Abstract
Linoleate triol esters are intermediates along the pathway of formation of the mammalian skin permeability barrier. In connection with the study of their involvement in barrier formation we required access to isomerically pure and defined samples of four linoleate triol esters. A common synthetic strategy was developed starting from isomeric alkynols derived from d-tartaric acid and 2-deoxy-d-ribose.
Keywords: Total synthesis, Stereochemistry, Fatty acids, Synthesis strategy
The permeability barrier of the mammalian epidermis is critical to sustainability of life as it provides defense against water loss and prevents the infiltration of harmful bacteria and toxins [1]. Human and mouse genetic studies identify an enzymatic pathway initiated by 12R-lipoxygenase (12R-LOX) and followed by epidermal lipoxygenase-3 (eLOX3) as critical to the formation of an intact epidermal barrier; genetic deficiencies are neonatal lethal in mice and lead to congenital ichthyosis (scaly skin) in afflicted human families [2,3]. 12R-LOX and eLOX3 have been shown to oxidize the essential fatty acid linoleate esterified in the skin-specific ceramide, Cer-EOS, and a subsequent epoxide hydrolase step forms EOS-linoleate-triols (Fig. 1) [4–6]. Overall the pathway presumably serves to convert a hydrophobic fatty acid to hydrophilic triols leading to membrane reorganization and further processing of the skin barrier [4].
Fig. 1.
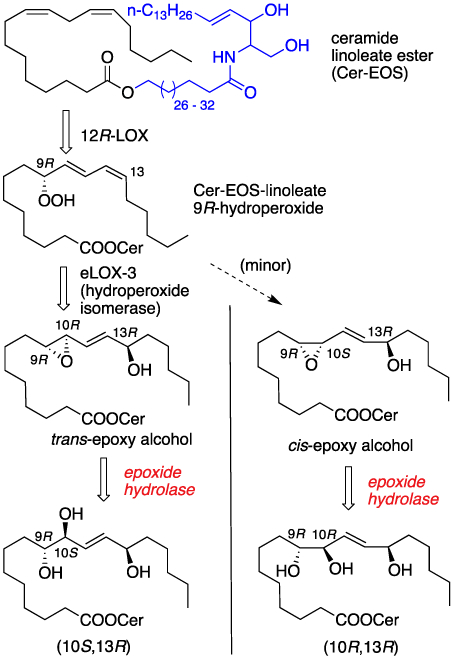
212R-LOX oxidation of linoleate ceramide to linoleate triol.
Key steps of triol formation are summarized in Fig. 1 starting from 12R-LOX mediated oxidation of linoleate ceramide ester to the corresponding linoleate 9R-hydroperoxide. Isomerization of the latter to an intermediate epoxy alcohol sets the stage for epoxide hydrolysis leading to isomeric linoleate triols. It is hypothesized that triol formation facilitates hydrolysis of the ceramide ester resulting in release of the omega hydroxyl group which then cross-link proteins by ester bond and ultimately skin barrier formation [4,5]. In order to support mechanistic studies of the epidermal LOX pathway we required defined samples of the four isomeric triol carboxylic acids, the hydrolytic products of the ceramide esters (Fig. 1). Below we describe the stereocontolled synthesis of these triols as their corresponding methyl esters utilizing a common synthetic strategy from isomeric starting materials. These specific epidermal triols are among the sixteen regio- and stereo isomers produced through autoxidation for which an LC-MS separation workflow was recently reported [7,8].
Common among lipid oxidation products are 1,2-oxygenated stereocenters, typically in the form of 1,2-diols with examples of all four possible stereochemical perturbations. As part of our synthetic program to provide investigators access to a broad spectrum of lipid metabolites we have established synthetic routes to access all four isomers of acetonide protected alkynols 2 (Fig. 2). Alkynols (9R, 10R)- and (9R, 10S)-2 are prepared starting from d-tartaric acid and L-2-deoxyribose respectively (Scheme 1). Alkynol (9R, 10R)-2 was prepared from d-tartaric acid in five steps by way of acetonide 3 (Scheme 1) [9]. Acetylide displacement of the triflate derived from alcohol 3 followed a slight modification of an earlier procedure reported by Mukai and Hanaoka [10]. Alkynol (9R, 10S)-2 was prepared in 2 steps from 2-deoxy-l-ribose employing a Colvin alkynylation of an intermediate lactol as reported by Brimble [11].
Fig. 2.
