Abstract
The powdered rhizome of turmeric has been extensively used in India and other South Asian cuisines, and is an integral part of Ayurvedic medicine for a broad range of conditions. In particular, curcumin, a major active component of turmeric, is one of the most studied botanicals for its anti-inflammatory, anti-oxidant and anti-cancer properties. Despite its well-documented therapeutic efficacy, for years the limited systemic bioavailability of curcumin has hindered its development as a potential therapeutic agent. However, recent introduction of unique extraction processes and various delivery methods has resulted in the development of new curcumin formulations and significantly improved its bioavailability. While these new formulations will no doubt expand curcumin’s therapeutic potential, there are notable inconsistencies surrounding curcumin’s bioavailability and corresponding bioactivity, raising some important questions. This article dissects various contributing factors of curcumin bioavailability to identify possible causes for the discrepancies associated with its bioactivity and discuss how these new curcumin formulations could further improve its clinical usefulness.
Keywords: Curcumin, Bioavailability, Bioactivity
INTRODUCTION
Turmeric, the powdered rhizome of the herb Curcuma longa, has been extensively used in India and other South Asian cuisines and is a notable ingredient in most curry powders. Turmeric is an integral part of the religious traditions and customs in Hindu culture. For more than six thousand years, turmeric has been used as a medicine in one of the oldest systems of traditional medicine, Ayurveda, for a wide range of conditions including biliary disorders, anorexia, coryza, cough, hepatic and rheumatic disorders, sprains and swelling caused by injury, and sinusitis. In the Unani system of medicine, turmeric has been used for conditions such as liver obstruction and jaundice, and has been applied externally for ulcers and inflammation. Curcumin is a major active component of turmeric, and is one of the most studied botanicals in terms of its anti-inflammatory, anti-oxidant and anti-cancer properties. Interest in this compound is growing and the number of annual reports on the molecule has surpassed one thousand for the last 2 years, with a total of more than nine thousand scientific studies published till date.
Despite thousands of in vivo studies demonstrating the therapeutic efficacy of curcumin, even at very low doses, limited systemic bioavailability has hindered its development as a potential therapeutic agent. Several clinical trials have found that administration of various doses of curcumin resulted in limited bioavailability in blood due to its poor absorption,1–4 which has curtailed its progress from laboratory to the clinic. Nevertheless, recently several clinical studies have shown effectiveness of curcumin in various diseases,5–7 while multiple clinical studies supplementing patients with curcumin are currently ongoing.8,9 Although some investigators have questioned the therapeutic use of curcumin against various diseases for lack of systemic bioavailability, substantial effort has already gone into improving its bioavailability, through introduction of unique extraction processes and various delivery systems.3 The prospect of improving the bioavailability of curcumin and the subsequent enhancement of its bioactivity could monumentally alter the landscape of the research field as this will open up new avenues for its therapeutic application in various chronic illnesses, including cancer. However, there are notable inconsistencies surrounding curcumin’s bioavailability and corresponding bioactivity, raising some important questions: (a) Is bioavailability the only factor driving curcumin’s bioactivity? (b) If bioavailability of curcumin was a singular critical issue, what is the basis of observed efficacy reported in hundreds and thousands of pre-clinical and clinical studies with curcumin? (c) Why does significant increase in curcumin bioavailability not correspond to increasing bioactivity of equal magnitude? On the flip side, if none of these concerns are reasonable, what are the other factors that might contribute to curcumin’s bioactivity? This article dissects some of these issues, and provides a perspective that will help better understand how curcumin exerts its bioactivity, and how various approaches aimed at enhancing its absorption may further improve its efficacy for various clinical purposes.
POTENTIAL FACTORS CONTRIBUTING TO LOW CURCUMIN BIOAVAILABILITY
Before we delve into possible causes of discrepancies between bioavailability and bioactivity of curcumin, it is important to identify major obstacles contributing to low systemic bioavailability of curcumin. The low bioavailability of curcumin is primarily caused by three factors: low aqueous solubility, poor absorption, and extensive metabolic conversion of the molecule. While low solubility in water stems from polarity and poor dissolution rate of curcumin, the lipophilic nature of curcumin is a major contributing factor for poor absorption. Interestingly, for gastrointestinal diseases, while most dietary compounds will be absorbed along the digestive tract and will not reach the colon, large intestinal curcumin concentration can be significantly increased through ingestion. In support of this, a previous clinical study demonstrated that daily consumption of 3.6 g of curcumin resulted in achieving curcumin concentration in the malignant colorectal tissues ranging from 7 to 20 nmol/g.10 Nevertheless, poor water solubility is a major problem that hinders delivery of curcumin to non-enteric organs.
