs were then run separately for males and females. Extreme outliers lower than 3 interquartile ranges below the first quartile or higher than 3 inter-quartile ranges above the third quartile were identified and removed. Differences were considered significant at p ≤ 0.05. Significant main effects and interactions were examined using post hoc pairwise comparisons with Šídák correction.
Pre-CUS CORT data were analyzed using a one-way ANOVA for the factor of prenatal treatment (C, PF, PAE); n = 23 for males, 24 for females. Post-CUS CORT levels (n = 7–8/treatment/CUS/sex) were analyzed using an ANOVA for the factors of prenatal treatment and CUS exposure (Non-CUS, CUS). Behavioral data (n = 7–8/treatment/CUS/sex) and mRNA mean gray values (n = 4–6/treatment/CUS/sex) were analyzed using an ANOVA for the factors of prenatal treatment and CUS exposure (Non-CUS, CUS-1, CUS-14). Further analyses utilized planned comparisons to test the a priori hypotheses that: 1) PAE will alter anxiety-like behavior and MR, GR, and CRHR1 mRNA expression; and 2) CUS will differentially alter anxiety-like behavior and mRNA expression of MR, GR, and CRHR1 mRNA in PAE compared to control animals.
3. Results
3.1. Corticosterone
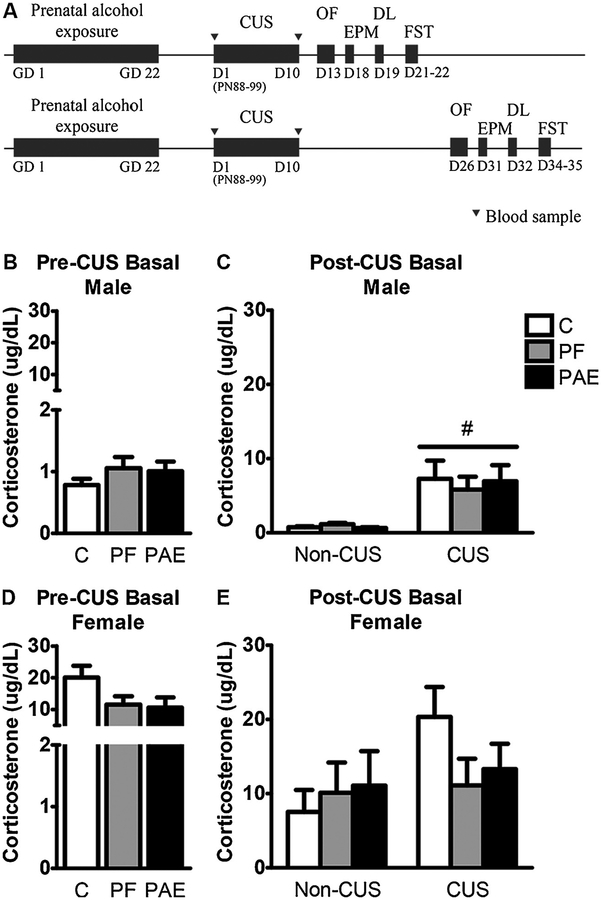
There were no differences among prenatal treatment groups for either males or females for pre- or post-CUS basal CORT levels. By contrast, CUS increased basal CORT levels [main effect of CUS (F1,64 = 10.769, p = 0.002)] in males but not females (Fig. 1).
Fig. 1.
Experimental design (A) and basal corticosterone levels pre- (B,D) and post-CUS (C,E). Bars represent the mean ± SEM (μg/dL) of basal corticosterone levels following prenatal alcohol exposure (PAE) and chronic unpredictable stress (CUS). # indicates a significant main effect of CUS (pre-CUS: n=23/prenatal treatment in males, n = 24/prenatal treatment in females; post-CUS: n= 7–8/prenatal treatment/sex for non-CUS, n=15–16/prenatal treatment/sex for CUS). GD = gestational day; D = day; PN = postnatal day; OF = open field test; EPM = elevated plus maze; DL = dark-light emergence test; FST = forced swim test.
3.2. Behavior
Open field (OF).
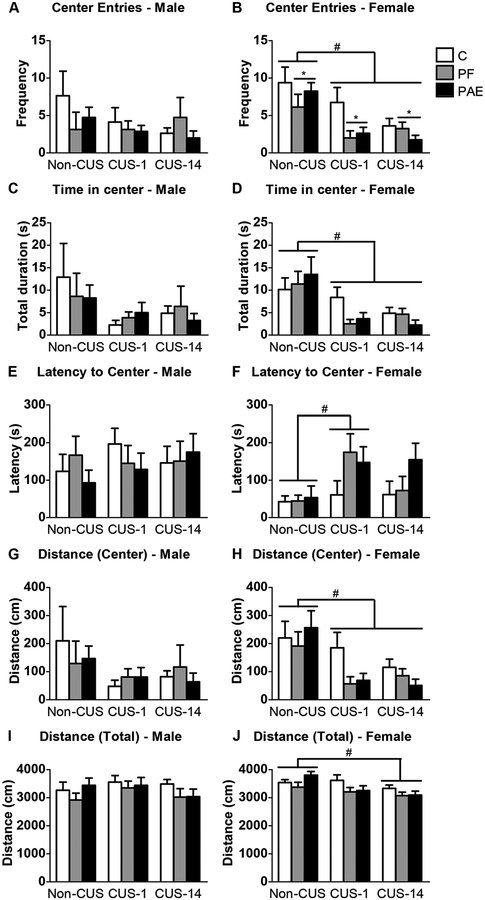
In males, there were no effects of prenatal treatment or CUS on any OF measure (Fig. 2A,C,E,G,I). By contrast, PAE and PF females, overall, showed fewer center entries than C females [main effect of treatment (F2,63 = 3.766, p = 0.029); Fig. 2B]. As well, CUS decreased the number of center entries, time spent in the center, and distance travelled in the center regardless of whether testing occurred immediately (CUS-1) or following a delay (CUS-14) [main effect of CUS for center frequency: F2,63 = 11.977, p < 0.001; center duration: F2,63 = 11.258, p < 0.001; center distance: F2,63 = 9.455, p < 0.001; Fig. 2B,D,H]. Additionally, latency to the first center entry was longer for CUS-1 than non-CUS animals overall [main effect of CUS (F2,63 = 3.864, p = 0.026); Fig. 2F], and distance travelled in the entire field was lower overall for CUS-14 females than non-CUS females [main effect of CUS (F2,63 = 5.583, p = 0.006); Fig. 2J].
