Abstract
Background:
Limited knowledge exists on inter-hospital variation in the utilization of short-term, non-durable mechanical circulatory support (MCS) for myocardial infarction (MI) complicated by cardiogenic shock (CS).
Methods and Results:
Hospitalizations for MI with CS in 2014 in a nationally representative all-payer database were included. The proportion of hospitalizations for MI with CS using MCS (MCS ratio) and in-hospital mortality were evaluated. Hospital characteristics and outcomes were compared across quartiles of MCS usage. Of 1,813 hospitals evaluated, 1,440 (79.4%) performed ≥10 PCIs annually. Of these, 1,064 [73.9%] had at least one code for MCS. Forty-one percent of hospitals did not use MCS. The median (interquartile range [IQR]) proportion of MCS use among admissions for MI with CS was 33.3% (0.0–50.0%). High MCS utilizing hospitals were larger (p <0.001). Eighty-five percent (2808/3301) of MCS use was intra-aortic balloon pump (IABP). There was significant variation in receipt of MCS at different hospitals (median OR of receiving MCS at two random hospitals: 1.58; 95% CI 1.45–1.70). Adjusted in-hospital mortality was not different across quartiles of MCS use (Q4 vs Q1; OR 0.95; 95% CI 0.77–1.16, p = 0.58).
Conclusions:
Wide variation exists in hospital use of MCS for MI with CS, unexplained by patient characteristics. The predominant form of MCS use is IABP. Risk-adjusted mortality rates were not different between higher and lower MCS-utilizing hospitals.
Keywords: Mechanical Circulatory Support, Myocardial Infarction, Cardiogenic Shock
INTRODUCTION
Cardiogenic shock (CS), the inability to maintain adequate perfusion due to failure of the pumping mechanism, is present in 3.5% of patients presenting with acute heart failure and 6.6% in those presenting with myocardial infarction (MI), and is associated with an in-hospital mortality rate as high as 70% (1, 2). The recent availability of temporary mechanical circulatory support (MCS) has led to an expansion of the therapeutic options for CS, allowing short-term circulatory support as a bridge to recovery, transplantation, or placement of a durable left ventricular assist device in selected patients (3). Despite increasing use of MCS devices overall, rises in CS hospitalization rates have outpaced growth in MCS use (4). Reasons for this discrepancy are unclear and may relate to changes in patient or disease characteristics or the inability of some hospitals to develop or augment capacity to place MCS. Additionally, few randomized trials exist evaluating the use of non-durable MCS in shock patients, leading to uncertainty in its true clinical benefit.
Given differences in hospital capabilities to use MCS as well as uncertainty in the current evidence supporting its use, we hypothesized that significant variation between hospitals may exist in the propensity to utilize MCS for treatment of MI complicated by CS. We therefore sought to 1) assess variation in MCS usage between hospitals in the United States among hospitalizations for myocardial infarction (MI) complicated by CS, 2) quantify the variability between hospitals in MCS use for MI complicated by shock and 3) assess whether those hospitals with the highest rates of MCS use had different outcomes compared with those with the lowest use.
METHODS
All data and materials are publicly available through the Healthcare Cost and Utilization Project (HCUP) and can be accessed at http://www.hcup-us.ahrq.gov/databases.jsp.
Study Population
All hospitalizations of adults ≥18 years old with diagnoses of CS (International Classification of Disease, Clinical Modification, version 9 [ICD-9-CM] code 785.51) with MI (ICD-9-CM 410.11–410.91) in any position in the National Inpatient Sample (NIS), 1/1/14–12/31/14, were included (5,6). Developed through the HCUP, the NIS contains patient-level claims on 35 million hospitalizations nationally, representing a 20% sample of all hospital discharges (7). The NIS is publicly available for researchers through the HCUP website (7). Only one year was evaluated as the hospitals in the NIS change annually (7). Hospitals performing <10 PCIs per year were excluded from the primary analysis to restrict the analysis to hospitals with cardiac catheterization laboratories, as hospitals without the capacity to perform PCI are likely unable to have the capacity to place MCS devices. To confirm the results in a database with more complete sampling, analyses were repeated in the 2015 Nationwide Readmissions Database (NRD), a database containing an 85% sample of inpatient claims from 27 states, representing 36 million discharges, roughly 56.6% of all US hospitalizations (7). Only January 1, 2015 to September 30, 2015 were analyzed in the NRD due to the transition to the International Classification of Diseases, Tenth Revision, Clinical Modification/Procedure Coding System (ICD-10-CM/PCS) on October 1, 2015. Due to extensive quality control by HCUP (7), there was minimal missing data in this analysis. Studies based on the NIS and NRD are exempt from Institutional Review Board approval at Beth Israel Deaconess Medical Center.
Exposures and Outcomes
The primary exposure of interest was receipt of MCS, defined as percutaneous and non-percutaneous devices, intra-aortic balloon pump, and extracorporeal membrane oxygenation. Percutaneous and non-percutaneous devices (ND-MCS) were defined using ICD-9-CM procedure codes 37.68 (percutaneous) or 37.60 and 37.65 (non-percutaneous). These codes would typically include percutaneous devices such as TandemHeart™ (CardiacAssist Inc., Pittsburgh, PA) and Impella® devices (Abiomed Inc., Danvers, MA) and non-percutaneous devices including Thoratec paracorporeal ventricular assist device (VAD) (Thoratec Inc., Pleasanton, CA), AB5000 (Abiomed Inc., Danvers, MA), BVS 5000 (Abiomed Inc., Danvers, MA), and CentriMag™ (Thoratec Inc., Pleasanton, CA) (3). Intra-aortic balloon counterpulsation (IABP) was defined using ICD-9-CM procedure code 37.61, extracorporeal membrane oxygenation (ECMO) using ICD-9-CM procedure code 39.65 (central ECMO), and percutaneous cardiopulmonary support (PCPS; peripheral ECMO) using code 39.66. Permanent ventricular assist devices were excluded (ICD-9-CM codes 37.52 and 37.66). The primary outcome was each hospital’s MCS ratio, defined as the proportion of hospitalizations for MI with CS that included use of MCS.