Likewise, even though there are varying reports on the parameters contributing to systemic absorption of curcumin, poor curcumin absorption is a well-recognized phenomenon.3,11,12 A previous study reported that 75% of curcumin fed to rodents was excreted in feces,11 while findings from another study utilizing radioactively labeled curcumin indicated that curcumin absorption was limited to 60–66% regardless of curcumin dose.12 Preclinical in vivo studies investigating the pharmacokinetics of curcumin show that only 0.13–1.35 µg/mL concentration of curcumin was present in the blood from 1 to 2 g/kg when gavaged.13,14 Although these studies confirm the low systemic bioavailability of curcumin relative to the amount delivered, not all physical characteristics of curcumin are detrimental. Curcumin has been shown to be detectable in the brain after oral administration15 and curcumin treatment in a mouse model of Alzheimer disease resulted in significant improvement in cognitive function.16 Ironically, the high lipophilic characteristic of curcumin hinders its water solubility, but still allows curcumin to cross the blood-brain barrier.
Understandably, limited solubility and absorption results in lower bioactivity of curcumin in non-enteric organs, but this issue is further complicated by the fact that there is a growing understanding that efficacy of curcumin may not be entirely due to the parent molecule, but in part may be due to its metabolites. The majority of curcumin is suggested to be metabolized in the liver and subsequently converted into curcumin conjugates and degradation products.11,17 This contention is partly because various preclinical animal studies and human clinical trials demonstrating curcumin-induced prevention or reversal of the disease state cannot completely be explained if curcumin absorption was the most important concern. Therefore, it is not unreasonable to speculate that some of the observed efficacy of curcumin may be contributed by its metabolites, which are often not measured in curcumin bioavailability studies. Collectively, these data provide a reasonable justification and rationale that moving forward we must recognize that bioavailability of curcumin should not be the sole focus, but we should consider absorption and bioavailability of both curcumin and its metabolites to target organs during the design of future clinical trials.
CURCUMIN CONJUGATES
While most recent researches have been focusing on the bioavailability of curcumin, it has been long known that curcumin undergoes rapid metabolism after administration.18 Curcumin absorbed in the general circulation is subjected to conjugations such as glucuronidation and sulfation primarily in the liver, but also at other tissue sites.2 Although initially dismissed as functionally irrelevant, curcumin conjugates are now being recognized to exert antitumorigenic as well as anti-inflammatory effects.19,20 Structurally, curcumin possesses easily accessible – OH and –OH3 sites to form conjugates.21 Therefore, it is quite possible that for those experiments reporting low curcumin bioavailability, the majority of curcumin could have been metabolized by the time plasma was collected. Both curcumin glucuronide and sulfate retain the basic chemical structure, except for a modified phenolic group (Figure 1). Given that the most active parts of the curcumin molecule, two electrophilic α, β-unsaturated carbonyl groups, are preserved in curcumin glucuronide and sulfate, theoretically these molecules should retain some bioactivity of the parent curcumin molecule. Moreover, unlike curcumin, these modified curcumin conjugates are water soluble, which explains why a substantial proportion of curcumin in the general circulation exists as conjugates.13 While conjugated curcumin achieves maximum plasma concentration around 1 hour after ingestion, a substantial amount of conjugated curcumin (up to a third of maximum concentration) was detected in plasma 24 hours post administration compared to only trace levels of non-conjugated curcumin detected in plasma.22 Although most studies support that curcumin conjugates maintain similar molecular properties as parent curcumin, whether the magnitude of their bioactivity is comparable to parent curcumin remains unclear. In fact, there are two schools of thought on this issue: one suggesting that these conjugates are less active, while the other claims that they are more potent compared to parent curcumin.23–26 The major biliary curcumin metabolite, tetra-hydrocurcumin (THC) appears to have weaker anti-inflammatory and anti-tumorigenic properties than parent curcumin in vitro,23 while a rodent study showed that THC had superior attenuation of chloroquine-mediated oxidative damage in rat kidney, in contrast to curcumin.26 In addition, curcumin sulfate has been shown to inhibit prostaglandin E2 (PGE2) production in colonic epithelial cells with lower potency than curcumin.27 Although more investigations are needed to clarify the role of curcumin conjugates, it appears that curcumin conjugates are an important piece of the puzzle, and are significant contributors towards the overall bioactivity of curcumin. Interestingly, piperine, a major component of black and long peppers, has been shown to inhibit enzymatic conjugation of curcumin allowing greater levels of unconjugated curcumin to be absorbed into portal blood circulation14 and increased curcumin tissue retention time,28 as illustrated in Figure 1. Therefore, piperine has been investigated as a potential systemic transporter of curcumin. While this co-delivery strategy with piperine is promising, there are concerns for potential adverse/negative interactions of such preparations with various pharmaceutical drugs, limiting the usefulness of such curcumin preparations in various disease conditions.29
Figure 1: Limiting and contributing factors to curcumin bioavailability.