Fig. 2.
Immediate and delayed effects of chronic unpredictable stress (CUS-1 and CUS-14) and prenatal alcohol exposure (PAE) on behaviors in the open field. Bars represent the mean ± SEM of frequency in center entries (A,B), time in the center (C,D), latency to center entry (E,F), distance travelled in the center (G,H), and total distance travelled in the field (I,J). * indicates a significant main effect of prenatal treatment: for B, PF and PAE are different from C; # indicates a significant main effect of CUS: CUS-1 and CUS-14 are different from non-CUS for B,D,H, CUS-1 is different from non-CUS for F, and CUS-14 is different from non-CUS for J (n=8/prenatal treatment/CUS condition/sex).
Dark-light emergence test (DL).
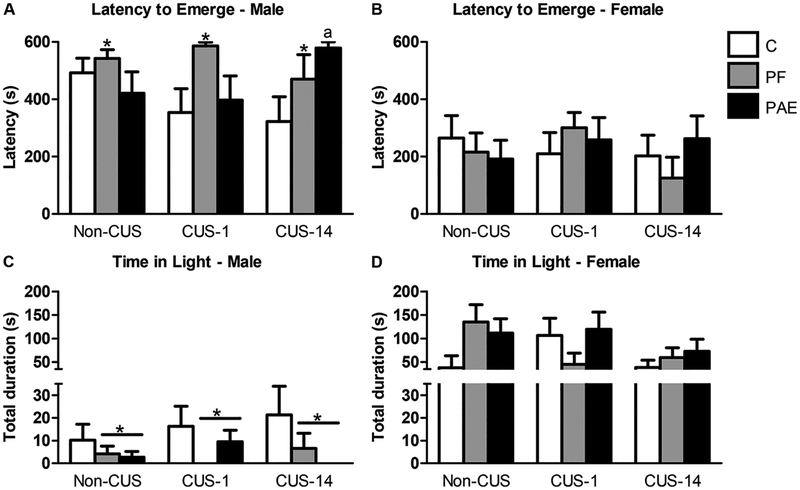
Latency to emerge was longer for PF than C males [main effect of treatment (F2,60 = 3.376, p = 0.041); Fig. 3A]. Also, both PAE and PF males spent less time in the light than C males [main effect of treatment (F2,60 = 3.255, p = 0.045); Fig. 3C]. Prenatal treatment had no effects in females (Fig. 3B,D).
Fig. 3.
Immediate and delayed effects of chronic unpredictable stress (CUS-1 and CUS-14) and prenatal alcohol exposure (PAE) on behaviors in the dark-light emergence test. Bars represent the mean ± SEM of latency to emerge from the dark (A,B) and time spent in the light compartment (C,D). * indicates a significant main effect of prenatal treatment: PF is different from C for B, and PF and PAE are different from C for C; a indicates that CUS-14 PAE is significantly different from CUS-14 C based on a priori comparisons (n=6–8/prenatal treatment/CUS condition/sex).
In addition, while ANOVA showed no effects of CUS in either males or females, a priori analyses revealed that CUS-14 PAE males showed longer latencies to emerge than CUS-14 C males (p = 0.015).
Elevated plus maze (EPM).
There were no effects of prenatal treatment or CUS in either males or females on the percent of time in the open arms and the frequency of closed arm entries (data not shown).
3.3. Receptor mRNA expression
3.3.1. CRHR1 mRNA expression
Medial prefrontal cortex (mPFC).
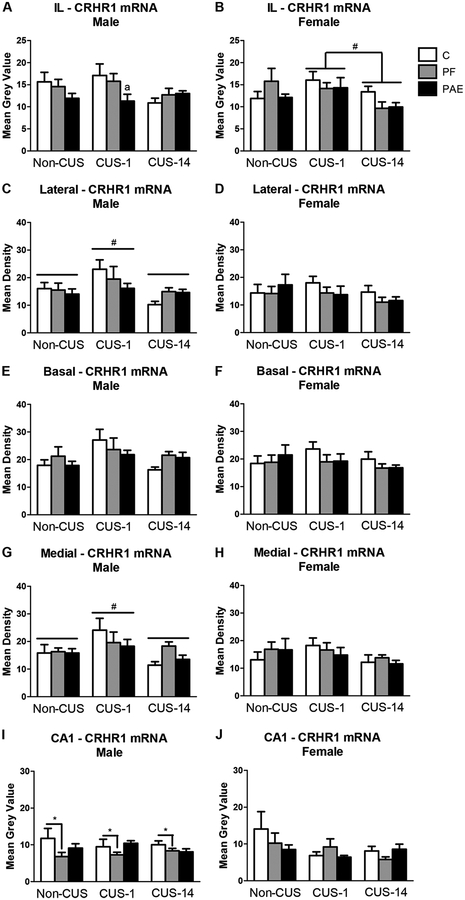
Neither prenatal treatment nor CUS altered CRHR1 mRNA expression in the mPFC or in the Cg1 and PrL, respectively, in either males or females. In the IL, however, CUS-1 females had higher CRHR1 mRNA expression than CUS-14 females [main effect of CUS (F2,42 = 4.125, p = 0.023); Fig. 4B], while CRHR1 mRNA expression was decreased in PAE compared to C males in the CUS-1 condition (a priori analysis, p = 0.02; Fig. 4A).
Fig. 4.
CRHR1 mRNA expression in the medial prefrontal cortex (mPFC), amgydala, and hippocampal formation in response to behavioral testing initiated 1- or 14-days following chronic unpredictable stress (CUS-1 and CUS-14, respectively) in adult male and female control (C), pair-fed (PF), and prenatal alcohol-exposed (PAE) rats. Bars represent mean grey values (mean ± SEM) of CRHR1 mRNA expression in the IL of the mPFC (A,B); lateral, basal, and medial nuclei of the amygdala (C-H); and CA1 subregion of the hippocampal formation (I,J). * indicates a significant main effect of prenatal treatment: PF is different from C for I; # indicates a significant main effect of CUS: CUS-1 is different from non-CUS and CUS-14 for C,G, and different from CUS-14 for B; a indicates that CUS-1 PAE is different from CUS-1 C based on a priori comparisons for A (n=4–6/prenatal treatment/CUS condition/sex).
