Abstract
Recent hospital-based cohort studies found the CHA2DS2-VASC score to be associated with ischemic stroke in individuals without atrial fibrillation (AF). Our aim was to determine the distribution of embolic and thrombotic strokes and association with the CHA2DS2-VASC score, among community-dwelling individuals without AF. We included participants from the Atherosclerosis Risk in Communities (ARIC) Study who attended visit 4 (1996-98) and had no prior AF, stroke, or anticoagulant use (n=10,671). During follow-up through 2008, incident AF cases (n=760) and participants who started warfarin were censored. Incident AF was ascertained from study ECGs and hospital discharge diagnosis codes, and stroke was physician-adjudicated. After 10 years of follow-up, 280 ischemic strokes were identified, of which 146 were thrombotic and 57 embolic. The hazard ratios (95% confidence intervals [CI]) for thrombotic stroke were 1 (reference), 1.71 (1.13-2.59), 2.92 (1.91-4.45), 3.22 (1.70-6.11), and 1.25 (0.17-9.09), with CHA2DS2-VASc scores of 0-1, 2, 3, 4, and ≥5, respectively. The hazard ratios (95% CI) for embolic stroke were 1 (ref), 4.91 (2.10-11.5), 7.07 (2.93-17.0), 14.8 (5.50-39.6), and 15.2 (3.16-73.3), with CHA2DS2-VASc scores of 0-1, 2, 3, 4, and ≥5, respectively. A receiver-operating characteristic model had a C statistic of 0.65 for ischemic stroke, 0.61 for thrombotic stroke, and 0.71 for embolic stroke. In conclusion, in community-dwelling individuals without AF, the CHA2DS2-VASc score can assess ischemic stroke risk and has good discriminatory capacity for embolic stroke.
Keywords: Ischemic stroke, CHA2DS2-VASc score, Epidemiology
Introduction
The CHA2DS2-VASc score (congestive heart failure, hypertension, age ≥75 years [doubled], diabetes, stroke/transient ischemic attack/thromboembolism [doubled], vascular disease, age 65-74 years, female sex is widely used to stratify the risk of ischemic stroke in nonvalvular atrial fibrillation (AF).1 Recent evidence suggests that the CHA2DS2-VASc score may also predict ischemic stroke in individuals without AF.2–6 These studies, however, were comprised of highly-selected individuals, often lacked adjudication of stroke, and did not account for the competing risk of death. Furthermore, the proportion of thrombotic versus embolic strokes were not reported, which could clarify the role for anticoagulation in those without AF. We therefore aimed to evaluate the association of the CHA2DS2-VASc score with incident ischemic stroke—paying particular attention to the association with thrombotic and embolic subtypes—among community-dwelling individuals without AF from the Atherosclerosis Risk in Communities (ARIC) study.
Methods
The ARIC study is a prospective community-based cohort study that was designed to investigate the causes of atherosclerosis and its clinical outcomes as well as variation in cardiovascular risk factors, medical care, and disease by race and sex.7 From 1987 to 1989 (ARIC study baseline), 15,792 adults (55% women, 45-64 years of age) from 4 US communities (Washington County, MD; suburbs of Minneapolis, MN; Jackson, MS; and Forsyth County, NC) were enrolled and underwent a home interview and clinic visit. After the visit 1 examination, there were 4 additional exams: visit 2 (1990–1992), visit 3 (1993–1995), visit 4 (1996–1998) and visit 5 (2011–2013). Refer to Supplemental Material for further details.