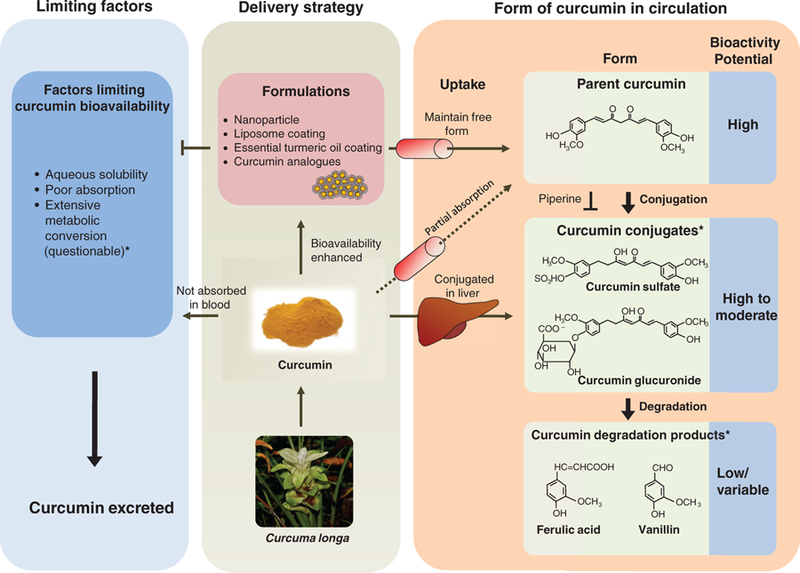
An illustration of potential delivery strategies to enhance curcumin bioavailability. Curcumin formulations result in enhancement of bioavailable parent curcumin in circulation, while standard curcumin is predominantly converted to curcumin conjugates in liver (middle rectangle) then subsequently degraded to curcumin degradation products. The right rectangle summarizes sequential curcumin metabolism in circulation and corresponding potential bioactivity of curcumin metabolites. Piperine can be co-administered to inhibit curcumin conjugation. The left rectangle summarizes factors limiting curcumin bioavailability. *Metabolic conversion could result in curcumin bioactivity through curcumin conjugates and degradation products.
CURCUMIN DEGRADATION PRODUCTS
Recently, it was shown that in addition to curcumin conjugates, curcumin degradation products such as ferulic acid and vanillin may also act as important mediators for its pharmacological effects.30 These curcumin degradation products structurally differ from curcumin conjugates, in that these molecules, including trans-6-(49-hydroxy-39-methoxyphenyl)-2,4-dioxo-5-hexenal, vanillin, ferulic acid, and feruloyl methane, are products of decomposition and reduction due to the unstable nature of curcumin in solution.31 Interestingly, ferulic acids and vanillin have been shown to possess similar pharmacological activity to curcumin in age-related diseases.32,33 In further support of this observation, other studies concluded that heating and alkali treatment of such curcumin degradation products did not alter their bioactivity, indicating that these degradation products appear to be a major contributing factor of curcumin’s bioactivity.34,35
Taken together, bioactivity of curcumin conjugates and degradation products may in part help explain the inconsistencies between bioactivity and bioavailability of curcumin, as well as newer, high-absorption, curcumin formulations that have been developed in recent years. While these formulations have successfully been able to enhance systemic bioavailability of curcumin (anywhere between 5- and 30-fold higher absorption), based upon the limited body of evidence thus far, the corresponding improvement in bioactivity of these curcumin formulations has generally been somewhat modest. Although properties of curcumin conjugates and degradation products will perhaps be better understood in future studies, there definitely seems to be ample room for improving curcumin’s bioactivity, considering a significant proportion of ingested curcumin is excreted into the feces. In order to harness the true potential of this botanical, we need to decipher the mechanism(s) by which curcumin and its metabolites exert bioactivity and incorporate this knowledge into the development of novel curcumin formulations. To provide a better understanding of this concept, we summarized potential bioactivity of curcumin metabolites in circulation and how curcumin delivery strategies can modify the profile of curcumin metabolites (Figure 1). There are already various curcumin formulations available in the market that utilize different strategies to enhance curcumin absorption and subsequently its bioavailability in systemic circulation. While some of these approaches work in a similar manner, it is important to understand the fundamental concepts underpinning the development of these formulations.
CURCUMIN FORMULATIONS
In an effort to improve systemic absorption and increase bioavailability of curcumin in the circulation, several formulations of curcumin have been developed over the last couple of decades. The underlying principles for each of these preparations are distinct in some instances; others have some degree of overlapping features as well.
NANOPARTICLE CURCUMIN
The use of curcumin formulations using nanoparticles is currently one of the most popular strategies in improving the bioavailability of curcumin. Although there are several variations of such formulations, including incorporation of polymers, phospholipids, and solid lipids, in principle these curcumin preparations are developed through partitioning/encapsulating curcumin into the hydrophobic lipid cores that not only improve the bioavailability of the product (curcumin, in this instance), but also increase its stability by protecting it from the outside environment. “Nanocurcumin” is a polymeric nanoparticle-encapsulated formulation of curcumin with a narrow size range distribution of approximately 50 nm. Unlike free curcumin, nanocurcumin is readily dispersed in aqueous media and has been shown to exert greater inhibition of nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB) in cancer cell lines, compared to its parent counterpart.36 NF-κB is a protein complex that controls DNA transcription, cytokine production, and cell survival. Incorrect regulation of NF-κB has been linked to cancer, and its inhibition is considered to be one of the important strategies for cancer prevention and treatment with various anti-cancer compounds.