Amygdala.
Prenatal treatment had no effects in either males or females on CRHR1 mRNA expression in the amygdala (Fig. 4). By contrast, CUS altered CRHR1 mRNA expression in the lateral and medial amygdala nuclei of males but not females, such that CUS-1 males showed higher mRNA expression than both non-CUS and CUS-14 males [main effect of CUS for lateral: F2,41 = 4.864, p = 0.013; medial: F2,41 = 4.596, p = 0.016; Fig. 4C,D,G,H].
Hippocampal formation.
CRHR1 mRNA expression was lower in the CA1 in PF than C males [main effect of treatment (F2,41 = 3.704, p = 0.033); Fig. 4I]. There were no effects of prenatal treatment or CUS on CRHR1 mRNA expression in the hippocampal formation in females (Fig. 4J).
3.3.2. MR mRNA expression
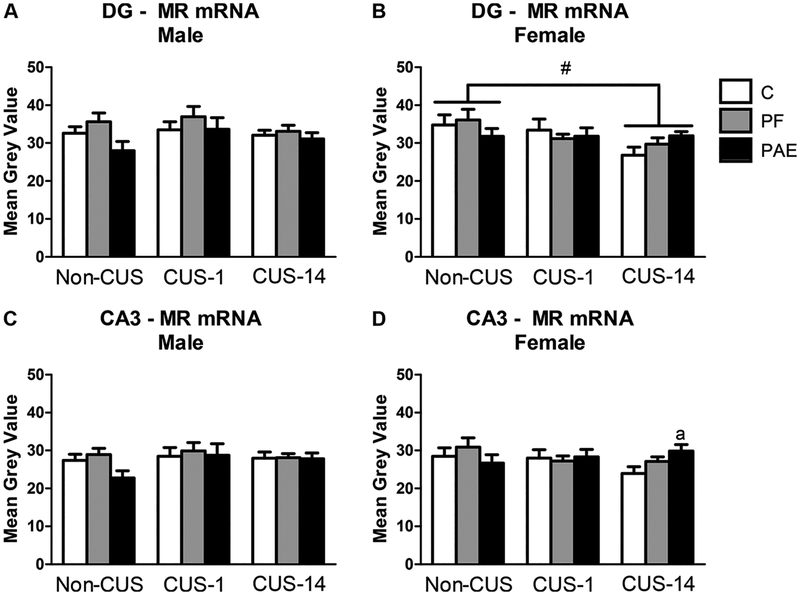
There were no effects of prenatal treatment in either males or females on MR mRNA expression in the hippocampal formation (Fig. 5). Further, while there were no effects of CUS in males, CUS-14 females had lower mRNA expression overall than non-CUS females in the DG [main effect of CUS (F2,44 = 3.543, p = 0.037); Fig. 5B]. In the CA3, a priori analyses revealed that CUS-14 PAE females had higher mRNA expression than CUS-14 C females (p = 0.038; Fig. 5D).
Fig. 5.
MR mRNA in the dentate gyrus (DG) and CA3 of the hippocampal formation in response to behavioral testing initiated 1- or 14-day following chronic unpredictable stress (CUS-1 and CUS-14, respectively) in adult male and female control (C), pair-fed (PF), and prenatal alcohol-exposed (PAE) rats. Bars represent mean grey values (mean ± SEM) of MR mRNA in the DG (A,B) and CA3 (C,D) subregions of the hippo-campal formation. # indicates a significant main effect of CUS: CUS-14 is different from non-CUS; a indicates that CUS-14 PAE is different from CUS-14 C based on a priori comparisons (n=5–6/prenatal treatment/CUS condition/sex).
3.3.3. GR mRNA expression
Medial prefrontal cortex.
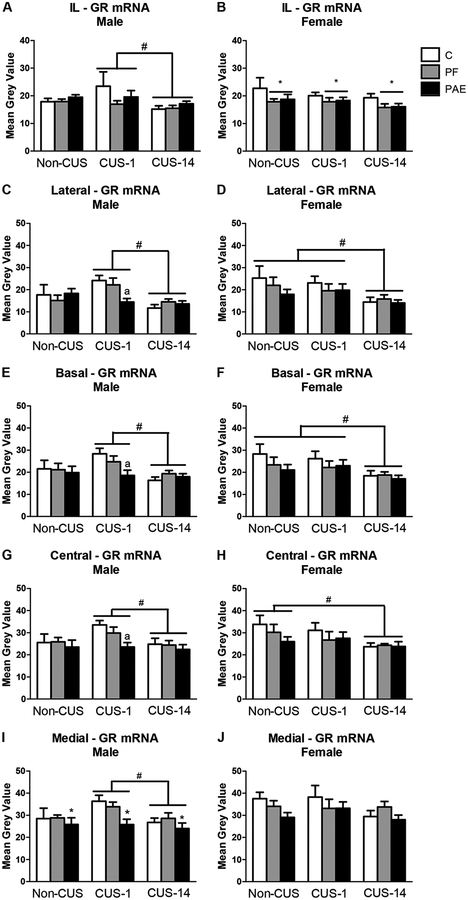
There were no effects of prenatal treatment or CUS in either males or females on GR mRNA expression in the Cg1 or PrL subregions of the mPFC (data not shown). However, in the IL, both PAE and PF females had decreased GR mRNA expression compared to C females [main effect of treatment (F2,40 = 3.389, p = 0.044); Fig. 6B]. Furthermore, CUS altered GR mRNA expression in males but not females such that CUS-1 males showed higher GR mRNA expression than CUS-14 males [main effect of CUS (F2,42 = 3.341, p = 0.045); Fig. 6A].
Fig. 6.