Covariates
Patient-level covariates included demographics and 29 Elixhauser clinical and procedural variables, determined at index hospitalization (Appendix eTable 1) (8). Elixhauser Comorbidity measures were designed to predict in-hospital mortality using administrative data (8–11). Additionally, five hospital level covariates were available in the NIS, determined from the American Hospital Association Annual Survey of Hospitals. These included hospital bed-size, ownership, location/teaching status, region, and total discharges. A hospital was considered a teaching hospital if it had an American Medical Association approved residency program, was a member of the Council of Teaching Hospitals, or had a ratio of full-time equivalent interns and residents to beds of 0.25 or higher (7).
Statistical Analysis
The distribution of hospital MCS ratios were plotted using a histogram. Patient level characteristics, rates of coronary angiography, revascularization, RHC, and in-hospital mortality of hospitalizations for CS with MI were compared across quartiles of MCS ratio via one-way analysis of variance (ANOVA) for continuous variables and Chi-squared tests for categorical variables. Similarly, hospital characteristics were compared across quartiles of MCS usage with ANOVA for continuous variables and Chi-squared tests for categorical variables. Given the large sample size, the normality assumptions for ANOVA were met based on the central-limit theorem and the Welch’s ANOVA test was used which does not assume equality of variances. A multivariable linear mixed effects model with a logit link was used to model receipt of MCS according to patient and hospital characteristics, using all variables, accounting for the complex sampling design of the NIS. Hospital was included as a random effect to account for clustering of CS hospitalizations by hospital. The variance of the random effect for hospital was used to create median odds ratios (MORs), adjusted for all patient level variables. The median odds ratio represents the ratio of the likelihood of an identical patient receiving MCS at one randomly selected hospital versus another.
Rates of in-hospital mortality were compared across quartiles of MCS use with a Chi-squared test. A multivariable mixed effects model with hospital site as a random effect was used to evaluate whether quartile of MCS was associated with in-hospital mortality, and the Wald test used to test significance. Additionally, we examined whether analyzing the effect of the MCS ratio on in-hospital mortality in the primary analysis as a continuous rather than categorical variable would alter results.
Finally, because small hospitals with few hospitalizations for MI with CS could have less stable MCS ratios, we repeated the analyses considering only hospitals performing ≥ 10 PCIs and having ≥ 10 hospitalizations for CS and MI to identify if results differed. Moreover, as several covariates could also be outcomes, we repeated the model for in-hospital mortality, restricting the analysis to covariates likely to be present on admission to identify if results differed. Additionally, we repeated the analysis excluding transfers into acute care hospitals as these patients may represent a different population. Finally, analyses were repeated in the 2015 NRD in order to test the sensitivity of the findings to examination in a fully sampled population. Due to HCUP privacy rules, values <10 are suppressed. All analyses were done with SAS version 9.4 (SAS Institute Inc., Cary, NC) at a two-tailed level of significance of p <0.05.
RESULTS
Unadjusted Hospital Characteristics
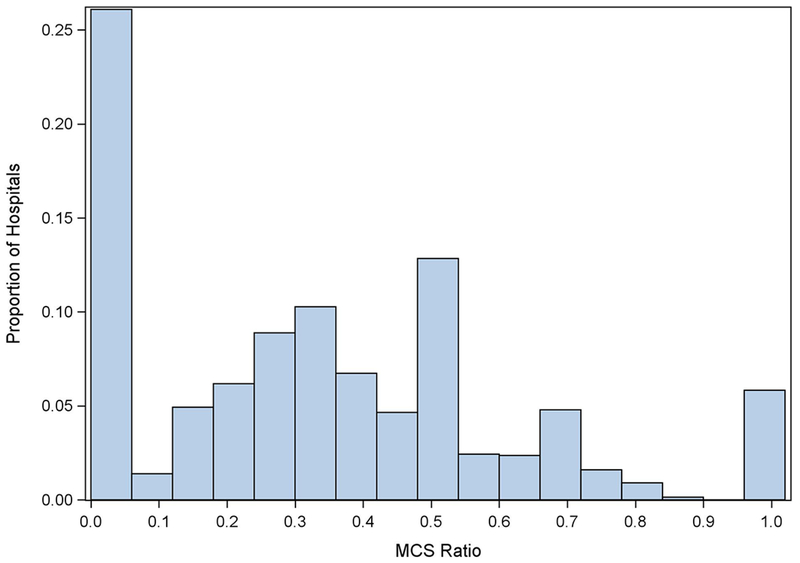
A total of 1,813 hospitals had at least one discharge for MI with CS, of which 1,440 (79.4%) performed ≥10 PCIs per year. Of the 1,440 hospitals included in the analytic cohort, 1,064 (73.9%) had at least one record of MCS usage. The hospital median rate of MCS use for MI with CS was 33.3%; IQR 0.0–50.0% (Figure). Overall, forty-one percent of hospitals admitting MI with CS patients did not perform MCS. Eighty-four hospitals (5.8%) included in the analysis performed MCS in all hospitalizations for MI with CS. The median (IQR) MCS ratio by quartile was 0.0% (0.0–0.0%) in Q1, 22.2% (16.7–25.0%) in Q2, 42.9% (33.3–50.0%) in Q3, and 67.7% (60.6–100.0%) in Q4 (Table 1). Compared with hospitals in the lowest quartile, hospitals in the highest quartile of MCS use were larger (Q4 vs. Q1; percentage classified as large [>45 to >350 beds depending on region, location, and teaching status]; 128 [47.1%] vs. 136 [36.2%]; p < 0.001) and had a greater number of discharges (Q4 vs. Q1; median [IQR] annual discharges; 2900 [1779–4522] vs. 2026 [1456–2930]; p <0.001). Additionally, more than half of MCS high utilizing hospitals were urban teaching hospitals compared to less than half of low utilizing hospitals (Q4 vs. Q1; 171 [62.9%] vs. 175 [46.5%]; p <0.001). High-utilizing MCS hospitals were less likely to be rural (Q4 vs. Q1; 16 [5.9%] vs. 45 [12.0%]; p < 0.001) or urban non-teaching hospitals (Q4 vs. Q1; 85 [31.3%] vs. 156 [41.5%]; p < 0.001). MCS utilization was numerically highest in the South (mean proportion of MCS utilizing hospitals: 37.8%) and least common in the Northeast (mean proportion of MCS utilizing hospitals: 14.3%).