Another type of nanoformulation, solid lipid nanoparticle (SLN) loaded with curcumin, has also shown significant potential in delivering improved curcumin concentrations to organs such as the brain.37 Supporting its superior performance, another in vivo study demonstrated that SLN-loaded curcumin had increased uptake in bone marrow, spleen and liver, and was still detectable 6 hours post-injection.38 SLN-based curcumin appears to be more suitable for potential clinical applications as it is less toxic compared with polymer based nanoparticles.39 Currently, nanoparticle preparations are perhaps among the most extensively investigated curcumin formulations in preclinical animal studies40 with a clinical study demonstrating substantially higher absorption efficiency than other curcumin formulations.41
Moreover, nanoparticle curcumin has been widely tested in preclinical models for non-cancer diseases including Alzheimer, hepatitis, cerebral ischemia, asthma, and heart failure.42–45 The success of these preclinical studies has built high hopes for many of the currently on-going clinical trials.
LIPOSOMAL CURCUMIN
Another common curcumin formulation aimed at increasing higher absorption and bioavailability involves liposomal coating of the active molecule. Liposomes are spherical bilayer vesicles that shield hydrophobic compounds (such as curcumin), and interact with aqueous environments to improve aqueous solubility. The liposomal delivery system is ideal for the lipophilic characteristics of curcumin, where curcumin is entrapped in a lipophilic liposomal core. An in vitro study demonstrated that dimyristoyl phosphatidylcholine (DMPC)-based liposomal formulated curcumin only required one-tenth of the parent curcumin dose to exert equivalent proliferation inhibition in prostate cancer cell lines.46 Similarly, curcumin formulated in dimyristoyl-sn-glycero-3-phosphocholine showed significant suppression of pancreatic tumor growth in a xenograft model.47 In addition, a recent study demonstrated that liposomal curcumin coated with a thin layer of synthesized chitosan derivative released curcumin in a sustained manner, while exerting selective cytotoxicity to melanoma cells.48 Although liposomal curcumin is a promising delivery system to increase bioavailability of curcumin, there is still substantial room to optimize its formulation to enhance bioavailability as well as stability.
ESSENTIAL TURMERIC OIL COATED CURCUMIN
There are many other promising novel approaches to enhance absorption and bioavailability of curcumin. One of such approach utilizes a proprietary extraction procedure of curcumin from turmeric, and subsequently blending extracted curcumin with the essential oils from turmeric roots by steam distillation. This simple yet elegant concept of delivering curcumin blended with turmeric essential oils (ETO-curcumin, primarily ar-turmerones), is based upon centuries-old concepts of Indian traditional system of Ayurveda, and the resulting curcumin product is all natural. ETO curcumin has been shown to have 7 to 10-fold higher bioavailability and shown to be retained longer in systemic circulation, compared with standard curcumin.49,50 In addition, a recent rodent study demonstrated that ETO-curcumin was superior to standard curcumin in suppressing chemically induced colitis.51 Even though this curcumin extract may not have the highest bioavailability compared with other formulations, its natural composition and unique blending with turmeric essential oils vs. synthetic lipids should provide superior consumer assurance on safety-related issues. Furthermore, several studies have shown that components of essential turmeric oil themselves have anti-tumorigenic activities.52,53 While additional studies are required to optimize the ratio between curcumin and ETO to further improve the absorption rate of ETO, several clinical studies have demonstrated that regular ETO-curcumin consumption improved diseases including arthritis, depression, and Alzheimer disease.54–56 Until other curcumin formulations have been rigorously tested to ensure safety with long-term use, ETO-curcumin appears to be the one of most reliable sources of curcumin currently available in the market.
CURCUMIN ANALOGUES
In another attempt to circumvent some of the challenges with poor solubility and bioavailability of curcumin, various synthetic analogues of curcumin have also been developed. Difluorinated-curcumin (CDF) is one such analogue. CDF is one of the fluorinated analogues of curcumin modified by Knoevenagel condensation, a process in which an active hydrogen compound is added to a carbonyl group followed by a dehydration reaction to eliminate a water molecule.57,58 CDF has been shown to have significantly improved stability over parent curcumin and has been shown to be effective in both in vitro and in vivo studies.59–61 In addition, CDF has been shown to be a potent epigenetic regulator modulating both well-established tumor suppressive and oncocogenic miRNAs in vitro.60,62 Considering that results of these preclinical studies clearly demonstrate impressive anti-tumorigenic capacity of CDF, it would be intriguing to find out whether this analogue is equally effective in clinical studies.