GR mRNA expression in the medial prefrontal cortex (mPFC) and amygdala in response to behavioral testing initiated 1- or 14-day following chronic unpredictable stress (CUS-1 and CUS-14, respectively) in adult male and female control (C), pair-fed (PF), and prenatal alcohol-exposed (PAE) rats. Bars represent mean grey values (mean ± SEM) of GR mRNA expression in the IL of the mPFC (A,B); and lateral, basal, medial, and central nuclei of the amygdala (C-J). * indicates a significant main effect of prenatal treatment: PF and PAE are different from C for B, and PAE is different from both PF and C for I; # indicates a significant main effect of CUS: CUS-1 is different from CUS-14 for A,C,E,G,I, CUS-14 is different from both non-CUS and CUS-1 for D,F, and CUS-14 is different from non-CUS for H; a indicates that CUS-1 PAE is different from CUS-1 C based on a priori comparisons for C,E,G (n=4–6/prenatal treatment/CUS condition/sex).
Amygdala.
PAE males overall had lower GR mRNA expression than C and PF males in the medial nucleus of the amygdala [main effect of treatment (F2,42 = 3.903, p = 0.028); Fig. 6I]. ANOVA also found that independent of prenatal treatment, CUS-1 males had higher GR mRNA expression than CUS-14 males in all nuclei of the amygdala [main effect of CUS for lateral: F2,42 = 6.726, p = 0.003; basal: F2,42 = 4.703, p = 0.014; central: F2,42 = 3.434, p = 0.042; medial: F2,42 = 3.479, p = 0.40; Fig. 6C,E,G,I]. However, a priori analysis revealed that PAE males in the CUS-1 condition had lower GR mRNA expression than CUS-1 C males in the lateral (p = 0.007), basal (p = 0.008), and central (p = 0.009) nuclei (Fig. 6C,E,G).
By contrast, there were no prenatal treatment effects in females, and CUS-14 females showed lower mRNA expression compared to CUS-1 and non-CUS females in the lateral and basal nuclei, and compared to non-CUS females in the central nucleus [main effect of CUS for lateral: F2,41 = 4.415, p = 0.018; basal: F2,41 = 4.184, p = 0.022; central: F2,41 = 3.382, p = 0.044; Fig. 6D,F,H].
Hippocampal formation.
There were no effects of prenatal treatment or CUS in either males or females on GR mRNA expression in the hippocampal formation (data not shown).
4. Discussion
PAE and CUS, independently and interactively, altered anxiety-like behavior and CRHR1, MR, and GR expression in a time- and sex-dependent manner. Specifically, PAE differentially increased anxiety-like behavior and decreased GR mRNA in males and females compared to their control counterparts. Furthermore, depending on the timing of testing, CUS unmasked alterations in anxiety-like behavior, as well as GR and CRHR1 mRNA expression in the mPFC and amygdala in PAE males, and MR mRNA expression in the hippocampal formation in PAE females compared to their C counterparts. That CUS differentially affected brain and behavioral outcome of PAE and C animals, and did so in a sexually-dimorphic manner, has important implications for understanding the etiology of psychopathology in individuals prenatally exposed to alcohol.
4.1. PAE differentially increased anxiety-like behavior and decreased expression of GR mRNA in males and females independent of CUS
The behavioral tests used in this study assessed anxiety-like behavior associated with the unconditioned response of rats in situations where there is a conflict between exploring novel environments and avoiding open spaces (Griebel and Holmes, 2013). Consistent with findings from previous studies, we found that PAE increased anxiety-like behavior, but the effects were sex- and test-dependent (Cullen et al., 2013; Hofmann et al., 2005; Raineki et al., 2016; Rouzer et al., 2017; Varlinskaya and Mooney, 2014). Specifically, regardless of CUS condition, PAE males showed greater anxiety-like behavior (less time in the light) compared to C males in the DL, while PAE females exhibited greater anxiety-like behavior (fewer center entries with no differences in locomotor activity) compared to C females in the OF. These findings support previous data indicating that PAE has lasting impacts and may increase anxiety-like behavior in adulthood (Famy et al., 1998; O’Connor and Paley, 2009; Pei et al., 2011). The neurobiology that underlies behavior in each of these tests is not fully understood (Griebel and Holmes, 2013). However, studies demonstrating that rodent strains exhibiting moderate to high degrees of anxiety-like behavior perform differently in a battery of anxiety tests (van der Staay et al., 2009; van Gaalen and Steckler, 2000), and that subtle differences are found in the effects of several classes of anxiolytics (e.g. 5-HT1A receptor agonists, adrenergic agents) on behavior in the EPM and DL (Bourin, 2015) provide support for the suggestion that different aspects of anxiety-like behavior may be measured by each test, and that acquiring results from a battery of tests is better than relying on findings from a single test. Together, our results suggest that PAE may alter different aspects of anxiety in males and females.
While there were no overall effects of PAE, independent of CUS, on CRHR1 and MR mRNA expression in either sex, PAE differentially decreased GR mRNA expression in males and females. In the medial amygdala, PAE males, but not females, showed lower GR mRNA expression than their C counterparts. By contrast, in the IL of the mPFC, PAE females, but not males, exhibited lower GR mRNA expression than their C counterparts. The role of GR in mediating HPA activity following stress varies by brain area. In the mPFC, GR activation is involved with glucocorticoid negative feedback, but in the amygdala, it provides feedforward regulation of the HPA axis (Herman et al., 2016; Myers et al., 2012). Therefore, although there was GR downregulation in both males and females, it is possible that PAE females may have deficits in negative feedback regulation while PAE males may have deficits in feedforward regulation of HPA activity. These findings are consistent with previous data showing that PAE differentially affects HPA activity/regulation in males and females (Glavas et al., 2007; Hellemans et al., 2010). Given that PAE males and females both showed increased anxiety-like behavior albeit in different tasks, a decrease in GR mRNA expression may, at least in part, contribute to this altered behavior, although different mechanisms may be involved in males and females.
4.2. Differential immediate vs. delayed effects of CUS on stress-related receptor expression and anxiety-like behavior in males and females
We examined both immediate and delayed effects of CUS on brain and behavior. This allowed us to gain insight into the changes that occur immediately following chronic stress, while basal HPA activity is likely increased, compared to changes during a recovery period, when HPA activity likely returns to basal levels. Overall, we found that CUS unmasked alterations in mRNA expression of CRHR1 and GR, but not MR, in the brain of PAE males following immediate testing post-CUS, whereas the effects of CUS on anxiety-like behavior were apparent only with delayed testing. By contrast, CUS had delayed effects on MR, but not CRHR1 and GR, mRNA expression in the brain of PAE females, and there were no specific effects of CUS on behavior. Moreover, there was an overall effect of CUS on brain and behavior outcomes of females, independent of prenatal treatment.