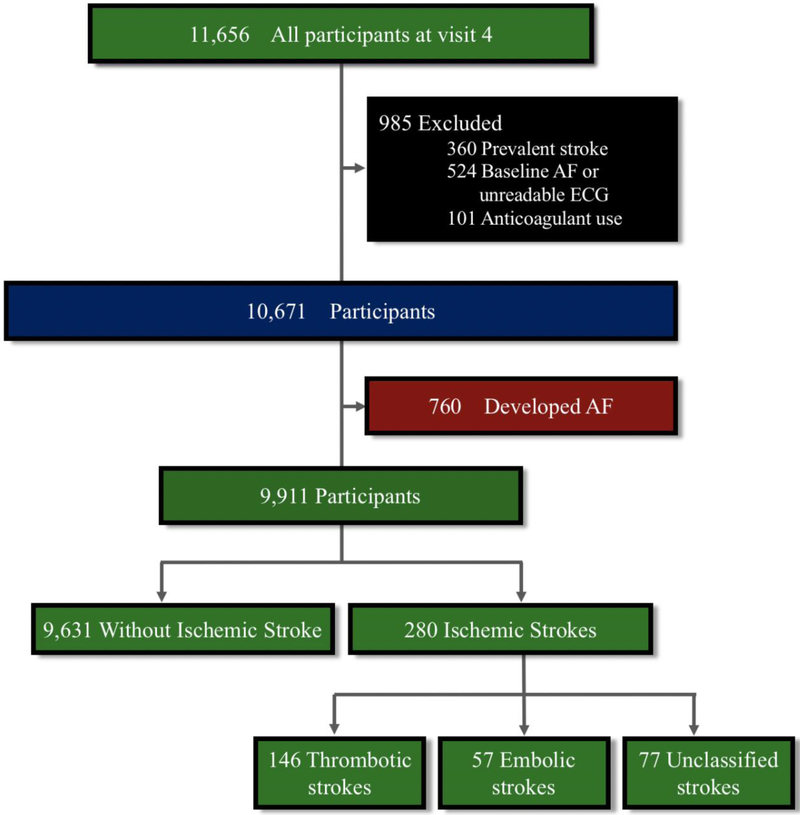
We included all ARIC participants who attended visit 4 (n = 11,656) (1996-1998). Visit 4 was used as the baseline because patients were now older and had more cardiovascular risk factors, yielding a wider spread of CHA2DS2-VASc scores which facilitated our analyses. Of the visit 4 attendees, we excluded those with prevalent AF or unreadable ECG (n= 524), prevalent stroke (n = 360), and prior anticoagulant use (n=101). After these exclusions, there were 10,671 participants for the main analysis. Participants were censored if they developed AF or began anticoagulant therapy during the follow-up period, regardless of the indication. Figure 1 illustrates the flow of study participants.
Figure 1.
Participant selection flowchart.
AF cases were identified from review of hospital discharge diagnoses.8,9 AF during follow-up was defined as International Classification of Disease 9th revision, Clinical Modification [ICD-9-CM] 427.31 or 427.32 diagnosis codes. Hospital diagnosis codes for AF ascertainment have been shown to have good positive predictive value of 98.6%.8,10
Stroke at visit 1 was defined as a self-reported history of physician-diagnosed stroke, while stroke after visit 1 was adjudicated.11 To identify subsequent stroke, cohort participants were followed over time through annual telephone interviews, field center examinations, surveillance of the ARIC community hospitals for all cohort members’ hospitalizations, and the review of death certificates, physician questionnaires, coroner/medical examiner reports, and informant interviews. The Supplemental Material explains how hospitalizations for possible validation of stroke were identified. Once identified, pertinent medical records from hospitalizations were copied and sent to a single nurse abstractor at a central ARIC office who abstracted each record for pertinent information (see Supplemental Material). Next, using criteria adopted from the National Survey of Stroke,12 strokes were classified by computer algorithm and categorized into 1 of 4 main types: SAH, intracerebral hemorrhage, thrombotic brain infarction, or embolic brain infarction (with carotid and non-carotid subtypes) (see Supplemental Material for computerized algorithm). In addition to a computer-determined diagnosis, cases were independently reviewed by a physician, who was provided with all pertinent details from the medical records. Disagreement between the computer and physician classification were adjudicated by a second physician-reviewer. Aside from a classification algorithm (see Supplemental Material), physicians also used their own discretion when the clinical picture deviated from the algorithm. Only “definite” but not “probable” ischemic strokes were further classified into thrombotic or embolic subtypes.
For this analysis, covariates included all CHA2DS2-VASc score components. Covariates were taken from visit 4. Definitions of the covariates are detailed in the Supplemental Material.
In our analysis, the crude 10-year incidence of stroke was calculated for each predictor and CHA2DS2-VASc score category. Hazard ratios to assess the association between each variable and stroke were calculated using univariate Cox proportional hazards models. To account for competing risk of death, 1-, 5-, and 10-year absolute risks for ischemic stroke stratified by CHA2DS2-VASC categories in participants using the Aalen-Johansen estimator (see Supplemental Material for more information).
Results
Of the 10,671 participants (mean age 62.7 years; 57% women; 23% non-whites) included in this 10-year analysis, 280 (2.6%) developed ischemic stroke. The mean follow-up was 9.3±1.9 years. Baseline clinical characteristics of the study are shown in Table 1, stratified by ischemic stroke. Participants who developed ischemic stroke were older and more likely to be non-white, non-female, and on aspirin compared to those who did not develop ischemic stroke. Additionally, participants who developed ischemic stroke had a higher prevalence of each variable of the CHA2DS2-VASc score (except female status).
Table 1.