Collectively, these approaches have been shown to improve the bioavailability of curcumin and also provide alternative delivery strategies for this natural medicine. Considering each formulation type possesses unique advantages over others, different delivery strategies can be used to target different organs. Furthermore, there are too many variables that must be taken into account for the identification of the most potent curcumin formulation required for a specific purpose. Therefore, until we have comprehensive comparison data between various curcumin formulations, it would be challenging to logically provide unbiased opinions as to which of these various curcumin formulation may be best in terms of not only its enhanced bioavailability, but more importantly, in terms of its bioefficacy in preventing and treating various acute and chronic diseases. In the end, it is even likely that each formulation may have preferred target uses, and affinity for different target organs. For example, specific composition of nanoparticle curcumin can be used to deliver curcumin to the brain, while a different type could be used for gastrointestinal diseases. Furthermore, these delivery strategies would allow better delivery of not only the parent form of curcumin, but also curcumin metabolites to the target organs.
TECHNICAL CHALLENGES WHEN CONSIDERING BIOLOGICAL EFFICACY OF CURCUMIN
Although there is a growing interest in enhancing and further improving the bioavailability of curcumin, one must be mindful that some of the data gathered on this topic also suffers from various technical challenges. Without a doubt, while detection methods for curcumin and its metabolites in circulation and tissues have improved significantly with the development of superior technologies, it is critical to acknowledge that detection of true levels of curcumin, its metabolites and conjugates all require careful standardization. Current inconsistencies in curcumin quantification are primarily due to its lack of stability, short retention time, and limited reports on curcumin’s metabolites and conjugates. These are important parameters that must be accounted for when making direct comparison between studies, and while contemplating comparison between various curcumin formulations. Furthermore, the majority of the studies investigating bioactivity of curcumin have failed to report bioavailability and/or tissue biodistribution of that particular curcumin preparation, making it impossible to determine how curcumin treatment influenced the outcome of the disease under investigation. Admittedly, controlling for both disease progression and bioavailability is challenging in preclinical in vivo settings, especially where diseases are chemically or genetically induced in the animals. Nevertheless, having both bioavailability and bioactivity in a study will provide seminal insights into how curcumin exerts its disease preventative or therapeutic effects.
Furthermore, the majority of the previous studies have utilized high performance liquid chromatography (HPLC)-based approaches for measuring curcumin and its metabolite levels. These technologies have the inherent limitation due to their detection sensitivities and their inability to distinguish between curcumin and its metabolites. Hence, use of highly sensitive detection devices such as liquid chromatography coupled with tandem mass spectrometry (LC-MS/MS) has shown significant improvement over traditional methodologies minimizing intra-day and inter-day variations.63 In addition, we need to be aware of limitations when interpreting data derived from clinical studies. Typically, a study investigating bioavailability of a botanical is carried out using a small number of volunteers (generally below 10 subjects). Since inter-individual variations of curcumin can be so large, it would be challenging to even replicate the research results from same subjects.
In conclusion, as we improve our understanding on how curcumin regulates signaling pathway involved in various diseases, it is imperative to identify which curcumin delivery strategy provides the best result for most diseases. In addition, it remains to be determined as to what is the minimum dose of curcumin required to see its beneficial effects in each disease condition, and whether there is a linear relationship between its bioavailability and bioefficacy. In view of the overwhelming body of compelling data for curcumin in thousands of studies, it is very possible that curcumin bioavailability may actually not even be a serious issue, if only a very small amount of this compound, its metabolites and/or conjugates are sufficient to exert their beneficial effect. After all we need to remind ourselves that the ultimate goal is not simply delivering the most curcumin/curcumin metabolites in circulation, but to prevent, mitigate, or treat a specific disease. Although the medicinal properties of curcumin were recognized and routinely exploited in some of the oldest systems of medicine in Southeast Asia for centuries, we can now support its medicinal use through all the scientific evidence we have gathered for its efficacy in the last few decades. We have come the full circle, and the time is ripe to give this age-old herb the due credit, and begin harnessing the benefits from the true potential of curcumin.
Acknowledgments
GRANT FUNDING
This work was supported by grants R01 CA72851, CA181572 and CA187956 from the National Cancer Institute, National Institutes of Health, a pilot grant from Charles A Sammons Cancer Center, and funds from the Baylor Research Institute to AG.
Footnotes
COMPETING INTERESTS
The authors declare they have no competing interests.