Specifically, we found that PAE males failed to show the typical upregulation of CRHR1 and GR mRNA expression shown by their control counterparts following immediate testing post-CUS. That is, CRHR1 mRNA expression in the lateral and medial nuclei of the amygdala was increased following immediate testing compared to both delayed testing and the non-CUS condition. GR mRNA expression was also higher following immediate compared to delayed testing in the IL, and in all nuclei of the amygdala. However, analyses indicate that CUS-induced increases in CRHR1 and GR mRNA expression were generally driven by C and PF males, with PAE males showing comparatively lower CRHR1 and GR mRNA expression in these brain areas. As noted, CRHR1 and GR have different roles in modulating HPA activity and behavior depending on where they are expressed (Henckens et al., 2016; Herman et al., 2016; Myers et al., 2012). Taken together, our findings suggest that PAE males may have deficits in modulating CRHR1- and GR-mediated HPA feedback and feedforward regulation, and in receptor autoregulation following CUS. Importantly, these results are consistent with our suggestion of a deficit in feedforward regulation in PAE males. Furthermore, given that basal CORT levels post-CUS were elevated similarly among groups, lower GR mRNA expression may indicate that PAE and C males respond differently to the same CUS-induced increase in basal CORT levels. Alternatively, as high CORT levels may downregulate GR (Saenz del Burgo et al., 2013), lower expression of GR mRNA in the mPFC and amygdala of PAE males immediately following CUS may reflect exposure to elevated CORT levels over the 10-day CUS period. This suggestion is consistent with previous findings, which demonstrate that CORT and/or ACTH levels are typically higher in PAE compared to control animals in response to a variety of stressors (reviewed in Hellemans et al., 2010).
Interestingly, in comparison to the brain, the effects of CUS on anxiety-like behavior in PAE males were apparent only with delayed testing. Specifically, PAE males showed increased anxiety-like behavior (longer latency to emerge) compared to C males when testing occurred following a recovery period, but not when testing occurred immediately after CUS. It is possible that the immediate changes in the brain set the stage for subsequent effects on behavior. For instance, both phosphorylation of trk B (the receptor for BDNF) and ERK (downstream kinase suggested to mediate antidepressant efficacy) were found to be decreased 2 weeks or 1 month, but not 1 day, following chronic CORT exposure (Gourley and Taylor, 2009). BDNF plays an important role in neuronal growth and survival, and in synaptogenesis and plasticity, and GR has been suggested to mediate the CORT-induced decrease in BDNF expression (Chen et al., 2017). In turn, altered BDNF expression has been suggested to contribute to the development of stress-related disorders, such as anxiety (Arango-Lievano et al., 2015). Therefore, a deficit in autoregulation of GRs in PAE males in response to CUS may have important implications for neuronal morphology and function, and may contribute to the delayed increase in anxiety-like behavior in PAE compared to C males.
By contrast to males, there were interactive effects of PAE and CUS on MR, but not GR and CRHR1, mRNA expression in females. MR mRNA expression in the CA3 subregion of the hippocampal formation was higher in PAE than C females following delayed testing post-CUS, suggesting that PAE females failed to show the typical downregulation of MR mRNA shown by their control counterparts following CUS, and thus may have a deficit in modulating MR expression. MR is involved in regulating both basal and stress-induced HPA activity, setting the threshold of HPA reactivity to stress, and maintaining high neuronal excitability (Joëls and de Kloet, 2017; ter Heegde et al., 2015). Furthermore, transgenic and pharmacological studies indicate that MR may be crucial for promoting neurogenesis, maintaining neuronal integrity, and preventing GR-mediated apoptosis (ter Heegde et al., 2015). Therefore, although we did not observe interactive effects of PAE and CUS on anxiety-like behavior in females, our finding that differential MR mRNA expression was unmasked in PAE compared to C females following a recovery period after CUS suggests that changes in the brain may continue to occur after stress exposure has ended, with implications for sensitivity and stress reactivity of the HPA axis, neurogenesis, and neuronal integrity in the long-term.
In addition, we found both immediate and delayed effects of CUS on receptor mRNA expression and anxiety-like behavior in females, independent of prenatal treatment. Expression of CRHR1 mRNA in the IL was higher following immediate compared to delayed testing post-CUS. By contrast, MR mRNA expression in the DG and GR mRNA expression in the amygdala were lower with delayed testing compared to both immediate testing and/or non-CUS condition. Anxiety-like behavior was increased overall (i.e. decreased frequency of center entries, time in center, latency to center, and distance travelled in center) following both immediate and delayed testing post-CUS. CRHR1 has been suggested to mediate the anxiogenic effects of CRH in the mPFC, as CRH infusion in this brain area, which expresses only CRHR1, promotes anxiety-like behavior (Jaferi and Bhatnagar, 2007). Therefore, increased CRHR1 expression may contribute, at least in part, to the increase in anxiety-like behavior following CUS among females. The delayed effects of CUS on MR and GR mRNA expression suggest that CUS may alter both basal and stress regulation of HPA activity, but that dysregulation may not manifest immediately. Taken together, it appears that CUS-induced alterations in CRHR1, MR and GR mRNA expression may underlie the increase in anxiety-like behavior among females. Furthermore, our finding of increased anxiety-like behavior following immediate testing post-CUS is consistent with previous studies on the short-term effects of CUS (see Willner, 2005), and our findings extend the literature by demonstrating that certain effects of CUS on behavior may be delayed. Moreover, that CUS may selectively increase anxiety-like behavior in females is consistent with clinical literature suggesting that anxiety may be almost twice as common in women than in men, possibly due to increased susceptibility to environmental stress (Altemus, 2006; Sandanger et al., 2004).