Figure.
Histogram demonstrating the proportion of hospitalizations for myocardial infarction and cardiogenic shock utilizing MCS by hospital. Ratio = Proportion of MCS use per hospitalization for MI with CS.
Table 1.
Characteristics of Hospitals Utilizing Mechanical Circulatory Support (MCS) for Myocardial Infarction Complicated by Cardiogenic Shock, Stratified by Quartile of MCS Use
| Hospital Variables | Quartile 1 (N = 376) | Quartile 2 (N = 335) | Quartile 3 (N = 457) | Quartile 4 (N = 272) | P-value* |
|---|---|---|---|---|---|
| MCS ratio (%)–median (IQR) | 0.0% (0.0-0.0%) | 22.2% (16.7-25.0%) | 42.9% (33.3-50.0%) | 67.7% (60.6-100.0%) | < 0.001 |
| Bedsize – no. (%) | < 0.001 | ||||
| Small | 103(27.4%) | 25(7.5%) | 76(16.6%) | 59(21.7%) | |
| Medium | 137(36.4%) | 115(34.3%) | 138(30.2%) | 85(31.3%) | |
| Large | 136(36.2%) | 195(58.2%) | 243(53.2%) | 128(47.1%) | |
| Hospital Ownership – no. (%) | 0.0051 | ||||
| Government | 45(12.0%) | 38(11.3%) | 31(6.8%) | 13(4.8%) | |
| Non-profit | 252(67.1%) | 242(72.2%) | 329(72.0%) | 207(76.1%) | |
| Private | 79(21.0%) | 55(16.4%) | 97(21.2%) | 52(19.1%) | |
| Location/teaching – no. (%) | < 0.001 | ||||
| Rural | 45(12.0%) | † | 29(6.4%) | 16(5.9%) | |
| Urban nonteaching | 156(41.5%) | 103(30.8%) | 134(29.2%) | 85(31.3%) | |
| Urban teaching | 175(46.5%) | 226(67.5%) | 294(64.3%) | 171(62.9%) | |
| Hospital region – no. (%) | 0.12 | ||||
| Northeast | 60(16.0%) | 44(13.1%) | 64(14.0%) | 37(13.6%) | |
| Midwest / North | 103(27.4%) | 80(23.9%) | 109(23.9%) | 89(32.7%) | |
| South | 145(38.6%) | 140(41.8%) | 174(38.1%) | 89(32.7%) | |
| West | 68(18.1%) | 71(21.2%) | 110(24.1%) | 57(21.0%) | |
| Total Discharges – median (IQR) | 2026(1456-2930) | 3822(2698-5440) | 3337(2223-4809) | 2900(1779-4522) | < 0.001 |
p-value for Chi-squared test across quartiles. N = number of hospitals per quartile. MCS Ratio = Proportion of MCS use per hospitalization for MI with CS.
Due to HCUP privacy rules, cell values <10 are suppressed.
Patient Characteristics by Quartile of Hospital MCS Use
There were a total of 10,438 hospitalizations for shock and MI of which 9,129 (87.5%) were included in the primary analysis (1,312 [12.6%] excluded due hospitalization at sites performing <10 PCIs per year [N = 730], resulting in transfer to an acute care hospital [N = 579], or missing [N <10]). Patient-level characteristics of all hospitalizations for MI with CS by receipt of MCS are listed in Appendixe Table 2. Of these 9,129 hospitalizations for shock and MI, only one MCS device was placed in 2972 (32.6%), two devices in 157 (1.7%), and three or more devices in <10. Of hospitalizations for MI with CS, none (0.0% [0/937]) received MCS in Q1, 725 (21.8% [725/3328]) received MCS in Q2, 1374 (41.8% [1374/3284]) received MCS in Q3, and 1035 (65.5% [1035/1580]) received MCS in Q4 (p < 0.001) (Table 2). Hospitalizations in high-utilizing hospitals were for patients who were younger (Q4 vs. Q1; mean (SD) age in years; 67.9 (13.0) vs. 71.5 (13.0); p < 0.001), less frequently admitted from the Emergency Department (Q4 vs. Q1; 945 [59.8%] vs. 741 [79.1%]; p < 0.001), and more likely to be privately insured (Q4 vs. Q1; 362 [22.9%] vs. 149 [15.9%]; p = 0.02). Hospitalizations at high utilizing hospitals were less likely to include a diagnosis of heart failure, renal failure, chronic pulmonary disease, pulmonary circulatory disorders, other neurologic disorders, weight loss, and fluid/electrolyte disorders but were more likely to have coagulopathy (Q4 vs. Q1: 347 [22.0%] vs. 129 [13.8%]). Other comorbidities were not significantly different between quartiles of MCS use.