REFERENCES
- 1.Sharma RA, McLelland HR, Hill KA, et al. Pharmacodynamic and pharmacokinetic study of oral Curcuma extract in patients with colorectal cancer. Clin Cancer Res 2001;7(7):1894–900. [PubMed] [Google Scholar]
- 2.Garcea G, Jones DJ, Singh R, et al. Detection of curcumin and its metabolites in hepatic tissue and portal blood of patients following oral administration. Br J Cancer 2004;90(5):1011–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Anand P, Kunnumakkara AB, Newman RA, Aggarwal BB. Bioavailability of curcumin: problems and promises. Mol Pharm 2007;4(6):807–18. [DOI] [PubMed] [Google Scholar]
- 4.Douglass BJ, Clouatre DL. Beyond yellow curry: assessing commercial curcumin absorption technologies. J Am Coll Nutr 2015;34(4):347–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Patil K, Guledgud MV, Kulkarni PK, et al. Use of curcumin mouthrinse in radio-chemotherapy induced oral mucositis patients: a pilot study. J Clin Diagn Res 2015;9(8):ZC59–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Parsons HA, Baracos VE, Hong DS, et al. The effects of curcumin (diferuloylmethane) on body composition of patients with advanced pancreatic cancer. Oncotarget 2016;7(15):20293–304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kuriakose MA, Ramdas K, Dey B, et al. A randomized double-blind placebo-controlled phase IIB trial of curcumin in oral leukoplakia. Cancer Prevent Res 2016;9(8):683–91. [DOI] [PubMed] [Google Scholar]
- 8.Irving GR, Iwuji CO, Morgan B, et al. Combining curcumin (C3-complex, Sabinsa) with standard care FOLFOX chemotherapy in patients with inoperable colorectal cancer (CUFOX): study protocol for a randomised control trial. Trials 2015;16:110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mahammedi H, Planchat E, Pouget M, et al. The new combination docetaxel, prednisone and curcumin in patients with castration-resistant prostate cancer: a pilot phase II study. Oncology 2016;90(2):69–78. [DOI] [PubMed] [Google Scholar]
- 10.Garcea G, Berry DP, Jones DJ, et al. Consumption of the putative chemopreventive agent curcumin by cancer patients: assessment of curcumin levels in the colorectum and their pharmacodynamic consequences. Cancer Epidemiol Biomarkers Prevent 2005;14(1):120–5. [PubMed] [Google Scholar]
- 11.Wahlstrom B, Blennow G. A study on the fate of curcumin in the rat. Acta Pharmacol Toxicol 1978;43(2):86–92. [DOI] [PubMed] [Google Scholar]
- 12.Ravindranath V, Chandrasekhara N. Absorption and tissue distribution of curcumin in rats. Toxicology 1980;16(3):259–65. [DOI] [PubMed] [Google Scholar]
- 13.Pan MH, Huang TM, Lin JK. Biotransformation of curcumin through reduction and glucuronidation in mice. Drug Metab Dispos 1999;27(4):486–94. [PubMed] [Google Scholar]
- 14.Shoba G, Joy D, Joseph T, et al. Influence of piperine on the pharmacokinetics of curcumin in animals and human volunteers. Planta Med 1998;64(4):353–6. [DOI] [PubMed] [Google Scholar]
- 15.Ryu EK, Choe YS, Lee KH, et al. Curcumin and dehydrozingerone derivatives: synthesis, radiolabeling, and evaluation for beta-amyloid plaque imaging. J Med Chem 2006;49(20):6111–9. [DOI] [PubMed] [Google Scholar]
- 16.Wang Y, Yin H, Li J, et al. Amelioration of beta-amyloid-induced cognitive dysfunction and hippocampal axon degeneration by curcumin is associated with suppression of CRMP-2 hyperphosphorylation. Neurosci Lett 2013; 557 Pt B:112–7. [DOI] [PubMed] [Google Scholar]
- 17.Hoehle SI, Pfeiffer E, Solyom AM, Metzler M. Metabolism of curcuminoids in tissue slices and sub-cellular fractions from rat liver. J Agric Food Chem 2006;54(3):756–64. [DOI] [PubMed] [Google Scholar]
- 18.Wahlang B, Pawar YB, Bansal AK. Identification of permeability-related hurdles in oral delivery of curcumin using the Caco-2 cell model. Eur J Pharm Biopharm 2011;77(2):275–82. [DOI] [PubMed] [Google Scholar]
- 19.Khopde SM, Priyadarsini KI, Guha SN, et al. Inhibition of radiation-induced lipid peroxidation by tetrahydrocurcumin: possible mechanisms by pulse radiolysis. Biosci Biotechnol Biochem 2000;64(3):503–9. [DOI] [PubMed] [Google Scholar]
- 20.Okada K, Wangpoengtrakul C, Tanaka T, et al. Curcumin and especially tetrahydrocurcumin ameliorate oxidative stress-induced renal injury in mice. J Nutr 2001;131(8):2090–5. [DOI] [PubMed] [Google Scholar]
- 21.Bansal SS, Goel M, Aqil F, et al. Advanced drug delivery systems of curcumin for cancer chemoprevention. Cancer Prevent Res 2011;4(8):1158–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Asai A, Miyazawa T. Occurrence of orally administered curcuminoid as glucuronide and glucuronide/sulfate conjugates in rat plasma. Life Sci 2000;67(23):2785–93. [DOI] [PubMed] [Google Scholar]