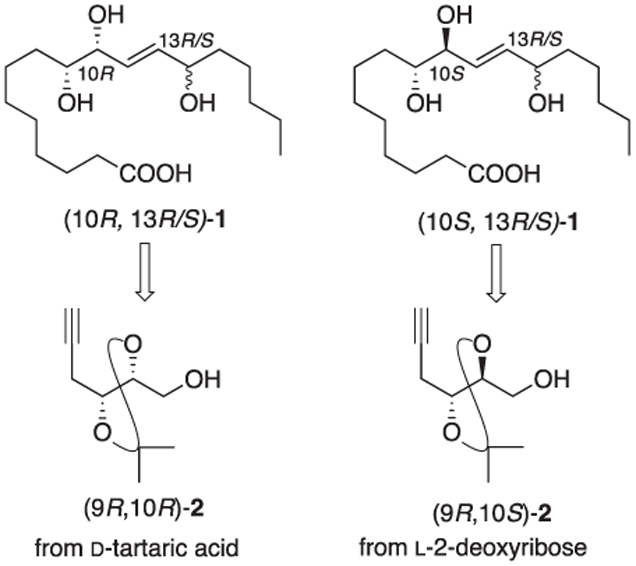
Synthetic strategy to access four linoleic acid triols starting isomer alkynols (2).
Scheme 1.
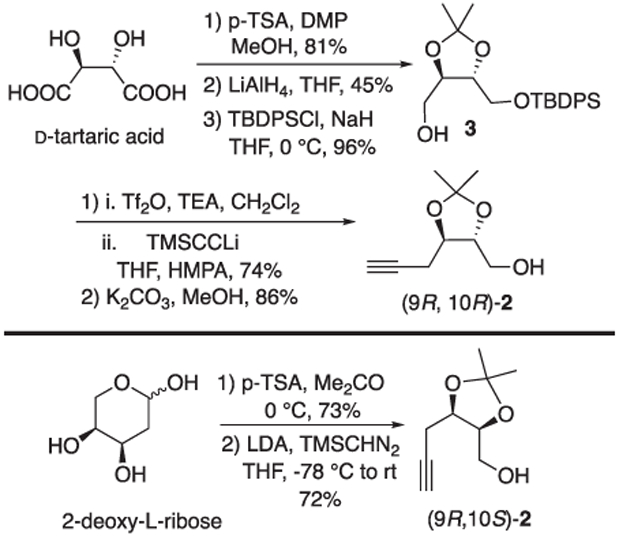
Syntheses of akynols (9R 10R)- and (9R, 10S)-2.
The synthesis of linoleic acid triols (9R, 10R, 13R)- and (9R, 10R, 13S)-1 started with a Sonagishira coupling of (9R, 10R)-2 and vinyl iodide 4 [12] to afford enyne 5 in 82% yield. Saturation of the enyne group of 5 under standard hydrogenation conditions (e.g. H2, Pd/C, EtOAc) proved variable often leading to incomplete reduction. In contrast, saturation of 5 by in situ generated nickel(0) under an atmosphere of hydrogen reproducibly delivered ester 6 in near quantitative yield. Dess-Martin periodinane oxidation of 6 was followed by condensation with the anion derived from ketophospho- nate 8 [13] to afford enone 9. Luche reduction of 9 provided a 1:1 mixture of (13R)- and (13S)-10, separated by flash chromatography. Alternatively, reduction of 9 using (S)-CBS catalyst was demonstrated to afford a 9:1 mixture of alcohols favoring (13R)-10. The configuration of the alcohols (13R)- and (13S)-10 were assigned using the Mosher ester analysis method, the major isomer produced from the (S)-CBS reduction was in agreement with related enone substrates [14]. Acetonide removal yielded the corresponding triol esters (9R, 10R, 13R)- and (9R, 10R, 13S)-11 (See Scheme 2). The individual esters were saponified with aqueous potassium hydroxide in methanol as needed for the analysis of epidermal lipid samples.
Scheme 2.
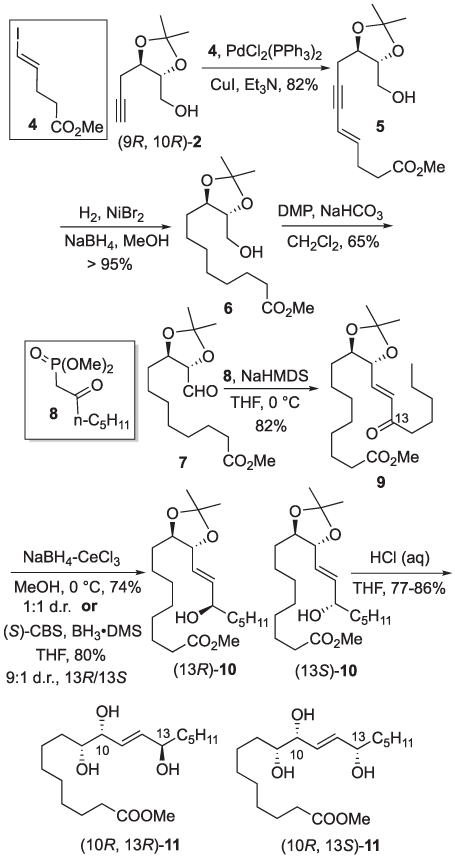
Synthesis of triol esters (10R, 13R)- and (10R, 13S)-11.