Baseline Characteristics of Participants without Atrial Fibrillation† in the Atherosclerosis Risk in communities (ARIC) Study at Visit 4 (1996-98), stratified by future ischemic stroke
| No AF (n= 10,671) |
Ischemic stroke (within 10 years) (n=280) |
No stroke (within 10 years) (n=l0,391) |
|
|---|---|---|---|
| Mean age (SD), years | 62.7(5.6) | 65.1 (5.6) | 62.6(5.6) |
| Non-white race | 2435 (23%) | 99 (35%) | 2336 (22%) |
| Female | 6039 (57%) | 134 (48%) | 5905 (57%) |
| Hypertension | 4936 (46%) | 187 (67%) | 4749 (46%) |
| Hyperlipidemia | 5897 (55%) | 158(56%) | 57339 (55%) |
| Diabetes mellitus | 1699(16%) | 89 (32%) | 1610(15%) |
| Heart failure | 470 (4%) | 24(9%) | 446 (4%) |
| Peripheral arterial disease / MI | 861 (8%) | 49(18%) | 812(8%) |
| Coronary heart disease | 788 (7%) | 26(16%) | 742 (7%) |
| CHA2DS2VASc score | 1.7(1.1) | 2.3 (1.1) | 1.7(1.1) |
| Aspirin use | 5968 (56%) | 184 (66%) | 5784 (56%) |
| Statin use | 1168(11%) | 35 (13%) | 1133(11%) |
AF is for atrial fibrillation; MI, myocardial infarction
Also excluding anticoagulant use at baseline (n=101)
Medication history was obtained by self-report of medication intake during the prior 2 weeks before Visit 4 and by reviewing medications brought by the participants to the Visit
Table 2 shows the crude 10-year incidence and hazards ratios for ischemic stroke, and thrombotic and embolic subtypes, for each CHA2DS2-VASc score category and individual component. With increasing CHA2DS2-VASc score, the 10-year incidence of ischemic stroke increased from 1.3 per 1000 person-years (score 0-1) to 8.9 per 1000 person-years (score ≥5). Similarly, the incidence for both thrombotic (except for score ≥ 5) and embolic subtypes increased with increasing CHA2DS2-VASc score. With increasing CHA2DS2-VASc score, the hazard ratios for ischemic stroke, thrombotic subtype (except for score ≥ 5), and embolic subtype also increased, although in a more exponential manner for embolic subtype compared to thrombotic subtype (more linearly). Univariate predictors for ischemic stroke included age 65 to 74, male sex, heart failure, hypertension, diabetes, vascular disease, and aspirin use; univariate predictors of thrombotic stroke included age 65 to 74, male sex, hypertension, diabetes, and vascular disease. Univariate predictors for embolic stroke included age 65 to 74, heart failure, hypertension, and diabetes.
Table 2.
Univariate analysis and incidence rates per 1,000 person-years for ischemic stroke, thrombotic stroke and embolic stroke in participants without atrial fibrillation,† Atherosclerosis Risk in Communities Study, (1996-2005)
| Ischemic stroke | Thrombotic stroke‡ | Embolic stroke‡ | ||||
|---|---|---|---|---|---|---|
| Events within 10 yrs | 280 | 146 | 57 | |||
| Incidence rates | 2.8 | 1.5 | 0.58 | |||
| Individual predictors | Incidence | HR (95% CI) | Incidence | HR (95% CI) | Incidence | HR (95% CI) |
| Age ≥75§ | -- | -- | -- | -- | -- | -- |
| Age 65-74 vs. <65 | 4.3 | 2.22(1.75-2.81) | 2.1 | 1.89(1.37-2.62) | 1.0 | 3.13 (1.82-5.39) |
| Male vs. female sex | 3.5 | 1.48(1.17-1.87) | 1.8 | 1.52(1.10-2.11) | 0.55 | 0.92(0.54-1.56) |
| Heart failure | 6.2 | 2.29(1.51-3.48) | 1.8 | 1.24(0.58-2.65) | 2.3 | 4.59 (2.25-9.36) |
| Hypertension | 4.2 | 2.42(1.89-3.11) | 1.9 | 1.78(1.28-2.48) | 0.91 | 3.09(1.73-5.51) |
| Hyperlipidemia | 2.9 | 1.04(0.82-1.32) | 1.6 | 1.15 (0.83-1.60) | 0.44 | 0.61 (0.37-1.03) |
| Diabetes mellitus | 6.0 | 2.64 (2.05-3.40) | 3.2 | 2.71 (1.91-3.83) | 1.2 | 2.62(1.50-4.59) |
| Vascular disease | 6.7 | 2.68 (1.97-3.65) | 2.7 | 2.02(1.26-3.24) | 1.1 | 2.07 (0.98-4.37) |
| Aspirin | 3.3 | 1.54(1.20-1.97) | 1.6 | 1.29(0.93-1.80) | 0.63 | 1.28 (0.75-2.18) |
| Statin use | 3.3 | 1.18(0.83-1.69) | 1.7 | 1.17(0.71-1.91) | 0.37 | 0.61 (0.22-1.69) |
| CHA2DS2-VASc | ||||||
| 0-1 reference | 1.3 | 1 (ref) | 0.91 | 1 (ref) | 0.15 | 1 (ref) |
| 2 | 3.3 | 2.54 (1.85-3.49) | 1.6 | 1.71 (1.13-2.59) | 0.72 | 4.91 (2.10-11.5) |
| 3 | 5.0 | 3.80 (2.73-5.28) | 2.6 | 2.92(1.91-4.45) | 1.0 | 7.07 (2.93-17.0) |
| 4 | 6.8 | 5.16 (3.30-8.06) | 2.9 | 3.22(1.70-6.11) | 2.2 | 14.8(5.50-39.6) |
| ≥5 | 8.9 | 6.84 (3.27-14.3) | 1.1 | 1.25 (0.17-9.09) | 2.2 | 15.2(3.16-73.3) |
| Abbreviations: HR, hazard ratios | ||||||
Excluding and censoring anticoagulant use at baseline and follow up
Incidence rates for thrombotic and embolic stroke includes “definite” stroke only; ischemic stroke incidence includes “definite” as well as “probable” stroke.