4.3. Pair-feeding
The PF group, which receives a reduced ration of a nutritionally optimal diet matched in amount to that of an alcohol-consuming partner (Weinberg, 1985, 1984), is used to control for the reduction in food intake typically observed in alcohol-consuming animals. However, pair-feeding is an imperfect control group as it cannot control for the nutritional effects of alcohol (e.g. absorption and utilization of nutrients). In addition, because they receive a reduced ration of food, these animals also tend to consume their entire daily ration within a few hours of diet presentation, and are thus food deprived and hungry until feeding the next day. Therefore, a component of prenatal stress is likely associated with the pair-feeding paradigm and effects on offspring represent, at least partially, the impact of combined effects of prenatal stress and the nutritional consequence of receiving a reduced food ration. Our behavioral data show that both PF males and females, regardless of CUS exposure, exhibited increased anxiety-like behavior compared to their respective C counterparts. Furthermore, we found that pair-feeding decreased CRHR1 mRNA expression in the CA1 in males and decreased GR mRNA expression in the IL in females. More importantly, however, while PF males and females may in a few cases show similar changes in brain and behavioral measures as PAE animals, in other cases they exhibit different mRNA expression and behavior. Taken together, these results indicate that while some effects of PAE and PF may overlap, the mechanisms underlying PAE and PF effects likely differ rather than represent a continuum of effects along the same pathway (Glavas et al., 2007).
4.4. Limitations
It is possible that mechanisms beyond those investigated in the present study might play a role in the functional effects of PAE that were observed. For example, while there were several instances where there were no effects of PAE on receptor mRNA expression, changes in receptor localization could also be an issue in the altered anxiety-like behavior observed. Indeed, it has been reported that while total levels of GR and MR were unchanged in unstressed adolescent PAE mice, nuclear localization of the receptors in the hippocampus and PFC was altered, and these impairments were associated with decreased hippocampus-dependent learning and memory, and inflexibility in frontal cortical-dependent reversal learning (Allan et al., 2014; Caldwell et al., 2014). Additionally, membrane-bound GR and MR are known to exert non-genomic effects distinct from those of cytoplasmic/nuclear receptors, in response to stress (Joëls and de Kloet, 2017; Rainville et al., 2017). Further investigations using an integrated approach in examining the complex molecular machinery of MR- and GR-mediated regulation of the HPA stress response following PAE will certainly enrich the current findings.
Another limitation in the present experimental design is that behavioral tests were conducted without counterbalancing for order of testing. The order of testing was found to have only a minor impact on behavioral responses, except when a more stressful behavioral test, such as FST, is done first or last (Blokland et al., 2012; McIlwain et al., 2001). Our typical practice is to order the tests from least to most stressful, e.g., FST is typically performed last. The possibility that PAE animals may respond differently from controls to the cumulative stress from a particular testing order remains to be determined and could provide additional insight into the outcomes we observed. As it stands, the present study does provide important information on brain and behavioral responses to the same cumulative stress experience, which has implications for the response to stress in general in individuals prenatally exposed to alcohol. It would be an interesting extension to the current findings to probe for possible prenatal treatment × order of testing × CUS exposure interactions to further evaluate the impact of PAE on anxiety-like behavior, which could enhance interpretations of the present results.
5. Conclusions
In the present study, we found that PAE and CUS independently increased anxiety-like behavior and altered MR, GR, and CRHR1 mRNA expression, and did so in a sexually dimorphic manner. In addition, exposure to CUS differentially unmasked alterations in anxiety-like behavior and receptor mRNA expression in the mPFC, amygdala, and hippocampal formation in PAE males and females. These results substantiate sex differences in vulnerability to stress and anxiety, and suggest that different mechanisms may underlie anxiety-like behavior in males and females. That the effects of CUS can be immediate or delayed underscores the importance of investigating the temporal effects of CUS for a more thorough understanding of the role of HPA activity in anxiety-like behavior. Overall, the changes observed in the receptor systems in brain areas involved with both stress and emotional regulation could potentially underlie the increase in anxiety-like behavior following PAE and CUS exposure in adulthood. Delineating the mechanisms by which PAE and CUS, independently and interactively, may contribute to the development of mental health issues, such as anxiety, will ultimately help establish novel or targeted interventions and treatments for affected individuals.
Acknowledgements
We thank all members of the Weinberg laboratory for their assistance with this study. We would like to especially thank Erin Morgan for her help with slicing brains, and Melissa Chiu for her help with analysis of the autoradiographic films.
Role of the funding sources
This work was supported by funding from the National Institutes of Health/National Institute on Alcohol Abuse and Alcoholism [grant numbers R37 AA007789 and R01 AA022460] and Kids Brain Health Network (Canadian Networks of Centers of Excellence) [grant 20R64153] to Joanne Weinberg; and Natural Sciences and Engineering Research Council of Canada and an IMPART Fellowship (Canadian Institute of Health Research STIHR) to Vivian Lam. Funding sources did not contribute to experimental design, collection, analysis or interpretation of the data, or decisions regarding submission.
Footnotes
Conflict of interest
None.