- 23.Sandur SK, Pandey MK, Sung B, et al. Curcumin, demethoxycurcumin, bisdemethoxycurcumin, tetrahydrocurcumin and turmerones differentially regulate anti-inflammatory and anti-proliferative responses through a ROS-independent mechanism. Carcinogenesis 2007;28(8):1765–73. [DOI] [PubMed] [Google Scholar]
- 24.Pfeiffer E, Hoehle SI, Walch SG, et al. Curcuminoids form reactive glucuronides in vitro. J Agric Food Chem 2007;55(2):538–44. [DOI] [PubMed] [Google Scholar]
- 25.Kim JM, Araki S, Kim DJ, et al. Chemopreventive effects of carotenoids and curcumins on mouse colon carcinogenesis after 1,2-dimethylhydrazine initiation. Carcinogenesis 1998;19(1):81–5. [DOI] [PubMed] [Google Scholar]
- 26.Pari L, Murugan P. Tetrahydrocurcumin: effect on chloroquine-mediated oxidative damage in rat kidney. Basic Clin Pharmacol Toxicol 2006;99(5):329–34. [DOI] [PubMed] [Google Scholar]
- 27.Ireson C, Orr S, Jones DJ, et al. Characterization of metabolites of the chemopreventive agent curcumin in human and rat hepatocytes and in the rat in vivo, and evaluation of their ability to inhibit phorbol esterinduced prostaglandin E2 production. Cancer Res 2001;61(3):1058–64. [PubMed] [Google Scholar]
- 28.Suresh D, Srinivasan K. Tissue distribution & elimination of capsaicin, piperine & curcumin following oral intake in rats. Indian J Med Res 2010;131:682–91. [PubMed] [Google Scholar]
- 29.Piyachaturawat P, Glinsukon T, Toskulkao C. Acute and subacute toxicity of piperine in mice, rats and hamsters. Toxicol Lett 1983;16(3–4):351–9. [DOI] [PubMed] [Google Scholar]
- 30.Ji HF, Shen L. Can improving bioavailability improve the bioactivity of curcumin? Trends Pharmacol Sci 2014;35:265–6. [DOI] [PubMed] [Google Scholar]
- 31.Wang YJ, Pan MH, Cheng AL, et al. Stability of curcumin in buffer solutions and characterization of its degradation products. J Pharm Biomed Anal 1997;15(12):1867–76. [DOI] [PubMed] [Google Scholar]
- 32.Sultana R Ferulic acid ethyl ester as a potential therapy in neurodegenerative disorders. Biochim Biophys Acta 2012;1822(5):748–52. [DOI] [PubMed] [Google Scholar]
- 33.Barone E, Calabrese V, Mancuso C. Ferulic acid and its therapeutic potential as a hormetin for age-related diseases. Biogerontology 2009;10(2):97–108. [DOI] [PubMed] [Google Scholar]
- 34.Kurien BT, Singh A, Matsumoto H, Scofield RH. Improving the solubility and pharmacological efficacy of curcumin by heat treatment. Assay Drug Dev Technol 2007;5(4):567–76. [DOI] [PubMed] [Google Scholar]
- 35.Kurien BT, Scofield RH. Curcumin/turmeric solubilized in sodium hydroxide inhibits HNE protein modification–an in vitro study. J Ethnopharmacol 2007;110(2):368–73. [DOI] [PubMed] [Google Scholar]
- 36.Bisht S, Feldmann G, Soni S, et al. Polymeric nanoparticle-encapsulated curcumin (“nanocurcumin”): a novel strategy for human cancer therapy. J Nanobiotechnol 2007;5:3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bawarski WE, Chidlowsky E, Bharali DJ, Mousa SA. Emerging nanopharmaceuticals. Nanomedicine 2008;4(4):273–82. [DOI] [PubMed] [Google Scholar]
- 38.Sou K, Inenaga S, Takeoka S, Tsuchida E. Loading of curcumin into macrophages using lipid-based nanoparticles. Int J Pharm 2008;352(1–2):287–93. [DOI] [PubMed] [Google Scholar]
- 39.Kaur IP, Bhandari R, Bhandari S, Kakkar V. Potential of solid lipid nanoparticles in brain targeting. J Control Release 2008;127(2):97–109. [DOI] [PubMed] [Google Scholar]
- 40.Naksuriya O, Okonogi S, Schiffelers RM, Hennink WE. Curcumin nanoformulations: a review of pharmaceutical properties and preclinical studies and clinical data related to cancer treatment. Biomaterials 2014;35(10):3365–83. [DOI] [PubMed] [Google Scholar]
- 41.Sunagawa Y, Hirano S, Katanasaka Y, et al. Colloidal submicron-particle curcumin exhibits high absorption efficiency–a double-blind, 3-way crossover study. J Nutr Sci Vitaminol (Tokyo) 2015;61(1):37–44. [DOI] [PubMed] [Google Scholar]
- 42.Cheng KK, Yeung CF, Ho SW, et al. Highly stabilized curcumin nanoparticles tested in an in vitro blood-brain barrier model and in Alzheimer’s disease Tg2576 mice. AAPS J 2013;15(2):324–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cutrin JC, Crich SG, Burghelea D, et al. Curcumin/Gd loaded apoferritin: a novel “theranostic” agent to prevent hepatocellular damage in toxic induced acute hepatitis. Mol Pharm 2013;10(5):2079–85. [DOI] [PubMed] [Google Scholar]