Insufficient data (only 1 patient was aged ≥75 at baseline)
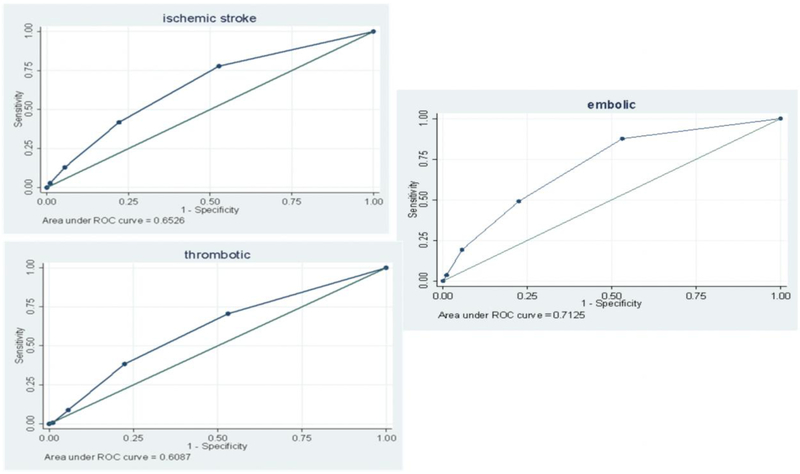
Figure 2 shows that the CHA2DS2-VASc score provided moderate discriminatory performance for ischemic and thrombotic stroke, but good discriminatory performance for embolic stroke. The C-statistic (95% CI) was 0.65 (0.62-0.68) for ischemic stroke, 0.61 (0.56-0.65) for thrombotic stroke, and 0.71 (0.65-0.77) for embolic stroke.
Figure 2.
Receiver operating characteristic curves for the CHA2DS2-VASc scores for prediction of ischemic stroke, thrombotic stroke, and embolic stroke in participants without atrial fibrillation.
Table 3 combines the 1- and 5-year crude incidences of ischemic stroke with the 10-year results. Similar to the 10-year follow up, there was an increasing risk in 1- and 5-year ischemic stroke with increasing CHA2DS2-VASc scores. Thrombotic and embolic subtypes were not calculated at 1 or 5 years due to relatively low event rates.
Table 3.