Declarations of interest
None
References
- Allan AM, Goggin SL, Caldwell KK, 2014. Prenatal alcohol exposure modifies glucocorticoid receptor subcellular distribution in the medial prefrontal cortex and impairs frontal cortex-dependent learning. PLoS One 9, e96200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altemus M, 2006. Sex differences in depression and anxiety disorders: potential biological determinants. Horm. Behav 50, 534–538. [DOI] [PubMed] [Google Scholar]
- Arango-Lievano M, Lambert WM, Bath KG, Garabedian MJ, Chao MV,Jeanneteau F, 2015. Neurotrophic-priming of glucocorticoid receptor signaling is essential for neuronal plasticity to stress and antidepressant treatment. Proc. Natl. Acad. Sci 112, 15737–15742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Binder EB, Nemeroff CB, 2010. The CRF system, stress, depression and anxiety - insights from human genetic studies. Mol. Psychiatry 15, 574–588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blokland A, ten Oever S, van Gorp D, van Draanen M, Schmidt T, Nguyen E, Krugliak A, Napoletano A, Keuter S, Klinkenberg I, 2012. The use of a test battery assessing affective behavior in rats: order effects. Behav. Brain Res 228, 16–21. [DOI] [PubMed] [Google Scholar]
- Bourin M, 2015. Animal models for screening anxiolytic-like drugs: a perspective. Dialogues Clin. Neurosci 17, 295–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brocardo PS, Boehme F, Patten A, Cox A, Gil-Mohapel J, Christie BR, 2012. Anxiety- and depression-like behaviors are accompanied by an increase in oxidative stress in a rat model of fetal alcohol spectrum disorders: protective effects of voluntary physical exercise. Neuropharmacology 62, 1607–1618. [DOI] [PubMed] [Google Scholar]
- Caldwell KK, Goggin SL, Tyler CR, Allan AM, 2014. Prenatal alcohol exposure is associated with altered subcellular distribution of glucocorticoid and miner-alocorticoid receptors in the adolescent mouse hippocampal formation. Alcohol. Clin. Exp. Res 38, 392–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caldwell KK, Goggin SL, Labrecque MT, Allan AM, 2015. The impact of prenatal alcohol exposure on hippocampal-dependent outcome measures is influenced by prenatal and early-life rearing conditions. Alcohol. Clin. Exp. Res 39, 631–639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen H, Lombès M, Le Menuet D, 2017. Glucocorticoid receptor represses brain-derived neurotrophic factor expression in neuron-like cells. Mol. Brain 10, 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cullen CL, Burne THJ, Lavidis NA, Moritz KM, 2013. Low dose prenatal ethanol exposure induces anxiety-like behaviour and alters dendritic morphology in the basolateral amygdala of rat offspring. PLoS One 8, e54924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Detke MJ, Rickels M, Lucki I, 1995. Active behaviors in the rat forced swimming test differentially produced by serotonergic and noradrenergic antidepressants. Psychopharmacology (Berl.) 121, 66–72. [DOI] [PubMed] [Google Scholar]
- Famy C, Streissguth APAP, Unis ASAS, 1998. Mental illness in adults with fetal alcohol syndrome or fetal alcohol effects. Am. J. Psychiatry 155, 552–554. [DOI] [PubMed] [Google Scholar]
- Fanselow MS, Dong H-W, 2010. Are the dorsal and ventral hippocampus functionally distinct structures? Neuron 65, 7–19. 10.1016/j.neuron.2009.11.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallagher JP, Orozco-Cabal LF, Liu J, Shinnick-Gallagher P, 2008. Synaptic physiology of central CRH system. Eur. J. Pharmacol 583, 215–225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallo PV, Weinberg J, 1981. Corticosterone rhythmicity in the rat: interactive effects of dietary restriction and schedule of feeding. J. Nutr 111, 208–218. [DOI] [PubMed] [Google Scholar]
- Glavas MM, Ellis L, Yu WK, Weinberg J, 2007. Effects of prenatal ethanol exposure on basal limbic-hypothalamic-pituitary-adrenal regulation: role of corticosterone. Alcohol. Clin. Exp. Res 31, 1598–1610. [DOI] [PubMed] [Google Scholar]
- Gourley SL, Taylor JR, 2009. Recapitulation and reversal of a persistent depression-like syndrome in rodents Curr. Protoc. Neurosci Chapter 9, Unit 9.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griebel G, Holmes A, 2013. 50 years of hurdles and hope in anxiolytic drug discovery. Nat. Rev. Drug. Discov 12, 667–687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hellemans KGC, Sliwowska JH, Verma P, Weinberg J, 2010. Prenatal alcohol exposure: fetal programming and later life vulnerability to stress, depression and anxiety disorders. Neurosci. Biobehav. Rev 34, 791–807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henckens MJAG, Deussing JM, Chen A, 2016. Region-specific roles of the corticotropin-releasing factor–urocortin system in stress. Nat. Rev. Neurosci 17, 636–651. [DOI] [PubMed] [Google Scholar]
- Herman JP, Watson SJ, Spencer RL, 1999. Defense of adrenocorticosteroid receptor expression in rat hippocampus: effects of stress and strain. Endocrinology 140, 3981–3991. 10.1210/endo.140.9.6962. [DOI] [PubMed] [Google Scholar]
- Herman JP, McKlveen JM, Ghosal S, Kopp B, Wulsin A, Makinson R, Scheimann J, Myers B, 2016. Regulation of the hypothalamic-pituitary-adrenocortical stress response. Comprehensive Physiology John Wiley & Sons, Inc, Hoboken, NJ, USA, pp. 603–621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofmann CE, Patyk IA, Weinberg J, 2005. Prenatal ethanol exposure: sex differences in anxiety and anxiolytic response to a 5-HT1A agonist. Pharmacol. Biochem. Behav 82, 549–558. [DOI] [PubMed] [Google Scholar]
- Holsboer F, Ising M, 2008. Central CRH system in depression and anxiety — evidence from clinical studies with CRH1 receptor antagonists. Eur. J. Pharmacol 583, 350–357. [DOI] [PubMed] [Google Scholar]
- Holsboer F, Ising M, 2010. Stress hormone regulation: biological role and translation into therapy. Annu. Rev. Psychol 61, 81–109. [DOI] [PubMed] [Google Scholar]
- Inda C, Armando NG, Dos Santos Claro PA, Silberstein S, 2017. Endocrinology and the brain: corticotropin-releasing hormone signaling. Endocr. Connect 6, R99–R120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobson L, 2014. Hypothalamic-pituitary-adrenocortical axis: neuropsychiatric aspects. Compr. Physiol 4, 715–738. [DOI] [PubMed] [Google Scholar]