Table 2.
Characteristics of Hospitalizations for Myocardial Infarction Complicated by Cardiogenic Shock by Quartile of MCS Use
| Patient Variables | Quartile 1 (N = 937) | Quartile 2 (N = 3328) | Quartile 3 (N = 3284) | Quartile 4 (N = 1580) | P-value* |
|---|---|---|---|---|---|
| Proportion of MCS use – no. (%) | 0.0 (0.0) | 725 (21.8) | 1374 (41.8) | 1035 (65.5) | < 0.001 |
| Age, mean (SD) | 71.5 (13.0) | 68.2 (13.1) | 68.1 (12.7) | 67.9 (13.0) | < 0.001 |
| Female – no. (%) | 383 (40.9) | 1258 (37.8) | 1232 (37.6) | 575 (36.4) | 0.16 |
| Admission from Emergency Department – no. (%) | 741 (79.1) | 2049 (61.6) | 2104 (64.1) | 945 (59.8) | < 0.001 |
| Elective admission – no. (%) | 57(6.1) | 235 (7.1) | 181 (5.5) | 109 (6.9) | 0.06 |
| Primary Insurance – no. (%) | 0.02 | ||||
| Medicare | 641 (68.6) | 2087 (62.9) | 2055 (62.7) | 971 (61.5) | |
| Medicaid | 78 (8.3) | 292 (8.8) | 288 (8.8) | 135 (8.6) | |
| Private | 149 (15.9) | 702 (21.1) | 670 (20.4) | 362 (22.9) | |
| Self-pay | 39 (4.2) | 155 (4.7) | 159 (4.9) | 61 (3.9) | |
| Heart Failure – no. (%) | 191 (20.4) | 576 (17.3) | 449 (13.7) | 184 (11.7) | < 0.001 |
| Valvular disease – no. (%) | 40 (4.3) | 160 (4.8) | 121 (3.7) | 61 (3.7) | 0.13 |
| Hypertension – no. (%) | 565 (60.3) | 2051 (61.6) | 2082 (63.5) | 1004 (63.5) | 0.17 |
| Renal Failure – no. (%) | 294 (31.4) | 985 (29.6) | 936 (28.5) | 419 (26.5) | 0.04 |
| Peripheral vascular disorders – no. (%) | 149 (15.9) | 563 (16.9) | 528 (16.1) | 241 (15.3) | 0.50 |
| Diabetes mellitus – no. (%) | 354 (37.8) | 1301 (39.1) | 1346 (41.0) | 616 (39.0) | 0.20 |
| Hypothyroidism – no. (%) | 105 (11.2) | 355 (10.7) | 342 (10.4) | 161(10.2) | 0.86 |
| Chronic pulmonary disease – no. (%) | 238 (25.4) | 862 (25.9) | 740 (22.6) | 332 (21.0) | < 0.001 |
| Pulmonary circulatory disorders – no. (%) | 47 (5.0) | 125 (3.8) | 73 (2.2) | 32 (2.0) | < 0.001 |
| Alcohol or Drug Abuse – no. (%) | 48 (5.1) | 230 (6.9) | 228 (7.0) | 95 (6.0) | 0.15 |
| Psychoses – no. (%) | 30 (3.2) | 88 (2.6) | 110 (3.3) | 32 (2.0) | 0.05 |
| Liver disease – no. (%) | 26 (2.8) | 102 (3.1) | 89 (2.7) | 36 (2.3) | 0.47 |
| Depression – no. (%) | 61 (6.5) | 235 (7.1) | 212 (6.5) | 100 (6.3) | 0.71 |
| Solid tumor without metastases – no. (%) | 30 (3.2) | 61 (1.8) | 67 (2.0) | 27 (1.7) | 0.05 |
| Lymphoma – no. (%) | † | 15 (0.4) | 20 (0.6) | 16 (1.0) | 0.11 |
| Metastatic cancer – no. (%) | 14 (1.5) | 54 (1.6) | 43 (1.3) | 16 (1.0) | 0.37 |
| Coagulopathy – no. (%) | 129 (13.8) | 657(19.7) | 628 (19.1) | 347 (22.0) | < 0.001 |
| Chronic blood loss anemia – no. (%) | 13 (1.4) | 47 (1.4) | 35 (1.1) | 16 (1.0) | 0.48 |
| Deficiency anemias – no. (%) | 233 (24.9) | 770 (23.1) | 777 (23.7) | 375 (23.7) | 0.74 |
| Paralysis – no. (%) | 35 (3.7) | 102 (3.1) | 130 (4.0) | 50 (3.2) | 0.20 |
| Other Neurologic Disorder – no. (%) | 107 (11.4) | 296 (8.9) | 278 (8.5) | 121 (7.7) | 0.01 |
| Peptic ulcer disease – no. (%) | 0 (0) | † | † | 0 (0) | 0.63 |
| Acquired Immune Deficiency Syndrome – no. (%) | 0 (0) | † | † | † | 0.30 |
| Rheumatoid arthritis / collagen vascular diseases – no. (%) | 20 (2.1) | 78 (2.3) | 80 (2.4) | 40 (2.5) | 0.93 |
| Weight loss – no. (%) | 94 (10.0) | 401 (12.5) | 304 (9.3) | 151 (9.6) | 0.0014 |
| Obesity – no. (%) | 131 (14.0) | 541 (16.3) | 474 (14.5) | 247(15.6) | 0.13 |
| Fluid and electrolyte disorders – no. (%) | 581 (62.0) | 2015(60.6) | 1906 (58.1) | 904 (57.2) | 0.02 |
p-value for ANOVA/Chi-squared test across quartiles. N = the number of hospitalizations for CS with MI.