Incidence rates per 1,000 person-years for ischemic stroke in participants without AF based on CHA2DS2-VASc risk score at 1 year, 5 years, and 10 years of follow-up,† Atherosclerosis Risk in Communities Study
| CHA2DS2-VASc score | |||||
|---|---|---|---|---|---|
| 0-1 | 2 | 3 | 4 | ≥5 | |
| No AF, Number of participants | 4976 | 3282 | 1800 | 498 | 115 |
| 1 Year of follow-up | |||||
| # of Ischemic stroke events | 4 | 13 | 5 | 6 | 4 |
| Person-years | 4959 | 3266 | 1790 | 489 | 114 |
| Incidence rate (95% CI) | 0.81 (0.27-1.92) | 3.98 (2.23-6.61) | 2.79 (1.06-6.12) | 12.3 (5.10-25.3) | 35.1 (11.7-83.4) |
| 5 Years of follow-up | |||||
| # of Ischemic stroke events | 29 | 57 | 38 | 15 | 6 |
| Person-years | 24407 | 15874 | 8660 | 2299 | 523 |
| Incidence rate (95% Cl) | 1.19 (0.81-1.68) | 3.59 (2.75-4.62) | 4.39 (3.15-5.96) | 6.52 (3.81-10.5) | 11.5 (4.8-23.6) |
| 10 Years of follow-up | |||||
| # of Ischemic stroke events | 62 | 101 | 81 | 28 | 8 |
| Person-years | 47370 | 30351 | 16310 | 4151 | 897 |
| Incidence rate (95% CI)‡ | 1.31 (1.01-1.67) | 3.33 (2.73-4.03) | 4.97 (3.97-6.14) | 6.75 (4.58-9.61) | 8.9(4.20-16.8) |
| Abbreviations: AF, atrial fibrillation; CI, confidence interval. | |||||
Excluding and censoring anticoagulant use at baseline and follow up
The data in this row was also reported in Table 2
The p-value for trend of the CHA2DS2-VASc score for ischemic stroke was <0.0001 for the 1, 5, and 10 year follow-up years
Sensitivity analyses were performed to assess the impact of aspirin and anticoagulant use on these results. Not excluding participants on anticoagulation at baseline or during follow up had a miniscule effect on the results (Supplemental Table I) as was also the case after adjusting the main analysis for aspirin (Supplemental Table II).
Taking into account the competing risk of death, the 1-year absolute risk of ischemic stroke was 0.1%, 0.4%, 0.3%, and 1.2%, and 3.5% for CHA2DS2-VASc scores of 0-1, 2, 3, 4 and 5, respectively. Absolute stroke risks at 5 years were 0.6%, 1.8%, 2.1%, 3%, and 5.2% for CHA2DS2-VASc scores 0-1, 2, 3, 4, and ≥5, respectively. Absolute stroke risks at 10 years were 1.2%, 3.1%, 4.6%, 5.8%, and 7%, for CHA2DS2-VASc scores 0-1, 2, 3, 4, and ≥5, respectively (Figure 3).
Figure 3.
Absolute ischemic stroke risks by CHA2DS2-VASc risk score during 1-, 5-, and 10- years of follow-up, accounting for competing risk of death.
Discussion
In this large cohort of middle-aged, community-dwelling individuals without AF, higher CHA2DS2-VASc scores were associated with higher risk of ischemic stroke, including both thrombotic and embolic subtypes. The embolic stroke risk, while low in participants with a CHA2DS2-VASc scores of 0-1, increased exponentially in the setting of cumulative risk factors, carrying a 5-fold greater risk at a score of 2 and a 15-fold greater risk at a score of 4. The risk of thrombotic stroke also increased with higher CHA2DS2-VASc score but more linearly than in embolic stroke. This suggests that, when combined, the risk factors of the CHA2DS2-VASc score have a potentiating effect that markedly increases embolic stroke risk. In addition, the CHA2DS2-VASc score demonstrated good discriminatory performance in the prediction of embolic stroke, and modest performances for ischemic and thrombotic stroke. The findings of this study suggest that the CHA2DS2-VASc score may be useful as a prediction tool for ischemic stroke, including embolic stroke, in people without AF.
For patients with AF, current guidelines recommend anticoagulation for a CHA2DS2-VASc of 2 (and higher), which confers a 2.2% per year stroke risk, although anticoagulation can be considered for a score of 1, which confers a 1.3% per year stroke risk.13 In our present study of non-AF individuals, after accounting for the competing risk of death, the 1-year absolute risks of ischemic stroke were 1.2% and 3.5% for CHA2DS2-VASc scores of 4 and ≥5, respectively. Further, we have demonstrated that embolic stroke risk—in which anticoagulation would be particularly useful—increases exponentially in non-AF participants with increasing CHA2DS2-VASc scores. Collectively, these findings suggest that antithrombotic therapy may be useful at a CHA2DS2-VASc score of ≥ 5 in non-AF patients, although further studies evaluating their effect would be needed.