- 44.Kakkar V, Muppu SK, Chopra K, Kaur IP. Curcumin loaded solid lipid nanoparticles: an efficient formulation approach for cerebral ischemic reperfusion injury in rats. Eur J Pharm Biophar 2013;85(3 Pt A):339–45. [DOI] [PubMed] [Google Scholar]
- 45.Sunagawa Y, Wada H, Suzuki H, et al. A novel drug delivery system of oral curcumin markedly improves efficacy of treatment for heart failure after myocardial infarction in rats. Biol Pharm Bull 2012;35(2):139–44. [DOI] [PubMed] [Google Scholar]
- 46.Thangapazham RL, Puri A, Tele S, et al. Evaluation of a nanotechnology-based carrier for delivery of curcumin in prostate cancer cells. Int J Oncol 2008;32(5):1119–23. [PMC free article] [PubMed] [Google Scholar]
- 47.Li L, Braiteh FS, Kurzrock R. Liposome-encapsulated curcumin: in vitro and in vivo effects on proliferation, apoptosis, signaling, and angiogenesis. Cancer 2005;104(6):1322–31. [DOI] [PubMed] [Google Scholar]
- 48.Karewicz A, Bielska D, Loboda A, et al. Curcumin-containing liposomes stabilized by thin layers of chitosan derivatives. Colloids Surf B Biointerfaces 2013;109:307–16. [DOI] [PubMed] [Google Scholar]
- 49.Shishu Maheshwari M. Comparative bioavailability of curcumin, tumeric, and BiocurcumaxTM in traditional vehicles using non-everted rat intestinal sac model. J Funct Foods 2010;2:60–5. [Google Scholar]
- 50.Antony B, Merina B, Iyer VS, et al. A pilot cross-over study to evaluate human oral bioavailability of BCM-95CG (Biocurcumax), a novel bioenhanced preparation of curcumin. Indian J Pharm Sci 2008;70(4):445–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Toden S, Theiss AL, Wang X, Goel A. Essential turmeric oils enhance anti-inflammatory efficacy of curcumin in dextran sulfate sodium-induced colitis. Sci Rep 2017;7(1):814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Aggarwal BB, Yuan W, Li S, Gupta SC. Curcumin-free turmeric exhibits anti-inflammatory and anticancer activities: Identification of novel components of turmeric. Mol Nutr Food Res 2013;57(9):1529–42. [DOI] [PubMed] [Google Scholar]
- 53.Liju VB, Jeena K, Kuttan R. An evaluation of antioxidant, anti-inflammatory, and antinociceptive activities of essential oil from Curcuma longa. L. Indian J Pharmacol 2011;43(5):526–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Chandran B, Goel A. A randomized, pilot study to assess the efficacy and safety of curcumin in patients with active rheumatoid arthritis. Phytother Res 2012;26(11):1719–25. [DOI] [PubMed] [Google Scholar]
- 55.Sanmukhani J, Satodia V, Trivedi J, et al. Efficacy and safety of curcumin in major depressive disorder: a randomized controlled trial. Phytother Res 2014;28:579–85. [DOI] [PubMed] [Google Scholar]
- 56.Baum L, Lam CW, Cheung SK, et al. Six-month randomized, placebo-controlled, double-blind, pilot clinical trial of curcumin in patients with Alzheimer disease. J Clin Psychopharmacol 2008;28(1):110–3. [DOI] [PubMed] [Google Scholar]
- 57.Padhye S, Yang H, Jamadar A, et al. New difluoro Knoevenagel condensates of curcumin, their Schiff bases and copper complexes as proteasome inhibitors and apoptosis inducers in cancer cells. Pharm Res 2009;26(8):1874–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Qiu X, Du Y, Lou B, et al. Synthesis and identification of new 4-arylidene curcumin analogues as potential anti-cancer agents targeting nuclear factor-kappaB signaling pathway. J Med Chem 2010;53(23):8260–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Bao B, Ali S, Banerjee S, et al. Curcumin analogue CDF inhibits pancreatic tumor growth by switching on suppressor microRNAs and attenuating EZH2 expression. Cancer Res 2012;72(1):335–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Bao B, Ali S, Kong D, et al. Anti-tumor activity of a novel compound-CDF is mediated by regulating miR-21, miR-200, and PTEN in pancreatic cancer. PLoS One 2011;6(3):e17850. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 61.Roy S, Yu Y, Padhye SB, et al. Difluorinated-curcumin (CDF) restores PTEN expression in colon cancer cells by down-regulating miR-21. PLoS One 2013;8(7):e68543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ali S, Ahmad A, Banerjee S, et al. Gemcitabine sensitivity can be induced in pancreatic cancer cells through modulation of miR-200 and miR-21 expression by curcumin or its analogue CDF. Cancer Res 2010;70(9):3606–17. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 63.Ramalingam P, Ko YT. A validated LC-MS/MS method for quantitative analysis of curcumin in mouse plasma and brain tissue and its application in pharmacokinetic and brain distribution studies. J Chromatogr B Analyt Technolog Biomed Life Sci 2014;969:101–8. [DOI] [PubMed] [Google Scholar]