Due to HCUP privacy rules, cell values <10 are suppressed.
Hospitalizations in high utilizing hospitals were significantly more likely to include procedures (Q4 vs. Q1; coronary angiography: 1165 [73.7%] vs. 440 [47.0%]; PCI: 822 [52.0%] vs. 335 [35.8%]; CABG: 309 [19.6%] vs. 38 [4.1%]; RHC: 93 [5.9%] vs. 13 [1.4%]; p-values all < 0.001) (Table 3). While IABP was most common overall (85.1% of all MCS use) patterns of device subtype use did not differ by quartile of MCS use (Table 4). While IABP use was consistent across quartiles of PCI utilization, non-IABP MCS use was positively associated with hospital PCI volume (p < 0.001) (AppendixeTable 3)
Table 3.
Rates of Cardiac Procedures by Quartile of MCS Utilization
| Procedure | Quartile 1 (N = 937) | Quartile 2 (N = 3328) | Quartile 3 (N = 3284) | Quartile 4 (N = 1580) | P-value* |
|---|---|---|---|---|---|
| Coronary angiography – no. (%) | 440 (47.0) | 1852 (55.6) | 2132 (65.0) | 1165 (73.7) | < 0.001 |
| Percutaneous Coronary Intervention – no. (%) | 335 (35.8) | 1256 (37.8) | 1448 (44.1) | 822 (52.0) | < 0.001 |
| Coronary artery bypass grafting – no. (%) | 38 (4.1) | 446 (13.4) | 545 (16.6%) | 309 (19.6) | < 0.001 |
| Total revascularization – no. (%) | 373(39.8) | 1702 (51.1) | 1993 (60.7) | 1133 (71.7) | < 0.001 |
| Right heart catheterization – no. (%) | 13 (1.4) | 126 (3.8) | 143 (4.4) | 93 (5.9) | < 0.001 |
p-value for Chi-squared test across quartiles. N = the number of hospitalizations for CS with MI.
Table 4.
Subtypes of MCS by Hospital MCS Utilization Quartile
| Procedure | Quartile 1 (N = 937) | Quartile 2 (N = 3328) | Quartile 3 (N = 3284) | Quartile 4 (N = 1580) | P-value* |
|---|---|---|---|---|---|
| ND-MCS (percutaneous) – no. (%) | 0.0 (0.0) | 82 (2.5) | 160 (4.9) | 110 (6.7) | < 0.001 |
| ND-MCS (non-percutaneous) – no. (%) | 0 (0.0) | † | † | † | 0.51 |
| IABP – no. (%) | 0 (0.0) | 628 (18.9) | 1234 (37.6) | 946 (59.9) | < 0.001 |
| ECMO – no. (%) | 0 (0.0) | 48 (1.4) | 50 (1.5) | 35 (2.2) | < 0.001 |
| PCPS – no. (%) | 0 (0.0) | † | † | † | 0.72 |
p-value for Chi-squared test across quartiles. N = the number of hospitalizations for CS and MI. MCS = Mechanical Circulatory Support. ND-MCS = non-durable mechanical circulatory support. IABP = Intra-aortic balloon pump. ECMO = centrally cannulated extracorporeal membrane oxygenation. PCPS = peripherally cannulated extracorporeal membrane oxygenation.
Due to HCUP privacy rules, cell values <10 are suppressed.
Predictors of MCS Use
Detailed odds ratios, 95% confidence intervals, and p-values for patient and hospital level factors associated with MCS utilization are listed in Appendix eTable 4. Age, renal failure, chronic obstructive pulmonary disease, and neurologic disorders were associated with decreased receipt of MCS, whereas receipt of coronary angiography, PCI, CABG and RHC were associated with an increased likelihood of MCS receipt. Hospital ownership was predictive of MCS use (p = 0.02), with hospitalization at government institutions being less likely to involve MCS (OR 0.72 compared to Private hospitals; 95% CI 0.55–0.93; p = 0.01). There was substantial variation between hospitals in MCS use after adjustment for patient factors (adjusted median odds ratio of receiving MCS at two randomly selected hospitals: 1.58; 95% CI 1.45–1.70)
In-hospital mortality
Among hospitals performing ≥10 PCIs per year, crude in-hospital mortality ranged from 38.6–46% and decreased according to quartile of MCS use (p < 0.001) (Table 5). After adjustment for patient, hospital, procedural variables, and device subtype, however, there was no significant relationship between hospital MCS utilization and in-hospital mortality (p = 0.78) (Appendix eTable 5). The MCS ratio as a continuous measure was not associated with adjusted in-hospital mortality (p = 0.68).
Table 5.
In-Hospital Mortality by Quartile (Crude and Adjusted)
| Quartile | Unadjusted In-hospital mortality – no. (%)* | Unadjusted Odds Ratio for In-Hospital Mortality | 95% CI | P-value |
|---|---|---|---|---|
| Quartile 1 (N = 937) | 431 (46.0) | - | - | - |
| Quartile 2 (N – 3327) | 1290 (38.8) | 0.74 | 0.64–0.86 | 0.03 |
| Quartile 3 (N = 3284) | 1266 (38.6) | 0.74 | 0.64–0.85 | 0.02 |
| Quartile 4 (N = 1579) | 615 (39.0) | 0.75 | 0.64–0.88 | 0.13 |
| Quartile | Adjusted In-hospital mortality – no. (%)*† | Adjusted Odds Ratio for In-Hospital Mortality† | 95% CI | P-value |
|---|---|---|---|---|
| Quartile 1 (N = 937) | 295 (31.5) | - | - | - |
| Quartile 2 (N – 3327) | 928 (27.9) | 0.99 | 0.82–1.19 | 0.87 |
| Quartile 3 (N = 3284) | 912 (27.8) | 1.05 | 0.86–1.28 | 0.63 |
| Quartile 4 (N = 1579) | 442 (28.0) | 0.95 | 0.77–1.16 | 0.58 |
Chi-squared p-value for unadjusted comparison across quartiles < 0.001.