The mechanisms by which CHA2DS2-VASc risk factors contribute to the pathogenesis of thromboembolism in AF are not well understood. Conventional wisdom holds that in the absence of the dysrhythmia of AF, these vascular risk factors per se do not increase the risk of thromboembolism; AF is often presumed to be a necessary instigating factor for thromboembolism. In our study, however, the association between increasing CHA2DS2-VASc score and increasing risk of embolic stroke was observed in the absence of AF. This observation suggests that these risk factors, in and of themselves, may be intrinsically pro-thrombotic. This is supported by studies showing that maintenance of sinus rhythm versus a rate-control strategy does not reduce the risk of stroke.14 Also, studies assessing subclinical AF in patients with implanted cardiac devices (e.g. pacemakers and defibrillators) have shown an increased risk of stroke with higher AF burden, but have failed to show a temporal relationship between the timing of AF events and stroke.15,16 Studies have also shown paroxysmal supraventricular tachycardias to be associated with stroke, although the mechanism and causality of this relationship remains unclear.17 Finally, ECG-defined left atrial abnormalities and left atrial enlargement have been shown to be associated with ischemic stroke, independent of AF.11,18,19
The principal strengths of our study include a prospective community-based investigation with meticulous physician-adjudication of ischemic stroke, including classification of thrombotic and embolic stroke. Other strengths include inclusion of community non-white participants, extensive measurement of covariates, and large number of participants. Several limitations need to be considered. First, the higher CHA2DS2-VASc categories had a small number of events, and thus were combined. Second, it is possible that non-hospitalized stroke events that were not validated in the study could influence the results, although underestimation of the rate of stroke is estimated to be small (<5%).20 Third, we recognize that the criteria for classifying embolic stroke were not as rigorous compared to those currently recommended, and the classification for cryptogenic stroke was not used in the study.21 Fourth, adjustment for aspirin did not affect our results, which was unexpected in that it should have been associated with fewer thrombotic strokes. Finally, AF was identified mostly from hospitalization discharges in ARIC and we could not include asymptomatic AF or AF managed exclusively in an outpatient setting. However, prior analysis within the ARIC cohort to determine the validity of hospital discharge diagnoses for AF reported 84% sensitivity and 98% specificity in AF ascertainment,9 and that incidence rates of AF in ARIC are consistent with other population-based studies.22–24
In conclusion, the CHA2DS2-VASc score may be used to assess the risk of ischemic stroke, including both thrombotic and embolic subtypes, in community-dwelling people without AF. Our findings will need to be replicated in other cohorts. If replicated, further research would be needed to determine whether anticoagulant use in certain individuals without AF but with high CHA2DS2-VASc risk scores would reduce the risk of ischemic stroke.
Supplementary Material
Acknowledgments
The authors thank the staff and participants of the ARIC study for their important contributions.
Sources of Funding
The Atherosclerosis Risk in Communities Study is carried out as a collaborative study supported by National Heart, Lung, and Blood Institute contracts (HHSN268201100005C, HHSN268201100006C, HHSN268201100007C, HHSN268201100008C, HHSN268201100009C, HHSN268201100010C, HHSN268201100011C, and HHSN268201100012C). Dr. Chen receives grant funding from the National Heart, Lung, and Blood Institute as PI of R01HL126637 and R01HL141288. This work was additionally supported by American Heart Association grant 16EIA26410001 (Alonso).
Footnotes
Disclosures
GYHL: Consultant for Bayer/Janssen, BMS/Pfizer, Medtronic, Boehringer Ingelheim, Novartis, Verseon and Daiichi-Sankyo. Speaker for Bayer, BMS/Pfizer, Medtronic, Boehringer Ingelheim, and Daiichi-Sankyo. No fees are directly received personally.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Lip GYH, Nieuwlaat R, Pisters R, Lane DA, Crijns HJGM. Refining Clinical Risk Stratification for Predicting Stroke and Thromboembolism in Atrial Fibrillation Using a Novel Risk Factor-Based Approach. Chest [Internet]. 2010. [cited 2018 January 13];137:263–272. Available from: http://www.ncbi.nlm.nih.gov/pubmed/19762550 [DOI] [PubMed] [Google Scholar]