Synthesis of the remaining pair of linoleic acid triols were prepared from alkynol (9R, 10S)-2 using the same series of reactions as employed for the (9R, 10R, 13R)- and (9R, 10R, 13S)-11 pair of esters (Scheme 3). Once again cross-coupling of (9R, 10S)-2 and vinyl iodide 4 was followed by hydrogenation over nickel(0) to afford alcohol 12. The latter was subjected to the earlier described oxidation-olefination sequence to give enone 13. Luche reduction again provided a separable mixture of allylic alcohols (13R)- and (13S)-14, assignment of C13 alcohol stereochemistry was again based upon Mosher ester analysis. Finally, acetonide hydrolysis (13R)- and (13S)-14 afforded methyl esters (10S, 13R)- and (10S, 13S)-11, respectively.
Scheme 3.
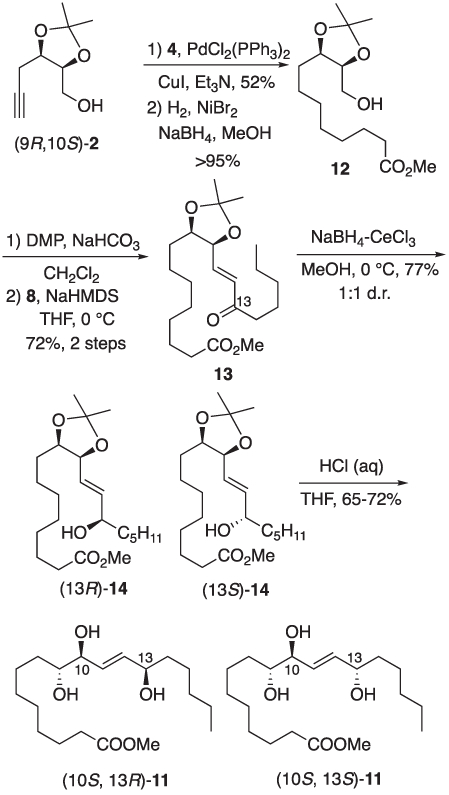
Synthesis of triol esters (10S, 13R)- and (10S, 13S)-11.
In summary, we have developed stereocontrolled routes to four linoleic ester triols. The synthetic products and intermediates will find utility in studies aimed at defining the process by which the mammalian epidermal permeability barrier is formed. Studies along this line will be reported in due course.
Acknowledgments
This work was supported by National Institute of General Medical Sciences Grant GM-115722 (G.A.S.), National Institute of Arthritis and Musculoskeletal and Skin Diseases Grant AR-51968 (A.R.B.), and National Institutes of Health Shared Resource Grant P30 CA068485.
Footnotes
Appendix A. Supplementary data
Supplementary data to this article can be found online at https://doi.org/10.1016/j.tetlet.2018.11.033.
References
- [1].Madison KC, J. Invest. Dermatol. 121 (2003) 231–241. [DOI] [PubMed] [Google Scholar]
- [2].Krieg P, Furstenberger G, Biochim. Biophys. Acta (2014) 390–400. [DOI] [PubMed] [Google Scholar]
- [3].Muñoz-Garcia A, Thomas CP, Keeney DS, Zheng Y, Brash AR, Biochim. Biophys. Acta (2014) 401–408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Zheng Y, Yin H, Boeglin WE, Elias PM, Crumrine D, Beier DR, Brash AR, J. Biol. Chem. 286 (2011) 24046–24056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Chiba T, Thomas CP, Boeglin WE, O’Donnell VB, Brash AR, J. Biol. Chem. 291 (2016) 14540–14554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Yamanishi H, Boeglin WE, Morisseau C, Davies RW, Sulikowski GA, Hammock BD, Brash AR, J. Lipid Res. 59 (2018) 684–695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Fuchs D, Hamberg M, Sköld CM, Wheelock AM, Wheelock CE, J. Lipid Res. 59 (2018) 2025–2033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].A racemic synthesis of linoleic acid triols has been reported, see: Tadario K, Hirukawa T, Yano T, M. Bull Chem. Soc Jpn 67 (1994) 839–842. [Google Scholar]
- [9].Iida H, Yamazaki N, Kibayashi C, J. Org. Chem. 52 (1987) 3337–3342. [Google Scholar]
- [10].Mukai C, Kim JS, Uchiyama M, Sakamoto S, Hanaoka MJ, Chem. Soc. Perk. Trans 1 (1998) 2903–2915. [Google Scholar]
- [11].Yuen TY, Brimble MA, Org. Lett. 14 (2012) 5154–5157. [DOI] [PubMed] [Google Scholar]
- [12].Reddy CR, Suman D, Rao NN, Helv. Chim. Acta 98 (2015) 967–972. [Google Scholar]
- [13].Hayashi K, Tanimoto H, Zhang H, Morimoto T, Nishiyama Y, Kakiuchi K, Org. Lett. 14 (2012) 5728–5731. [DOI] [PubMed] [Google Scholar]
- [14].Pandya BA, Snapper ML, J. Org. Chem. 73 (2008) 3754–3758. [DOI] [PubMed] [Google Scholar]