Adjusted for age, sex, all patient and hospital variables, receipt of coronary angiography, PCI, CABG, RHC, and device subtype. Omnibus p-value for effect of quartile on in-hospital mortality = 0.78. N = the number of hospitalizations for CS witd MI.
Sensitivity Analysis
Among hospitals excluded from the primary analysis due to performing <10 PCIs per year, no MCS devices were used and 155 (21.1%) of hospitalizations for MI with CS resulted in transfer to an acute care facility. Hospitals excluded from the primary analysis had a low rate of procedures (coronary angiography: 63 [8.6%]; PCI: 20 [2.7%]; CABG: <10; RHC: <10). The overall in-hospital hospital mortality for hospitalizations for CS and MI was 43.6% (N = 320) among hospitals performing <10 PCIs per year.
When limiting the population to hospitals with both ≥10 PCIs per year and ≥10 cases of MI with shock per year, median MCS utilization rates remained 36.4% (IQR 25.0%−46.7%) with significant unexplained variation (adjusted median odds ratio of receiving MCS at two randomly selected hospitals: 1.57; 95% CI 1.41–1.72) (Appendix eFigure 1). Both unadjusted and adjusted in-hospital mortality rates were not significantly different between quartiles of MCS use (adjusted p = 0.78) (Appendix eTables 6–7). Restricting the analysis to variables likely to be present on only admission (i.e. excluding heart failure, chronic pulmonary disease, coagulopathy, paralysis, other neurologic disorder, and fluid and electrolyte disorders), results were again unchanged, with neither quartile of MCS ratio (p = 0.85) nor MCS ratio (p = 0.83) was associated with in-hospital mortality. Additionally, excluding transfers in to an acute care hospital did not change the relationship of MCS use and mortality (quartile of MCS ratio: p = 0.14; MCS ratio: p = 0.52). When these analyses were repeated in the NRD (85% sample), the findings were consistent: neither quartile of MCS use (p = 0.33) or MCS ratio (p = 0.23) were associated with adjusted in-hospital mortality in separate models. A total of 38.7% of hospitals admitting patients with CS with MI in the NRD did not use MCS, with median (IQR) use of 30.0% (17.6–62.5%) (Appendix eTable 8 and eFigure 2).
DISCUSSION
While recent advances in short-term non-durable MCS devices have made cardiopulmonary support more widely available to patients with myocardial infarction complicated by CS, utilization of MCS nationally has lagged behind rates of CS (4). Our study demonstrates wide variation in MCS use CS and MI between hospitals, unexplained by patient factors. IABP use represented the vast majority of MCS devices. Overall MCS usage rates were under 50% in the majority of hospitals admitting shock patients. Although crude in-hospital mortality was lowest in high MCS utilizing hospitals, this was no longer evident after adjustment for patient and hospital factors.
There is a paucity of data on hospital variation in MCS use for patients presenting with MI and shock. Prior studies have confirmed significant hospital variation in IABP use for patients undergoing high-risk PCI and CABG (12–13) as well as ECMO use in the setting of shock (14–16), but information on hospital variation among all subtypes of MCS use is limited, as is literature on the extent to which the observed variation is related to patient and hospital characteristics. In a prior study of only individuals receiving PCI in the National Cardiovascular Data Registry (NCDR), Sandhu et al. reported that non-IABP MCS utilization was less than 5% for over half of NCDR participating hospitals and greater than 20% in less than one tenth of all hospitals. As the NCDR is limited to participating centers and those with cardiac catheterization laboratories, it is limited in its assessment of overall national trends in CS care (17). Our study found that forty-one percent of hospitals nationally and 26.1% of PCI-capable hospitals who admitted patients with CS did not implant MCS devices. While these hospitals may admit less severe forms of CS, not requiring MCS, the high observed in-hospital mortality rate for CS and MI among these hospitals argues otherwise. These findings may also indicate the lack of capacity to provide such care, either due to lack of provider training or appropriate facilities, or uncertainty about the benefits of MCS in this setting.
At PCI-capable hospitals, one-third of hospitalizations for MI with shock received MCS, with significant variation between hospitals and regions. Despite large observed variation in MCS use, after adjustment for patient and hospital characteristics, only hospital ownership was significantly different between high and low MCS utilizing hospitals, with government hospitals being less likely to use MCS, possibly owing to differences in insurance or practice characteristics. The continued predominance of IABP use despite multiple trials not demonstrating a mortality benefit (18–20) may reflect continued uncertainty about the data or a desire to help high-risk patients with whatever means possible.