- 2.Peguero JG, Issa O, Podesta C, Elmahdy HM, Santana O, Lamas GA. Usefulness of the CHA2DS2VASc Score to Predict Postoperative Stroke in Patients Having Cardiac Surgery Independent of Atrial Fibrillation. Am J Cardiol [Internet]. 2015. [cited 2018 January 13];115:758–762. Available from: http://www.ncbi.nlm.nih.gov/pubmed/25616533 [DOI] [PubMed] [Google Scholar]
- 3.Lip GYH, Lin H-J, Chien K-L, Hsu H-C, Su T-C, Chen M-F, Lee Y-T. Comparative assessment of published atrial fibrillation stroke risk stratification schemes for predicting stroke, in a non-atrial fibrillation population: The Chin-Shan Community Cohort Study. Int J Cardiol [Internet]. 2013. [cited 2018 January 13];168:414–419. Available from: http://www.ncbi.nlm.nih.gov/pubmed/23073283 [DOI] [PubMed] [Google Scholar]
- 4.Melgaard L, Gorst-Rasmussen A, Lane DA, Rasmussen LH, Larsen TB, Lip GYH. Assessment of the CHA 2 DS 2 -VASc Score in Predicting Ischemic Stroke, Thromboembolism, and Death in Patients With Heart Failure With and Without Atrial Fibrillation. JAMA [Internet]. 2015. [cited 2018 January 13];314:1030 Available from: http://www.ncbi.nlm.nih.gov/pubmed/26318604 [DOI] [PubMed] [Google Scholar]
- 5.Ntaios G, Lip GYH, Makaritsis K, Papavasileiou V, Vemmou A, Koroboki E, Savvari P, Manios E, Milionis H, Vemmos K. CHADS2, CHA2DS2-VASc, and long-term stroke outcome in patients without atrial fibrillation. Neurology [Internet]. 2013. [cited 2018 January 13];80:1009–1017. Available from: http://www.ncbi.nlm.nih.gov/pubmed/23408865 [DOI] [PubMed] [Google Scholar]
- 6.Mitchell LB, Southern DA, Galbraith D, Ghali WA, Knudtson M, Wilton SB, APPROACH investigators. Prediction of stroke or TIA in patients without atrial fibrillation using CHADS 2 and CHA 2 DS 2 -VASc scores. Heart [Internet]. 2014. [cited 2018 January 13];100:1524–1530. Available from: http://www.ncbi.nlm.nih.gov/pubmed/24860007 [DOI] [PubMed] [Google Scholar]
- 7.The Atherosclerosis Risk in Communities (ARIC) Study: design and objectives. The ARIC investigators. Am J Epidemiol [Internet]. 1989. [cited 2018 January 13];129:687–702. Available from: http://www.ncbi.nlm.nih.gov/pubmed/2646917 [PubMed] [Google Scholar]
- 8.Alonso A, Agarwal SK, Soliman EZ, Ambrose M, Chamberlain AM, Prineas RJ, Folsom AR. Incidence of atrial fibrillation in whites and African-Americans: The Atherosclerosis Risk in Communities (ARIC) study. Am Heart J [Internet]. 2009. [cited 2018 January 13];158:111–117. Available from: http://www.ncbi.nlm.nih.gov/pubmed/19540400 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Soliman EZ, Prineas RJ, Case LD, Russell G, Rosamond W, Rea T, Sotoodehnia N, Post WS, Siscovick D, Psaty BM, Burke GL. Electrocardiographic and clinical predictors separating atherosclerotic sudden cardiac death from incident coronary heart disease. Heart [Internet]. 2011. [cited 2018 January 13];97:1597–1601. Available from: http://www.ncbi.nlm.nih.gov/pubmed/21775508 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bengtson LGS, Kucharska-Newton A, Wruck LM, Loehr LR, Folsom AR, Chen LY, Rosamond WD, Duval S, Lutsey PL, Stearns SC, Sueta C, Yeh H-C, Fox E, Alonso A. Comparable ascertainment of newly-diagnosed atrial fibrillation using active cohort follow-up versus surveillance of centers for medicare and medicaid services in the atherosclerosis risk in communities study. PLoS One [Internet]. 2014. [cited 2018 January 13];9:e94321 Available from: http://www.ncbi.nlm.nih.gov/pubmed/24727837 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Maheshwari A, Norby FL, Soliman EZ, Koene RJ, Rooney MR, O’Neal WT, Alonso A, Chen LY. Abnormal P-Wave Axis and Ischemic Stroke. Stroke [Internet]. 2017. [cited 2018 January 13];48:2060–2065. Available from: http://www.ncbi.nlm.nih.gov/pubmed/28626057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.The National Survey of Stroke. National Institute of Neurological and Communicative Disorders and Stroke. Stroke [Internet]. [cited 2018. September 23];12:I1–91. Available from: http://www.ncbi.nlm.nih.gov/pubmed/7222163 [PubMed] [Google Scholar]