- Jaferi A, Bhatnagar S, 2007. Corticotropin-releasing hormone receptors in the medial prefrontal cortex regulate hypothalamic-pituitary-adrenal activity and anxiety-related behavior regardless of prior stress experience. Brain Res 1186, 212–223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joëls M, de Kloet ER, 2017. 30 years of the mineralocorticoid receptor: the brain mineralocorticoid receptor: a saga in three episodes. J. Endocrinol 234, T49–T66. [DOI] [PubMed] [Google Scholar]
- Lam VYY, Raineki C, Takeuchi LE, Ellis L, Woodward TS, Weinberg J, 2018. Chronic stress alters behavior in the forced swim test and underlying neural activity in animals exposed to alcohol prenatally: sex- and time-dependent effects. Front. Behav. Neurosci 12, 42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lan N, Yamashita F, Halpert AG, Ellis L, Yu WK, Viau V, Weinberg J, 2006. Prenatal ethanol exposure alters the effects of gonadectomy on hypothalamic-pituitary-adrenal activity in male rats. J. Neuroendocrinol 18, 672–684. [DOI] [PubMed] [Google Scholar]
- Lan N, Hellemans KGC, Ellis L, Weinberg J, 2015. Exposure to chronic mild stress differentially alters corticotropin-releasing hormone and arginine vasopressin mRNA expression in the stress-responsive neurocircuitry of male and female rats prenatally exposed to alcohol. Alcohol. Clin. Exp. Res 39, 2414–2421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matuszewich L, Karney JJ, Carter SR, Janasik SP, O’Brien JL, Friedman RD, 2007. The delayed effects of chronic unpredictable stress on anxiety measures. Physiol. Behav 90, 674–681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McIlwain KL, Merriweather MY, Yuva-Paylor LA, Paylor R, 2001. The use of behavioral test batteries: effects of training history. Physiol. Behav 73, 705–717. [DOI] [PubMed] [Google Scholar]
- Myers B, McKlveen JM, Herman JP, 2012. Neural regulation of the stress response: the many faces of feedback. Cell. Mol. Neurobiol 32, 683–694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nestler EJ, Barrot M, DiLeone RJ, Eisch AJ, Gold SJ, Monteggia LM, 2002Neurobiology of depression. Neuron 34, 13–25. [DOI] [PubMed] [Google Scholar]
- O’Connor MJ, Paley B, 2009. Psychiatric conditions associated with prenatal alcohol exposure. Dev. Disabil. Res. Rev 15, 225–234. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C, 2004. The Rat Brain in Stereotaxic Coordinates, 5th ed ElsevierAcademic Press. [Google Scholar]
- Pei J, Denys K, Hughes J, Rasmussen C, 2011. Mental health issues in fetal alcohol spectrum disorder. J. Ment. Health 20, 438–448. [DOI] [PubMed] [Google Scholar]
- Raineki C, Chew L, Mok P, Ellis L, Weinberg J, 2016. Short- and long-term effects of stress during adolescence on emotionality and HPA function of animals exposed to alcohol prenatally. Psychoneuroendocrinology 74, 13–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raineki C, Ellis L, Weinberg J, 2018. Impact of adolescent stress on the expression of stress-related receptors in the hippocampus of animals exposed to alcohol prenatally. Hippocampus 28, 201–216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rainville JR, Weiss GL, Evanson N, Herman JP, Vasudevan N, Tasker JG, 2017. Membrane-initiated nuclear trafficking of the glucocorticoid receptor in hypothalamic neurons. Steroids ahead of print [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rouzer SK, Cole JM, Johnson JM, Varlinskaya EI, Diaz MR, 2017. Moderate maternal alcohol exposure on gestational day 12 impacts anxiety-like behavior in offspring. Front. Behav. Neurosci 11, 183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saenz del Burgo L, Cortés R, Mengod G, Montaña M, García del Caño G, Sallés J, 2013. Chronic effects of corticosterone on GIRK1–3 subunits and 5-HT1A receptor expression in rat brain and their reversal by concurrent fluoxetine treatment. Eur. Neuropsychopharmacol 23, 229–239. [DOI] [PubMed] [Google Scholar]
- Sandanger I, Nygård JF, Sørensen T, Moum T, 2004. Is women’s mental health more susceptible than men’s to the influence of surrounding stress? Soc. Psychiatry Psychiatr. Epidemiol 39, 177–184. [DOI] [PubMed] [Google Scholar]
- Sliwowska JH, Lan N, Yamashita F, Halpert AG, Viau V, Weinberg J, 2008. Effects of prenatal ethanol exposure on regulation of basal hypothalamic–pituitary–adrenal activity and hippocampal 5-HT1A receptor mRNA levels in female rats across the estrous cycle. Psychoneuroendocrinology 33, 1111–1123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strange BA, Witter MP, Lein ES, Moser EI, 2014. Functional organization of the hippocampal longitudinal axis. Nat. Rev. Neurosci 15, 655–669. 10.1038/nrn3785. [DOI] [PubMed] [Google Scholar]
- ter Heegde F, De Rijk RH, Vinkers CH, 2015. The brain mineralocorticoid receptor and stress resilience. Psychoneuroendocrinology 52, 92–110. [DOI] [PubMed] [Google Scholar]
- Uban KA, Comeau WL, Ellis LA, Galea LAM, Weinberg J, 2013. Basal regulation of HPA and dopamine systems is altered differentially in males and females by prenatal alcohol exposure and chronic variable stress. Psychoneuroendocrinology 38, 1953–1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Staay FJ, Schuurman T, van Reenen CG, Korte SM, 2009. Emotional reactivity and cognitive performance in aversively motivated tasks: a comparison between four rat strains. Behav. Brain Funct 5, 50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Gaalen MM, Steckler T, 2000. Behavioural analysis of four mouse strains in an anxiety test battery. Behav. Brain Res 115, 95–106. [DOI] [PubMed] [Google Scholar]
- Varlinskaya EI, Mooney SM, 2014. Acute exposure to ethanol on gestational day 15 affects social motivation of female offspring. Behav. Brain Res 261, 106–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veenit V, Riccio O, Sandi C, 2014. CRHR1 links peripuberty stress with deficits in social and stress-coping behaviors. J. Psychiatr. Res 53, 1–7. [DOI] [PubMed] [Google Scholar]
- Weinberg J, 1984. Nutritional issues in perinatal alcohol exposure. Neurobehav. Toxicol. Teratol 6, 261–269. [PubMed] [Google Scholar]
- Weinberg J, 1985. Effects of ethanol and maternal nutritional status on fetal development. Alcohol. Clin. Exp. Res 9, 49–55. [DOI] [PubMed] [Google Scholar]
- Wilcoxon JS, Kuo AG, Disterhoft JF, Redei EE, 2005. Behavioral deficits associated with fetal alcohol exposure are reversed by prenatal thyroid hormone treatment: a role for maternal thyroid hormone deficiency in FAE. Mol. Psychiatry 10, 961–971. [DOI] [PubMed] [Google Scholar]
- Willner P, 2005. Chronic mild stress (CMS) revisited: consistency and behaviouralneurobiological concordance in the effects of CMS. Neuropsychobiology 52, 90–110. [DOI] [PubMed] [Google Scholar]