While IABP represented 85.1% of MCS utilization, there was growth in ECMO and ND-MCS use, though currently rates remain <9% even in the highest volume MCS hospitals. This low uptake may be due to concerns about high device cost, slow technology adoption, or skepticism about the efficacy of these devices for treatment of shock. As the volume of non-IABP MCS grows, it is reasonable to evaluate variation in use and outcomes separately from other forms of MCS. While IABP use was widespread prior to the publication of trials demonstrating lack of mortality benefit to IABP therapy in shock (18–20), its relative use has declined (4). Whether or not the displacement of IABP by newer technology will result in improved outcomes is unclear this time. In the current study, in-hospital mortality was not different between quartiles of MCS use. Given the recently expanded indications for MCS use in non-MI shock and high-risk PCI (21), our study highlights the need for further investigation into the efficacy of MCS for CS and for clear evidence-based guidelines for initiation of MCS therapy in MI to improve outcomes and reduce inappropriate hospital variation. Given limited data supporting the use of IABP in MI and CS (18–20), the observed wide variation in IABP utilization suggests the need for evidence-based guidelines on MCS initiation. While the European Society of Cardiology 2014 guidelines for revascularization list routine IABP use as a Class III indication (22), the absence of such a recommendation in US guidelines could contribute to the observed variation in IABP use.
There are several limitations of the current study worth note. First, as the study was retrospective and limited in the number of variables ascertained, causality cannot be inferred, and less variability may exist in MCS use after incorporation of unmeasured variables. Second, the timing of MCS relative to other diagnoses noted on discharge or the development of MI and shock cannot be discerned, given limited granularity of the database, though a sensitivity analysis restricting the model to only variables likely to be present on admission did not identify any changes to the results. Third, inaccuracies in coding may reduce precision in the interpretation of results. Similarly, inaccuracy of coding for procedures or sampling variability may affect national estimates of cardiac procedural usage. Fourth, as the hospitals sampled in the NIS differ annually, the observed findings may not fully reflect national trends in other years. Fifth, as only inpatient data are available in the NIS, only in-hospital outcomes could be evaluated. Sixth, there was insufficient hospital level variation in subtypes of MCS to evaluate in-hospital outcomes according to device subtype. Seventh, as cost data in the NIS is extrapolated from hospital charges, no cost data are presented in the current manuscript.
CONCLUSIONS
There is significant variation between hospitals in the use of MCS for treatment of MI complicated by cardiogenic shock, with IABP use still most common. In-hospital mortality is not different between quartiles of MCS use after adjustment for patient and hospital factors. More outcomes data and detailed evidence-based guidelines on the optimal clinical conditions for MCS use are needed.
Supplementary Material
WHAT IS KNOWN.
Despite increasing use of mechanical circulatory support (MCS) in hospitalizations involving cardiogenic shock (CS), rates of CS have increased out of proportion to use of MCS nationally.
Reasons for this discrepancy are unclear, but may in part be related to questions about device efficacy, differences in physician experience, and institutional availability.
The inter-hospital variation in use of MCS and its impact on CS outcomes is unknown.
WHAT THIS STUDY ADDS.
Wide variation in use of MCS exists across hospitals, unexplained by patient or hospital characteristics.
Despite wide differences in utilization, outcomes for MI hospitalizations complicated by CS were similar at high and low MCS utilizing hospitals. Intra-aortic balloon pumps represented the dominant form of MCS used during the study period.
ACKNOWLEDGEMENTS:
SOURCES OF FUNDING: Dr. Yeh reports investigator-initiated grant funding for the current submission from Abiomed. Dr. Strom is funded by a grant from the American Heart Association (18CDA34110267), outside the submitted work. Dr. Yeh is funded by a grant from the National, Heart, Lung, and Blood Institute (1R01HL136708–01), outside the submitted work. Dr. Yeh reports additional grant support from Abbott Vascular, Abiomed, Astra Zeneca and Boston Scientific and, and consulting fees from Abbott Vascular, Asahi Intecc, Boston Scientific, Medtronic, and Teleflex, outside the submitted work.
DISCLOSURES: Dr. Yeh has received funding for investigator initiated research studies from Abiomed, for the current submission (Significant). All authors have nothing to disclose.
REFERENCES
- 1.Adams KF Jr., Fonarow GC, Emerman CL, LeJemtel TH, Costanzo MR, Abraham WT, Berkowitz RL, Galvao M, Horton DP; ADHERE Scientific Advisory Committee and Investigators. Characteristics and outcomes of patients hospitalized for heart failure in the United States: Rationale, design, and preliminary observations from the first 100,000 cases in the Acute Decompensated Heart Failure National Registry (ADHERE). American Heart Journal 2005;149:209–216. [DOI] [PubMed] [Google Scholar]
- 2.Babaev A, Frederick PD, Pasta DJ, Every N, Sichrovsky T, Hochman JS; NRMI Investigators. Trends in Management and Outcomes of Patients With Acute Myocardial Infarction Complicated by Cardiogenic Shock. JAMA 2005;294:448. [DOI] [PubMed] [Google Scholar]
- 3.Stretch R, Sauer CM, Yuh DD, Bonde P. National Trends in the Utilization of Short-Term Mechanical Circulatory Support. Journal of the American College of Cardiology 2014;64:1407–1415. [DOI] [PubMed] [Google Scholar]