- 13.January CT, Wann LS, Alpert JS, Calkins H, Cigarroa JE, Cleveland JC, Conti JB, Ellinor PT, Ezekowitz MD, Field ME, Murray KT, Sacco RL, Stevenson WG, Tchou PJ, Tracy CM, Yancy CW, ACC/AHA Task Force Members. 2014 AHA/ACC/HRS Guideline for the Management of Patients With Atrial Fibrillation: Executive Summary: A Report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and the Heart Rhythm Society. Circulation [Internet]. 2014. [cited 2018 January 13];130:2071–2104. Available from: http://www.ncbi.nlm.nih.gov/pubmed/24682348 [DOI] [PubMed] [Google Scholar]
- 14.Al-Khatib SM, Allen LaPointe NM, Chatterjee R, Crowley MJ, Dupre ME, Kong DF, Lopes RD, Povsic TJ, Raju SS, Shah B, Kosinski AS, McBroom AJ, Sanders GD. Rate- and Rhythm-Control Therapies in Patients With Atrial Fibrillation. Ann Intern Med [Internet]. 2014. [cited 2018 January 23];160:760 Available from: http://annals.org/article.aspx?doi=10.7326/M13-1467 [DOI] [PubMed] [Google Scholar]
- 15.Daoud EG, Glotzer T V, Wyse DG, Ezekowitz MD, Hilker C, Koehler J, Ziegler PD, TRENDS Investigators. Temporal relationship of atrial tachyarrhythmias, cerebrovascular events, and systemic emboli based on stored device data: a subgroup analysis of TRENDS. Hear Rhythm [Internet]. 2011. [cited 2018 February 3];8:1416–23. Available from: http://linkinghub.elsevier.com/retrieve/pii/S1547527111004966 [DOI] [PubMed] [Google Scholar]
- 16.Brambatti M, Connolly SJ, Gold MR, Morillo CA, Capucci A, Muto C, Lau CP, Van Gelder IC, Hohnloser SH, Carlson M, Fain E, Nakamya J, Mairesse GH, Halytska M, Deng WQ, Israel CW, Healey JS, ASSERT Investigators. Temporal Relationship Between Subclinical Atrial Fibrillation and Embolic Events. Circulation [Internet]. 2014. [cited 2018 February 3];129:2094–2099. Available from: http://www.ncbi.nlm.nih.gov/pubmed/24633881 [DOI] [PubMed] [Google Scholar]
- 17.Kamel H, Elkind MS V., Bhave PD, Navi BB, Okin PM, Iadecola C, Devereux RB, Fink ME. Paroxysmal Supraventricular Tachycardia and the Risk of Ischemic Stroke. Stroke [Internet]. 2013. [cited 2018 September 23];44:1550–1554. Available from: http://www.ncbi.nlm.nih.gov/pubmed/23632982 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kamel H, Soliman EZ, Heckbert SR, Kronmal RA, Longstreth WT, Nazarian S, Okin PM. P-Wave Morphology and the Risk of Incident Ischemic Stroke in the Multi-Ethnic Study of Atherosclerosis. Stroke [Internet]. 2014. [cited 2018 January 24];45:2786–2788. Available from: http://www.ncbi.nlm.nih.gov/pubmed/25052322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tiwari S, Løchen M-L, Jacobsen BK, Hopstock LA, Nyrnes A, Njølstad I, Mathiesen EB, Schirmer H. CHA 2 DS 2 -VASc score, left atrial size and atrial fibrillation as stroke risk factors in the Tromsø Study. Open Hear [Internet]. 2016. [cited 2018 February 3];3:e000439 Available from: http://www.ncbi.nlm.nih.gov/pubmed/27621829 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sacco RL, Boden-Albala B, Gan R, Chen X, Kargman DE, Shea S, Paik MC, Hauser WA. Stroke incidence among white, black, and Hispanic residents of an urban community: the Northern Manhattan Stroke Study. Am J Epidemiol [Internet]. 1998. [cited 2018 January 13];147:259–68. Available from: http://www.ncbi.nlm.nih.gov/pubmed/9482500 [DOI] [PubMed] [Google Scholar]
- 21.Kamel H, Healey JS. Cardioembolic Stroke. Circ Res [Internet]. 2017. [cited 2018 September 23];120:514–526. Available from: https://www.ahajournals.org/doi/10.1161/CIRCRESAHA.116.308407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Benjamin EJ, Levy D, Vaziri SM, D’Agostino RB, Belanger AJ, Wolf PA. Independent risk factors for atrial fibrillation in a population-based cohort. The Framingham Heart Study. JAMA [Internet]. 1994. [cited 2018 January 13];271:840–4. Available from: http://www.ncbi.nlm.nih.gov/pubmed/8114238 [PubMed] [Google Scholar]
- 23.Psaty BM, Manolio TA, Kuller LH, Kronmal RA, Cushman M, Fried LP, White R, Furberg CD, Rautaharju PM. Incidence of and risk factors for atrial fibrillation in older adults. Circulation [Internet]. 1997. [cited 2018 January 13];96:2455–61. Available from: http://www.ncbi.nlm.nih.gov/pubmed/9337224 [DOI] [PubMed] [Google Scholar]
- 24.Miyasaka Y, Barnes ME, Gersh BJ, Cha SS, Bailey KR, Abhayaratna WP, Seward JB, Tsang TSM. Secular Trends in Incidence of Atrial Fibrillation in Olmsted County, Minnesota, 1980 to 2000, and Implications on the Projections for Future Prevalence. Circulation [Internet]. 2006. [cited 2018 January 13];114:119–125. Available from: http://www.ncbi.nlm.nih.gov/pubmed/16818816 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.