- 4.Strom JB, Zhao Y, Shen C, Chung M, Pinto DS, Popma JJ, Yeh RW. National Trends, Predictors of Use, and In-Hospital Outcomes in the Mechanical Circulatory Support for Cardiogenic Shock. EuroIntervention. 2018;13:e2152–e2159. [DOI] [PubMed] [Google Scholar]
- 5.Bush M, Sturmer T, Stearns SC, Simpson RJ Jr, Brookhart MA, Rosamond W, Kucharska-Newton AM. Position matters: Validation of medicare hospital claims for myocardial infarction against medical record review in the atherosclerosis risk in communities study. Pharmacoepidemiol Drug Saf. 2018;27:1085–1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Joynt KE, Blumenthal DM, Orav EJ, Resnic FS, Jha AK. Association of public reporting for percutaneous coronary intervention with utilization and outcomes among Medicare beneficiaries with acute myocardial infarction. JAMA. 2012;308:1460–1468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.HCUP Databases. Healthcare Cost and Utilization Project (HCUP) Available at: www.hcup-us.ahrq.gov/databases.jsp. Accessed September 14, 2017. [Google Scholar]
- 8.Moore BJ, White S, Washington R, Coenen N, Elixhauser A. Identifying Increased Risk of Readmission and In-hospital Mortality Using Hospital Administrative Data. Medical Care 2017;55:698–705. [DOI] [PubMed] [Google Scholar]
- 9.Austin SR, Wong Y- N, Uzzo RG, Beck JR, Egleston BL. Why Summary Comorbidity Measures Such As the Charlson Comorbidity Index and Elixhauser Score Work. Medical Care 2015;53:e65–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Baldwin L- M, Klabunde CN, Green P, Barlow W, Wright G. In Search of the Perfect Comorbidity Measure for Use With Administrative Claims Data. Medical Care 2006;44:745–753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ghali WA, Hall RE, Rosen AK, Ash AS, Moskowitz MA. Searching for an improved clinical comorbidity index for use with ICD-9-CM administrative data. J Clin Epidemiol 1996;49:273–278. [DOI] [PubMed] [Google Scholar]
- 12.Curtis JP, Rathore SS, Wang Y, Chen J, Nallamothu BK, Krumholz HM. Use and effectiveness of intra-aortic balloon pumps among patients undergoing high risk percutaneous coronary intervention: insights from the National Cardiovascular Data Registry. Circ Cardiovasc Quality Outcomes 2012;5:21–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ghali WA, Ash AS, Hall RE, Moskowitz MA. Variation in hospital rates of intraaortic balloon pump use in coronary artery bypass operations. Ann Thorac Surg. 1999;67:441–445. [DOI] [PubMed] [Google Scholar]
- 14.McCarthy FH, McDermott KM, Sragan D, Hoedt A, Kini V, Atluri P, Gaffey A, Szeto WY, Acker MA, Desai ND. Unconventional Volume-Outcome Associations in Adult Extracorporeal Membrane Oxygenation in the United States. Ann Thorac Surg. 2016;102:489–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sauer CM, Yuh DD, Bonde P. Extracorporeal membrane oxygenation use has increased by 433% in adults in the United States from 2006 to 2011. ASAIO J. 2015;61:31–6. [DOI] [PubMed] [Google Scholar]
- 16.Barbaro RP, Odetola FO, Kidwell KM, Paden ML, Bartlett RH, Davis MM, Annich GM. Association of hospital-level volume of extracorporeal membrane oxygenation. Analysis of the extracorporeal life support organization registry. Am J Respir Crit Care Med. 2015;191:894–901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sandhu A, McCoy LA, Negi SI, Hameed I, Atri P, Al’Aref SJ, Curtis J, McNulty E, Anderson HV, Shroff A, Menegus M, Swaminathan RV, Gurm H, Messenger J, Wang T, Bradley SM. Use of Mechanical Circulatory Support in Patients Undergoing Percutaneous Coronary Intervention. Circulation 2015;132:1243–1251. [DOI] [PubMed] [Google Scholar]
- 18.Thiele H, Zeymer U, Neumann FJ, Ferenc M, Olbrich HG, Hausleiter J, Richardt G, Hennersdorf M, Empen K, Fuernau G, Desch S, Eitel I, Hambrecht R, Fuhrmann J, Bohm M, Ebelt H, Schneider S, Schuler G, Werdan K. Intraaortic balloon support for myocardial infarction with cardiogenic shock. N Engl J Med. 2012;367:1287–96. [DOI] [PubMed] [Google Scholar]
- 19.Prondzinsky R, Lemm H, Swyter M, Wegener N, Unverzagt S, Carter JM, Russ M, Schlitt A, Buerke U, Christoph A, Schmidt H, Winkler M, Thiery J, Werdan K, Buerke M. Intra-aortic balloon counterpulsation in patients with acute myocardial infarction complicated by cardiogenic shock: the prospective, randomized IABP SHOCK Trial for attenuation of multiorgan dysfunction syndrome. Crit Care Med. 2010;38:152–60. [DOI] [PubMed] [Google Scholar]
- 20.Sjauw KD, Engstrom AE, Vis MM, van der Schaaf RJ, Baan J Jr., Koch KT, de Winter RJ, Piek JJ, Tijssen JG, Henriques JP. A systematic review and meta-analysis of intra-aortic balloon pump therapy in ST-elevation myocardial infarction: should we change the guidelines? Eur Heart J. 2009;30:459–68. [DOI] [PubMed] [Google Scholar]
- 21.Ibrahim NG. Recently Approved Devices: Impella Ventricular Support Systems - P140003/S018. Food and Drug Administration; 2018. February 7 Available from: https://www.accessdata.fda.gov/cdrh_docs/pdf14/P140003S018A.pdf. [Google Scholar]
- 22.Windecker S, Kolh P, Alfonso F, Collet JP, Cremer J, Falk V, Filippatos G, Hamm C, Head SJ, Juni P, Kappetein AP, Kastrati A, Knuuti J, Landmesser U, Laufer G, Neumann FJ, Richter DJ, Schauerte P, Sousa Uva M, Stefanini GG, Taggart DP, Torraca L, Valgimigli M, Wijns W, Witskowski A. 2014 ESC/EACTS Guidelines on myocardial revascularization: The Task Force on Myocardial Revascularization of the European Society of Cardiology (ESC) and the European Association for Cardio-Thoracic Surgery (EACTS)Developed with the special contribution of the European Association of Percutaneous Cardiovascular Interventions (EAPCI). Eur Heart J. 2014;35:2541–619. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.